Multiple-Reaction Monitoring Tandem Mass Method for Determination of Phenolics and Water-Soluble Vitamins in Eccoilopus formosanus
Abstract
1. Introduction
2. Results and Discussion
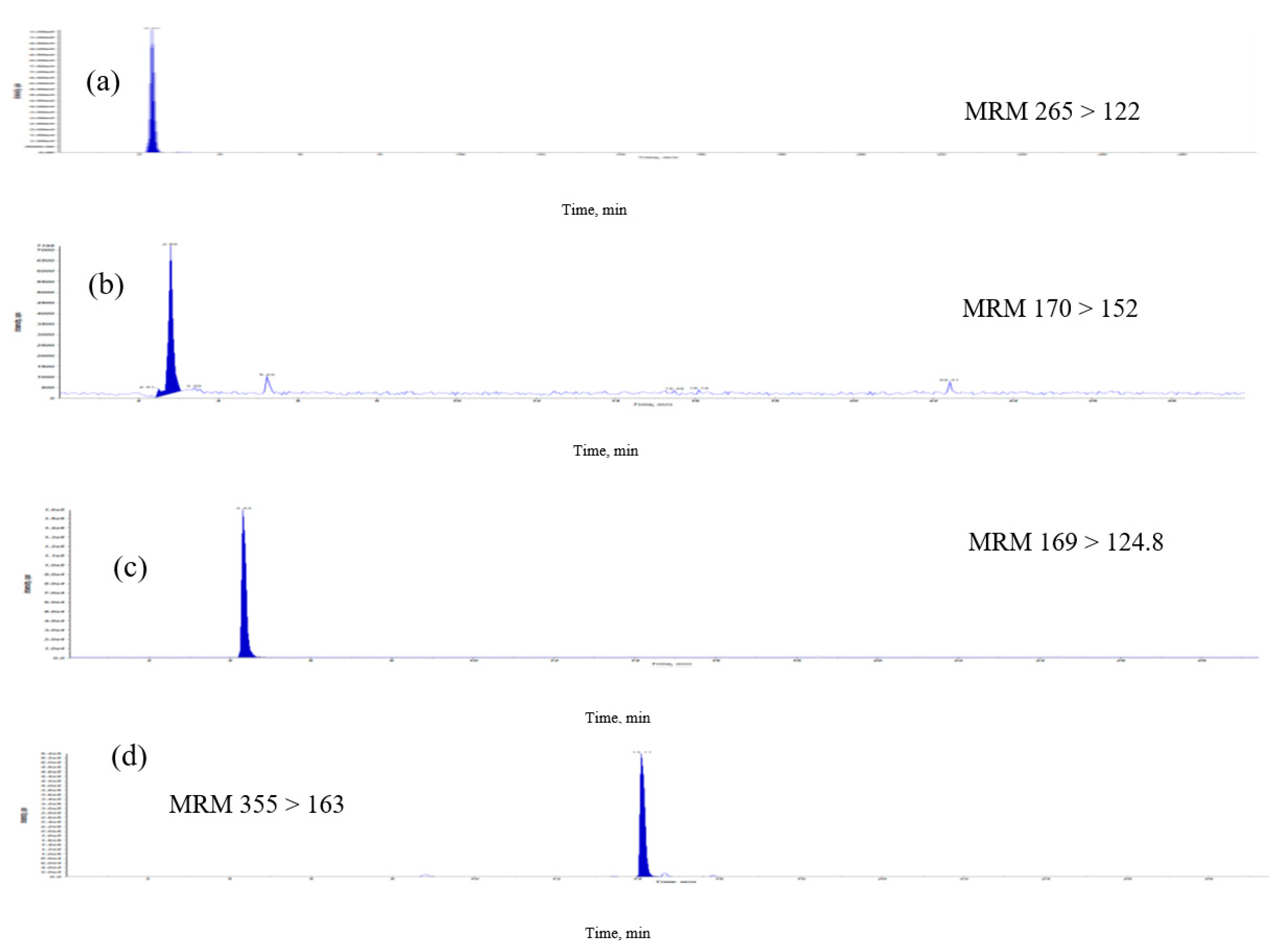
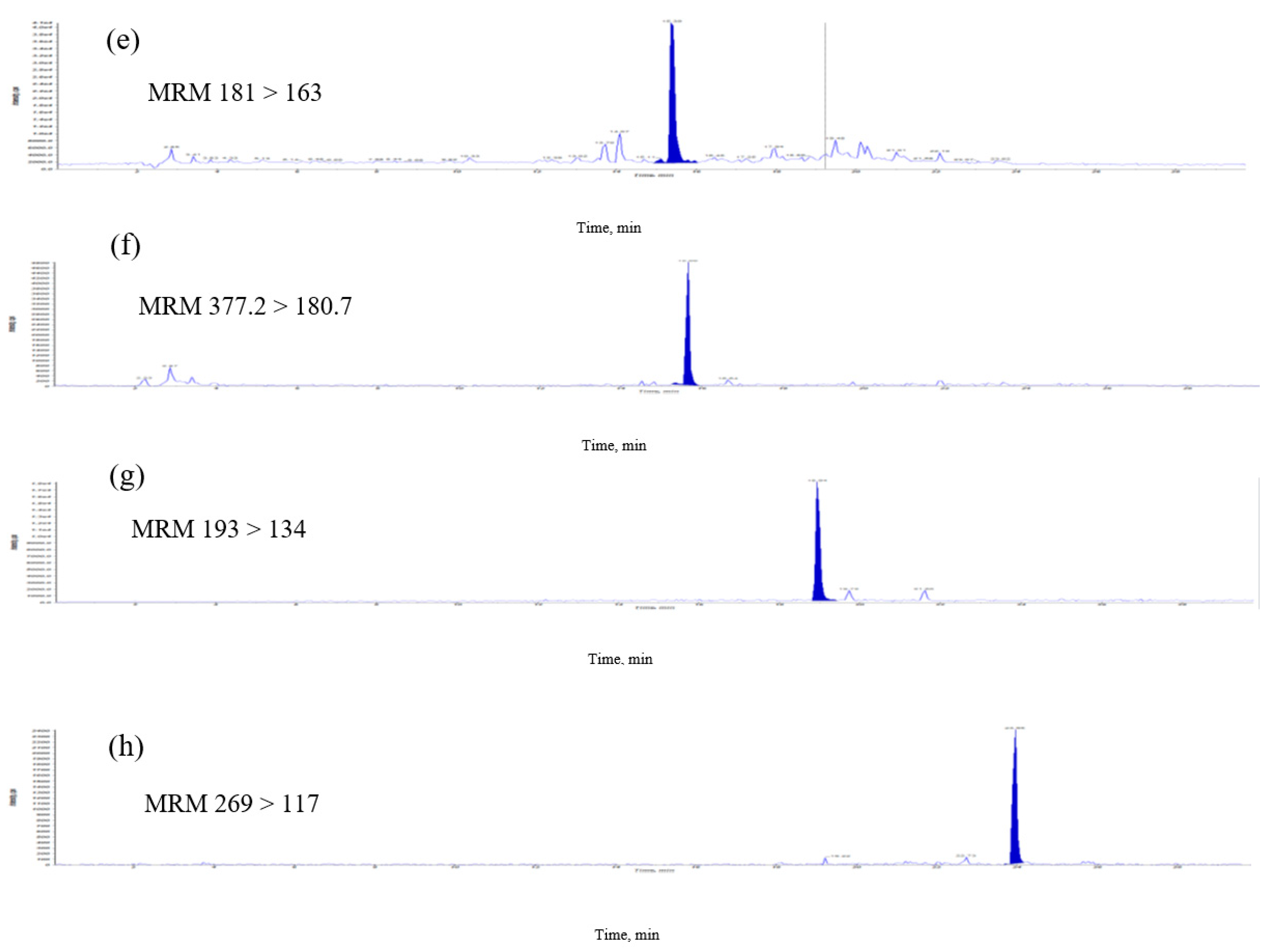
2.1. Analysis of Eight Signature Compounds by High-Performance Liquid Chromatography Electrospray Ionization Mass Spectrometry with Multiple Reaction Monitoring
2.2. The Antioxidant Activity, α-Glucosidase Inhibition Activity and Content of Total Polyphenols
3. Materials and Methods
3.1. Reagents
3.2. Plant Collection, Identification and Extraction
3.3. HPLC-ESI-MS Analysis of the Extract
3.4. Method Validation and Quantitation of Phenolic Acid and Water-Soluble Vitamins
3.5. Determination of Total Polyphenol Content
3.6. DPPH Radical Scavenging Activity
3.7. α-Glucosidase Inhibition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hadimani, N.A.; Malleshi, N.G. Studies on milling, physicochemical properties, nutrient composition and dietary fiber contemt of millets. J. Food Sci. Technol. 1993, 30, 17–20. [Google Scholar]
- Thompson, L.U. Potential health benefits and problems associated with ant-nutrients in foods. Food Res. Int. 1993, 26, 131–149. [Google Scholar] [CrossRef]
- Saleh, A.S.; Zhang, Q.; Chen, J.; Shen, Q. Millet grains: Nutritional quality, processing, and potential health benefits. Compr. Rev. Food Sci. Food Saf. 2013, 12, 281–295. [Google Scholar] [CrossRef]
