High-Performance Cataluminescence Sensor Based on Nanosized V2O5 for 2-Butanone Detection
Abstract
1. Introduction
2. Results and Discussion
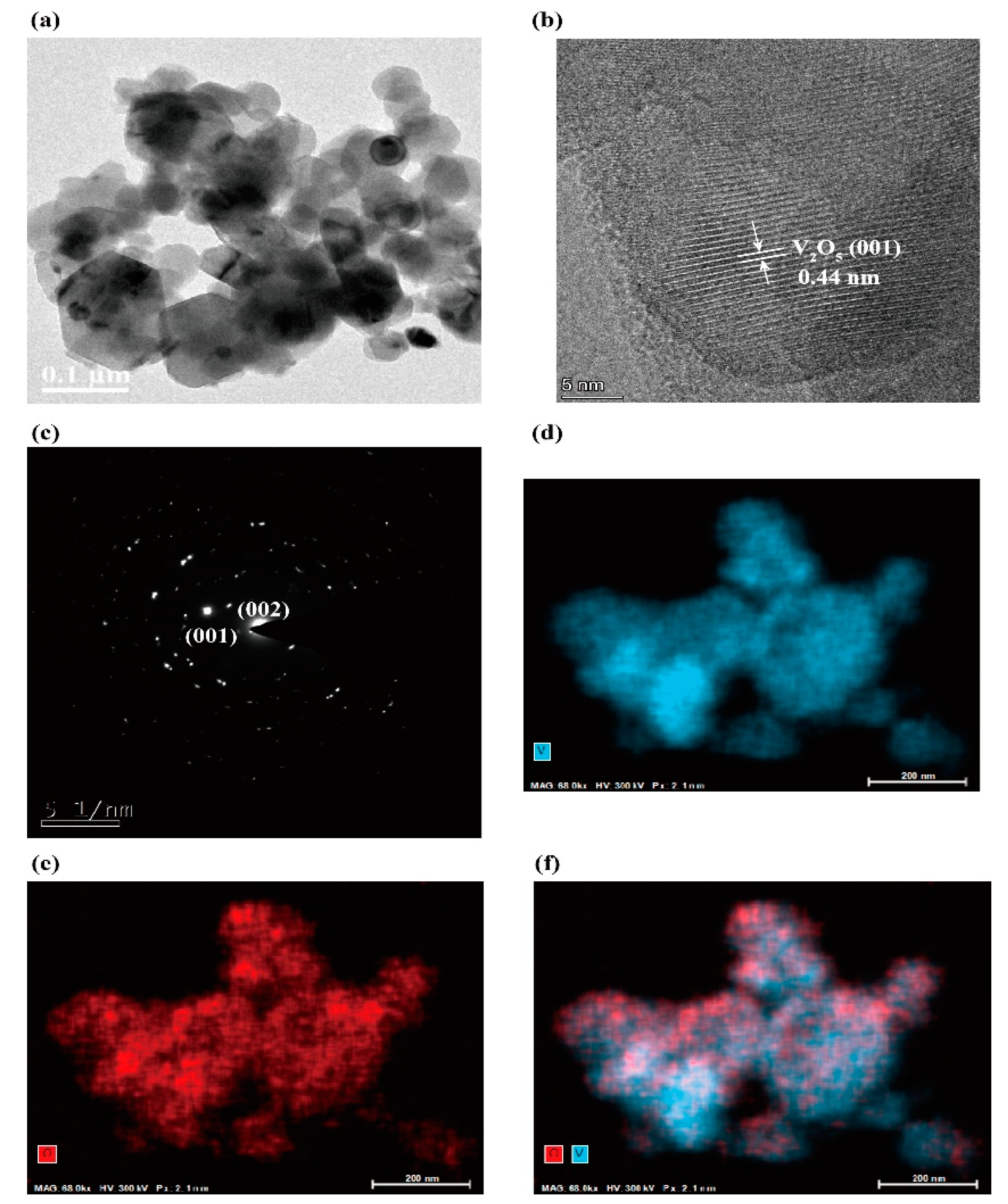
2.1. Characterizations of Nanosized V2O5
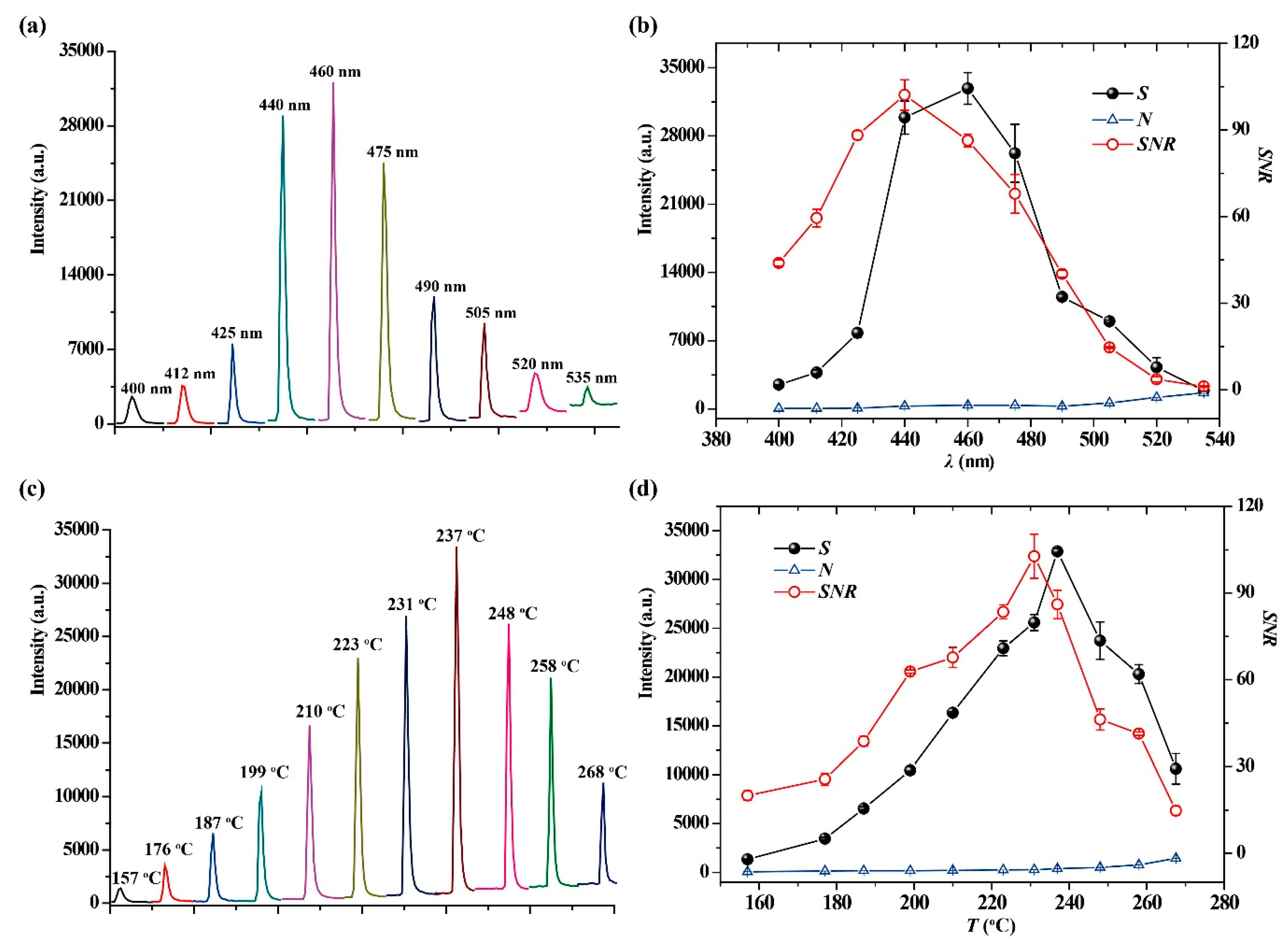
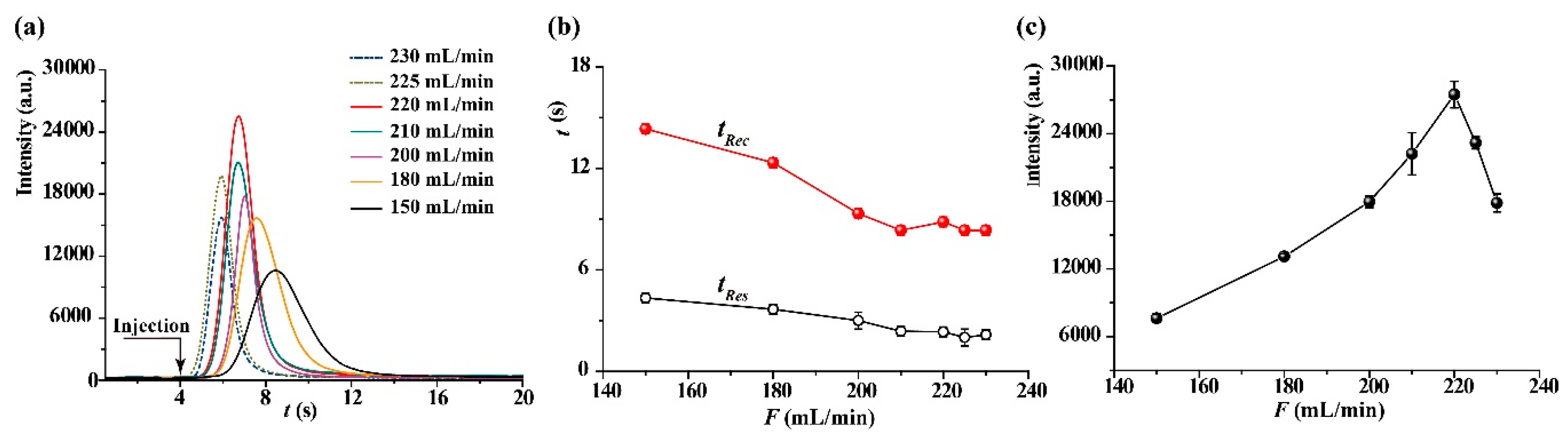
2.2. Optimization of Sensing Conditions
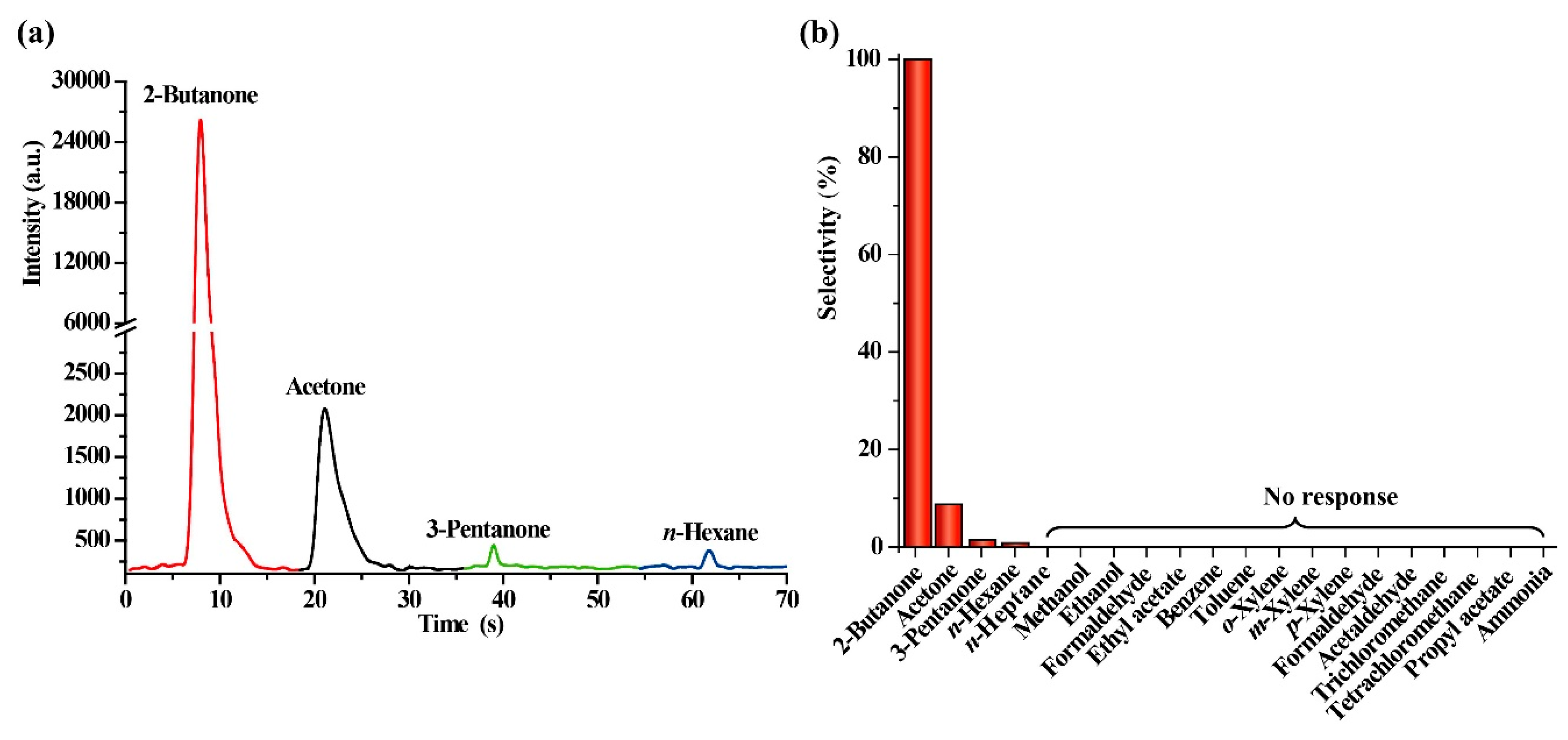
2.3. Evaluation of Selectivity
