Improved, Odorless Access to Benzo[1,2-d;4,5-d′]- bis[1,3]dithioles and Tert-butyl Arylsulfides via C-S Cross Coupling
Abstract
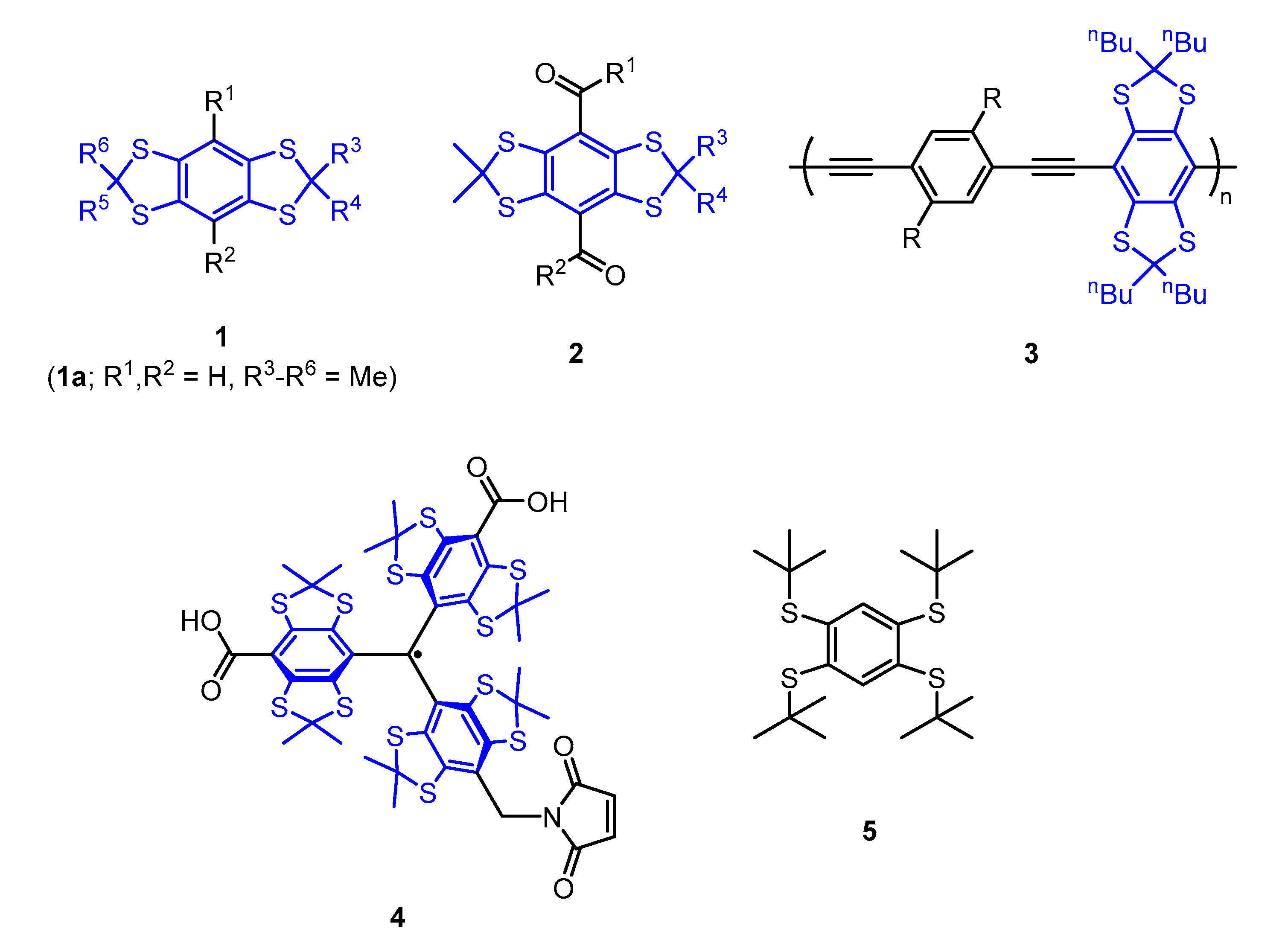
1. Introduction
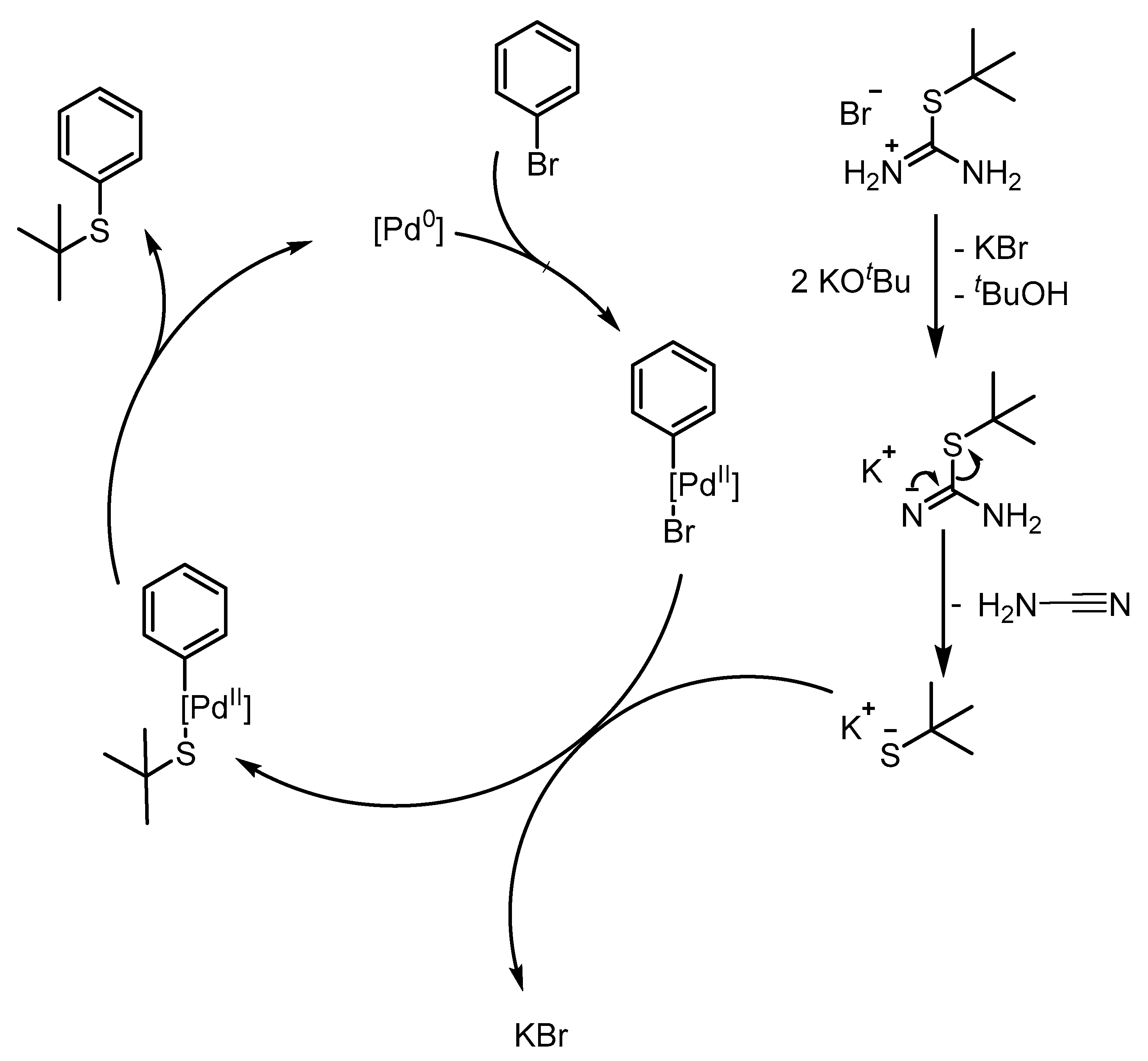
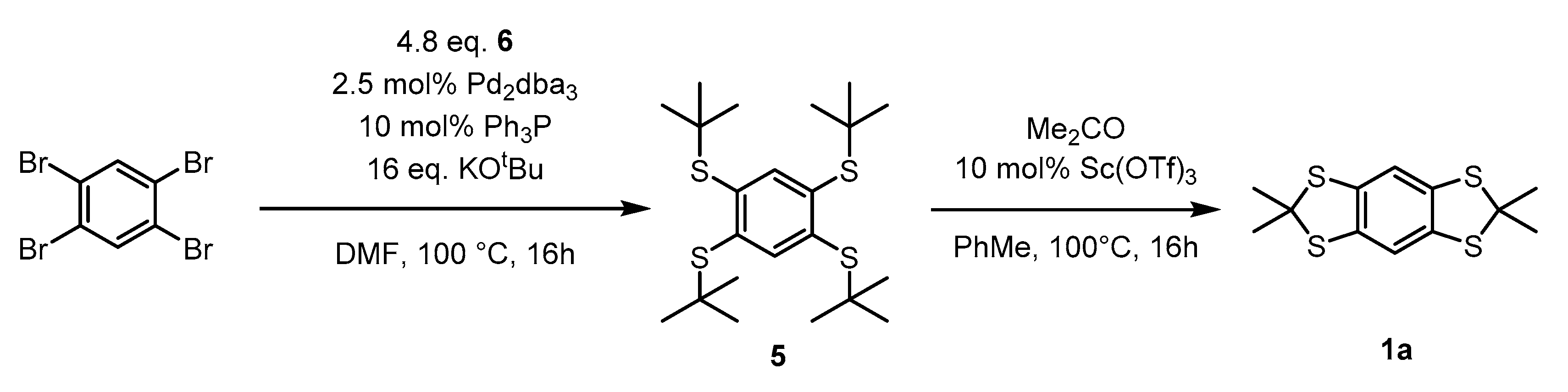
2. Results and Discussion
3. Experimental
3.1. General
3.2. Synthetic Procedures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wessig, P.; Freyse, D.; Schuster, D.; Kelling, A. Fluorescent Dyes with Large Stokes Shifts Based on Benzo[1,2-d:4,5-d′]Bis([1,3]Dithiole) (“S4-DBD Dyes”). Eur. J. Org. Chem. 2020, 2020, 1732–1744. [Google Scholar] [CrossRef]
- Hell, S.W.; Wichmann, J. Breaking the Diffraction Resolution Limit by Stimulated Emission: Stimulated-Emission-Depletion Fluorescence Microscopy. Opt. Lett. 1994, 19, 780. [Google Scholar] [CrossRef] [PubMed]
- Dane, E.L.; King, S.B.; Swager, T.M. Conjugated Polymers That Respond to Oxidation with Increased Emission. J. Am. Chem. Soc. 2010, 132, 7758–7768. [Google Scholar] [CrossRef]
- Reddy, T.J.; Iwama, T.; Halpern, H.J.; Rawal, V.H. General Synthesis of Persistent Trityl Radicals for EPR Imaging of Biological Systems. J. Org. Chem. 2002, 67, 4635–4639. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liu, Y.; Borbat, P.; Zweier, J.L.; Freed, J.H.; Hubbell, W.L. Pulsed ESR Dipolar Spectroscopy for Distance Measurements in Immobilized Spin Labeled Proteins in Liquid Solution. J. Am. Chem. Soc. 2012, 134, 9950–9952. [Google Scholar] [CrossRef] [PubMed]