- Ganguly, S.; Kumar, T.; Mantha, S.; Panda, K. Simultaneous determination of black tea-derived catechins and theaflavins in tissues of tea consuming animals using ultra-performance liquid-chromatography tandem mass spectrometry. PLoS ONE 2016, 11, e0163498. [Google Scholar] [CrossRef] [PubMed]
- Illiano, A.; Arpino, V.; Pinto, G.; Berti, A.; Verdoliva, V.; Peluso, G.; Pucci, P.; Amoresano, A. Multiple Reaction Monitoring tandem mass spectrometry approach for the identification of biological fluids at crime scene investigations. Anal. Chem. 2018, 90, 5627–5636. [Google Scholar] [CrossRef] [PubMed]
- Fontanarosa, C.; Pane, F.; Sepe, N.; Pinto, G.; Trifuoggi, M.; Squillace, M.; Errico, F.; Usiello, A.; Pucci, P.; Amoresano, A. Quantitative determination of free d-Asp, l-Asp and N-methyl-d-aspartate in mouse brain tissues by chiral separation and Multiple Reaction Monitoring tandem mass spectrometry. PLoS ONE 2017, 12, e0179748. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T.; Furukawa, M.; Asoh, M.; Kanno, T.; Kojima, T.; Yonekubo, A. Fat-soluble and water-soluble vitamin contents of breast milk from Japanese women. J. Nutr. Sci. Vitaminol. 