Effects of High Intensity Ultrasound on Physiochemical and Structural Properties of Goat Milk β-Lactoglobulin
Abstract
1. Introduction
2. Results and Discussion
2.1. Comparison between Native Bovine Milk β-Lactoglobulin and Goat Milk β-Lactoglobulin Using Reversed Phase High Performance Liquid Chromatography (RP-HPLC) and Sodium Dodecyl Sulfate (SDS) Polyacrylamide Gel Electrophoresis (PAGE)
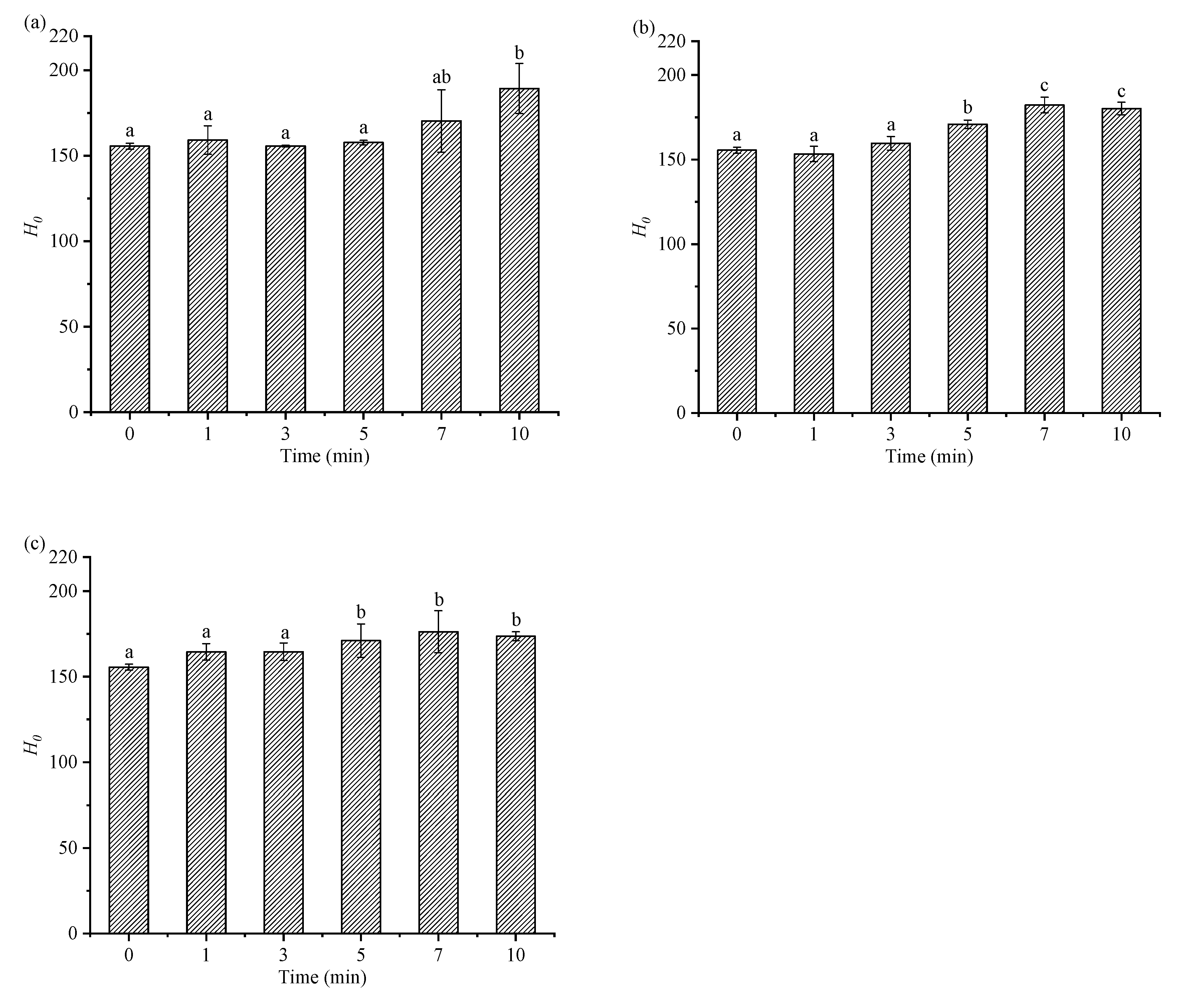
2.2. Effects of HIU Treatment on Surface Hydrophobicity Index of Goat Milk β-Lactoglobulin
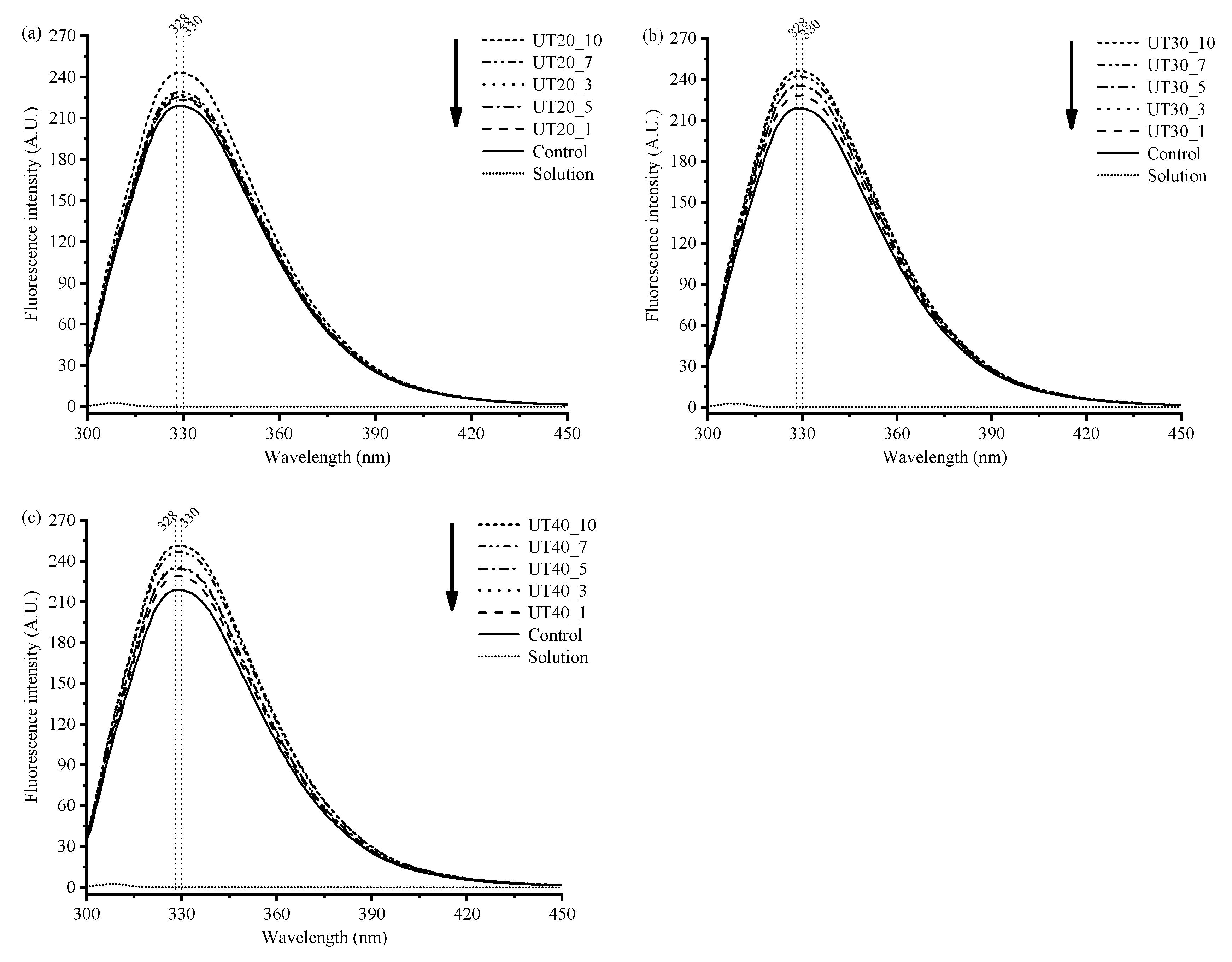
2.3. Effects of HIU Treatment on Intrinsic Fluorescence of Goat Milk β-Lactoglobulin
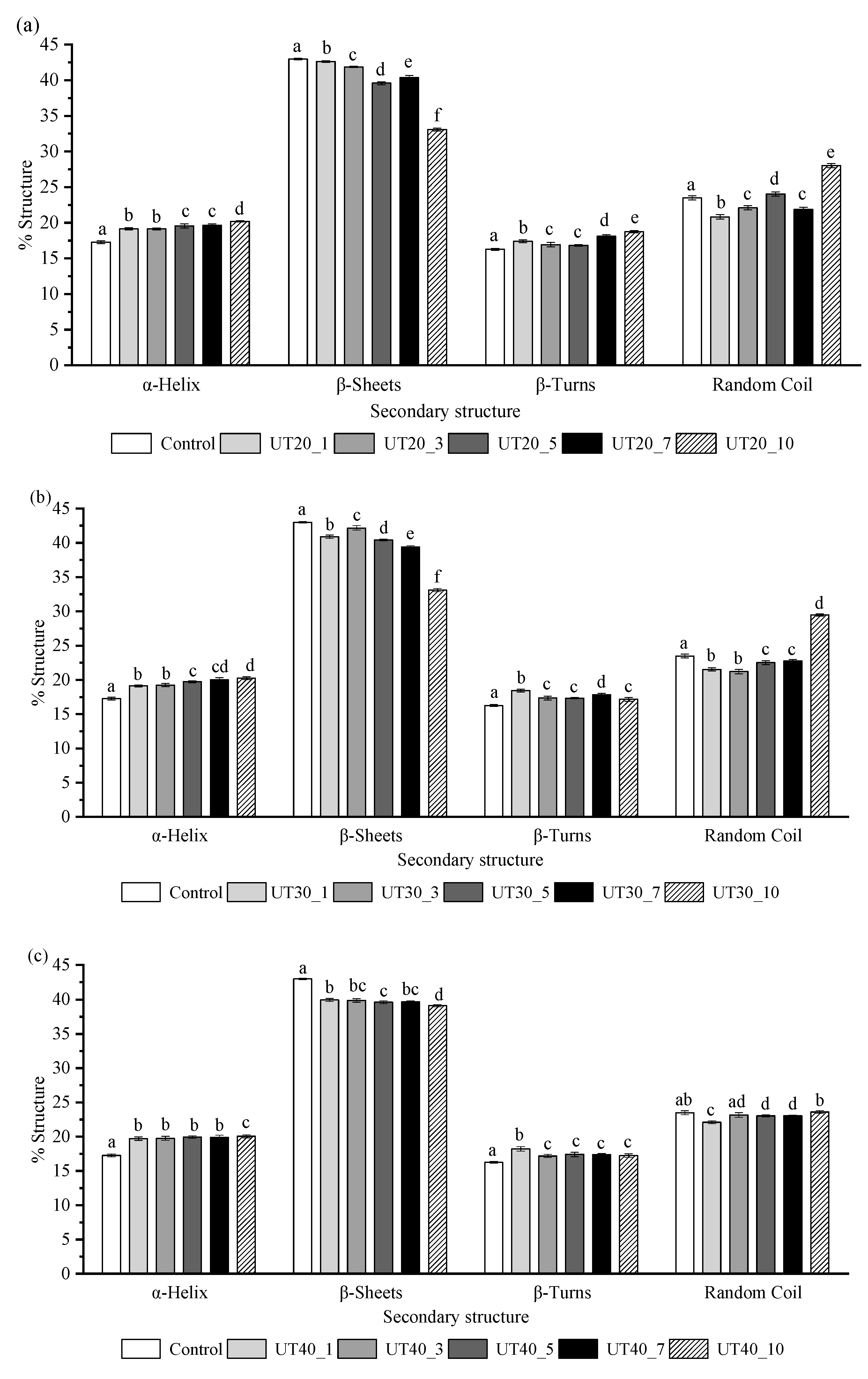
2.4. Effects of HIU Treatment on the Secondary Structure of Goat Milk β-Lactoglobulin
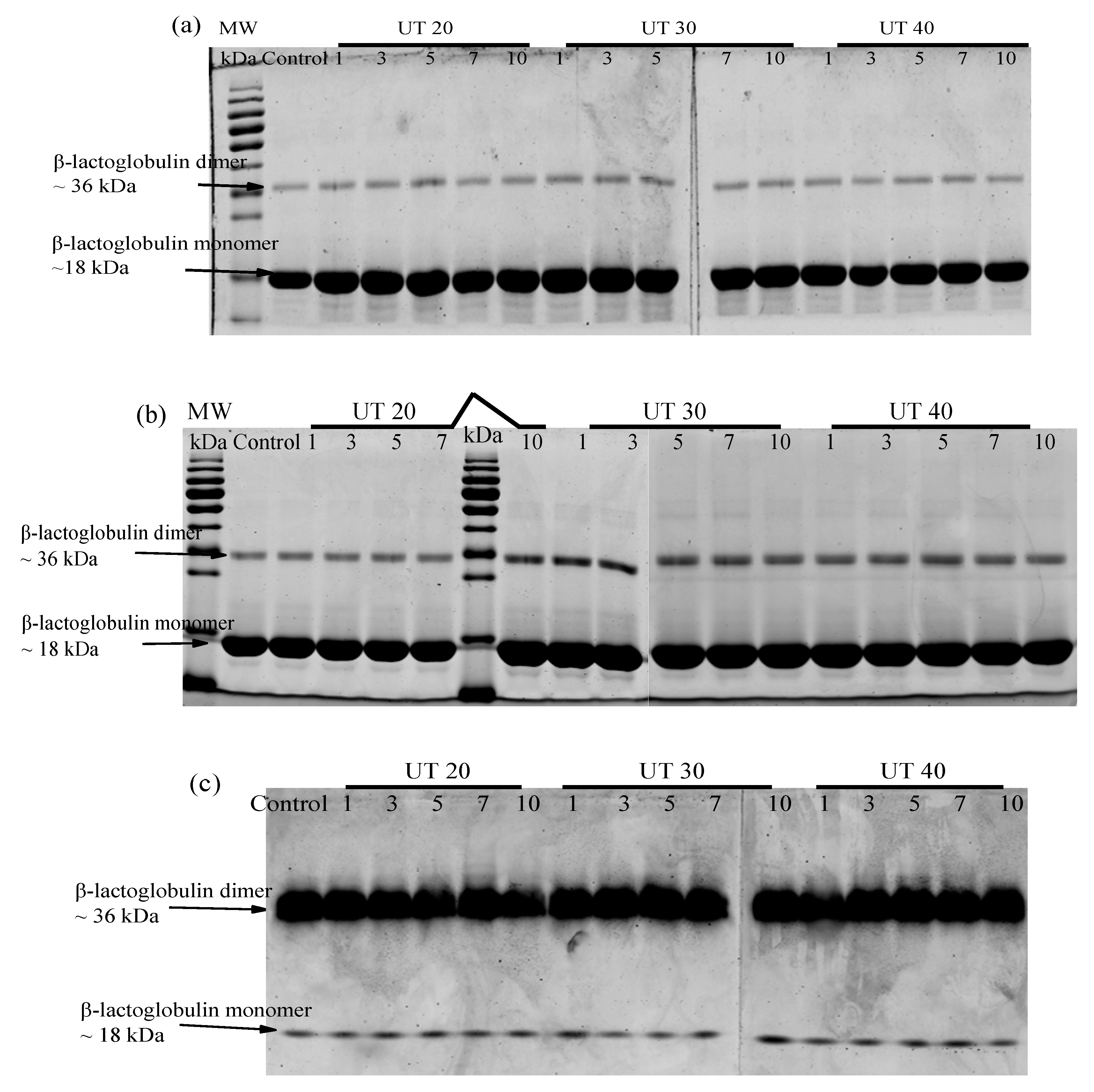
2.5. Effects of HIU Treatment on Molecular Weight of Goat Milk β-Lactoglobulin
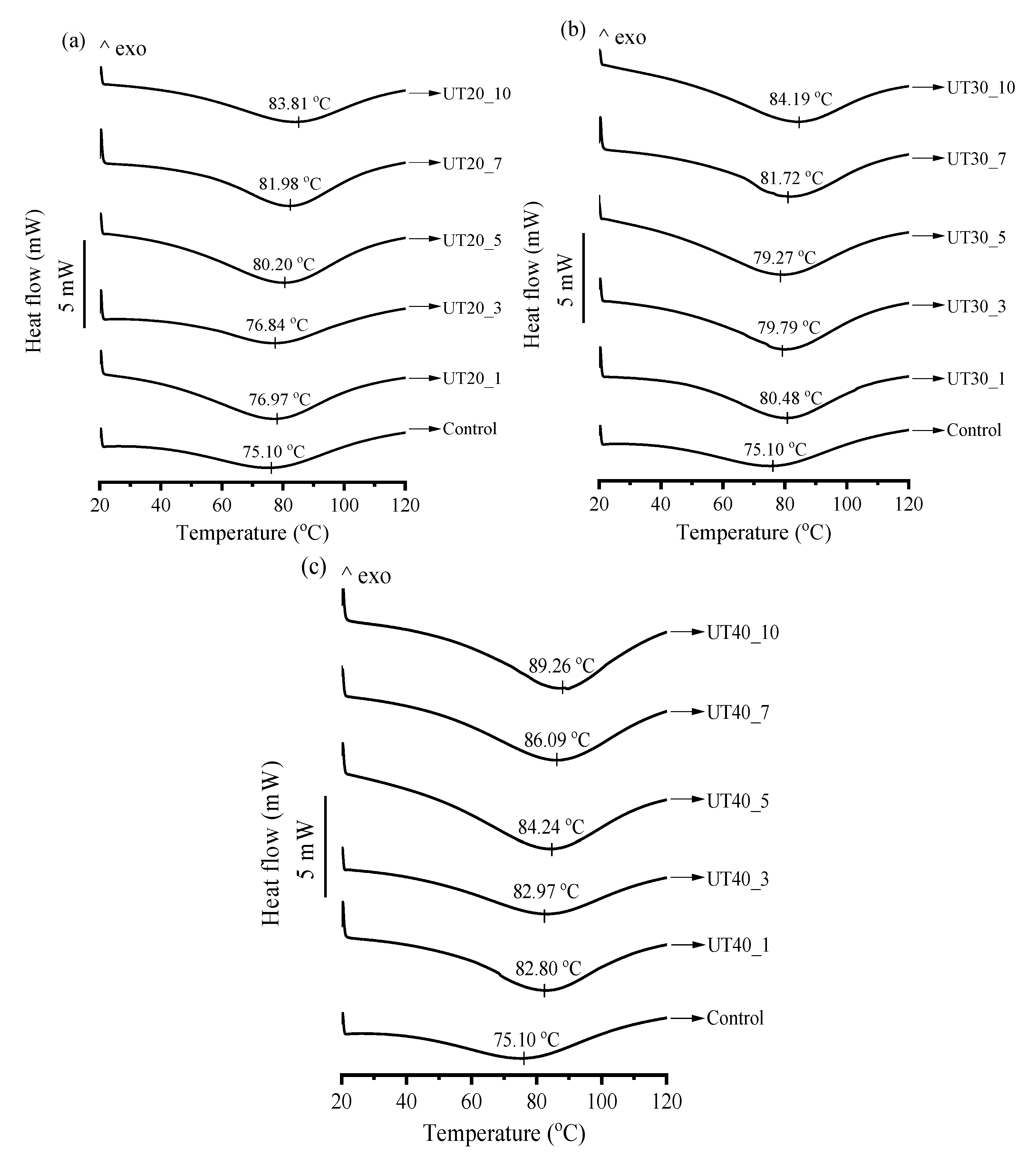
2.6. Effects of HIU Treatment on Thermal Properties of Goat Milk β-Lactoglobulin
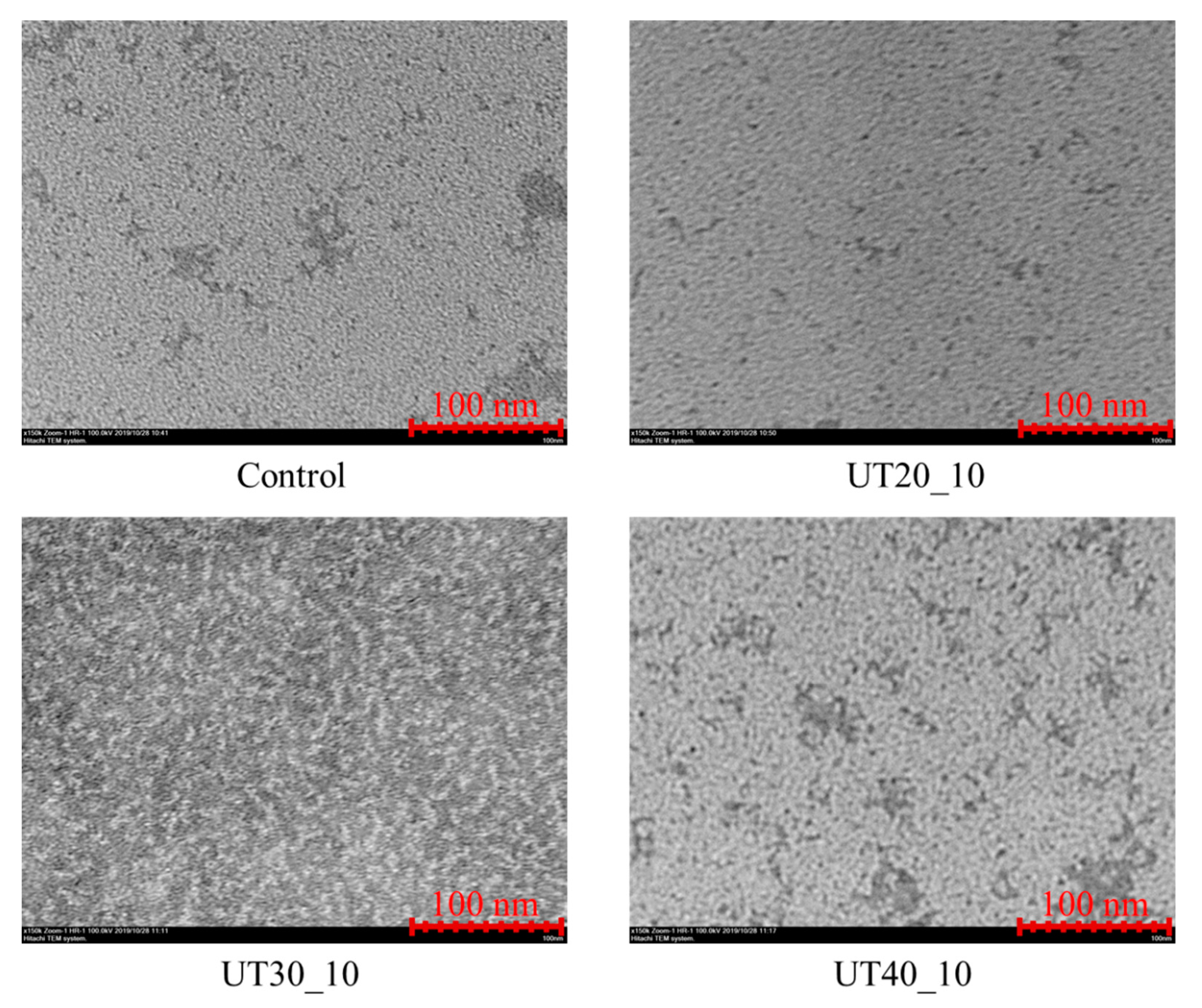
2.7. Effects of HIU Treatment on Microstructure of Goat Milk β-Lactoglobulin
3. Materials and Methods
3.1. Materials
3.2. Preparation of Goat Milk β-Lactoglobulin
3.3. Analysis of β-Lactoglobulin by Reversed Phase High Performance Liquid Chromatography (RP-HPLC)
3.4. High Intensity Ultrasound (HIU) Treatment on Goat Milk β-Lactoglobulin
3.5. Surface Hydrophobicity Measurements
3.6. Intrinsic Fluorescence Spectra
