Optimization with Response Surface Methodology of Microwave-Assisted Conversion of Xylose to Furfural
Abstract
1. Introduction
2. Results
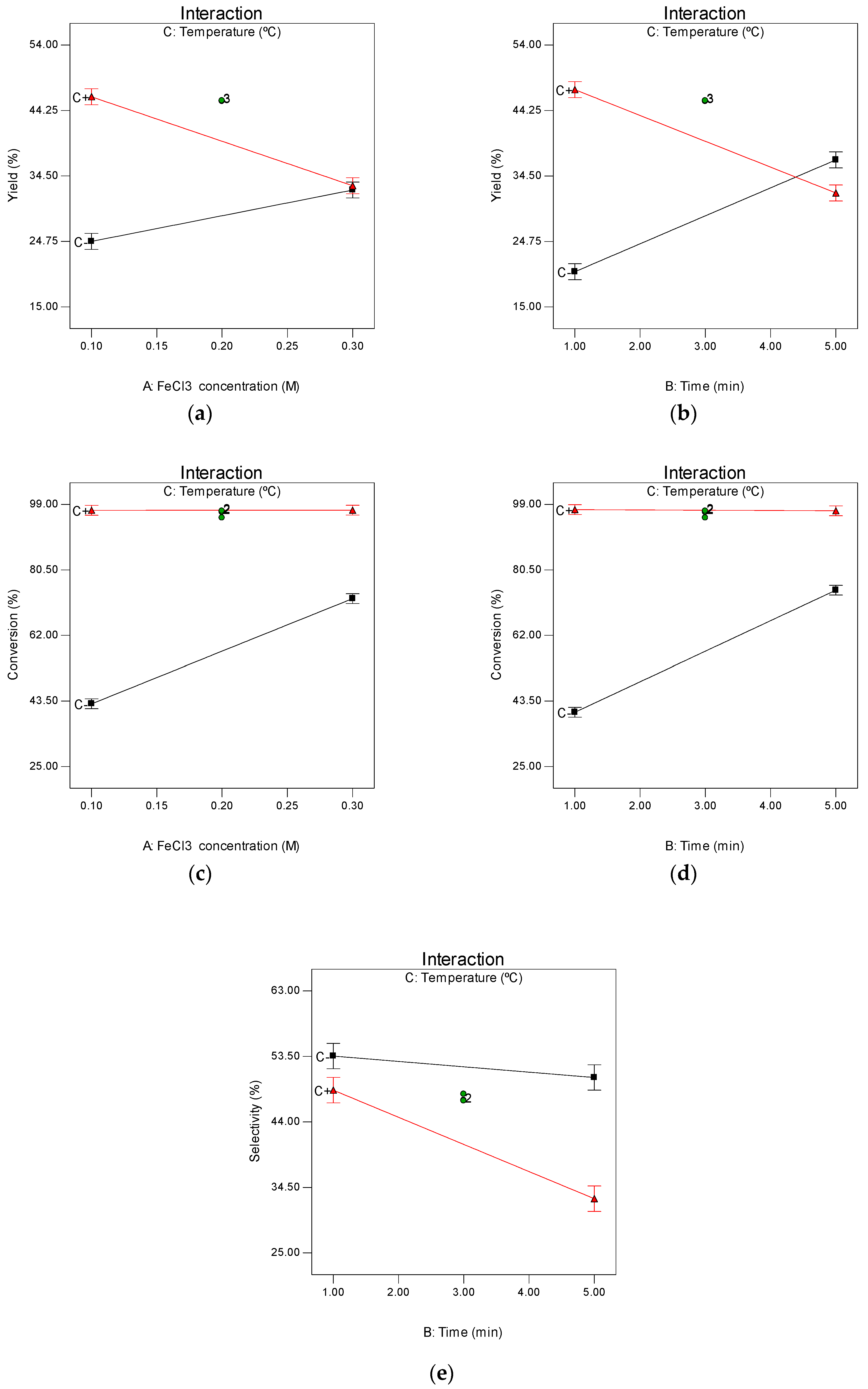
2.1. Two-Level Factorial Design
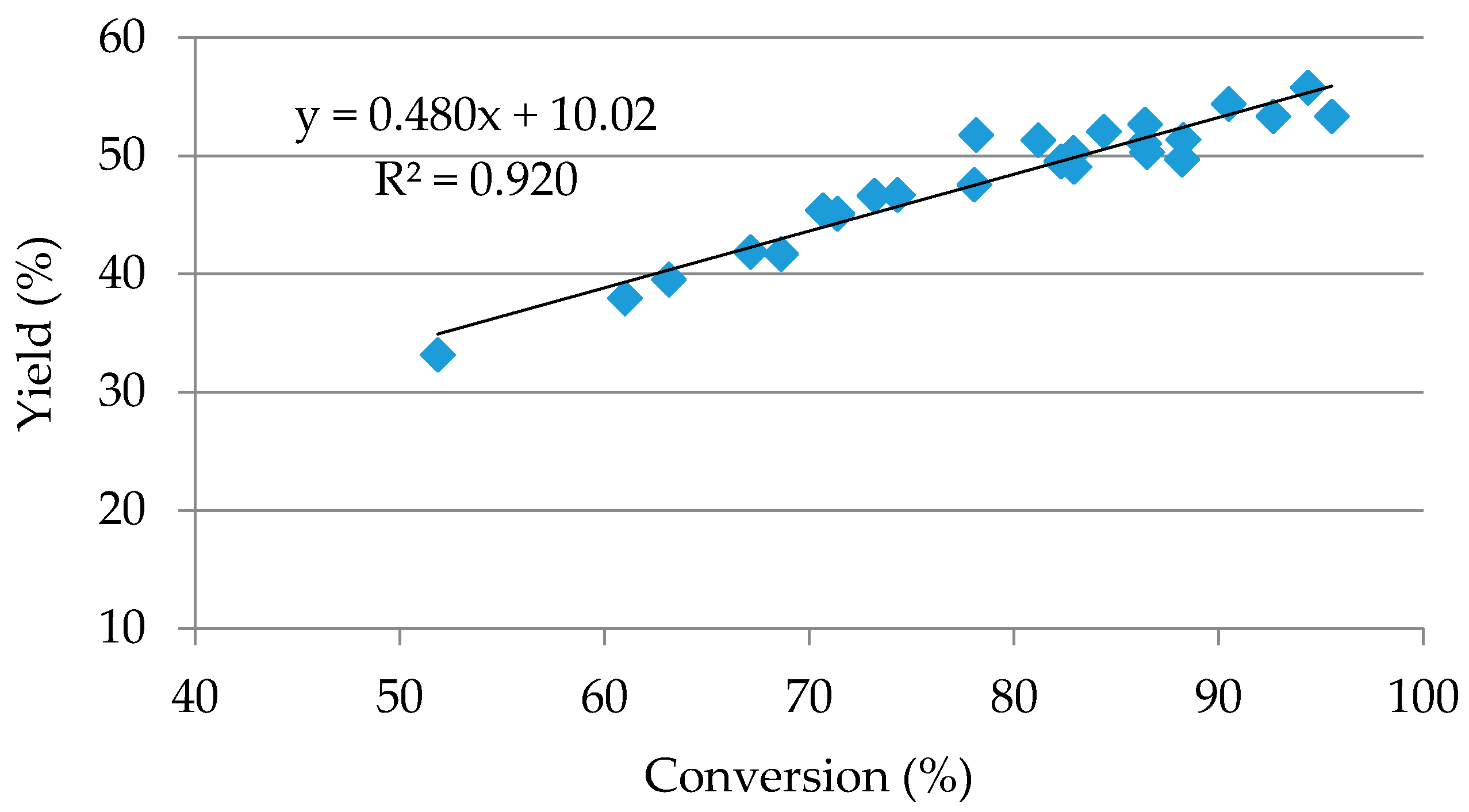
2.2. Central Composite Design
3. Discussion
Overview of the Production of Furfural from Xylose
4. Materials and Methods
4.1. Chemical Reagents
4.2. General Procedure for Dehydration Treatment
4.3. Methodology Based on the Design of Experiments
4.4. Analysis of the Liquid Fractions and Quantification of the Yield, Selectivity, and Conversion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Machado, G.; Leon, S.; Santos, F.; Lourega, R.; Dullius, J.; Mollmann, M.E.; Eichler, P. Literature Review on Furfural Production from Lignocellulosic Biomass. Nat. Resour. 2016, 07, 115–129. [Google Scholar] [CrossRef]
- Cai, C.M.; Zhang, T.; Kumar, R.; Wyman, C.E. Integrated furfural production as a renewable fuel and chemical platform from lignocellulosic biomass: Furfural production from lignocellulosic biomass. J. Chem. Technol. Biotechnol. 2014, 89, 2–10. [Google Scholar] [CrossRef]
- Dias, A.S.; Lima, S.; Pillinger, M.; Valente, A.A. Acidic cesium salts of 12-tungstophosphoric acid as catalysts for the dehydration of xylose into furfural. Carbohydr. Res. 2006, 341, 2946–2953. [Google Scholar] [CrossRef] [PubMed]
- Rong, C.; Ding, X.; Zhu, Y.; Li, Y.; Wang, L.; Qu, Y.; Ma, X.; Wang, Z. Production of furfural from xylose at atmospheric pressure by dilute sulfuric acid and inorganic salts. Carbohydr. Res. 2012, 350, 77–80. [Google Scholar] [CrossRef]
- Sánchez, C.; Serrano, L.; Andres, M.A.; Labidi, J. Furfural production from corn cobs autohydrolysis liquors by microwave technology. Ind. Crops Prod. 2013, 42, 513–519. [Google Scholar] [CrossRef]
- Montané, D.; Salvadó, J.; Torras, C.; Farriol, X. High-temperature dilute-acid hydrolysis of olive stones for furfural production. Biomass Bioenerg. 2002, 22, 295–304. [Google Scholar] [CrossRef]
- Mesa, L.; Morales, M.; González, E.; Cara, C.; Romero, I.; Castro, E.; Mussatto, S.I. Restructuring the processes for furfural and xylose production from sugarcane bagasse in a biorefinery concept for ethanol production. Chem. Eng. Process. 2014, 85, 196–202. [Google Scholar] [CrossRef]
- Sádaba, I.; Ojeda, M.; Mariscal, R.; Granados, M.L. Silica-poly(styrenesulphonic acid) nanocomposites for the catalytic dehydration of xylose to furfural. Appl. Catal. B Environ. 2014, 150–151, 421–431. [Google Scholar] [CrossRef]
- Agirrezabal-Telleria, I.; Gandarias, I.; Arias, P.L. Heterogeneous acid-catalysts for the production of furan-derived compounds (furfural and hydroxymethylfurfural) from renewable carbohydrates: A review. Catal. Today 2014, 234, 42–58. [Google Scholar] [CrossRef]
- Le Guenic, S.; Delbecq, F.; Ceballos, C.; Len, C. Microwave-assisted dehydration of D-xylose into furfural by diluted inexpensive inorganic salts solution in a biphasic system. J. Mol. Catal. A Chem. 2015, 410, 1–7. [Google Scholar] [CrossRef]