2005, 51, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Hampel, D.; York, E.R.; Allen, L.H. Ultra-performance liquid chromatography tandem mass-spectrometry (UPLC-MS/MS) for the rapid, simultaneous analysis of thiamin, riboflavin, flavin adenine dinucleotide, nicotinamide and pyridoxal in human milk. J. Chromatogr. B 2012, 903, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Yagi, T.; Iwamoto, S.; Mizuseki, R.; Furuya, M.; Nakayama, K. Contents of all forms of vitamin B6, pyridoxine-beta-glucoside and 4-pyridoxic acid in mature milk of Japanese women according to 4-pyridoxolactone-conversion high performance liquid chromatography. J. Nutr. Sci. Vitaminol. 2013, 59, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Laddomada, B.; Caretto, S.; Mita, G. Wheat Bran Phenolic Acids: Bioavailability and Stability in Whole Wheat-Based Foods. Molecules 2015, 20, 15666–15685. [Google Scholar] [CrossRef] [PubMed]
- Lillioja, S.; Neal, A.L.; Tapsell, L.; Jacobs, D.R. Whole Grains, Type 2 Diabetes, Coronary Heart Disease, and Hypertension: Links to the Aleurone preferred over Indigestible Fiber. BioFactors Oxf. Engl. 2013, 39, 242–258. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Nakamura, K.; Kayahara, H. Analysis of phenolic compounds in white rice, brown rice, and germinated brown rice. J. Agric. Food Chem. 2004, 52, 4808–4813. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zhang, G.; Pan, J.; Wang, Y. α-Glucosidase inhibition by luteolin: Kinetics, interaction and molecular docking. Int. J. Biol. Macromol. 