3.7. Fourier Transform Infrared Spectra (FTIR)
3.8. Native and Sodium Dodecyl Sulfate (SDS) Polyacrylamide Gel Electrophoresis (PAGE) of Treated and Untreated Samples
3.9. Differential Scanning Calorimetry (DSC)
3.10. Transmission Electron Microscopy (TEM)
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Marengo, M.; Miriani, M.; Ferranti, P.; Bonomi, F.; Iametti, S.; Barbiroli, A. Structural changes in emulsion-bound bovine beta-lactoglobulin affect its proteolysis and immunoreactivity. BBA-Proteins Proteom. 2016, 1864, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Maux, S.; Bouhallab, S.; Giblin, L.; Brodkorb, A.; Croguennec, T. Bovine beta-lactoglobulin/fatty acid complexes: Binding, structural, and biological properties. Dairy Sci. Technol. 2014, 94, 409–426. [Google Scholar] [CrossRef] [PubMed]
- Shafaei, Z.; Ghalandari, B.; Vaseghi, A.; Divsalar, A.; Haertle, T.; Saboury, A.A.; Sawyer, L. Beta-Lactoglobulin: An efficient nanocarrier for advanced delivery systems. Nanomed-Nanotechnol. 2017, 13, 1685–1692. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, C.; Das, K.P. Thermal unfolding and refolding of beta-lactoglobulin. An intrinsic andextrinsic fluorescence study. Eur. J. Biochem. 2000, 267, 3957–3964. [Google Scholar] [CrossRef] [PubMed]
