Biodegradation of Phenol by Rhodococcus sp. Strain SKC: Characterization and Kinetics Study
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Rhodococcus sp. SKC
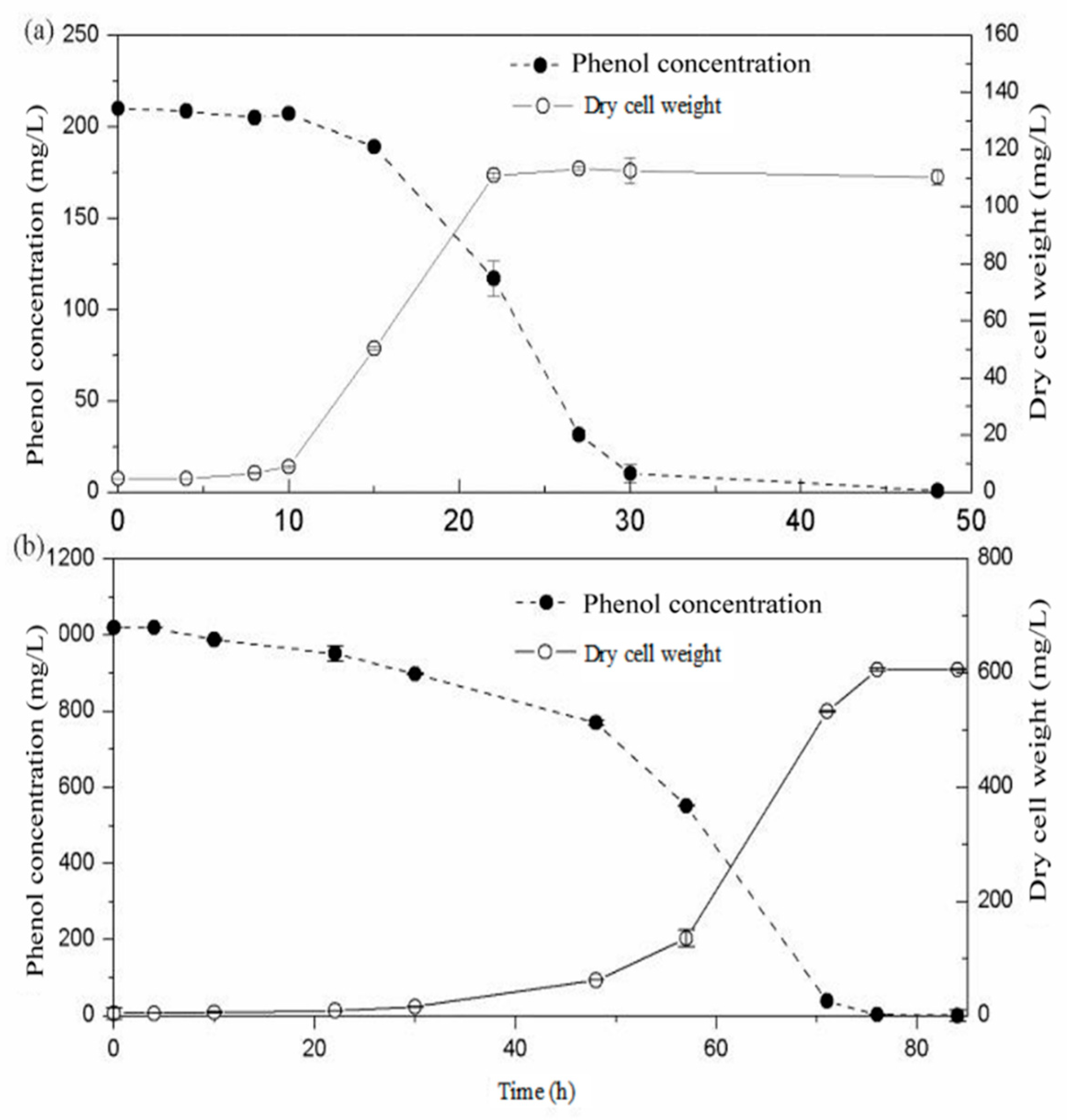
2.2. Biodegradation of Phenol as the Sole Carbon Substrate
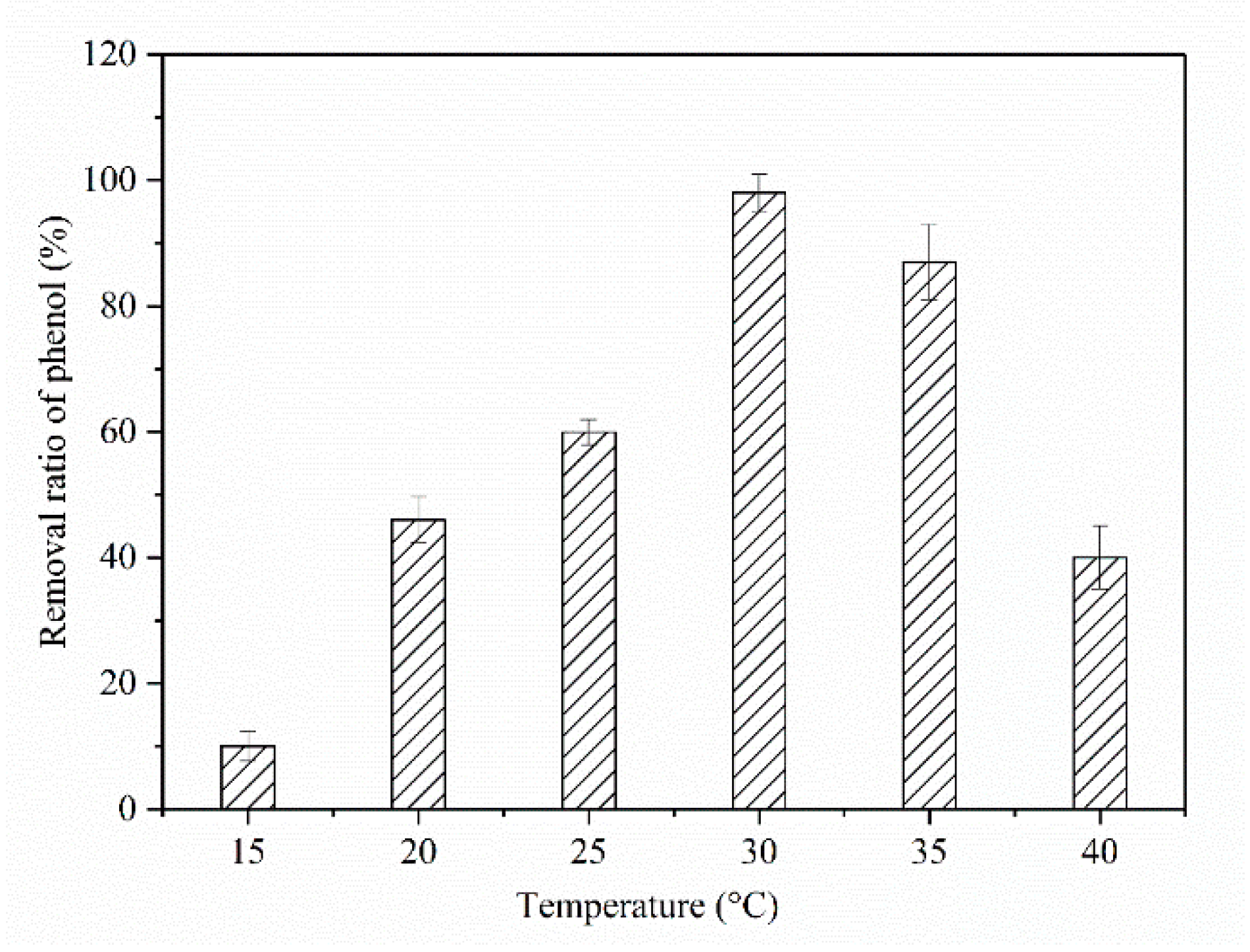
2.3. Effect of Temperature and Initial pH Value on Phenol Biodegradation
2.4. The Growth Kinetics
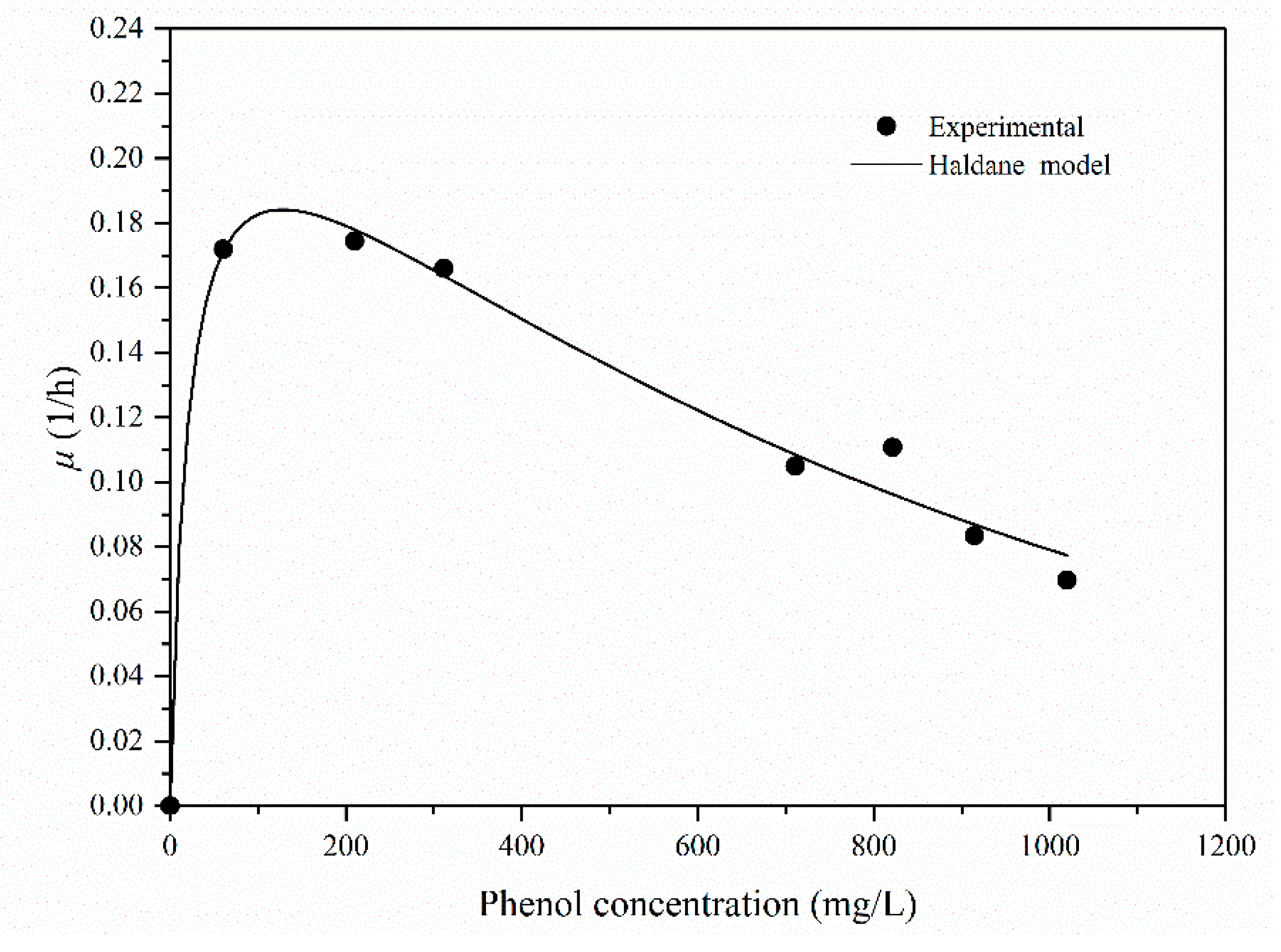
2.5. The Substrate-Inhibition Model
2.6. Yield Factor
3. Materials and Methods
3.1. Chemicals
3.2. Isolation and Identification of Strain
3.3. Experimental
3.4. Analytical Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eyal, K.; Yasmin, R.; Martin, E. Phenol biodegradation by bacterial culturesencapsulated in 3D microfiltration-membrane capsules. Environ. Technol. 2019, 1–9. [Google Scholar] [CrossRef]
- Wei, X.; Gilevska, T.; Wetzig, F.; Dorer, C. Characterization of phenol and cresol biodegradation by compound-specific stableisotope analysis. Environ. Pollut. 2016, 210, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Nawawi, N.M.; Shukor, M.Y.; Ibrahim, A.L. Characterization of Phenol-Degrading Bacteria: A Review. Selangor Sci. Technol. Rev. 2017, 1, 71–83. [Google Scholar]
- Nogina, T.; Fomina, M.; Dumanskaya, T. A new Rhodococcus aetherivorans strain isolated from lubricant-contaminated soil as a prospective phenol-biodegrading agent. Appl. Microbiol. Biotechnol. 2020, 104, 1–15. [Google Scholar]