- Padilla-Rascón, C.; Ruiz, E.; Romero, I.; Castro, E.; Oliva, J.M.; Ballesteros, I.; Manzanares, P. Valorisation of olive stone by-product for sugar production using a sequential acid/steam explosion pretreatment. Ind. Crops Prod. 2020, 148, 112279. [Google Scholar] [CrossRef]
- Kim, S.B.; Lee, J.H.; Yang, X.; Lee, J.; Kim, S.W. Furfural production from hydrolysate of barley straw after dilute sulfuric acid pretreatment. Korean J. Chem. Eng. 2015, 32, 2280–2284. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, H.; Wang, P.; Li, Y. Production of furfural from xylose, xylan and corncob in gamma-valerolactone using FeCl3·6H2O as catalyst. Bioresour. Technol. 2014, 151, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Marcotullio, G.; De Jong, W. Chloride ions enhance furfural formation from d-xylose in dilute aqueous acidic solutions. Green Chem. 2010, 12, 1739. [Google Scholar] [CrossRef]
- Nie, Y.; Hou, Q.; Li, W.; Bai, C.; Bai, X.; Ju, M. Efficient Synthesis of Furfural from Biomass Using SnCl4 as Catalyst in Ionic Liquid. Molecules 2019, 24, 594. [Google Scholar] [CrossRef] [PubMed]
- Romero, I.; Ruiz, E.; Castro, E. Pretreatment with Metal Salts. In Biomass Fractionation Technologies for a Lignocellulosic Feedstock Based Biorefinery; Elsevier: Amsterdam, The Netherlands, 2016; pp. 209–227. ISBN 978-0-12-802323-5. [Google Scholar]
- Weingarten, R.; Cho, J.; Conner, W.C., Jr.; Huber, G.W. Kinetics of furfural production by dehydration of xylose in a biphasic reactor with microwave heating. Green Chem. 2010, 12, 1423. [Google Scholar] [CrossRef]
- Hricovíniová, Z. Xylans are a valuable alternative resource: Production of d-xylose, d-lyxose and furfural under microwave irradiation. Carbohydr. Polym. 2013, 98, 1416–1421. [Google Scholar] [CrossRef]
- Yemiş, O.; Mazza, G. Acid-catalyzed conversion of xylose, xylan and straw into furfural by microwave-assisted reaction. Bioresour. Technol. 2011, 102, 7371–7378. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Feng, J.; Pan, H. Maximizing utilization of poplar wood by microwave-assisted pretreatment with methanol/dioxane binary solvent. Bioresour. Technol. 2020, 300, 122657. [Google Scholar] [CrossRef]
- Mikulski, D.; Kłosowski, G. Microwave-assisted dilute acid pretreatment in bioethanol production from wheat and rye stillages. Biomass Bioenerg. 2020, 136, 105528. [Google Scholar] [CrossRef]
- Benko, Z.; Andersson, A.; Gáspár, M.; Réczey, K.; Stålbrand, H. Heat extraction of corn fiber hemicellulose. Appl. Biochem. Biotechnol. 2007, 136, 14. [Google Scholar]
- Mashuni; Hamid, F.H.; Muzuni; Kadidae, L.O.; Jahiding, M.; Ahmad, L.O.; Saputra, D. The determination of total phenolic content of cocoa pod husk based on microwave-assisted extraction method. AIP Conf. Proc. 2020, 2243, 030013. [Google Scholar] [CrossRef]