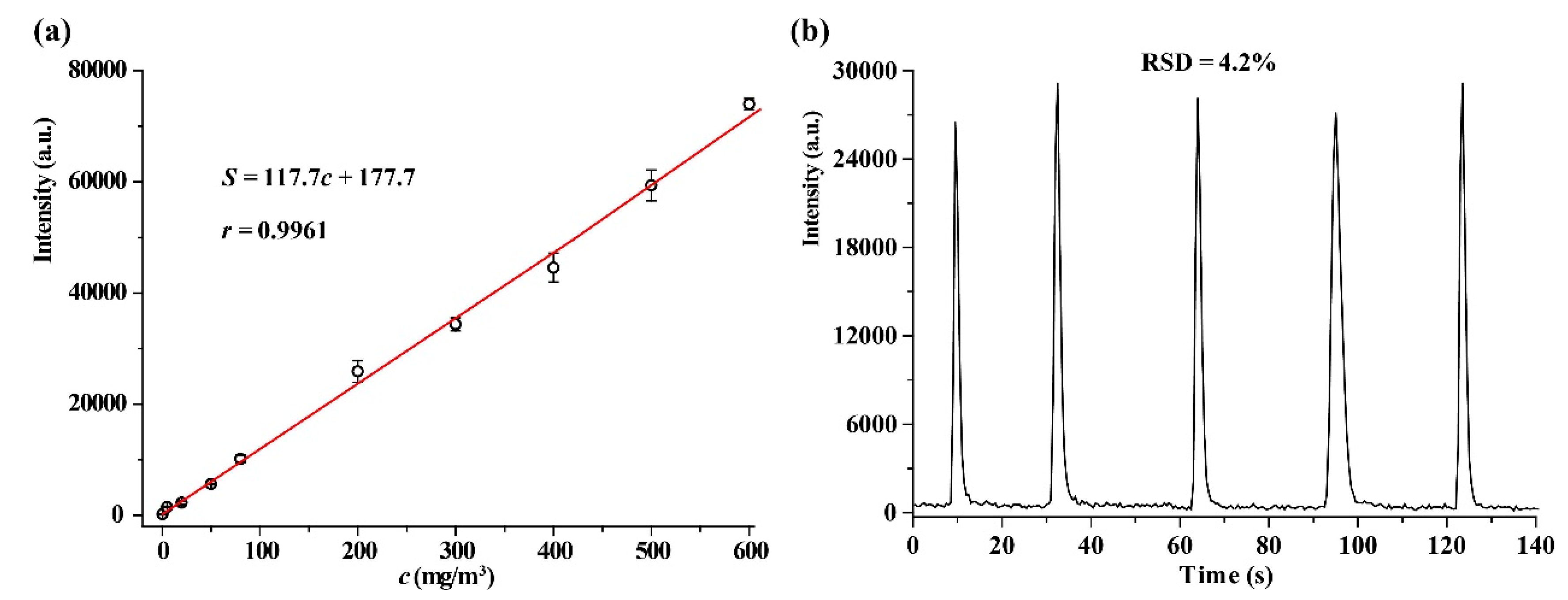
2.4. Analytical Characteristics
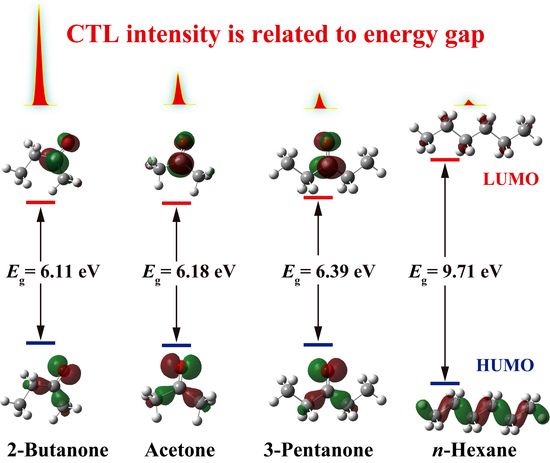
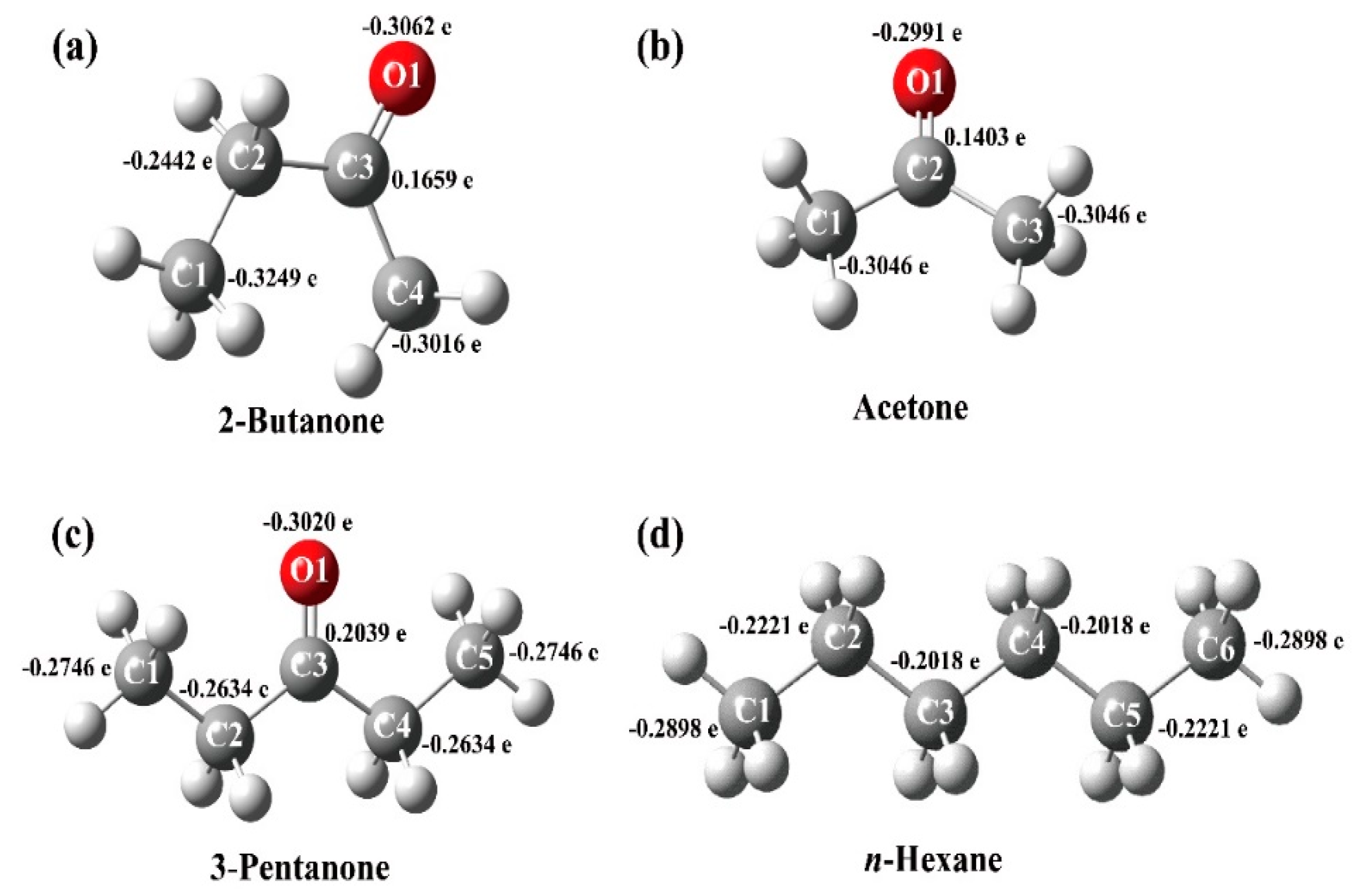
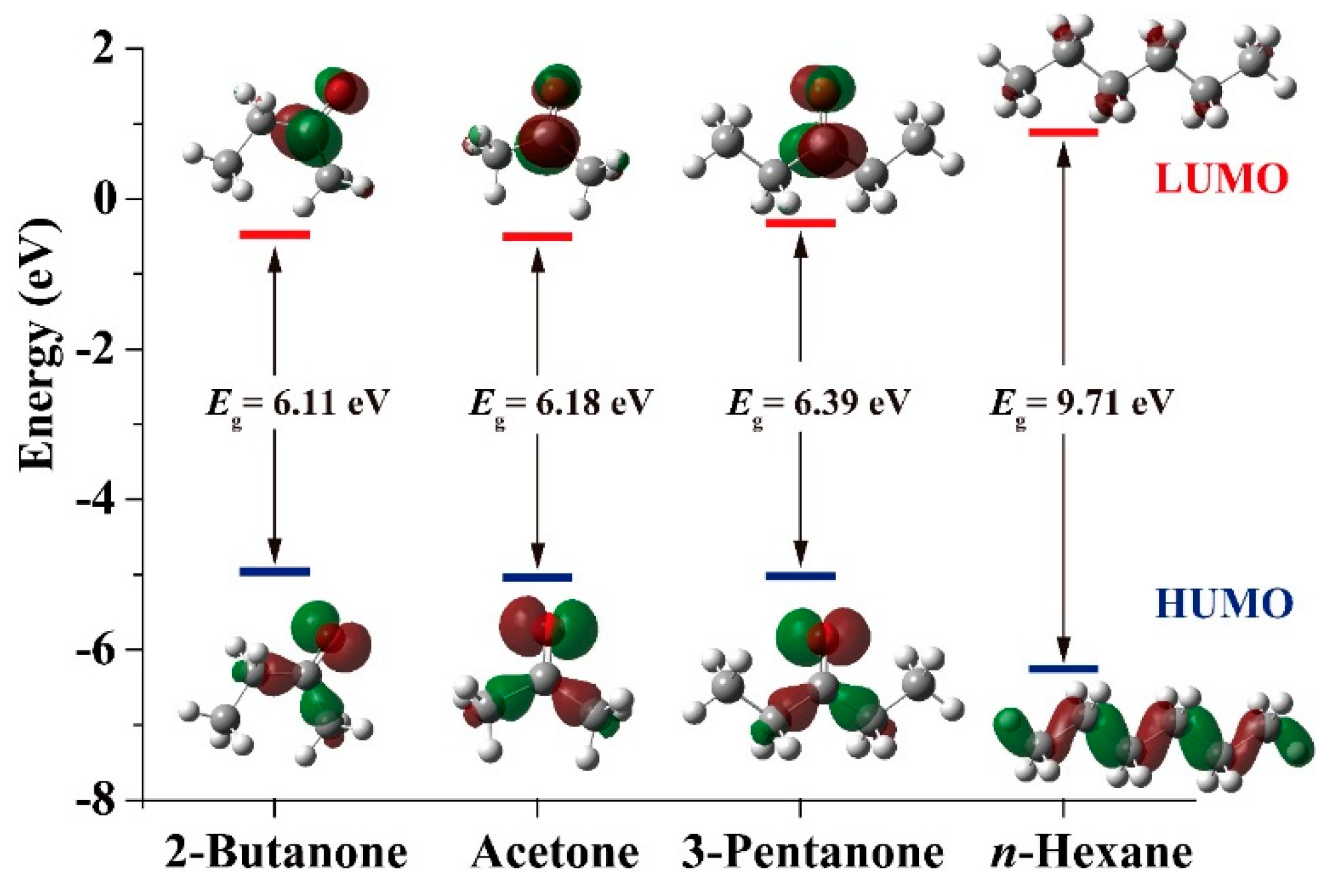
2.5. Gas-Sensing Selectivity Mechanism
3. Experimental Section
3.1. Synthesis and Characterizations of Nanosized V2O5
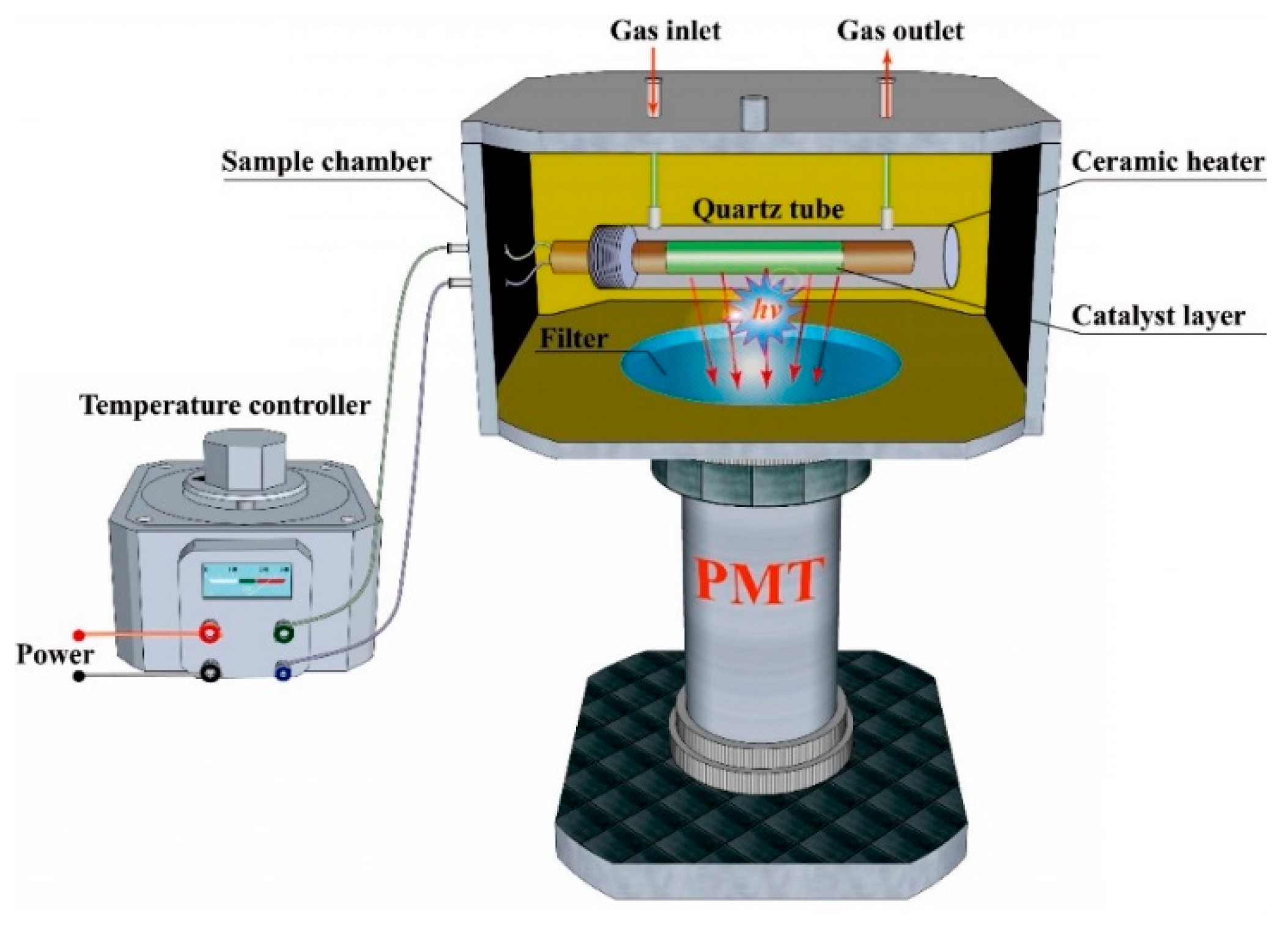
3.2. Fabrication of the Sensor System
3.3. Methods for Quantum Calculations