- Anurova, M.N.; Bakhrushina, E.O.; Demina, N.B.; Panteleeva, E.S. Modern Preservatives of Microbiological Stability. Pharm. Chem. J. 2019, 53, 564–571. [Google Scholar] [CrossRef]
- El-Naas, M.; Al-Muhtaseb, S.A.; Makhlouf, S. Biodegradation of phenol by Pseudomonas putida immobilized in polyvinyl alcohol (PVA) gel. J. Hazard. Mater. 2009, 164, 720–725. [Google Scholar] [CrossRef]
- Duan, W.; Meng, F.; Cui, H. Ecotoxicity of phenol and cresols to aquatic organisms: A review. Ecotoxicol. Environ. Saf. 2018, 157, 441–456. [Google Scholar] [CrossRef]
- Li, M.; Wang, X.; Lu, C. Nitrated phenols and the phenolic precursors in the atmosphere in urban Jinan, China. Sci. Total Environ. 2020, 714, 1–9. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, H.; He, Y.T. Application and Mechanism of Sludge-Based Activated Carbon for Phenol and Cyanide Removal from Bio-Treated Effluent of Coking Wastewater. Processes 2020, 8, 82. [Google Scholar] [CrossRef]
- Mohammadreza, K.; Tania, G.; Maria, E.C. Enhanced biodegradation of phenolic wastewaters with acclimatized activated sludge A kinetic study. Chem. Eng. J. 2019, 378, 122186. [Google Scholar]
- Singh, N.; Balomajumder, C. Biodegradation of phenol and cyanide by Pseudomonas putida MTCC 1194: An experimental and modeling study. Desalin. Water Treat. 2016, 57, 28426–28435. [Google Scholar] [CrossRef]