- Ji, Q.; Yu, X.; Yagoub, A.E.-G.A.; Chen, L.; Zhou, C. Efficient removal of lignin from vegetable wastes by ultrasonic and microwave-assisted treatment with ternary deep eutectic solvent. Ind. Crops Prod. 2020, 149, 112357. [Google Scholar] [CrossRef]
- Riansa-ngawong, W.; Prasertsan, P. Optimization of furfural production from hemicellulose extracted from delignified palm pressed fiber using a two-stage process. Carbohydr. Res. 2011, 346, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Li, P.; Bo, D.; Chang, H. The optimization of formic acid hydrolysis of xylose in furfural production. Carbohydr. Res. 2012, 357, 53–61. [Google Scholar] [CrossRef]
- Yang, W.; Li, P.; Bo, D.; Chang, H.; Wang, X.; Zhu, T. Optimization of furfural production from d-xylose with formic acid as catalyst in a reactive extraction system. Bioresour. Technol. 2013, 133, 361–369. [Google Scholar] [CrossRef]
- Lamminpää, K.; Tanskanen, J. Study of furfural formation using formic acid. In Proceedings of the 8th World Congress of Chemical Engineering, Montréal, QC, Canada, 23 August 2009. [Google Scholar]
- Lamminpää, K.; Ahola, J.; Tanskanen, J. Acid-catalysed xylose dehydration into furfural in the presence of kraft lignin. Bioresour. Technol. 2015, 177, 94–101. [Google Scholar] [CrossRef]
- Rasmussen, H.; Sørensen, H.R.; Meyer, A.S. Formation of degradation compounds from lignocellulosic biomass in the biorefinery: Sugar reaction mechanisms. Carbohydr. Res. 2014, 385, 45–57. [Google Scholar] [CrossRef]
- Peleteiro, S.; Rivas, S.; Alonso, J.L.; Santos, V.; Parajó, J.C. Furfural production using ionic liquids: A review. Bioresour. Technol. 2016, 202, 181–191. [Google Scholar] [CrossRef]
- Jeong, G.H.; Kim, E.G.; Kim, S.B.; Park, E.D.; Kim, S.W. Fabrication of sulfonic acid modified mesoporous silica shells and their catalytic performance with dehydration reaction of d-xylose into furfural. Micropor. Mesopor. Mat. 2011, 144, 134–139. [Google Scholar] [CrossRef]
- O’Neill, R.; Ahmad, M.N.; Vanoye, L.; Aiouache, F. Kinetics of Aqueous Phase Dehydration of Xylose into Furfural Catalyzed by ZSM-5 Zeolite. Ind. Eng. Chem. Res. 2009, 48, 4300–4306. [Google Scholar] [CrossRef]
- Xiouras, C.; Radacsi, N.; Sturm, G.; Stefanidis, G.D. Furfural Synthesis from d -Xylose in the Presence of Sodium Chloride: Microwave versus Conventional Heating. Chem. Sus. Chem. 2016, 9, 2159–2166. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Liu, S.; Abu-Omar, M.M.; Mosier, N.S. Selective Conversion of Biomass Hemicellulose to Furfural Using Maleic Acid with Microwave Heating. Energ. Fuel 2012, 26, 1298–1304. [Google Scholar] [CrossRef]
- Möller, M.; Schröder, U. Hydrothermal production of furfural from xylose and xylan as model compounds for hemicelluloses. RSC Adv. 2013, 3, 22253. [Google Scholar] [CrossRef]
- Antal, M.J.; Leesomboon, T.; Mok, W.S.; Richards, G.N. Mechanism of formation of 2-furaldehyde from d-xylose. Carbohydr. Res. 1991, 217, 71–85. [Google Scholar] [CrossRef]