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Akif, A.; ARI, P.E.; Yenïsoy-Karakas, S. Source characterization and risk assessment of occupational exposure to volatile organic compounds (VOCs) in a barbecue restaurant. Build. Environ. 2020, 174, 106791–106798. [Google Scholar]
- Wan, K.C.; Yang, J.L.; Wang, D.; Wang, X.Y. Graphene Oxide@3D Hierarchical SnO2 nanofiber/nanosheets nanocomposites for Highly Sensitive and Low-Temperature formaldehyde detection. Molecules 2020, 25, 35. [Google Scholar] [CrossRef]
- Wang, H.W.; Wang, D.; Tian, L.; Li, H.J.; Wang, P.; Ou, N.Q.; Wang, X.Y.; Yang, J.H. Graphene-like porous ZnO/graphene oxide nanosheets for high-performance acetone vapor detection. Molecules 2019, 24, 522. [Google Scholar] [CrossRef]
- Liu, X.H.; Qin, X.X.; Ji, H.M.; Wang, M.J. An enhanced butanone sensing performance of Er0.7Yb0.3FeO3 material with the proper electronic structure. J. Alloy. Compd. 2019, 772, 263–271. [Google Scholar] [CrossRef]
- Bos, P.M.; de Mik, G.; Bragt, P.C. Critical review of the toxicity of methyl n-butyl ketone: Risk from occupational exposure. Am. J. Ind. Med. 1991, 20, 175–194. [Google Scholar] [CrossRef]
- Hazardous Substance Fact Sheet: Methyl Ethyl Ketone. Available online: https://www.nj.gov/health/eoh/rtkweb/documents/fs/1258.pdf (accessed on 2 August 2002).
