Anti-Melanogenesis Activity of 6-O-Isobutyrylbritannilactone from Inula britannica on B16F10 Melanocytes and In Vivo Zebrafish Models
Abstract
1. Introduction
2. Results and Discussion
2.1. Extraction and Isolation of IBL
2.2. Cytotoxicity of IBL in B16F10 Cells
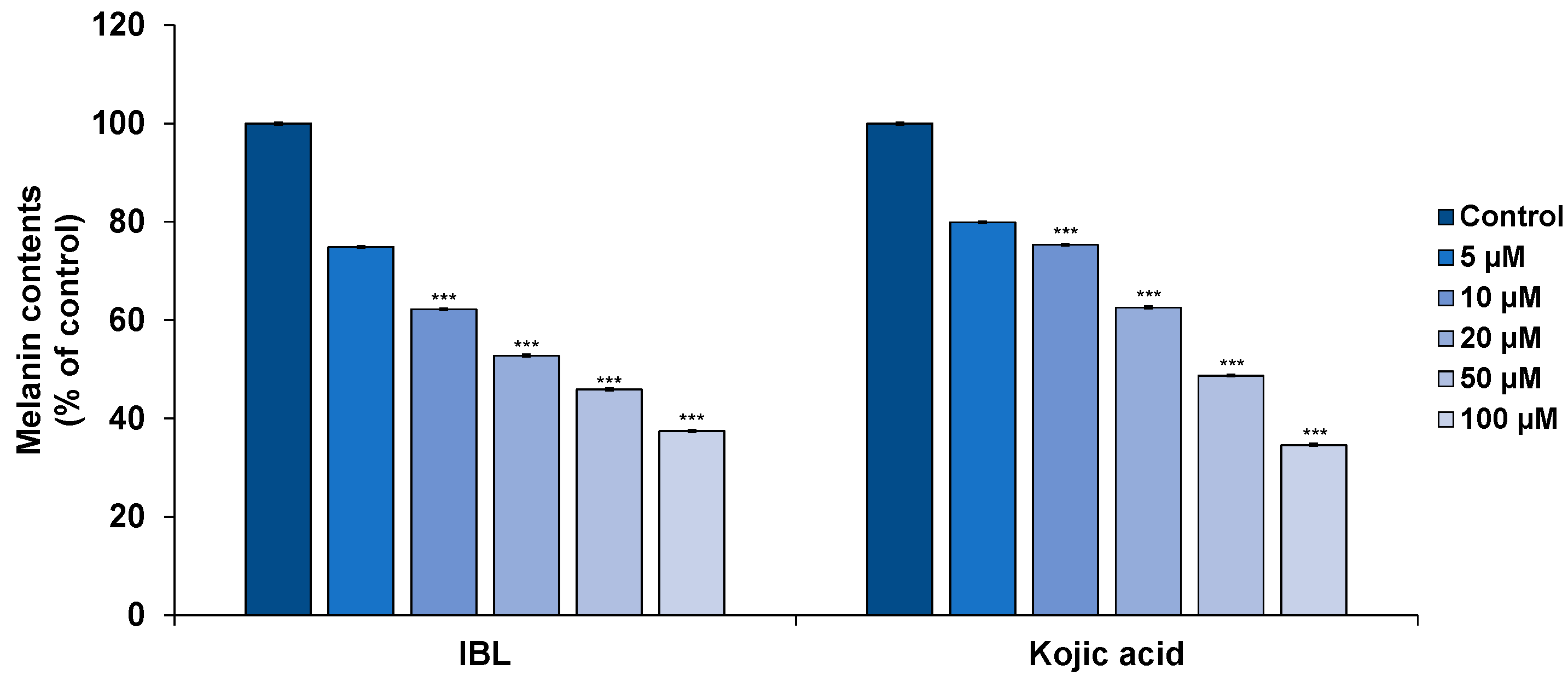
2.3. Effects of IBL on Melanin Synthesis
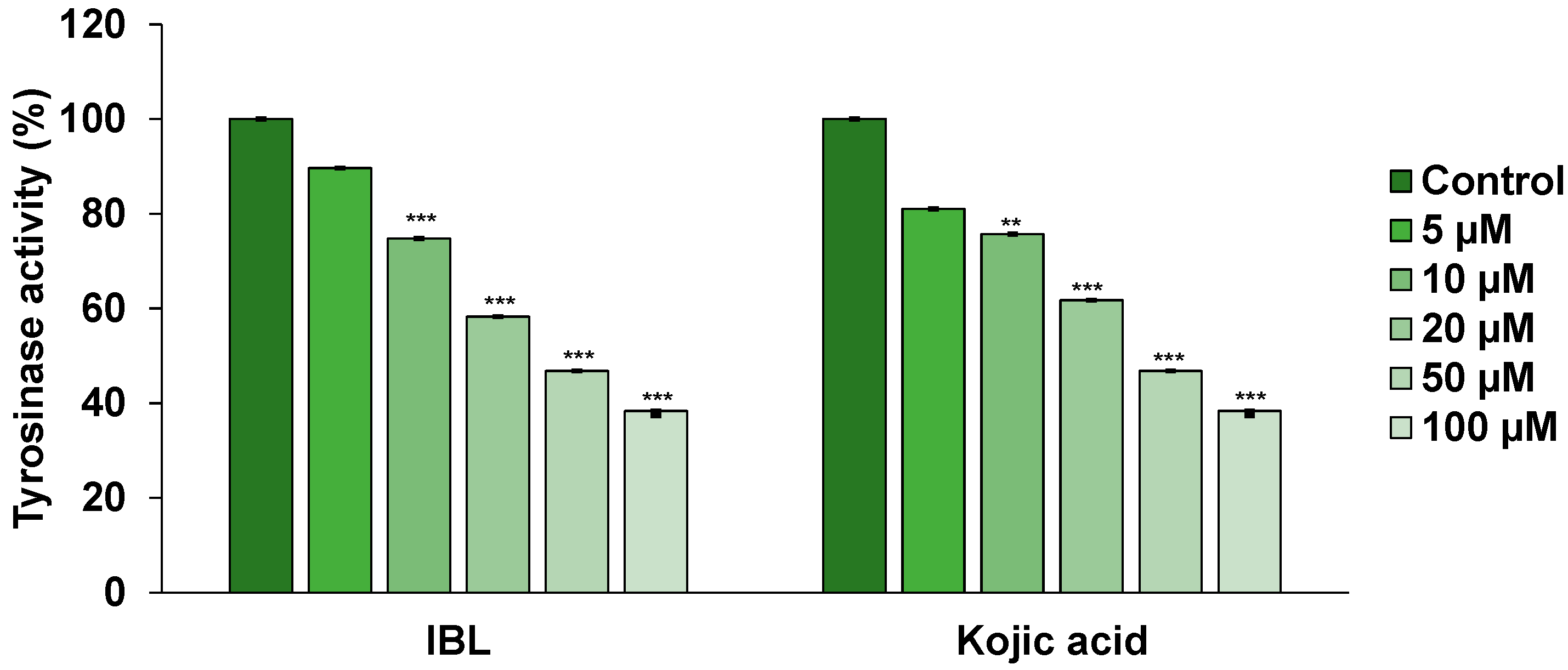
2.4. Effects of IBL on Tyrosinase Activity
2.5. Effects of IBL on Melanogenesis-Related Gene Expression
2.6. Effects of IBL on the Protein Expression of Tyrosinase, Trp1, Trp2, and MITF
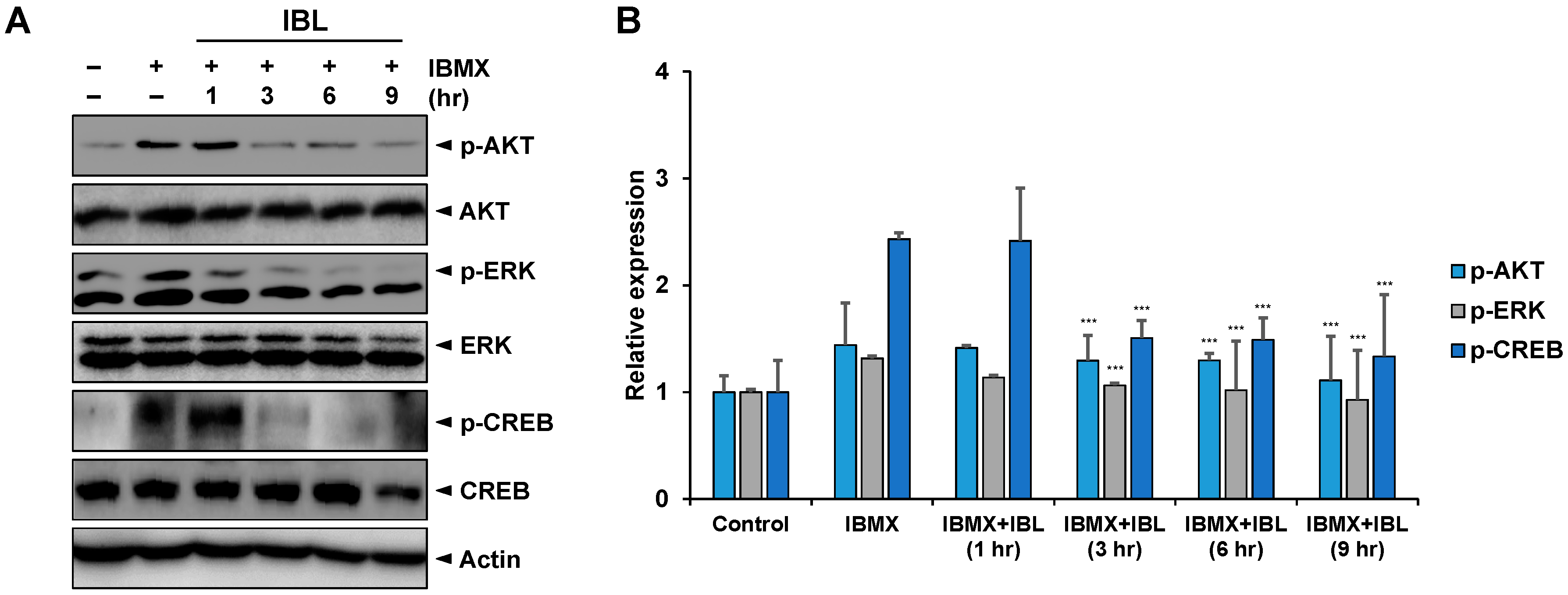
2.7. Effects of IBL on the Expression of Melanogenesis-Related Proteins
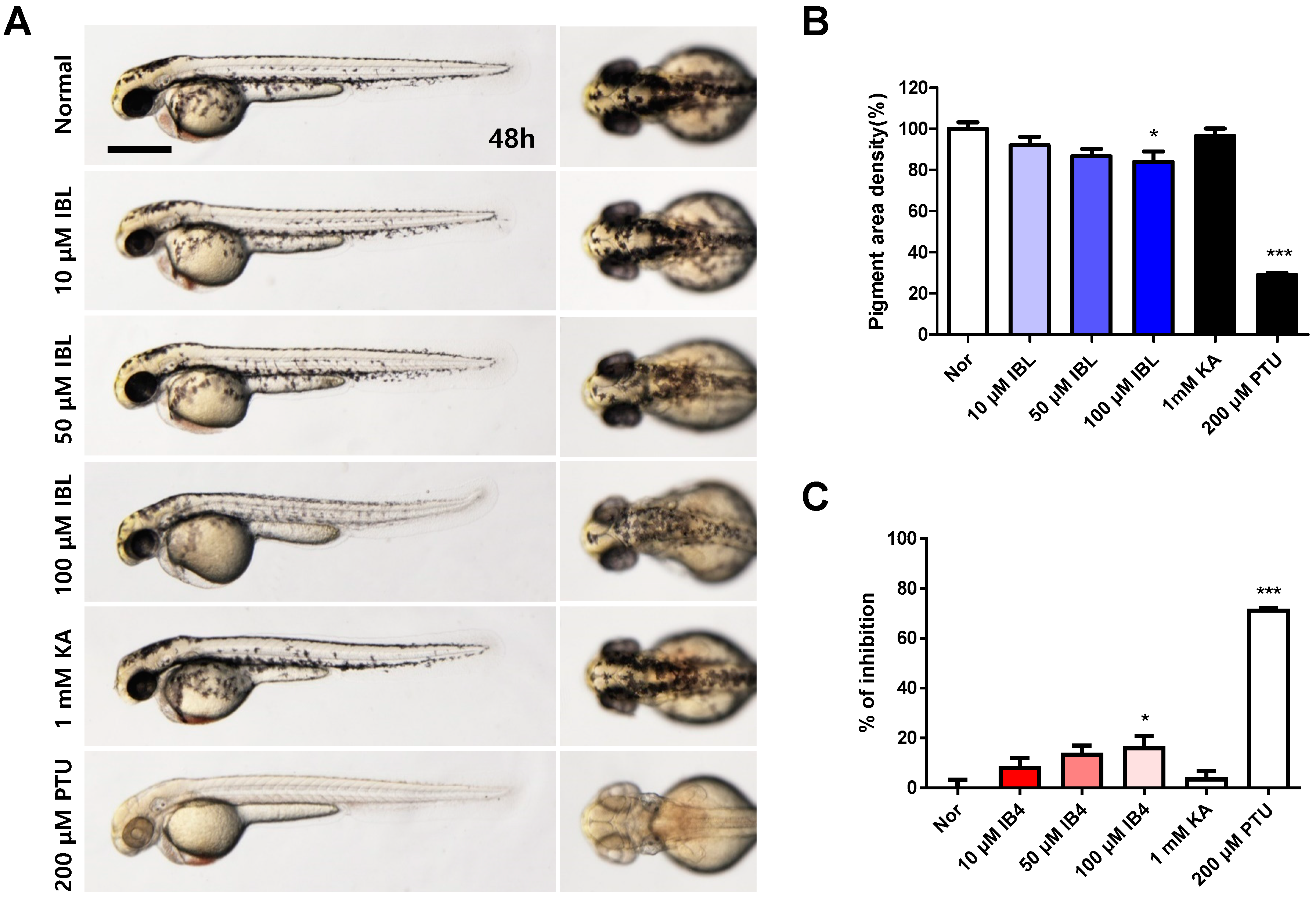
2.8. Effects of IBL on Melanin Pigmentation in Zebrafish Embryos
3. Materials and Methods
3.1. Plant Material
3.2. Extraction and Isolation
3.3. 6-O-Isobutyrylbritannilactone
3.4. Cell Cultures
3.5. Cell Viability Assay
3.6. Measurement of Melanin Content
3.7. Tyrosinase Activity
3.8. Western Blot Analysis
3.9. Reverse Transcription-Polymerase Chain Reaction
3.10. Maintenance of Zebrafish
3.11. Chemical Treatment of Zebrafish Embryos and Imaging
3.12. Quantitative Measurement of Melanocytes and Statistical Analysis
3.13. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mort, R.L.; Jackson, I.J.; Elizabeth Patton, E. The melanocyte lineage in development and disease. Development 2015, 142, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Costin, G.-E.; Hearing, V.J. Human skin pigmentation: Melanocytes modulate skin color in response to stress. FASEB J. 2007, 21, 976–994. [Google Scholar] [CrossRef]
- Park, H.Y.; Kosmadaki, M.; Yaar, M.; Gilchrest, B.A. Cellular mechanisms regulating human melanogenesis. Cell. Mol. Life Sci. 2009, 66, 1493–1506. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef] [PubMed]