- Hongsiri, W.; Danon, B.; Jong, W. de Kinetic Study on the Dilute Acidic Dehydration of Pentoses toward Furfural in Seawater. Ind. Eng. Chem. Res. 2014, 53, 5455–5463. [Google Scholar] [CrossRef]
- Mellmer, M.A.; Sener, C.; Gallo, J.M.R.; Luterbacher, J.S.; Alonso, D.M.; Dumesic, J.A. Solvent Effects in Acid-Catalyzed Biomass Conversion Reactions. Angew. Chem. Int. Ed. 2014, 53, 11872–11875. [Google Scholar] [CrossRef]
- Gallo, J.M.R.; Alonso, D.M.; Mellmer, M.A.; Yeap, J.H.; Wong, H.C.; Dumesic, J.A. Production of Furfural from Lignocellulosic Biomass Using Beta Zeolite and Biomass-Derived Solvent. Top. Catal. 2013, 56, 1775–1781. [Google Scholar] [CrossRef]
- Gürbüz, E.I.; Gallo, J.M.R.; Alonso, D.M.; Wettstein, S.G.; Lim, W.Y.; Dumesic, J.A. Conversion of Hemicellulose into Furfural Using Solid Acid Catalysts in γ-Valerolactone. Angew. Chem. Int. Ed. 2013, 52, 1270–1274. [Google Scholar] [CrossRef]
- Kim, S.B.; You, S.J.; Kim, Y.T.; Lee, S.; Lee, H.; Park, K.; Park, E.D. Dehydration of D-xylose into furfural over H-zeolites. Korean J. Chem. Eng. 2011, 28, 710–716. [Google Scholar] [CrossRef]
- Dias, A.S.; Pillinger, M.; Valente, A.A. Liquid phase dehydration of d-xylose in the presence of Keggin-type heteropolyacids. Appl. Catal. A Gen. 2005, 285, 126–131. [Google Scholar] [CrossRef]
- Lam, E.; Majid, E.; Leung, A.C.W.; Chong, J.H.; Mahmoud, K.A.; Luong, J.H.T. Synthesis of Furfural from Xylose by Heterogeneous and Reusable Nafion Catalysts. Chem. Sus. Chem. 2011, 4, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, V.; Pinar, A.B.; Sandler, S.I.; Vlachos, D.G.; Lobo, R.F. Xylose Isomerization to Xylulose and its Dehydration to Furfural in Aqueous Media. ACS Catal. 2011, 1, 1724–1728. [Google Scholar] [CrossRef]
- Binder, J.B.; Blank, J.J.; Cefali, A.V.; Raines, R.T. Synthesis of Furfural from Xylose and Xylan. Chem. Sus. Chem. 2010, 3, 1268–1272. [Google Scholar] [CrossRef] [PubMed]
- Lima, S.; Neves, P.; Antunes, M.M.; Pillinger, M.; Ignatyev, N.; Valente, A.A. Conversion of mono/di/polysaccharides into furan compounds using 1-alkyl-3-methylimidazolium ionic liquids. Appl. Catal. A Gen. 2009, 363, 93–99. [Google Scholar] [CrossRef]
- Zhang, Z.; Du, B.; Quan, Z.-J.; Da, Y.-X.; Wang, X.-C. Dehydration of biomass to furfural catalyzed by reusable polymer bound sulfonic acid (PEG-OSO3H) in ionic liquid. Catal. Sci. Technol. 2014, 4, 633. [Google Scholar] [CrossRef]
- Le Guenic, S.; Gergela, D.; Ceballos, C.; Delbecq, F.; Len, C. Furfural Production from d-Xylose and Xylan by Using Stable Nafion NR50 and NaCl in a Microwave-Assisted Biphasic Reaction. Molecules 2016, 21, 1102. [Google Scholar] [CrossRef]
- Peleteiro, S.; da Costa Lopes, A.M.; Garrote, G.; Parajó, J.C.; Bogel-Łukasik, R. Simple and Efficient Furfural Production from Xylose in Media Containing 1-Butyl-3-Methylimidazolium Hydrogen Sulfate. Ind. Eng. Chem. Res. 2015, 54, 8368–8373. [Google Scholar] [CrossRef]