- Yang, Y.Q.; Wang, X.D.; Yi, G.Y.; Li, H.M.; Shi, C.; Sun, G.; Zhang, Z.Y. Hydrothermal synthesis of Co3O4/ZnO hybrid nanoparticles for triethylamine detection. Nanomaterials 2019, 9, 1599. [Google Scholar] [CrossRef]
- Wang, J.; Shi, W.N.; Sun, X.; Wu, F.; Li, Y.; Hou, Y.X. Enhanced photo-assisted acetone gas sensor and efficient photocatalytic degradation using Fe-doped hexagonal and monoclinic WO3 phase-junction. Nanomaterials 2020, 10, 398. [Google Scholar] [CrossRef]
- Li, G.J.; Cheng, Z.X.; Xiang, Q.; Yan, L.M.; Wang, X.H.; Xu, J.Q. Bimetal Pd Au decorated SnO2 nanosheets based gas sensor with temperature-dependent dual selectivity for detecting formaldehyde and acetone. Sens. Actuators B 2019, 283, 590–601. [Google Scholar] [CrossRef]
- Lin, T.T.; Lv, X.; Hu, Z.N.; Xu, A.S.; Feng, C.H. Semiconductor metal oxides as chemoresistive sensors for detecting volatile organic compounds. Sensors 2019, 19, 233. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.R.; Min, L.; Wen, X.Y.; Ho, H.P.; Lu, H.F. Nanostructured ZnO/Ag film prepared by magnetron sputtering method for fast response of ammonia gas detection. Molecules 2020, 25, 1899. [Google Scholar] [CrossRef] [PubMed]
- Gulevich, D.; Rumyantseva, M.; Gerasimov, E.; Khmelevsky, N.; Tsvetkova, E.; Gaskov, A. Synergy effect of Au and SiO2 modification on SnO2 sensor properties in VOCs detection in humid air. Nanomaterials 2020, 10, 813. [Google Scholar] [CrossRef] [PubMed]
- Drobek, M.; Kim, J.H.; Bechelany, M.; Vallicari, C.; Julbe, A.; Kim, S.S. MOF-based membrane encapsulated ZnO nanowires for enhanced gas sensor selectivity. ACS Appl. Mater. Inter. 2016, 8, 8323–8328. [Google Scholar] [CrossRef] [PubMed]
- Kondalkar, V.V.; Duy, L.T.; Seo, H.; Lee, K. Nanohybrids of Pt-functionalized Al2O3/ZnO core-shell nanorods for high-performance MEMS-based acetylene gas sensor. ACS Appl. Mater. Inter. 2019, 11, 25891–25900. [Google Scholar] [CrossRef] [PubMed]
- Constantinoiu, I.; Viespe, C. Development of Pd/TiO2 porous layers by pulsed laser deposition for surface acoustic wave H2 gas sensor. Nanomaterials 2020, 10, 760. [Google Scholar] [CrossRef]
- Viespe, C.; Miu, D. Characteristics of surface acoustic wave sensors with nanoparticles embedded in polymer sensitive layers for VOC detection. Sensors 2018, 18, 2401. [Google Scholar] [CrossRef]
- Leong, A.; Saha, T.; Swamy, V.; Ramakrishnan, N. A langasite crystal microbalance coated with graphene oxide-platinum nanocomposite as a volatile organic compound sensor: Detection and discrimination characteristics. Sensors 2020, 20, 334. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, L.; Xing, H.Y.; Bai, P.H.; Liu, B.; Wang, C.J.; Lei, K.; Wang, H.; Peng, S.; Yang, S. Gas sensing of ordered and disordered structure SiO2 and their adsorption behavior based on quartz crystal microbalance. Sens. Actuators B 2020, 305, 127479–127485. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Shi, J.J.; Zhang, Z.Y.; Zhang, C.; Zhang, X.R. Development of a gas sensor utilizing chemiluminescence on nanosized titanium dioxide. Anal. Chem. 2002, 74, 120–124. [Google Scholar] [CrossRef]
- Yang, F.X.; Gu, C.X.; Liu, B.N.; Hou, C.J.; Zhou, K.W. Pt-activated Ce4La6O17 nanocomposites for formaldehyde and carbon monoxide sensor at low operating temperature. J. Alloy. Compd. 2019, 787, 173–179. [Google Scholar] [CrossRef]
- Luo, M.; Shao, K.; Long, Z.; Wang, L.X.; Peng, C.H.; Ouyang, J.; Na, N. A paper-based plasma-assisted cataluminescence sensor for ethylene detection. Sens. Actuators B 2017, 240, 132–141. [Google Scholar] [CrossRef]
- Wang, S.; Yuan, Z.Q.; Zhang, L.J.; Lin, Y.J.; Lu, C. Recent advances in cataluminescence-based optical sensing systems. Analyst 2017, 142, 1415–1428. [Google Scholar] [CrossRef] [PubMed]
- Na, N.; Liu, H.Y.; Han, J.; Han, F.F.; Liu, H.L.; Ouyang, J. Plasma-assisted cataluminescence sensor array for gaseous hydrocarbons discrimination. Anal. Chem. 2012, 84, 4830–4836. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.K.; Wang, D.; Wu, Y.J.; Hu, Y.H.; Chen, J.Y.; He, J.C.; Wang, J.X. A Cataluminescence Sensor based on NiO nanoparticles for sensitive detection of acetaldehyde. Molecules 2020, 25, 1097. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wei, C.D.; Song, H.J.; Yang, Y.S.; Xue, Y.; Deng, D.Y.; Lv, Y. Cataluminescence coupled with photoassisted technology: A highly efficient metal-free gas sensor for carbon monoxide. Anal. Chem. 2019, 91, 13158–13164. [Google Scholar] [CrossRef]