- Shevelev, G.Y.; Krumkacheva, O.A.; Lomzov, A.A.; Kuzhelev, A.A.; Rogozhnikova, O.Y.; Trukhin, D.V.; Troitskaya, T.I.; Tormyshev, V.M.; Fedin, M.V.; Pyshnyi, D.V.; et al. Physiological-Temperature Distance Measurement in Nucleic Acid Using Triarylmethyl-Based Spin Labels and Pulsed Dipolar EPR Spectroscopy. J. Am. Chem. Soc. 2014, 136, 9874–9877. [Google Scholar] [CrossRef]
- Jassoy, J.J.; Heubach, C.A.; Hett, T.; Bernhard, F.; Haege, F.R.; Hagelueken, G.; Schiemann, O. Site Selective and Efficient Spin Labeling of Proteins with a Maleimide-Functionalized Trityl Radical for Pulsed Dipolar EPR Spectroscopy. Molecules 2019, 24, 2735. [Google Scholar] [CrossRef]
- Yang, Y.; Pan, B.B.; Tan, X.; Yang, F.; Liu, Y.; Su, X.C.; Goldfarb, D. In-Cell Trityl-Trityl Distance Measurements on Proteins. J. Phys. Chem. Lett. 2020, 11, 1141–1147. [Google Scholar] [CrossRef]
- Fleck, N.; Heubach, C.A.; Hett, T.; Haege, F.R.; Bawol, P.P.; Baltruschat, H.; Schiemann, O. SLIM: A Short-Linked, Highly Redox-Stable Trityl Label for High-Sensitivity In-Cell EPR Distance Measurements. Angew. Chem. Int. Ed. 2020, 59, 9767–9772. [Google Scholar] [CrossRef]
- Jassoy, J.J.; Berndhäuser, A.; Duthie, F.; Kühn, S.P.; Hagelueken, G.; Schiemann, O. Versatile Trityl Spin Labels for Nanometer Distance Measurements on Biomolecules In Vitro and within Cells. Angew. Chem. Int. Ed. 2017, 56, 177–181. [Google Scholar] [CrossRef]
- Epel, B.; Haney, C.R.; Hleihel, D.; Wardrip, C. Electron Paramagnetic Resonance Oxygen Imaging of a Rabbit Tumor Using Localized Spin Probe Delivery. Med. Phys. 2010, 37, 2553–2559. [Google Scholar] [CrossRef] [PubMed]
- Bobko, A.A.; Dhimitruka, I.; Zweier, J.L.; Khramtsov, V.V. Trityl Radicals as Persistent Dual Function PH and Oxygen Probes for in Vivo Electron Paramagnetic Resonance Spectroscopy and Imaging: Concept and Experiment. J. Am. Chem. Soc. 2007, 129, 7240–7241. [Google Scholar] [CrossRef] [PubMed]