- Suhaila, Y.N.; Hasdianty, A.; Maegala, N.M. Biotransformation using resting cells of Rhodococcus UKMP-5M for phenol degradation. Biocatal. Agric. Biotechnol. 2019, 21, 101309. [Google Scholar] [CrossRef]
- Zhou, J.; Yu, X.; Ding, C.; Wang, Z.; Zhou, Q.; Pao, H.; Cai, W. Optimization of phenol degradation by Candida tropicalis Z-04 using Plackett-Burman design and response surface methodology. J. Environ. Sci. 2011, 23, 22–30. [Google Scholar] [CrossRef]
- Arif, N.M.; Ahmad, S.A.; Syed, M.A.; Shukor, M.Y. Isolation and characterization of aphenol-degrading Rhodococcus sp. strain AQ5NOL 2 KCTC 11961BP. J. Basic Microbiol. 2013, 53, 9–19. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Y.F.; Yang, L.Y.; Ji, R.; Miao, A.J. Transformation of tetrabromobisphenol A by Rhodococcus jostii RHA1: Effects of heavy metals. Chemosphere 2018, 196, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Wang, Y.; Qian, P.Y. Optimization of phthalic acid batch biodegradation and the use of modified Richards model for modelling degradation. Int. Biodeterior. Biodegrad. 2004, 53, 57–63. [Google Scholar] [CrossRef]
- He, Z.; Niu, C.; Lu, Z. Individual or synchronous biodegradation of di-n-butyl phthalate and phenol by Rhodococcus ruber strain DP-2. J. Hazard. Mater. 2014, 273, 104–109. [Google Scholar] [CrossRef]