- Wan, X.Y.; Song, H.J.; Zhao, D.; Zhang, L.C.; Lv, Y. A Y-doped metal-organic framework-based cataluminescence gas sensor for isobutanol. Sens. Actuators B 2014, 201, 413–419. [Google Scholar] [CrossRef]
- Li, Z.H.; Xi, W.; Lu, C. Hydrotalcite-supported gold nanoparticle catalysts as a low temperature cataluminescence sensing platform. Sens. Actuators B 2015, 219, 354–360. [Google Scholar] [CrossRef]
- Zheng, J.Z.; Zhang, T.M.; Zeng, H.J.; Guo, W.; Zhao, B.; Sun, Y.H.; Li, Y.Y.; Jiang, L. Multishelled hollow structures of yttrium oxide for the highly selective and ultrasensitive detection of methanol. Small 2019, 15, 1804688–1804696. [Google Scholar] [CrossRef]
- Datta, S.; Jo, C.S.; De Volder, M.; Torrente-Murciano, L. Morphological control of nanostructured V2O5 by deep eutectic solvents. ACS Appl. Mater. Inter. 2020, 12, 18803–18812. [Google Scholar] [CrossRef]
- Zhang, Z.T.; Wu, G.D.; Ji, H.P.; Chen, D.L.; Xia, D.C.; Gao, K.K.; Xu, J.F.; Mao, B.; Yi, S.S.; Zhang, L.Y. 2D/1D V2O5 nanoplates anchored carbon nanofibers as efficient separator interlayer for highly stable lithium–sulfur battery. Nanomaterials 2020, 10, 705. [Google Scholar] [CrossRef]
- Jin, W.; Yan, S.L.; An, L.; Chen, W.; Yang, S.; Zhao, C.X.; Dai, Y. Enhancement of ethanol gas sensing response based on ordered V2O5 nanowire microyarns. Sens. Actuators B 2015, 206, 284–290. [Google Scholar] [CrossRef]
- Yin, H.H.; Song, C.Q.; Wang, Z.L.; Shao, H.B.; Li, Y.; Deng, H.H.; Ma, Q.L.; Yu, K. Self-assembled vanadium oxide nanoflakes for p-type ammonia sensors at room temperature. Nanomaterials 2019, 9, 317. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, Y.; Mani, G.K.; Ponnusamy, D.; Shankar, P.; Kulandaisamy, A.J.; Tsuchiya, K.; Rayappan, J.B.B.; Reddy, M.R. V2O5 nanofibers: Potential contestant for high performance xylene sensor. J. Alloy.Compd. 2018, 731, 805–812. [Google Scholar] [CrossRef]
- Peng, F.; Sun, Y.; Yu, W.W.; Lu, Y.; Hao, J.M.; Cong, R.; Ge, M.Y.; Shi, J.C.; Dai, N. Studies on sensing properties and mechanism of CuO nanoparticles to H2S gas. Nanomaterials 2020, 10, 774. [Google Scholar] [CrossRef] [PubMed]
- Rafiqi, F.A.; Rather, M.S.; Majid, K. Doping polyaniline with copper bisglycinate [Cu(gly)2]—Synthesis, characterization and thermal study. Synth. Met. 2013, 171, 32–38. [Google Scholar] [CrossRef]
- Bushroa, A.R.; Rahbari, R.G.; Masjuki, H.H.; Muhamad, M.R. Approximation of crystallite size and microstrain via XRD line broadening analysis in TiSiN thin films. Vacuum 2012, 86, 1107–1112. [Google Scholar] [CrossRef]
- Li, L.; Yang, H.K.; Moon, B.K.; Choi, B.C.; Jeong, J.H.; Kim, K.H. Photoluminescent properties of Ln2O3: Eu3+ (Ln = Y, Lu and Gd) prepared by hydrothermal process and sol-gel method. Mater. Chem. Phys. 2010, 119, 471–477. [Google Scholar] [CrossRef]
- Breysse, M.; Claudel, B.; Faure, L.; Guenin, M.; Williams, R.J.; Wolkenstein, T. Chemiluminescence during the catalysis of carbon monoxide oxidation on a thoria surface. J. Catal. 1976, 45, 137–144. [Google Scholar] [CrossRef]
- Mondal, B.; Basumatari, B.; Das, J.; Roychaudhury, C.; Saha, H.; Mukherjee, N. ZnO-SnO2 based composite type gas sensor for selective hydrogen sensing. Sens. Actuators B 2014, 194, 389–396. [Google Scholar] [CrossRef]
- Weng, Y.Y.; Zhang, L.C.; Zhu, W.; Lv, Y. One-step facile synthesis of coral-like Zn-doped SnO2 and its cataluminescence sensing of 2-butanone. J. Mater. Chem. A 2015, 3, 7132–7138. [Google Scholar] [CrossRef]
- Zito, C.A.; Perfecto, T.M.; Oliveira, T.N.; Volanti, D.P. Bicone-like ZnO structure as high-performance butanone sensor. Mater. Lett. 2018, 223, 142–145. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, H.; Xu, M.K.; Shen, Z.R.; Wei, Q. A WO3 nanorod-Cr2O3 nanoparticle composite for selective gas sensing of 2-butanone. Chin. Chem. Lett. 2018, 29, 538–542. [Google Scholar] [CrossRef]