- vom Stein, T.; Grande, P.M.; Leitner, W.; Domínguez de María, P. Iron-Catalyzed Furfural Production in Biobased Biphasic Systems: From Pure Sugars to Direct Use of Crude Xylose Effluents as Feedstock. Chem. Sus. Chem. 2011, 4, 1592–1594. [Google Scholar] [CrossRef]
- Campos Molina, M.J.; Mariscal, R.; Ojeda, M.; López Granados, M. Cyclopentyl methyl ether: A green co-solvent for the selective dehydration of lignocellulosic pentoses to furfural. Bioresour. Technol. 2012, 126, 321–327. [Google Scholar] [CrossRef]
- Wang, W.; Li, H.; Ren, J.; Sun, R.; Zheng, J.; Sun, G.; Liu, S. An efficient process for dehydration of xylose to furfural catalyzed by inorganic salts in water/dimethyl sulfoxide system. Chinese J. Catal. 2014, 35, 741–747. [Google Scholar] [CrossRef]
- Li, H.; Ren, J.; Zhong, L.; Sun, R.; Liang, L. Production of furfural from xylose, water-insoluble hemicelluloses and water-soluble fraction of corncob via a tin-loaded montmorillonite solid acid catalyst. Bioresour. Technol. 2015, 176, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Tao, F.; Song, H.; Chou, L. Efficient process for the conversion of xylose to furfural with acidic ionic liquid. Can. J. Chem. 2011, 89, 83–87. [Google Scholar] [CrossRef]
- Serrano-Ruiz, J.C.; Campelo, J.M.; Francavilla, M.; Romero, A.A.; Luque, R.; Menéndez-Vázquez, C.; García, A.B.; García-Suárez, E.J. Efficient microwave-assisted production of furfural from C5 sugars in aqueous media catalysed by Brönsted acidic ionic liquids. Catal. Sci. Technol. 2012, 2, 1828. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, C.-W.; Abu-Omar, M.M. Synthesis of Furfural from Xylose, Xylan, and Biomass Using AlCl3⋅6 H2O in Biphasic Media via Xylose Isomerization to Xylulose. Chem. Sus. Chem. 2012, 5, 405–410. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |


| Run | Factors | Responses | ||||||
|---|---|---|---|---|---|---|---|---|
| A: FeCl3 (M) | B: Time (min) | C: Temp. (°C) | Xylose Consumed (g/L) | Furfural (g/L) | Yield(%) | Conversion (%) | Selectivity (%) | |
| 2 | 0.3 | 1 | 170 | 16.68 | 4.79 | 24.87 | 55.61 | 44.73 |
| 4 | 0.1 | 5 | 200 | 29.33 | 7.84 | 38.99 | 97.77 | 39.88 |
| 6 | 0.1 | 5 | 170 | 18.11 | 6.52 | 33.87 | 60.38 | 56.10 |
| 7 | 0.3 | 1 | 200 | 29.44 | 7.96 | 41.13 | 98.13 | 41.91 |
| 8 | 0.3 | 5 | 170 | 26.75 | 7.72 | 39.93 | 89.15 | 44.79 |
| 9 | 0.1 | 1 | 200 | 29.07 | 10.35 | 53.59 | 96.91 | 55.30 |
| 10 | 0.1 | 1 | 170 | 7.52 | 3.02 | 15.63 | 25.06 | 62.38 |
| 11 | 0.3 | 5 | 200 | 28.99 | 4.82 | 24.96 | 96.63 | 25.83 |
| 1 | 0.2 | 3 | 185 | 29.04 | 8.91 | 45.61 | 96.80 | 47.12 |
| 3 | 0.2 | 3 | 185 | 29.13 | 9.05 | 45.70 | 97.12 | 47.06 |
| 5 | 0.2 | 3 | 185 | 28.55 | 9.16 | 45.71 | 95.18 | 48.02 |
| Response | Model (Coded Terms) | p-Value | R2 | CV (%) | SD (%) |
|---|---|---|---|---|---|
| Yield (%) | Yield = 34.12 − 1.40·A + 0.32·B + 5.55·C − 5.22·AC − 8.01·BC | <0.0001 | 0.997 | 2.38 | 0.89 |