- Bobko, A.A.; Dhimitruka, I.; Komarov, D.A.; Khramtsov, V.V. Dual-Function pH and Oxygen Phosphonated Trityl Probe. Anal. Chem. 2012, 84, 6054–6060. [Google Scholar] [CrossRef] [PubMed]
- Poncelet, M.; Driesschaert, B. A 13C-labelled Triarylmethyl Radical as an EPR Spin Probe Highly Sensitive to Molecular Tumbling. Angew. Chem. Int. Ed. 2020. accepted article. [Google Scholar] [CrossRef]
- Mathies, G.; Caporini, M.A.; Michaelis, V.K.; Liu, Y.; Hu, K.N.; Mance, D.; Zweier, J.L.; Rosay, M.; Baldus, M.; Griffin, R.G. Efficient Dynamic Nuclear Polarization at 800 MHz/527 GHz with Trityl-Nitroxide Biradicals. Angew. Chem. Int. Ed. 2015, 54, 11770–11774. [Google Scholar] [CrossRef]
- Ardenkjær-Larsen, J.H.; Fridlund, B.; Gram, A.; Hansson, G.; Hansson, L.; Lerche, M.H.; Servin, R.; Thaning, M.; Golman, K. Increase in Signal-to-Noise Ratio of >10,000 Times in Liquid-State NMR. PNAS 2003, 100, 10158–10163. [Google Scholar] [CrossRef]
- Fleck, N.; Hett, T.; Brode, J.; Meyer, A.; Richert, S.; Schiemann, O. C–C Cross-Coupling Reactions of Trityl Radicals: Spin Density Delocalization, Exchange Coupling, and a Spin Label. J. Org. Chem. 2019, 84, 3293–3303. [Google Scholar] [CrossRef]
- Nolden, O.; Fleck, N.; Lorenzo, E.R.; Wasielewski, M.R.; Schiemann, O.; Gilch, P.; Richert, S. Excitation energy transfer and exchange-mediated quartet state formation in porphyrin-trityl systems. Chem. Eur. J. 2020. accepted article. [Google Scholar] [CrossRef]
- Dirk, C.W.; Cox, S.D.; Wellman, D.E.; Wudl, F. Isolation and Purification of Benzene-1,2,4,5-Tetrathiol. J. Org. Chem. 1985, 50, 2395–2397. [Google Scholar] [CrossRef]