- Del Bino, S.; Duval, C.; Bernerd, F. Clinical and biological characterization of skin pigmentation diversity and its consequences on UV impact. Int. J. Mol. Sci. 2018, 19, 2668. [Google Scholar] [CrossRef] [PubMed]
- Borovanskỳ, J.; Riley, P.A. Melanins and Melanosomes: Biosynthesis, Structure, Physiological and Pathological Functions; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011; ISBN 9783527636150. [Google Scholar]
- Gillbro, J.M.; Olsson, M.J. The melanogenesis and mechanisms of skin-lightening agents—Existing and new approaches. Int. J. Cosmet. Sci. 2011, 33, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Wakamatsu, K. Quantitative analysis of eumelanin and pheomelanin in humans, mice, and other animals: A comparative review. Pigment Cell Res. 2003, 16, 523–531. [Google Scholar] [CrossRef]
- Han, H.J.; Park, S.K.; Kang, J.Y.; Kim, J.M.; Yoo, S.K.; Heo, H.J. Anti-Melanogenic Effect of Ethanolic Extract of Sorghum bicolor on IBMX–Induced Melanogenesis in B16/F10 Melanoma Cells. Nutrients 2020, 12, 832. [Google Scholar] [CrossRef]
- Kim, C.S.; Noh, S.G.; Park, Y.; Kang, D.; Chun, P.; Chung, H.Y.; Jung, H.J.; Moon, H.R. A Potent Tyrosinase Inhibitor, (E)-3-(2,4-Dihydroxyphenyl)-1-(thiophen-2-yl)prop-2-en-1-one, with Anti-Melanogenesis Properties in α-MSH and IBMX-Induced B16F10 Melanoma Cells. Molecules 2018, 23, 2725. [Google Scholar] [CrossRef]
- Park, S.Y.; Jin, M.L.; Kim, Y.H.; Kim, Y.; Lee, S.J. Aromatic-turmerone inhibits α-MSH and IBMX-induced melanogenesis by inactivating CREB and MITF signaling pathways. Arch. Dermatol. Res. 2011, 303, 737–744. [Google Scholar] [CrossRef]
- D’Mello, S.A.N.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling pathways in melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.C.; Ko, J.H.; Kang, H.K.; Kim, S.; Kang, C.I.; Lee, J.N.; Park, S.M.; Hyun, C.G. Antimelanogenic effects of polygonum tinctorium flower extract from traditional Jeju fermentation via upregulation of extracellular signal-regulated kinase and protein kinase B activation. Int. J. Mol. Sci. 2018, 19, 2895. [Google Scholar] [CrossRef] [PubMed]