- Yaacob, N.S.; Mohamad, R.; Ahmad, S.A.; Abdullah, H.; Ibrahim, A.L.; Ariff, A.B. The influence of different modes of bioreactor operation on the efficiency of phenol degradation by Rhodococcus UKMP-5M. Rend. Lincei 2016, 27, 749–760. [Google Scholar] [CrossRef]
- Paisio, C.E.; Talano, M.A.; González, P.S.; Busto, V.D.; Talou, J.R.; Agostini, E. Isolation and characterization of a Rhodococcus strain with phenol-degrading ability and its potential use for tannery effluent biotreatment. Environ. Sci. Pollut. Res. 2012, 19, 3430–3439. [Google Scholar] [CrossRef]
- Peng, S.S.; Ling, N.S.; Rohana, A. Kinetics of biodegradation of phenol and p-nitrophenol by acclimated activated sludge. J. Phys. Sci. 2018, 29, 107–113. [Google Scholar] [CrossRef]
- Li, J.; Gu, J.D.; Pan, L. Transformation of dimethyl phthalate, dimethyl isophthalate and dimethyl terephthalate by Rhodococcus rubber Sa and modeling the processes using the modified Gompertz model. Int. Biodeterior. Biodegrad. 2005, 55, 223–232. [Google Scholar] [CrossRef]
- Othman, A.R.; Rahim, M.B.H.A. Modelling the Growth Inhibition Kinetics of Rhodotorula sp. strain MBH23 (KCTC 11960BP) on Acrylamide. Bioremediation Sci. Technol. Res. 2019, 7, 20–25. [Google Scholar]
- Liu, H.; Yu, Q.J.; Wan, G.; Ye, F.; Cong, Y. Biodegradation of phenol at high concentration by a novel yeast Trichosporon montevideense PHE1. Process. Biochem. 2011, 46, 1678–1681. [Google Scholar] [CrossRef]