- Loch, J.I.; Bonarek, P.; Polit, A.; Ries, D.; Dziedzicka-Wasylewska, M.; Lewinski, K. Binding of 18-carbon unsaturated fatty acids to bovine beta-lactoglobulin-structural and thermodynamic studies. Int. J. Biol. Macromol. 2013, 57, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Loch, J.I.; Bonarek, P.; Polit, A.; Swiatek, S.; Czub, M.; Ludwikowska, M.; Lewinski, K. Conformational variability of goat beta-lactoglobulin: Crystallographic and thermodynamic studies. Int. J. Biol. Macromol. 2015, 72, 1283–1291. [Google Scholar] [CrossRef]
- Townend, R.; Basch, J.J. Physicochemical comparison of goat beta-lactoglobulin with the bovine varieties. Arch. Biochem. Biophys. 1968, 126, 59–66. [Google Scholar] [CrossRef]
- Mercadante, D.; Melton, L.D.; Norris, G.E.; Loo, T.S.; Williams, M.A.; Dobson, R.C.; Jameson, G.B. Bovine beta-lactoglobulin is dimeric under imitative physiological conditions: Dissociation equilibrium and rate constants over the pH range of 2.5–7.5. Biophys. J. 2012, 103, 303–312. [Google Scholar] [CrossRef]
- Soria, A.C.; Villamiel, M. Effect of ultrasound on the technological properties and bioactivity of food: A review. Trends Food Sci. Tech. 2010, 21, 323–331. [Google Scholar] [CrossRef]
- Khatkar, A.B.; Kaur, A.; Khatkar, S.K.; Mehta, N. Characterization of heat-stable whey protein: Impact of ultrasound on rheological, thermal, structural and morphological properties. Ultrason. Sonochem. 2018, 49, 333–342. [Google Scholar] [CrossRef]