- Yasumoto, K.; Yokoyama, K.; Shibata, K.; Tomita, Y.; Shibahara, S. Microphthalmia-associated transcription factor as a regulator for melanocyte-specific transcription of the human tyrosinase gene. Mol. Cell. Biol. 1995, 15, 1833. [Google Scholar] [CrossRef] [PubMed]
- Yasumoto, K.I.; Yokoyama, K.; Takahashi, K.; Tomita, Y.; Shibahara, S. Functional analysis of microphthalmia-associated transcription factor in pigment cell-specific transcription of the human tyrosinase family genes. J. Biol. Chem. 1997, 272, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef]
- Niu, C.; Aisa, H.A. Upregulation of Melanogenesis and Tyrosinase Activity: Potential Agents for Vitiligo. Molecules 2017, 22, 1303. [Google Scholar] [CrossRef]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef]
- Chang, T.S. Natural melanogenesis inhibitors acting through the down-regulation of tyrosinase activity. Materials (Basel) 2012, 5, 1661–1685. [Google Scholar] [CrossRef]
- Khan, A.L.; Hussain, J.; Hamayun, M.; Gilani, S.A.; Ahmad, S.; Rehman, G.; Kim, Y.H.; Kang, S.M.; Lee, I.J. Secondary metabolites from Inula britannica L. and their biological activities. Molecules 2010, 15, 1562–1577. [Google Scholar] [CrossRef]
- Choo, S.J.; Ryoo, I.J.; Kim, K.C.; Na, M.; Jang, J.H.; Ahn, J.S.; Yoo, I.D. Hypo-pigmenting effect of sesquiterpenes from Inula britannica in B16 melanoma cells. Arch. Pharm. Res. 2014, 37, 567–574. [Google Scholar] [CrossRef]
- Jang, D.K.; Jung, S.H.; Jeong, J.H.; Yoo, H.M.; Lee, I.S.; Shin, H.S. The Antimelanogenic Effect of Inularin Isolated from Flowers of Inula britannica on B16F10 Melanoma Cells and Zebrafish Embryos. J. Microbiol. Biotechnol. 2020, 30, 749–752. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.D.; Ding, L.F.; Tu, W.C.; Yang, H.; Su, J.; Peng, L.Y.; Li, Y.; Zhao, Q.S. Bioactive sesquiterpenoids from the flowers of Inula japonica. Phytochemistry 2016, 129, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.J.; Jin, H.Z.; Zhu, J.X.; Fu, J.J.; Zeng, Q.; Cheng, X.R.; Zhu, Y.; Shan, L.; Zhang, S.D.; Pan, Y.X.; et al. New sesquiterpenes from Inula japonica Thunb. with their inhibitory activities against LPS-induced NO production in RAW264.7 macrophages. Tetrahedron 2010, 66, 9379–9388. [Google Scholar] [CrossRef]
- Serre, C.; Busuttil, V.; Botto, J.M. Intrinsic and extrinsic regulation of human skin melanogenesis and pigmentation. Int. J. Cosmet. Sci. 2018, 40, 328–347. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Tseng, T.S.; Hsiao, N.W.; Lin, Y.L.; Wen, Z.H.; Tsai, C.C.; Lee, Y.C.; Lin, H.H.; Tsai, K.C. Discovery of highly potent tyrosinase inhibitor, T1, with significant anti-melanogenesis ability by zebrafish in vivo assay and computational molecular modeling. Sci. Rep. 2015, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Liu, W.; Zhu, D.; Cao, Y.; Tang, A.; Gong, G.; Su, H. Natural skin-whitening compounds for the treatment of melanogenesis (Review). Exp. Med. 2020, 20, 173–185. [Google Scholar] [CrossRef]
- Olivares, C.; Jiménez-Cervantes, C.; Lozano, J.A.; Solano, F.; García-Borrón, J.C. The 5,6-dihydroxyindole-2-carboxylic acid (DHICA) oxidase activity of human tyrosinase. Biochem. J. 2001, 354, 131–139. [Google Scholar] [CrossRef]
- Boissy, R.E.; Sakai, C.; Zhao, H.; Kobayashi, T.; Hearing, V.J. Human tyrosinase related protein-1 (TRP-1) does not function as a DHICA oxidase activity in contrast to murine TRP-1. Exp. Derm. 1998, 7, 198–204. [Google Scholar] [CrossRef]
- Panzella, L.; Ebato, A.; Napolitano, A.; Koike, K. The late stages of melanogenesis: Exploring the chemical facets and the application opportunities. Int. J. Mol. Sci. 2018, 19, 1753. [Google Scholar] [CrossRef]