| Conversion (%) | Conversion = 77.45 + 7.43·A + 8.53·B + 19.91·C − 7.41·AC − 8.69·BC | <0.0001 | 0.999 | 1.26 | 1.04 |
| Selectivity (%) | Selectivity = 46.36 − 7.05·A − 4.71·B − 5.63·C − 3.16·BC | <0.0001 | 0.988 | 3.18 | 1.48 |
| Run | A: FeCl3 (M) | B: Time (min) | C: Temp. (°C) | Consumed Xylose (g/L) | Furfural (g/L) | Yield (%) | Conversion (%) | Selectivity (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.040 | 0.75 | 195 | 21.24 | 8.73 | 45.39 | 70.68 | 64.22 |
| 2 | 0.1 | 1 | 190 | 23.60 | 10.01 | 51.77 | 78.16 | 66.23 |
| 4 | 0.05 | 0.5 | 200 | 25.43 | 10.04 | 52.07 | 84.40 | 61.69 |
| 5 | 0.05 | 1 | 190 | 20.31 | 8.11 | 41.87 | 67.14 | 62.36 |
| 6 | 0.1 | 1 | 200 | 28.88 | 10.32 | 53.35 | 95.54 | 55.84 |
| 7 | 0.075 | 1.10 | 195 | 26.66 | 9.93 | 51.38 | 88.28 | 58.20 |
| 8 | 0.075 | 0.75 | 202.07 | 28.45 | 10.76 | 55.78 | 94.37 | 59.11 |
| 9 | 0.075 | 0.75 | 187.93 | 19.07 | 7.64 | 39.55 | 63.15 | 62.63 |
| 11 | 0.1 | 0.5 | 200 | 27.97 | 10.30 | 53.35 | 92.68 | 57.56 |
| 12 | 0.040 | 0.75 | 195 | 22.05 | 8.99 | 46.64 | 73.18 | 63.73 |
| 14 | 0.110 | 0.75 | 195 | 26.64 | 9.61 | 49.69 | 88.22 | 56.33 |
| 15 | 0.075 | 0.40 | 195 | 21.56 | 8.72 | 45.10 | 71.38 | 63.19 |
| 17 | 0.05 | 0.5 | 190 | 15.57 | 6.37 | 33.16 | 51.85 | 63.96 |
| 18 | 0.110 | 0.75 | 195 | 25.98 | 9.67 | 50.30 | 86.51 | 58.15 |
| 19 | 0.075 | 0.75 | 187.93 | 18.32 | 7.29 | 37.95 | 61.00 | 62.22 |
| 20 | 0.075 | 0.40 | 195 | 22.31 | 8.97 | 46.68 | 74.31 | 62.82 |
| 21 | 0.1 | 0.5 | 190 | 20.73 | 8.06 | 41.71 | 68.63 | 60.78 |
| 22 | 0.05 | 1 | 200 | 26.09 | 10.18 | 52.67 | 86.40 | 60.95 |
| 23 | 0.075 | 0.75 | 202.07 | 27.28 | 10.50 | 54.41 | 90.50 | 60.12 |
| 25 | 0.075 | 1.10 | 195 | 26.06 | 9.86 | 51.05 | 86.35 | 59.12 |
| 3 | 0.075 | 0.75 | 195 | 25.08 | 9.73 | 50.27 | 82.91 | 60.63 |
| 10 | 0.075 | 0.75 | 195 | 25.05 | 9.49 | 49.08 | 82.94 | 59.18 |
| 13 | 0.075 | 0.75 | 195 | 24.73 | 9.53 | 49.54 | 82.30 | 60.19 |
| 16 | 0.075 | 0.75 | 195 | 24.50 | 9.91 | 51.33 | 81.20 | 63.22 |
| 24 | 0.075 | 0.75 | 195 | 23.54 | 9.18 | 47.56 | 78.06 | 60.93 |
| Response | Model (Coded and Real Terms) | p-Value | R2 | CV (%) | SD (%) |
|---|---|---|---|---|---|
| Yield (%) | (Coded) = 49.10 + 1.98·A + 2.15·B + 5.57·C − 2.06·AC − 2.27·BC − 0.54·A2 − 1.09·C2 (Real) = −2345.52 + 3423.72·A + 362.70·B + 20.67·C − 16.48·AC − 1.82·BC − 871.20·A2 − 0.04·C2 | <0.0001 | 0.970 | 2.38 | 1.14 |
| Conversion (%) | (Coded) = 80.34 + 5.55·A + 4.41·B + 11.20·C − 1.30·AC − 2.50·BC − 1.78·C2 (Real) = 3533.74 + 2246.99·A + 406.90·B + 32.25·C − 10.38·AC − 2.00·BC − 0.07·C2 | <0.0001 | 0.977 | 2.46 | 1.95 |
| Selectivity (%) | (Coded) = 60.49 − 2.23·A − 1.19·B − 1.07·C (Real) = 112.51 − 89.22·A − 4.74·B − 0.21·C | <0.0001 | 0.911 | 1.26 | 0.77 |
| Maximise | A: FeCl3 (M) | B: Time (min) | C: Temp. (°C) | Yield (%) | Conversion (%) | Selectivity (%) |
|---|---|---|---|---|---|---|
| Yield | 0.07 | 0.5 | 200 | 53.71 | 87.53 | 60.77 |