2014, 64, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.O.; Chun, O.K.; Kim, Y.J.; Moon, H.Y.; Lee, C.J. Quantification of polyphenolics and their antioxidant capacity in fresh plums. J. Agric. Food Chem. 2003, 51, 6509–6515. [Google Scholar] [CrossRef] [PubMed]
- Apea-Bah, F.B.; Minnaar, A.; Bester, M.J.; Duodu, K.G. Sorghum-cowpea composite porridge as a functional food, Part II: Antioxidant properties as affected by simulated in vitro gastrointestinal digestion. Food Chem. 2016, 197, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Pistia-Brueggeman, G.; Hollingsworth, R.I. The use of the o-nitrophenyl group as a protecting/activating group for 2-acetamido-2-deoxyglucose. Carbohydr. Res. 2003, 338, 455–458. [Google Scholar] [CrossRef]
Sample Availability: Samples of the Eccoilopus formosanus are not available from the authors. |

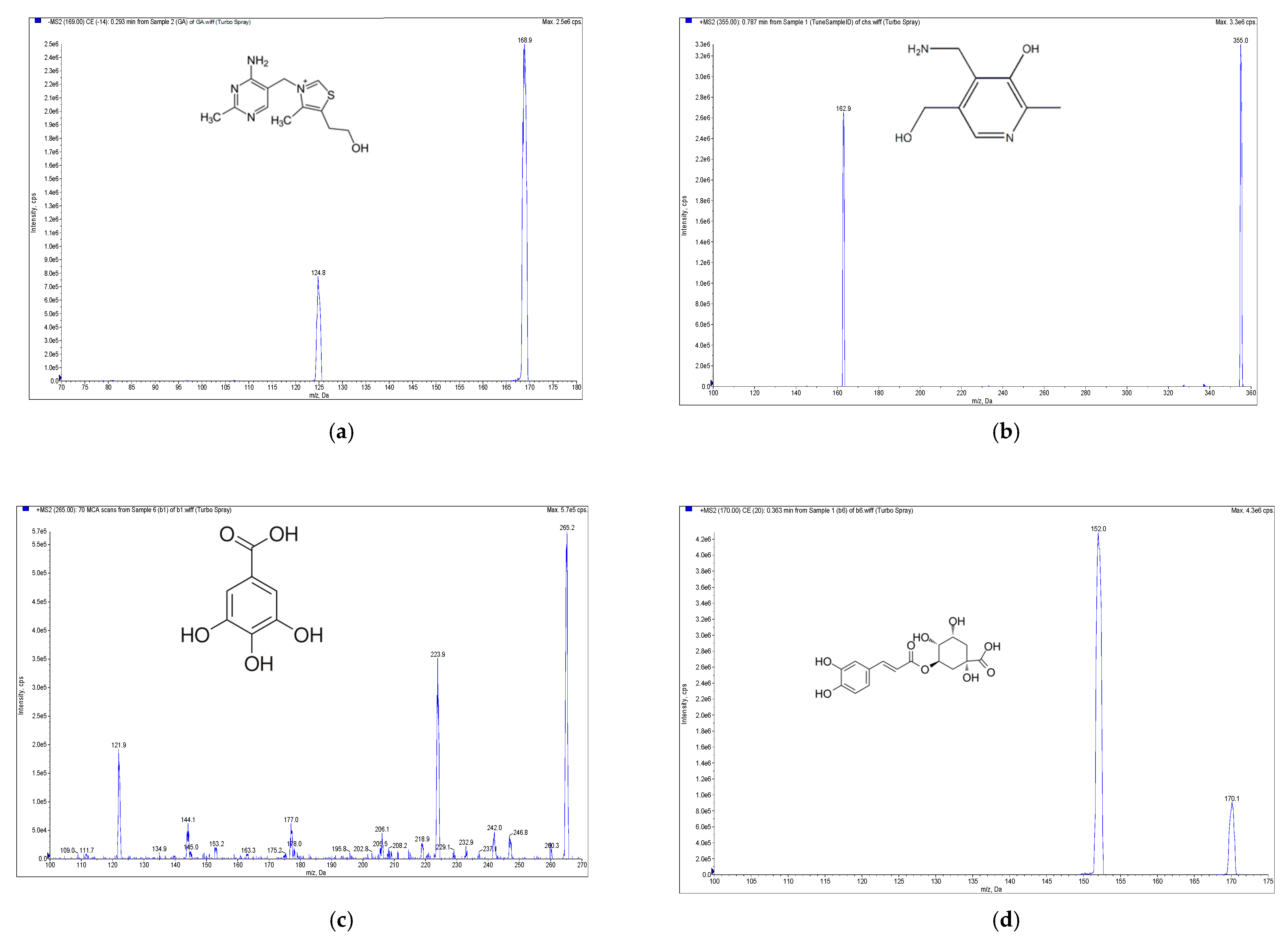
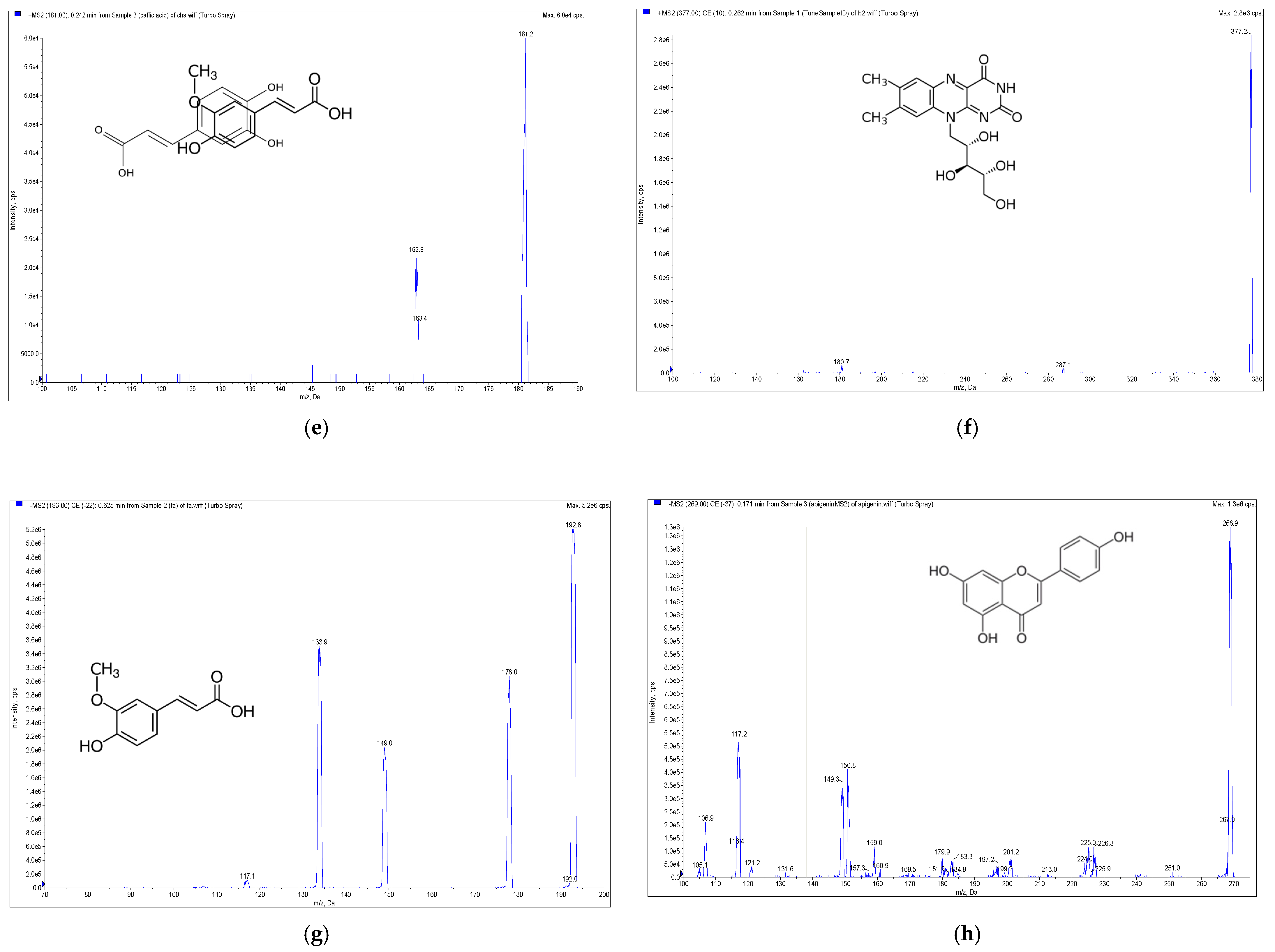
| Compound | MRM Transition (m/z) | DP (V) | CE (eV) | CXP (V) |
|---|---|---|---|---|
| Thiamine | 265 >122 | 62 | 22 | 18 |
| Pyridoxamin | 170 > 152 | 70 | 28 | 7 |
| Gallic acid | 169 > 124.8 | −29 | −23 | −27 |
| Chlorogenic acid | 355 >163 | 66 | 14 | 10 |
| Caffeic acid | 181 >163 | 45 | 17 | 9 |
| Riboflavin | 377.2 >180.7 | 50 | 20 | 10 |
| Ferulic acid | 193 > 134 | −36 | −20 | −4.5 |
| Apigenin | 269 > 117 | −90 | −52 | −15 |