- Chandrapala, J.; Zisu, B.; Palmer, M.; Kentish, S.; Ashokkumar, M. Effects of ultrasound on the thermal and structural characteristics of proteins in reconstituted whey protein concentrate. Ultrason. Sonochem. 2011, 18, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Wang, C.N.; Guo, M.R. Changes in structure and antioxidant activity of beta-lactoglobulin by ultrasound and enzymatic treatment. Ultrason. Sonochem. 2018, 43, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Pang, X.; Lu, J.; Liu, L.; Zhang, S.; Lv, J. Effect of high intensity ultrasound pretreatment on functional and structural properties of micellar casein concentrates. Ultrason. Sonochem. 2018, 47, 10–16. [Google Scholar] [CrossRef]
- Hu, H.; Wu, J.; Li, E.C.Y.; Zhu, L.; Zhang, F.; Xu, X.; Fan, G.; Wang, L.; Huang, X.; Pan, S. Effects of ultrasound on structural and physical properties of soy protein isolate (SPI) dispersions. Food Hydrocolloid. 2013, 30, 647–655. [Google Scholar] [CrossRef]
- Jiang, S.; Ding, J.; Andrade, J.; Rababah, T.M.; Almajwal, A.; Abulmeaty, M.M.; Feng, H. Modifying the physicochemical properties of pea protein by pH-shifting and ultrasound combined treatments. Ultrason. Sonochem. 2017, 38, 835–842. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, J.; Li, Y.; Wang, Z.; Liang, J.; Wang, R.; Chen, Y.; Ma, W.; Qi, B.; Zhang, M. Effects of ultrasound on the structure and physical properties of black bean protein isolates. Food Res. Int. 2014, 62, 595–601. [Google Scholar] [CrossRef]
- Sun, Y.J.; Chen, J.H.; Zhang, S.W.; Li, H.J.; Lu, J.; Liu, L.; Su, Y.L.; Cui, W.M.; Ge, W.P.; Lv, J.P. Effect of power ultrasound pre-treatment on the physical and functional properties of reconstituted milk protein concentrate. J. Food Eng. 2014, 124, 11–18. [Google Scholar]