| Conversion | 0.1 | 1 | 200 | 52.84 | 95.93 | 56.01 |
| Selectivity | 0.05 | 0.5 | 190 | 33.44 | 53.61 | 64.98 |
| Yield and selectivity | 0.05 | 0.5 | 200 | 53.24 | 83.59 | 62.84 |
| Yield and conversion | 0.1 | 1 | 200 | 52.84 | 95.93 | 56.01 |
| Yield, conversion, and selectivity | 0.05 | 0.5 | 200 | 53.24 | 83.59 | 62.84 |
| Star point experimental average | 0.075 | 0.75 | 202.07 | 55.10 | 92.43 | 59.62 |
| Predict star point | 0.075 | 0.75 | 202.07 | 54.81 | 92.62 | 58.98 |
| Out of range | 0.05 | 0.5 | 210 | 64.35 | 99.34 | 60.70 |
| Experimental out of range | 0.05 | 0.5 | 210 | 57.12 | 98.51 | 57.98 |
| Xylose | Solvent | Catalyst | Temperature/Time | Heating | Furfural Yield (%) | Ref. |
|---|---|---|---|---|---|---|
| 30 g/L= 200 mM | H2O | MSHS-SO3H (3.3 g/L) | 190 °C/1 h | Autoclave | 43.5 | [32] |
| 10 wt% | H2O | H-ZSM-5 (catalyst-xylose ratio, 0.3 w/w) | 200 °C/20 min | Autoclave | 46 | [33] |
| 35 mM | H2O | HCl (50 mM)-NaCl (850 mM) | 200 °C/5 min | Oil bath | 81.3 | [14] |
| 35 mM | H2O | HCl (50 mM)-NaCl (3.5 wt% = 599 mM) | 200 °C/440 s (7.3 min) | Microwave | 76 | [34] |
| 740 mM | H2O | HCl (100 mM) | 170 °C/30 min | Microwave | 40 | [17] |
| 667 mM | H2O | HCl (100 mM) | 180 °C/30 min | Microwave | 39 | [19] |
| 67 mM = 10 g/L | H2O | Maleic acid (250 mM) | 200 °C/28 min | Microwave | 67 | [35] |
| 57 mM | H2O | Not used | 200 °C/60 min | Microwave | 49 | [36] |
| 30 g/L | H2O | H2SO4 (2% w/v)-FeCl3 (0.05 M) | 210 °C/0.5 min | Microwave | 57.1 | Present study |
| 67 mM | H2O | Formic acid (30 wt%) | 200 °C/20 min | Oven preheating (360–420 °C) and a fluidized sand bath | 65 | [28] |
| 200 mM | H2O | Formic acid (30 wt%) | 200 °C/20 min | Oven preheating (360–420 °C) and a fluidized sand bath | 56.8 | [28] |
| 30 g/L | H2O | Formic acid (30 wt%) | 180 °C/80 min | Oven preheating (360–420 °C) and a fluidized sand bath | ~63.8 | [29] |
| 30 g/L | H2O | H2SO4 (0.2 wt%) | 180 °C/80 min | Oven preheating (360–420 °C) and a fluidized sand bath | ~56.2 | [29] |
| 18 wt% | H2O | H2SO4 (20 mM) | 250 °C/1 min | Supercritical flow reactor system | 64 | [37] |
| 50 mM | Seawater (salts (26,46 g/kg) | HCl (50 mM) | 200 °C/10 min | Oil bath | 71.7 | [38] |
| 150 mM | GVL | H2SO4 | 175 °C | Not specified | 75 | [39] |
| 2.4 wt% | GVL | FeCl3·6H2O (0.6 wt%) | 180 °C/9 min | Oil bath | 83.6 | [13] |
| 2 wt% | GVL-H2O (10 wt% H2O) | H2SO4 (0.05 M) | 170 °C/15 min | Oil bath | 87 | [40] |
| 2 wt% | GVL-H2O (10 wt% H2O) | H-Mordenite | 175 °C/2 h | Oil bath | 81 | [41] |
| 2 wt% | GVL-H2O (10 wt% H2O) | H-Beta (3.75 wt%) | 160 °C/1 h | Oil bath | 71 | [40] |
| 200 mM | DMSO | H-Mordenite (100 g/L) | 140 °C/4 h | Autoclave | 39 | [42] |
| 200 mM | DMSO | MP34CsPW (30 g/L) | 140 °C/4 h | Oil bath | 45 | [3] |
| 200 mM | DMSO | H3PW12O40 (PW) (20 g/L) | 140°C/4 h | Oil bath | 67 | [43] |
| 9.1 wt% | DMSO | Nafion 117 (20 wt% of initial xylose) | 150 °C/2 h | Oil bath | 60 | [44] |