| Compound | Retention Time (tR) | Regression Equation | R2 | Range (μg/mL) | LOQ (μg/mL) | Precision (%) | Recovery (%) |
|---|---|---|---|---|---|---|---|
| Thiamine | 2.32 | y = 9062.1x−2446.1 | 0.995 | 1–50 | 0.3 | 2.3 | 94.1 |
| Pyridoxamin | 2.81 | y = 2191.6x−695.89 | 0.995 | 1–50 | 0.2 | 1.7 | 81.5 |
| Gallic acid | 4.30 | y= 2915.1x−3240 | 0.996 | 1–50 | 1.2 | 3.5 | 90.5 |
| Chlorogenic acid | 14.08 | y = 1058.3x−60.5 | 0.997 | 10–200 | 1.6 | 1.5 | 91.2 |
| Caffeic acid | 15.39 | y = 5695.4−1165.5 | 0.999 | 1–50 | 0.9 | 1.4 | 86.7 |
| Riboflavin | 15.66 | y =1415.8+1248.6 | 0.995 | 1–50 | 0.1 | 1.2 | 85.5 |
| Ferulic acid | 18.34 | y = 934.5−2352.1 | 0.996 | 10–200 | 1.5 | 1.9 | 82.3 |
| Apigenin | 23.98 | y = 3276.6x−658.1 | 0.997 | 1–50 | 0.5 | 2.9 | 92.1 |
| Sample | Thiamine | Pyridoxamin | Gallic Acid | Chlorogenic Acid | Caffeic Acid | Riboflavin | Ferulic Acid | Apigenin |
|---|---|---|---|---|---|---|---|---|
| Extract of E. formosanus | 4.6 | 1.5 | 11.0 | 179.1 | 65.2 | 2.1 | 145.3 | 15.2 |
| Samples | Yield (%) | Total Phenolic Content (mg GAE/g, DW) |
|---|---|---|
| 30% ethanol | 37.1 ± 0.3 | 2.5 ± 0.3 |
| 50% ethanol | 45.5 ± 0.2 | 3.1 ± 0.1 |
| 70% ethanol | 40.4 ± 0.6 | 2.9 ± 0.5 |
| Samples | DPPH Radical Scavenging Activity, IC50 Value (µg/mL) | α-Glucosidase Inhibition Activity, IC50 Value (µg/mL) |
|---|---|---|
| E. formosanus extract | 120.7 ± 0.3 | 136.9 ± 0.5 |