- Hintz, H.; Vanas, A.; Klose, D.; Jeschke, G.; Godt, A. Trityl Radicals with a Combination of the Orthogonal Functional Groups Ethyne and Carboxyl: Synthesis without a Statistical Step and EPR Characterization. J. Org. Chem. 2019, 84, 3304–3320. [Google Scholar] [CrossRef]
- Devos, M.; Patte, F.; Rouault, J.; Laffort, P.; Van Gemert, L.J. Human Olfactory Thresholds; Oxford University Press: Oxford, UK, 1990. [Google Scholar]
- Müller, D.; Adelsberger, K.; Imming, P. Organic Preparations with Molar Amounts of Volatile Malodorous Thiols. Synth. Commun. 2013, 43, 1447–1454. [Google Scholar] [CrossRef]
- Hartwig, J.F. Carbon−Heteroatom Bond-Forming Reductive Eliminations of Amines, Ethers, and Sulfides. Acc. Chem. Res. 1998, 31, 852–860. [Google Scholar] [CrossRef]
- Xu, J.; Liu, R.Y.; Yeung, C.S.; Buchwald, S.L. Monophosphine Ligands Promote Pd-Catalyzed C–S Cross-Coupling Reactions at Room Temperature with Soluble Bases. ACS Catal. 2019, 9, 6461–6466. [Google Scholar] [CrossRef]
- Migita, T.; Shimizu, T.; Asami, Y.; Shiobara, J.; Kato, Y.; Kosugi, M. The Palladium Catalyzed Nucleophilic Substitution of Aryl Halides by Thiolate Anions. Bull. Chem. Soc. Jpn. 1980, 53, 1385–1389. [Google Scholar] [CrossRef]
- Itoh, T.; Mase, T. A General Palladium-Catalyzed Coupling of Aryl Bromides/Triflates and Thiols. Org. Lett. 2004, 6, 4587–4590. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, M.A.; Shen, Q.; Hartwig, J.F. A General and Long-Lived Catalyst for the Palladium-Catalyzed Coupling of Aryl Halides with Thiols. J. Am. Chem. Soc. 2006, 128, 2180–2181. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, M.A.; Hartwig, J.F. A General, Efficient, and Functional-Group-Tolerant Catalyst System for the Palladium-Catalyzed Thioetherification of Aryl Bromides and Iodides. J. Org. Chem. 2009, 74, 1663–1672. [Google Scholar] [CrossRef]
- Murata, M.; Buchwald, S.L. A General and Efficient Method for the Palladium-Catalyzed Cross-Coupling of Thiols and Secondary Phosphines. Tetrahedron 2004, 60, 7397–7403. [Google Scholar] [CrossRef]
- Park, N.; Park, K.; Jang, M.; Lee, S. One-Pot Synthesis of Symmetrical and Unsymmetrical Aryl Sulfides by Pd-Catalyzed Couplings of Aryl Halides and Thioacetates. J. Org. Chem. 2011, 76, 4371–4378. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, W.-Y.; Chen, S.-C.; He, M.-Y.; Chen, Q. A Highly Efficient Palladium-Catalyzed One-Pot Synthesis of Unsymmetrical Aryl Alkyl Thioethers under Mild Conditions in Water. Adv. Synth. Catal. 2012, 354, 839–845. [Google Scholar] [CrossRef]
- Bowman, R.W.; Burchell, C.J.; Kilian, P.; Slawin, A.M.Z.; Wormald, P.; Woollins, J.D. Investigations on Organo–Sulfur–Nitrogen Rings and the Thiocyanogen Polymer,(SCN) x. Chem. Eur. J. 2006, 12, 6366–6381. [Google Scholar] [CrossRef] [PubMed]
- Masquelin, T.; Delgado, Y.; Baumlé, V. A facile preparation of a combinatorial library of 2, 6-disubstituted triazines. Tet. Lett. 1998, 39, 5725–5726. [Google Scholar] [CrossRef]
- Urquhart, G.; Gates, J.; Connor, R. n-Dodecyl (lauryl) mercaptane. Org. Synth. 1941, 21, 36. [Google Scholar] [CrossRef]
- Luzzio, F.A. Decomposition of S-Alkylisothiouronium Salts Under Anhydrous Conditions - Application to a Facile Preparation of Nonsymmetrical Dialkyl Sulfides. Synth. Commun. 1984, 14, 209–214. [Google Scholar] [CrossRef]