- Chen, Y.S.; Lee, S.M.; Lin, C.C.; Liu, C.Y. Hispolon decreases melanin production and induces apoptosis in melanoma cells through the downregulation of tyrosinase and microphthalmia-associated transcription factor (MITF) expressions and the activation of caspase-3, -8 and -9. Int. J. Mol. Sci. 2014, 15, 1201–1215. [Google Scholar] [CrossRef]
- Alam, M.B.; Bajpai, V.K.; Lee, J.I.; Zhao, P.; Byeon, J.H.; Ra, J.S.; Majumder, R.; Lee, J.S.; Yoon, J.I.; Rather, I.A.; et al. Inhibition of melanogenesis by jineol from Scolopendra subspinipes mutilans via MAP-Kinase mediated MITF downregulation and the proteasomal degradation of tyrosinase. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Harashima, A.; Kato, K.; Gu, L.; Motomura, Y.; Otsuka, R.; Maeda, K. Degradation of tyrosinase by melanosomal pH change and a new mechanism of whitening with propylparaben. Cosmetics 2017, 4, 43. [Google Scholar] [CrossRef]
- Ando, H.; Ichihashi, M.; Hearing, V.J. Role of the ubiquitin proteasome system in regulating skin pigmentation. Int. J. Mol. Sci. 2009, 10, 4428–4434. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lee, E.J.; Ahn, Y.; Park, S.J.; Kim, S.H.; Oh, S.H. A chemical compound from fruit extract of Juglans mandshurica inhibits melanogenesis through p-ERK-associated MITF degradation. Phytomedicine 2019, 57, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Oka, M.; Nagai, H.; Ando, H.; Fukunaga, M.; Matsumura, M.; Araki, K.; Ogawa, W.; Miki, T.; Sakaue, M.; Tsukamoto, K.; et al. Regulation of melanogenesis through phosphatidylinositol 3-kinase-Akt pathway in human G361 melanoma cells. J. Invest. Derm. 2000, 115, 699–703. [Google Scholar] [CrossRef]
- Huang, H.C.; Chang, S.J.; Wu, C.Y.; Ke, H.J.; Chang, T.M. [6]-Shogaol inhibits -MSH-induced melanogenesis through the acceleration of ERK and PI3K/Akt-mediated MITF degradation. Biomed Res. Int. 2014, 2014, 1–9. [Google Scholar]
- Yoon, H.S.; Lee, S.R.; Ko, H.C.; Choi, S.Y.; Park, J.G.; Kim, J.K.; Kim, S.J. Involvement of extracellular signal-regulated kinase in nobiletin-induced melanogenesis in murine B16/F10 melanoma cells. Biosci. Biotechnol. Biochem. 2007, 71, 1781–1784. [Google Scholar] [CrossRef]