| Thiamine | >500 | >500 |
| Pyridoxamin | >500 | >500 |
| Gallic acid | 21.1 ± 0.3 | 59.2± 0.6 |
| Chlorogenic acid | 12.7 ± 0.5 | 9.1 ± 0.5 |
| Caffeic acid | 25.3 ± 0.3 | 7.2 ± 0.2 |
| Riboflavin | 155.7 ± 0.4 | >500 |
| Ferulic acid | 45.2 ± 0.3 | 107.1 ± 0.9 |
| Apigenin | 31.6 ± 0.9 | 79.1 ± 0.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.-S.; Yu, H.-S.; Yen, C.-H.; Liaw, E.-T. Multiple-Reaction Monitoring Tandem Mass Method for Determination of Phenolics and Water-Soluble Vitamins in Eccoilopus formosanus. Molecules 2020, 25, 3632. https://doi.org/10.3390/molecules25163632
Huang H-S, Yu H-S, Yen C-H, Liaw E-T. Multiple-Reaction Monitoring Tandem Mass Method for Determination of Phenolics and Water-Soluble Vitamins in Eccoilopus formosanus. Molecules. 2020; 25(16):3632. https://doi.org/10.3390/molecules25163632
Chicago/Turabian StyleHuang, Ho-Shin, Hsu-Sheng Yu, Chia-Hung Yen, and Ean-Tun Liaw. 2020. "Multiple-Reaction Monitoring Tandem Mass Method for Determination of Phenolics and Water-Soluble Vitamins in Eccoilopus formosanus" Molecules 25, no. 16: 3632. https://doi.org/10.3390/molecules25163632
APA StyleHuang, H.-S., Yu, H.-S., Yen, C.-H., & Liaw, E.-T. (2020). Multiple-Reaction Monitoring Tandem Mass Method for Determination of Phenolics and Water-Soluble Vitamins in Eccoilopus formosanus. Molecules, 25(16), 3632. https://doi.org/10.3390/molecules25163632