- ELee, G.L.Y.; Ahmad, S.; Yasid, N.A. Biodegradation of phenol by cold-adapted bacteria from Antarctic soils. Polar Biol. 2018, 41, 553–562. [Google Scholar]
- Sun, Q.; Bai, Y.; Zhao, C.; Xiao, Y.; Wen, D.; Tang, X. Aerobic biodegradation characteristics and metabolic products of quinoline by a Pseudomonas strain. Bioresour. Technol. 2009, 100, 5030–5036. [Google Scholar] [CrossRef] [PubMed]
- Acikgoz, E.; Birgul, O. Phenol biodegradation by halophilic archaea. Int. Biodeterior. Biodegrad. 2016, 107, 140–146. [Google Scholar] [CrossRef]
- Zhai, Z.; Wang, H.; Yan, S.; Yao, J. Biodegradation of phenol at high concentration by a novel bacterium: Gulosibacter sp. YZ4. J. Chem. Technol. Biotechnol. 2012, 87, 105–111. [Google Scholar] [CrossRef]
- Shahryari, S.; Zahiri, H.S.; Haghbeen, K. High phenol degradation capacity of a newly characterized Acinetobacter sp. SA01: Bacterial cell viability and membrane impairment in respect to the phenol toxicity. Ecotoxicol. Environ. Saf. 2018, 164, 455–466. [Google Scholar] [CrossRef]
- Su, X.; Li, S.; Cai, J. Aerobic degradation of 3,3′,4,4′-tetrachlorobiphenyl by a resuscitated strain Castellaniella sp. SPC4: Kinetics model and pathway for biodegradation. Sci. Total Environ. 2019, 688, 917–925. [Google Scholar] [CrossRef]