- Yun, W.J.; Kim, E.Y.; Park, J.E.; Jo, S.Y.; Bang, S.H.; Chang, E.J.; Chang, S.E. Microtubule-associated protein light chain 3 is involved in melanogenesis via regulation of MITF expression in melanocytes. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Hwang, Y.S.; Oh, S.W.; Park, S.H.; Lee, J.; Yoo, J.A.; Kwon, K.; Park, S.J.; Kim, J.; Yu, E.; Cho, J.Y.; et al. Melanogenic effects of maclurin are mediated through the activation of cAMP/PKA/CREB and p38 MAPK/CREB signaling pathways. Oxid. Med. Cell. Longev. 2019, 2019, 9827519. [Google Scholar] [CrossRef]
- Molina, D.M.; Grewal, S.; Bardwell, L. Characterization of an ERK-binding domain in microphthalmia-associated transcription factor and differential inhibition of ERK2-mediated substrate phosphorylation. J. Biol. Chem. 2005, 280, 42051–42060. [Google Scholar] [CrossRef]
- Lee, S.E.; Park, S.H.; Oh, S.W.; Yoo, J.A.; Kwon, K.; Park, S.J.; Kim, J.; Lee, H.S.; Cho, J.Y.; Lee, J. Beauvericin inhibits melanogenesis by regulating cAMP/PKA/CREB and LXR-α/p38 MAPK–mediated pathways. Sci. Rep. 2018, 8, 14958. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.S.; Yun, H.Y.; Baek, K.J.; Kwon, N.S.; Park, K.C.; Kim, D.S. Okadaic acid suppresses melanogenesis via proteasomal degradation of tyrosinase. Biol. Pharm. Bull. 2013, 36, 1503–1508. [Google Scholar] [CrossRef]
- Taylor, W.F.; Moghadam, S.E.; Farimani, M.M.; Ebrahimi Marzieh Tabefam, S.N.; Jabbarzadeh, E. A multi-targeting natural compound with growth inhibitory and anti-angiogenic properties re-sensitizes chemotherapy resistant cancer. PLoS ONE 2019, 14, e0218125. [Google Scholar] [CrossRef]