| 10 wt% | DMSO | HCl (0.1 M)-Sn-beta | 110 °C/3 h | Not specified | 14.3 | [45] |
| 10 wt% | DMA | CrCl2 (6 mol% of xylose)-LiBr (10 wt%) | 100 °C/4 h | Oil bath | 56 | [46] |
| 20 wt% | [emin]Br | SnCl4 (10 mol% of xylose) | 130 °C/1 h | Oil bath | 71.1 | [15] |
| 100 g/L | [emim]HSO4 | Not used | 100 °C/30 min | Oil bath | 62 | [47] |
| 37.5 g/L | [bmim]PF6 | PEG-OSO3H (50 mM)-MnCl2 (75 mM) | 120 °C/18 min | Not specified | 75 | [48] |
| Xylose | Solvent | Catalyst | Temperature/Time | Heating | Furfural Yield (%) | Ref. |
|---|---|---|---|---|---|---|
| 1.85 wt% | [bmim]HSO4-MIBK (1:4.4, w/w) | Not used | 140 °C/4 h | Oil bath | 80.3 | [50] |
| 400 mmol/L H2O | H2O-2MTHF (1:1, v/v) | FeCl3 (80 mM)-NaCl (20 wt%) | 140 °C/4 h | Oil bath | 71 | [51] |
| 4 wt% H2O phase | H2O-CPME (1:2.33, v/v) | H2SO4 (1 wt% H2O phase)-NaCl (40 wt% H2O phase) | 170 °C/100 min | Oil bath | 100 | [52] |
| 1.25 mol/L H2O | H2O-CPME (1:3, v/v) | FeCl3 (5.08 g/L)-NaCl (18.13 g/L) | 170 °C/20 min | Microwave | 74 | [10] |
| 1 mol/L H2O | H2O-CPME (1:3, v/v) | NaCl (23.75 g/L)-Nafion NR50 (23.75 g/L) | 170 °C/40 min | Microwave | 80 | [49] |
| 200 g/L H2O | H2O-DMSO (1:1, v/v) | SnCl4 (catalyst/xylose molar ratio 0.5) | 130 °C/6 h | Oil bath | 63 | [53] |
| 10 wt% H2O phase | H2O-DMSO-SBP (5:1:5, v/v/v) | Sn-MMT (xylose-catalyst, 5:1 w/w)-NaCl (satured solution, aprox. 36 g/100 g of H2O) | 180 °C/30 min | Autoclave | 76.8 | [54] |
| 1 g/L,5 mL H2O | H2O-MIBK (1.5:8, v/v) | [Sbmim]HSO4 (0.5g/1.5 mL H2O) | 150 °C/25 min | Autoclave | 91.4 | [55] |
| 740 mmol/L H2O | H2O-MIBK (1:1, w/w) | HCl (0.1 mol/L H2O) | 170 °C/30 min | Microwave | 80 | [17] |
| 10 wt% of H2O | H2O-THF (1:2, w/w) | [SbPy]BF4 (100 wt% of initial xylose) | 180 °C/1 h | Microwave | 85 | [56] |
| 250 mmol/L H2O | H2O-THF (1:3, v/v) | AlCl3-6H2O (25 mM)-NaCl (1.5 M) | 140 °C/45 min | Microwave | 75 | [57] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padilla-Rascón, C.; Romero-García, J.M.; Ruiz, E.; Castro, E. Optimization with Response Surface Methodology of Microwave-Assisted Conversion of Xylose to Furfural. Molecules 2020, 25, 3574. https://doi.org/10.3390/molecules25163574
Padilla-Rascón C, Romero-García JM, Ruiz E, Castro E. Optimization with Response Surface Methodology of Microwave-Assisted Conversion of Xylose to Furfural. Molecules. 2020; 25(16):3574. https://doi.org/10.3390/molecules25163574
Chicago/Turabian StylePadilla-Rascón, Carmen, Juan Miguel Romero-García, Encarnación Ruiz, and Eulogio Castro. 2020. "Optimization with Response Surface Methodology of Microwave-Assisted Conversion of Xylose to Furfural" Molecules 25, no. 16: 3574. https://doi.org/10.3390/molecules25163574
APA StylePadilla-Rascón, C., Romero-García, J. M., Ruiz, E., & Castro, E. (2020). Optimization with Response Surface Methodology of Microwave-Assisted Conversion of Xylose to Furfural. Molecules, 25(16), 3574. https://doi.org/10.3390/molecules25163574