- Jambrak, A.R.; Lelas, V.; Mason, T.J.; Krešić, G.; Badanjak, M. Physical properties of ultrasound treated soy proteins. J. Food Eng. 2009, 93, 386–393. [Google Scholar] [CrossRef]
- Jambrak, A.R.; Mason, T.J.; Lelas, V.; Herceg, Z.; Herceg, I.L. Effect of ultrasound treatment on solubility and foaming properties of whey protein suspensions. J. Food Eng. 2008, 86, 281–287. [Google Scholar] [CrossRef]
- MH, A.E.S.; El-Shibiny, S.; Salem, A. Factors affecting the functional properties of whey protein products: A review. Food Rev. Int. 2009, 25, 251–270. [Google Scholar]
- Resendiz-Vazquez, J.A.; Ulloa, J.A.; Urias-Silvas, J.E.; Bautista-Rosales, P.U.; Ramirez-Ramirez, J.C.; Rosas-Ulloa, P.; Gonzalez-Torres, L. Effect of high-intensity ultrasound on the technofunctional properties and structure of jackfruit (Artocarpus heterophyllus) seed protein isolate. Ultrason. Sonochem. 2017, 37, 436–444. [Google Scholar] [CrossRef]
- Halder, U.C.; Chakraborty, J.; Das, N.; Bose, S. Tryptophan dynamics in the exploration of micro-conformational changes of refolded beta-lactoglobulin after thermal exposure: A steady state and time-resolved fluorescence approach. J. Photoch. Photobio. B 2012, 109, 50–57. [Google Scholar] [CrossRef]