- Sun, L.R.; Zhou, W.; Zhang, H.M.; Guo, Q.S.; Yang, W.; Li, B.J.; Sun, Z.H.; Gao, S.H.; Cui, R.J. Modulation of Multiple Signaling Pathways of the Plant-Derived Natural Products in Cancer. Front. Oncol. 2019, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, L.X.; Ouyang, L.; Cheng, Y.; Liu, B. Plant natural compounds: Targeting pathways of autophagy as anti-cancer therapeutic agents. Cell Prolif. 2012, 45, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Choi, T.Y.; Kim, J.H.; Ko, D.H.; Kim, C.H.; Hwang, J.S.; Ahn, S.; Kim, S.Y.; Kim, C.D.; Lee, J.H.; Yoon, T.J. Zebrafish as a new model for phenotype-based screening of melanogenic regulatory compounds. Pigment Cell Res. 2007, 20, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.D.; Chen, H.J.; Wang, C.S.; Lum, C.C.; Wu, S.P.; Lin, S.P.; Cheng, K.C. Extract of ganoderma formosanum mycelium as a highly potent tyrosinase inhibitor. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, D.K.; Pham, C.H.; Lee, I.S.; Jung, S.-H.; Jeong, J.H.; Shin, H.-S.; Yoo, H.M. Anti-Melanogenesis Activity of 6-O-Isobutyrylbritannilactone from Inula britannica on B16F10 Melanocytes and In Vivo Zebrafish Models. Molecules 2020, 25, 3887. https://doi.org/10.3390/molecules25173887
Jang DK, Pham CH, Lee IS, Jung S-H, Jeong JH, Shin H-S, Yoo HM. Anti-Melanogenesis Activity of 6-O-Isobutyrylbritannilactone from Inula britannica on B16F10 Melanocytes and In Vivo Zebrafish Models. Molecules. 2020; 25(17):3887. https://doi.org/10.3390/molecules25173887
Chicago/Turabian StyleJang, Dae Kil, Chau Ha Pham, Ik Soo Lee, Seung-Hyun Jung, Ji Hye Jeong, Han-Seung Shin, and Hee Min Yoo. 2020. "Anti-Melanogenesis Activity of 6-O-Isobutyrylbritannilactone from Inula britannica on B16F10 Melanocytes and In Vivo Zebrafish Models" Molecules 25, no. 17: 3887. https://doi.org/10.3390/molecules25173887
APA StyleJang, D. K., Pham, C. H., Lee, I. S., Jung, S.-H., Jeong, J. H., Shin, H.-S., & Yoo, H. M. (2020). Anti-Melanogenesis Activity of 6-O-Isobutyrylbritannilactone from Inula britannica on B16F10 Melanocytes and In Vivo Zebrafish Models. Molecules, 25(17), 3887. https://doi.org/10.3390/molecules25173887