- Sprague, J.M.; Johnson, T.B. The Preparation of Alkyl Sulfonyl Chlorides from Isothioureas. J. Am. Chem. Soc. 1937, 59, 1837–1840. [Google Scholar] [CrossRef]
- Stevens, H.P. IX—Thiocarbamide Hydrochloride. J. Chem. Soc. Trans. 1902, 81, 79–81. [Google Scholar] [CrossRef]
- Feutrill, G.; Mirrington, R. Reactions with Thioethoxide Ion in Dimethylformamide. Selective Demethylation of Aryl Methyl Ethers. Aust. J. Chem. 1972, 25, 1719. [Google Scholar] [CrossRef]
- Hartwig, J.F. Organotransition Metal. Chemistry. From Bonding to Catalysis; University Science Books: Mill Valley, CA, USA, 2010. [Google Scholar]
- Labban, A.K.S.; Marcus, Y. The Solubility and Solvation of Salts in Mixed Nonaqueous Solvents. 1. Potassium Halides in Mixed Aprotic Solvents. J. Solution Chem. 1991, 20, 221–232. [Google Scholar] [CrossRef]
- Niemeyer, Z.L.; Milo, A.; Hickey, D.P.; Sigman, M.S. Parameterization of Phosphine Ligands Reveals Mechanistic Pathways and Predicts Reaction Outcomes. Nat. Chem. 2016, 8, 610–617. [Google Scholar] [CrossRef]
- Birkholz, M.N.; Freixa, Z.; Van Leeuwen, P. Bite Angle Effects of Diphosphines in C–C and C–X Bond Forming Cross Coupling Reactions. Chem. Soc. Rev. 2009, 38, 1099–1118. [Google Scholar] [CrossRef]
- Dickens, M.J.; Gilday, J.P.; Mowlem, T.J.; Wddowson, D.A. Transition metal mediated thiation of aromatic rings. Tetrahedron 1991, 47, 8621–8634. [Google Scholar] [CrossRef]
- Masato, Y.; Sakauchi, N.; Sato, A. Iminopyrdine Derivates and Use Thereof. Patent WO 2009131245, 29 October 2009. [Google Scholar]