- Chandrapala, J.; Zisu, B.; Kentish, S.; Ashokkumar, M. The effects of high-intensity ultrasound on the structural and functional properties of α-Lactalbumin, β-Lactoglobulin and their mixtures. Food Res. Int. 2012, 48, 940–943. [Google Scholar] [CrossRef]
- Ciuciu, A.M.S.; Aprodu, I.; Alexe, P.; Stănciuc, N. Thermally driven interactions between β-lactoglobulin and retinol acetate investigated by fluorescence spectroscopy and molecular modeling methods. Dairy Sci. Technol. 2016, 96, 405–423. [Google Scholar] [CrossRef]
- Chen, W.; Wang, W.; Ma, X.; Lv, R.; Balaso Watharkar, R.; Ding, T.; Ye, X.; Liu, D. Effect of pH-shifting treatment on structural and functional properties of whey protein isolate and its interaction with (-)-epigallocatechin-3-gallate. Food Chem. 2019, 274, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Stanic-Vucinic, D.; Stojadinovic, M.; Atanaskovic-Markovic, M.; Ognjenovic, J.; Gronlund, H.; Van Hage, M.; Lantto, R.; Sancho, A.I.; Velickovic, T.C. Structural changes and allergenic properties of beta-lactoglobulin upon exposure to high-intensity ultrasound. Mol. Nutr. Food Res. 2012, 56, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Zhang, Q.X.; Lu, R.R. Identification and analysis of the function of surface layer proteins from three Lactobacillus strains. Ann. Microbiol. 2018, 68, 207–216. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, C.; Li, T.; Sun, D.; Gao, H.; Gao, Z.; Mu, Z. Effect of ultrasound on the structure and functional properties of transglutaminase-crosslinked whey protein isolate exposed to prior heat treatment. Int. Dairy J. 2019, 88, 79–88. [Google Scholar] [CrossRef]