- Christen, P.; Vega, A.; Casalot, L.; Simon, G.; Auria, R. Kinetics of aerobic phenol biodegradation by the acidophilic and hyper thermophilic archaeon Sulfolobus solfataricus 98/2. Biochem. Eng. J. 2012, 62, 56–61. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Wang, C.; Wang, P. Growth kinetics and phenol biodegradation of psychrotrophic Pseudomonas putida LY1. Bioresour. Technol. 2010, 101, 6740–6744. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Q.; Yan, J. Isolation and characterization of a novel phenol degrading bacterial strain WUST-C1. Ind. Eng. Chem. Res. 2013, 52, 258–265. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

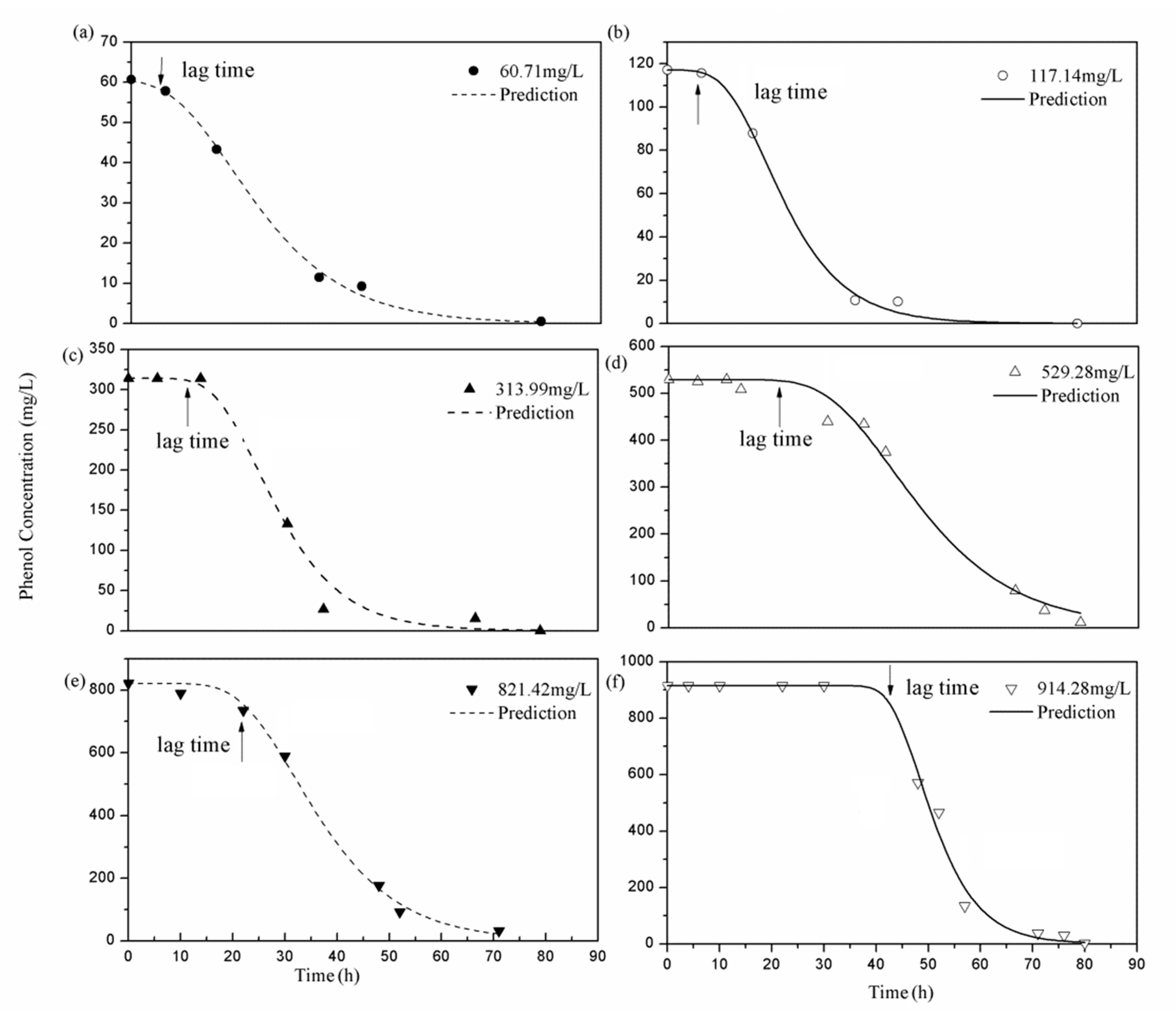

| Si (mg/L) | Rm [mg/(L·h)] | λ (h) | R2 | p-Value |
|---|---|---|---|---|
| 60.71 | 3.09 | 4.56 | 0.997 | p < 0.001 |
| 117.14 | 8.67 | 6.62 | 0.997 | p < 0.001 |
| 313.99 | 18.48 | 11.77 | 0.989 | p < 0.01 |
| 529.28 | 21.00 | 21.80 | 0.988 | p < 0.001 |
| 821.42 | 27.92 | 21.14 | 0.996 | p < 0.001 |
| 914.28 | 55.79 | 42.46 | 0.991 | p < 0.001 |
| Bacterial Strain | pH | Temperature (°C) | Si (mg/L) | Smax (mg/L) | μmax (L/h) | Ks (mg/L) | Ki (mg/L) | Yield (g/g) | References |
|---|---|---|---|---|---|---|---|---|---|
| Sulfolobus solfataricus 98/2 | 3.5 | 80 | 745.0 | 157.50 | 0.09 | 77.70 | 319.40 | 0.55 | 30 |
| Pseudomonas putida LY1 | 7.2 | 25 | 800.0 | 50.00 | 0.27 | 24.40 | 127.40 | 0.77 | 31 |