- Xuelei, Y.; Zhulun, W.; Athena, S.; Cardozo, M.; DeGraffenreid, M.; Di, Y.; Fan, P.; He, X.; Jaen, J.C.; Labelle, M.; et al. The synthesis and SAR of novel diarylsulfone 11β-HSD1 inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 7071–7075. [Google Scholar] [CrossRef]
- Rogozhnikova, O.Y.; Vasiliev, V.G.; Troitskaya, T.I.; Trukhin, D.V.; Mikhalina, T.V.; Halpern, H.J.; Tormyshev, V.M. Generation of Trityl Radicals by Nucleophilic Quenching of Tris(2,3,5,6-Tetrathiaaryl)-methyl Cations and Practical and Convenient Large-Scale Synthesis of Persistent Tris(4-Carboxy-2,3,5,6-Tetrathiaaryl)Methyl Radical. Eur. J. Org. Chem. 2013, 2013, 3347–3355. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
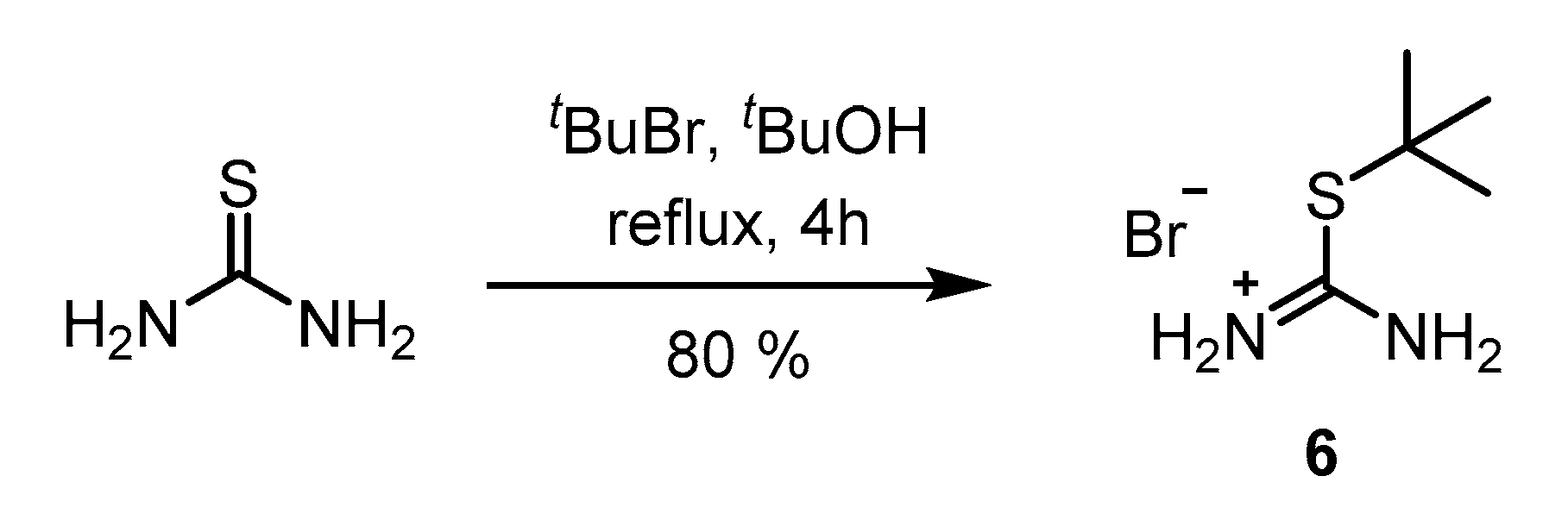
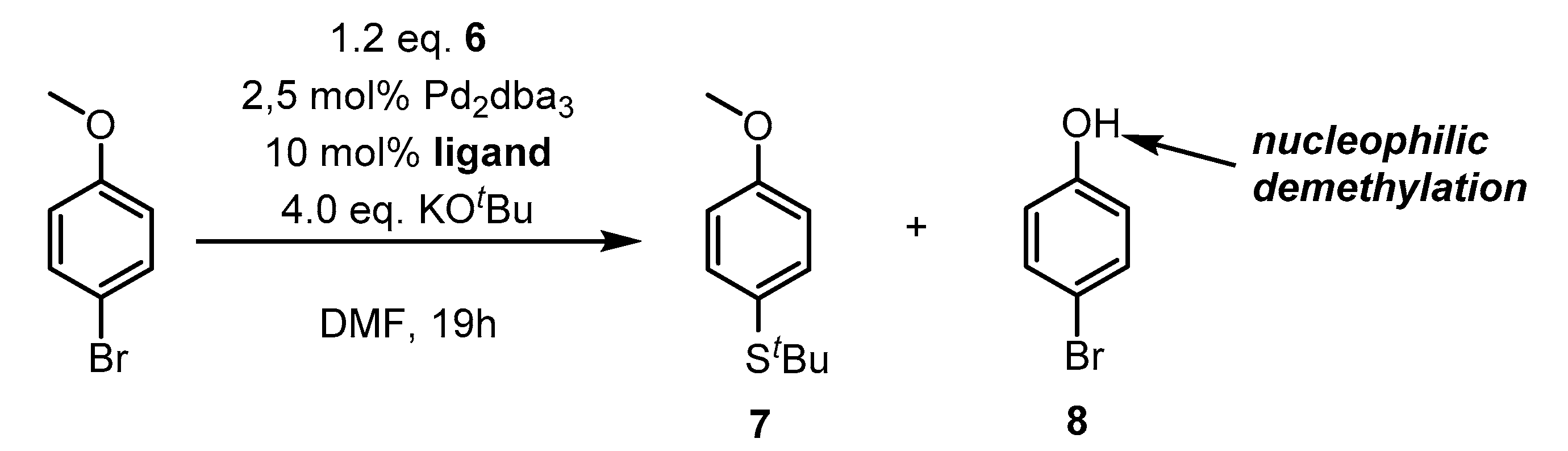
| No. | T [°C] | Ligand | Conversion * [%] | 7 * [%] | 8 * [%] | Selectivity to 7 [%] |
|---|---|---|---|---|---|---|
| 1 | 50 | Ph3P | 23 | 14 | 9 | 59 |
| 2 | 50 | XPhos | 7 | 0 | 7 | 0 |
| 3 | 50 | XantPhos | 0 | 0 | 0 | 0 |
| 4 | 80 | Ph3P | 100 | 100 | 0 | 100 |
| 5 | 80 | dppf | 69 | 34 | 35 | 49 |
| 6 | 80 | XantPhos | 38 | 2 | 36 | 4.8 |
| 7 | 80 | XPhos | 43 | 0 | 43 | 0 |
| 8 | 80 | BrettPhos | 45 | 0 | 45 | 0 |
| 9 | 80 | SPhos | 62 | 0 | 62 | 0 |
| 10 | 80 | nBu3P | 78 | 61 | 17 | 78 |
| 11 | 80 | none | 59 | 0 | 59 | 0 |
| 12 ** | 80 | none | 72 | 0 | 72 | 0 |
| 13 ** | 80 | SPhos | 65 | 0 | 65 | 0 |
| No. | Base | Solvent | Conversion * [%] | 7 * [%] | 8 * [%] | Selectivity to 7 [%] |
|---|---|---|---|---|---|---|
| 1 | KOtBu | DMF | 100 | 100 | 0 | 100 |
| 2 | KOtBu | nBuOH | 61 | 61 | 0 | 100 |
| 3 | Cs2CO3 | DMF | 19 | 19 | 0 | 100 |
| 4 | K2CO3 | DMF | 42 | 42 | 0 | 100 |