- Silva, M.; Zisu, B.; Chandrapala, J. Influence of low-frequency ultrasound on the physico-chemical and structural characteristics of milk systems with varying casein to whey protein ratios. Ultrason. Sonochem. 2018, 49, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Tammineedi, C.V.R.K.; Choudhary, R.; Perez-Alvarado, G.C.; Watson, D.G. Determining the effect of UV-C, high intensity ultrasound and nonthermal atmospheric plasma treatments on reducing the allergenicity of α-casein and whey proteins. LWT-Food Sci. Technol. 2013, 54, 35–41. [Google Scholar] [CrossRef]
- Yang, W.H.; Tu, Z.C.; Wang, H.; Li, X.; Tian, M. High-intensity ultrasound enhances the immunoglobulin (Ig)G and IgE binding of ovalbumin. J. Sci. Food Agr. 2017, 97, 2714–2720. [Google Scholar] [CrossRef] [PubMed]
- Park, H.W.; Kim, D.Y.; Shin, W.S. Fucoidan improves the structural integrity and the molecular stability of beta-lactoglobulin. Food Sci. Biotechnol. 2018, 27, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Aschaffenburg, R.; Drewry, J. Improved method for the preparation of crystalline beta-lactoglobulin and alpha-lactalbumin from cow’s milk. Biochem. J. 1957, 65, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Bonfatti, V.; Grigoletto, L.; Cecchinato, A.; Gallo, L.; Carnier, P. Validation of a new reversed-phase high-performance liquid chromatography method for separation and quantification of bovine