- Shah, K.W.; Li, W. A review on catalytic nanomaterials for volatile organic compounds VOC removal and their applications for healthy buildings. Nanomaterials 2019, 9, 910. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Morales, Y. HOMO−LUMO gap as an index of molecular size and structure for polycyclic aromatic hydrocarbons (PAHs) and asphaltenes: A theoretical study. I. J. Mater. Chem. A 2002, 106, 11283–11308. [Google Scholar] [CrossRef]
- Yoo, R.; Li, D.M.; Rim, H.J.; Cho, S.; Lee, H.; Lee, W. High sensitivity in Al-doped ZnO nanoparticles for detection of acetaldehyde. Sens. Actuators B 2018, 266, 883–888. [Google Scholar] [CrossRef]
- Jia, Q.; Ji, H.M.; Wang, D.H.; Bai, X.; Sun, X.H.; Jin, Z.G. Exposed facets induced enhanced acetone selective sensing property of nanostructured tungsten oxide. J. Mater. Chem. A 2014, 2, 13602–13611. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
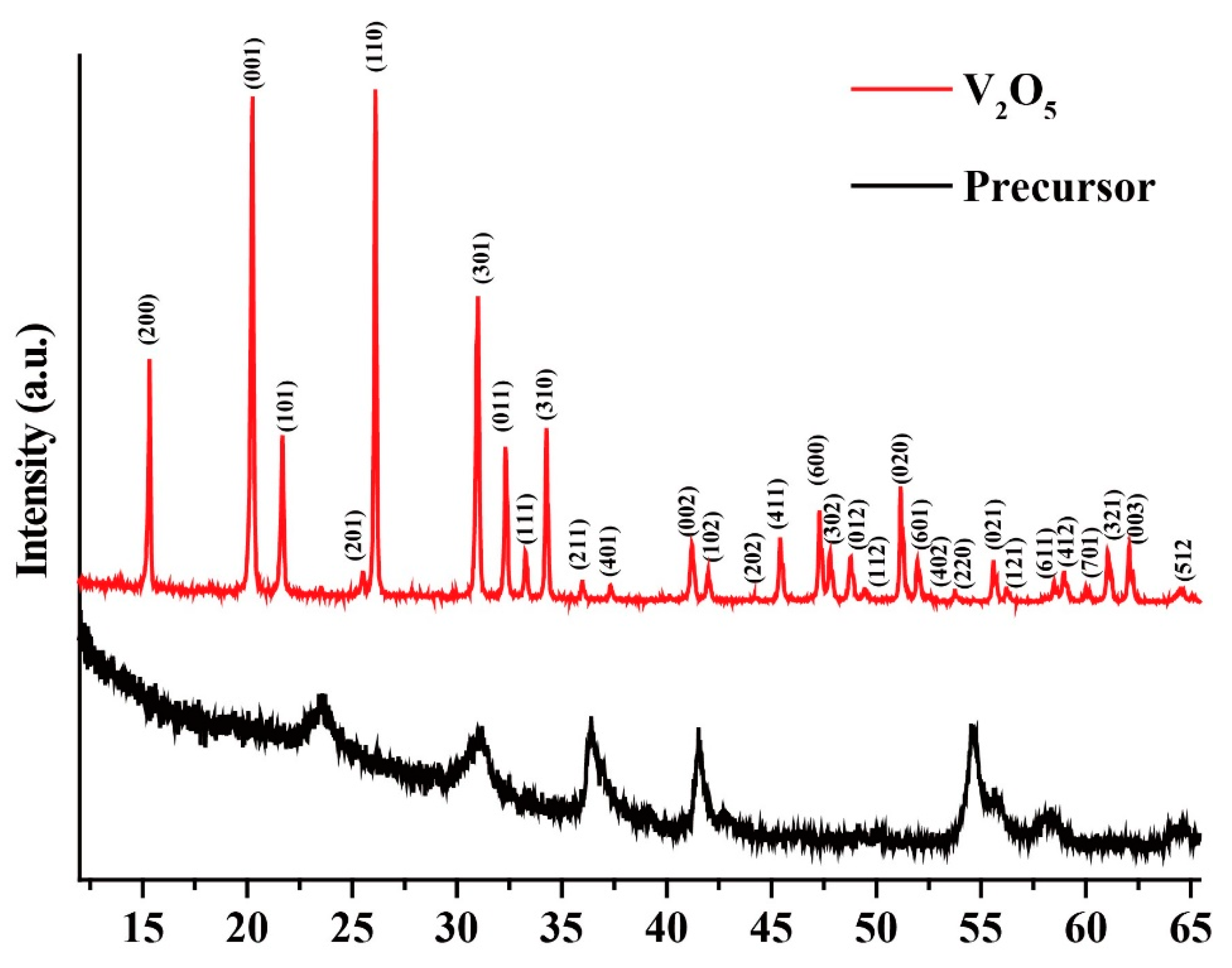
| No. | 2θ (o) | Crystal Planes | β (o) | D (nm) | d-Spacing (Å) | δ × 10−3 (nm−2) | ε × 10−3 |
|---|---|---|---|---|---|---|---|
| 1 | 15.33 | (200) | 0.2812 | 28.51 | 5.777 | 1.231 | 9.119 |
| 2 | 20.29 | (001) | 0.3049 | 26.47 | 4.373 | 1.427 | 7.434 |
| 3 | 21.68 | (101) | 0.2726 | 29.67 | 4.096 | 1.136 | 6.212 |
| 4 | 25.43 | (201) | 0.6638 | 12.27 | 3.500 | 6.643 | 12.84 |
| 5 | 26.09 | (110) | 0.2572 | 31.71 | 3.412 | 0.994 | 4.842 |
| 6 | 30.99 | (301) | 0.2598 | 31.73 | 2.883 | 0.993 | 4.088 |
| 7 | 32.33 | (011) | 0.2517 | 32.86 | 2.767 | 0.926 | 3.789 |
| 8 | 33.30 | (111) | 0.2407 | 34.44 | 2.689 | 0.843 | 3.513 |
| 9 | 34.38 | (310) | 0.3570 | 23.29 | 2.606 | 1.843 | 5.035 |
| 10 | 36.01 | (211) | 0.1961 | 37.80 | 2.492 | 0.551 | 2.632 |
| 11 | 37.35 | (401) | 0.1351 | 83.92 | 2.406 | 0.260 | 1.744 |
| 12 | 40.13 | (311) | 0.6619 | 12.78 | 2.245 | 6.125 | 7.907 |
| 13 | 41.22 | (002) | 0.2392 | 35.49 | 2.189 | 0.794 | 2.775 |
| 14 | 41.94 | (102) | 0.1983 | 42.91 | 2.152 | 0.543 | 2.257 |
| 15 | 44.25 | (202) | 0.5745 | 14.93 | 2.045 | 4.488 | 6.166 |
| 16 | 45.39 | (411) | 0.2626 | 32.79 | 1.997 | 0.930 | 2.740 |
| 17 | 47.33 | (600) | 0.2868 | 32.51 | 1.919 | 1.093 | 2.855 |
| 18 | 47.84 | (302) | 0.2932 | 29.64 | 1.900 | 1.138 | 2.884 |
| 19 | 48.78 | (012) | 0.2805 | 39.56 | 1.865 | 1.034 | 2.699 |
| 20 | 49.51 | (112) | 0.2962 | 90.94 | 1.840 | 1.146 | 2.803 |
| 21 | 51.20 | (020) | 0.2663 | 33.08 | 1.783 | 0.914 | 2.425 |
| 22 | 52.05 | (601) | 0.2992 | 29.55 | 1.756 | 1.145 | 2.673 |
| 23 | 52.59 | (402) | 0.5593 | 15.84 | 1.739 | 3.984 | 4.939 |
| 24 | 53.74 | (220) | 1.4643 | 6.08 | 1.704 | 27.03 | 12.61 |
| 25 | 55.56 | (021) | 0.2473 | 36.31 | 1.653 | 0.758 | 2.048 |
| 26 | 56.22 | (121) | 0.2986 | 91.35 | 1.635 | 1.099 | 2.439 |
| 27 | 58.46 | (611) | 0.0980 | 95.82 | 1.577 | 0.116 | 0.764 |
| 28 | 59.00 | (412) | 0.2296 | 39.76 | 1.564 | 0.632 | 1.770 |
| 29 | 60.10 | (701) | 4.4027 | 2.08 | 1.538 | 230.1 | 33.21 |
| 30 | 61.19 | (321) | 0.3442 | 26.81 | 1.514 | 1.391 | 2.540 |
| 31 | 62.09 | (003) | 0.2417 | 38.36 | 1.494 | 0.680 | 1.752 |
| 32 | 64.58 | (512) | 1.0553 | 8.91 | 1.442 | 12.610 | 7.287 |
| Average values | 30.46 | 2.330 | 9.832 | 5.275 | |||
| Principle | Materials | Temperature (°C) | Linear Rage (mg/m3) | LOD (mg/m3) | References |
|---|---|---|---|---|---|
| CTL | V2O5 | 231 | 0.5–600 | 0.2 | Present work |
| CTL | Zn-doped SnO2 | 241 | 2310–92570 | 600 | [40] |
| Electrochemistry | ZnO | 400 | 5.9–295 | 1.2 | [41] |
| Electrochemistry | WO3-Cr2O3 | 205 | 14.75–295 | ND | [42] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.-K.; Wang, J.-X.; Cao, H. High-Performance Cataluminescence Sensor Based on Nanosized V2O5 for 2-Butanone Detection. Molecules 2020, 25, 3552. https://doi.org/10.3390/molecules25153552
Zhang R-K, Wang J-X, Cao H. High-Performance Cataluminescence Sensor Based on Nanosized V2O5 for 2-Butanone Detection. Molecules. 2020; 25(15):3552. https://doi.org/10.3390/molecules25153552
Chicago/Turabian StyleZhang, Run-Kun, Jing-Xin Wang, and Hua Cao. 2020. "High-Performance Cataluminescence Sensor Based on Nanosized V2O5 for 2-Butanone Detection" Molecules 25, no. 15: 3552. https://doi.org/10.3390/molecules25153552
APA StyleZhang, R.-K., Wang, J.-X., & Cao, H. (2020). High-Performance Cataluminescence Sensor Based on Nanosized V2O5 for 2-Butanone Detection. Molecules, 25(15), 3552. https://doi.org/10.3390/molecules25153552