| 5 ** | K2CO3 | DMF | 0 | 0 | 0 | 0 |
| 6 | K3PO4 | DMF | 43 | 43 | 0 | 100 |
| 7 ** | K3PO4 | DMF | 12 | 12 | 0 | 100 |
| 8 | KOtBu *** | DMF | 22 | 22 | 0 | 100 |
| No. | Substrate | Catalyst | T [°C] | Yield * [%] |
|---|---|---|---|---|
| 1 * | 1,4-diiodobenzene | Ph3P/Pd2dba3 | 80 | 100 |
| 2 * | 1,4-diiodobenzene | Ph3P/Pd2dba3 | 50 | 100 |
| 3 | 4-nitrobromobenzene | Ph3P/Pd2dba3 | 25 | 100 |
| 4 | 4-nitrobromobenzene | none | 25 | 100 |
| 5 ** | 4-chloroansiole | Ph3P/Pd2dba3 | 80 | 0 |
| 6 ** | 4-chloroanisole | none | 80 | 0 |
| 7 ** | 4-fluoroanisole | Ph3P/Pd2dba3 | 80 | 0 |
| 8 ** | 4-fluoroanisole | none | 80 | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kopp, K.; Schiemann, O.; Fleck, N. Improved, Odorless Access to Benzo[1,2-d;4,5-d′]- bis[1,3]dithioles and Tert-butyl Arylsulfides via C-S Cross Coupling. Molecules 2020, 25, 3666. https://doi.org/10.3390/molecules25163666
Kopp K, Schiemann O, Fleck N. Improved, Odorless Access to Benzo[1,2-d;4,5-d′]- bis[1,3]dithioles and Tert-butyl Arylsulfides via C-S Cross Coupling. Molecules. 2020; 25(16):3666. https://doi.org/10.3390/molecules25163666
Chicago/Turabian StyleKopp, Kevin, Olav Schiemann, and Nico Fleck. 2020. "Improved, Odorless Access to Benzo[1,2-d;4,5-d′]- bis[1,3]dithioles and Tert-butyl Arylsulfides via C-S Cross Coupling" Molecules 25, no. 16: 3666. https://doi.org/10.3390/molecules25163666
APA StyleKopp, K., Schiemann, O., & Fleck, N. (2020). Improved, Odorless Access to Benzo[1,2-d;4,5-d′]- bis[1,3]dithioles and Tert-butyl Arylsulfides via C-S Cross Coupling. Molecules, 25(16), 3666. https://doi.org/10.3390/molecules25163666