milk protein genetic variants. J. Chromatogr. A 2008, 1195, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.B.; Wang, C.N.; Zhang, Y.C.; Liu, T.T.; Lv, J.P.; Shen, X.; Guo, M.R. Effects of gamma radiation on microbial, physicochemical, and structural properties of whey protein model system. J. Dairy Sci. 2018, 101, 4879–4890. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds pure goat milk β-lactoglobulins are available from the authors. |

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Wang, C.; Sun, X.; Zhao, Z.; Guo, M. Effects of High Intensity Ultrasound on Physiochemical and Structural Properties of Goat Milk β-Lactoglobulin. Molecules 2020, 25, 3637. https://doi.org/10.3390/molecules25163637
Zhou X, Wang C, Sun X, Zhao Z, Guo M. Effects of High Intensity Ultrasound on Physiochemical and Structural Properties of Goat Milk β-Lactoglobulin. Molecules. 2020; 25(16):3637. https://doi.org/10.3390/molecules25163637
Chicago/Turabian StyleZhou, Xinhui, Cuina Wang, Xiaomeng Sun, Zixuan Zhao, and Mingruo Guo. 2020. "Effects of High Intensity Ultrasound on Physiochemical and Structural Properties of Goat Milk β-Lactoglobulin" Molecules 25, no. 16: 3637. https://doi.org/10.3390/molecules25163637
APA StyleZhou, X., Wang, C., Sun, X., Zhao, Z., & Guo, M. (2020). Effects of High Intensity Ultrasound on Physiochemical and Structural Properties of Goat Milk β-Lactoglobulin. Molecules, 25(16), 3637. https://doi.org/10.3390/molecules25163637