| Pseudomonas putida MTCC | 7.0 | 30 | 1000.0 | 64.60 | 0.25 | 0.13 | 12.60 | 0.65 | 11 |
| Pseudomonas WUST-C1 | 7.0 | 35 | 1600.0 | - | 2.50 | 48.70 | 100.60 | - | 32 |
| Rhodococcus sp. strain AQ5NOL 2. | 7.0 | 27 | 2000.0 | - | 0.11 | 99.03 | 354.0 | - | 14 |
| Rhodococcus sp. SKC | 7.0 | 30 | 1019.0 | 123.45 | 0.19 | 36.40 | 418.79 | 0.61 | This work |
| Si (mg/L) | 60.71 | 117.14 | 313.99 | 529.28 | 821.42 | 914.28 |
| Y (mg biomass/mg substrate) | 0.39 | 0.47 | 0.61 | 0.56 | 0.57 | 0.53 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, Y.; Li, C.; Song, X.; Yang, Y. Biodegradation of Phenol by Rhodococcus sp. Strain SKC: Characterization and Kinetics Study. Molecules 2020, 25, 3665. https://doi.org/10.3390/molecules25163665
Wen Y, Li C, Song X, Yang Y. Biodegradation of Phenol by Rhodococcus sp. Strain SKC: Characterization and Kinetics Study. Molecules. 2020; 25(16):3665. https://doi.org/10.3390/molecules25163665
Chicago/Turabian StyleWen, Yujuan, Chaofan Li, Xiaoming Song, and Yuesuo Yang. 2020. "Biodegradation of Phenol by Rhodococcus sp. Strain SKC: Characterization and Kinetics Study" Molecules 25, no. 16: 3665. https://doi.org/10.3390/molecules25163665
APA StyleWen, Y., Li, C., Song, X., & Yang, Y. (2020). Biodegradation of Phenol by Rhodococcus sp. Strain SKC: Characterization and Kinetics Study. Molecules, 25(16), 3665. https://doi.org/10.3390/molecules25163665






