Streptomyces sp. Strain MUSC 125 from Mangrove Soil in Malaysia with Anti-MRSA, Anti-Biofilm and Antioxidant Activities
Abstract
1. Introduction
2. Results
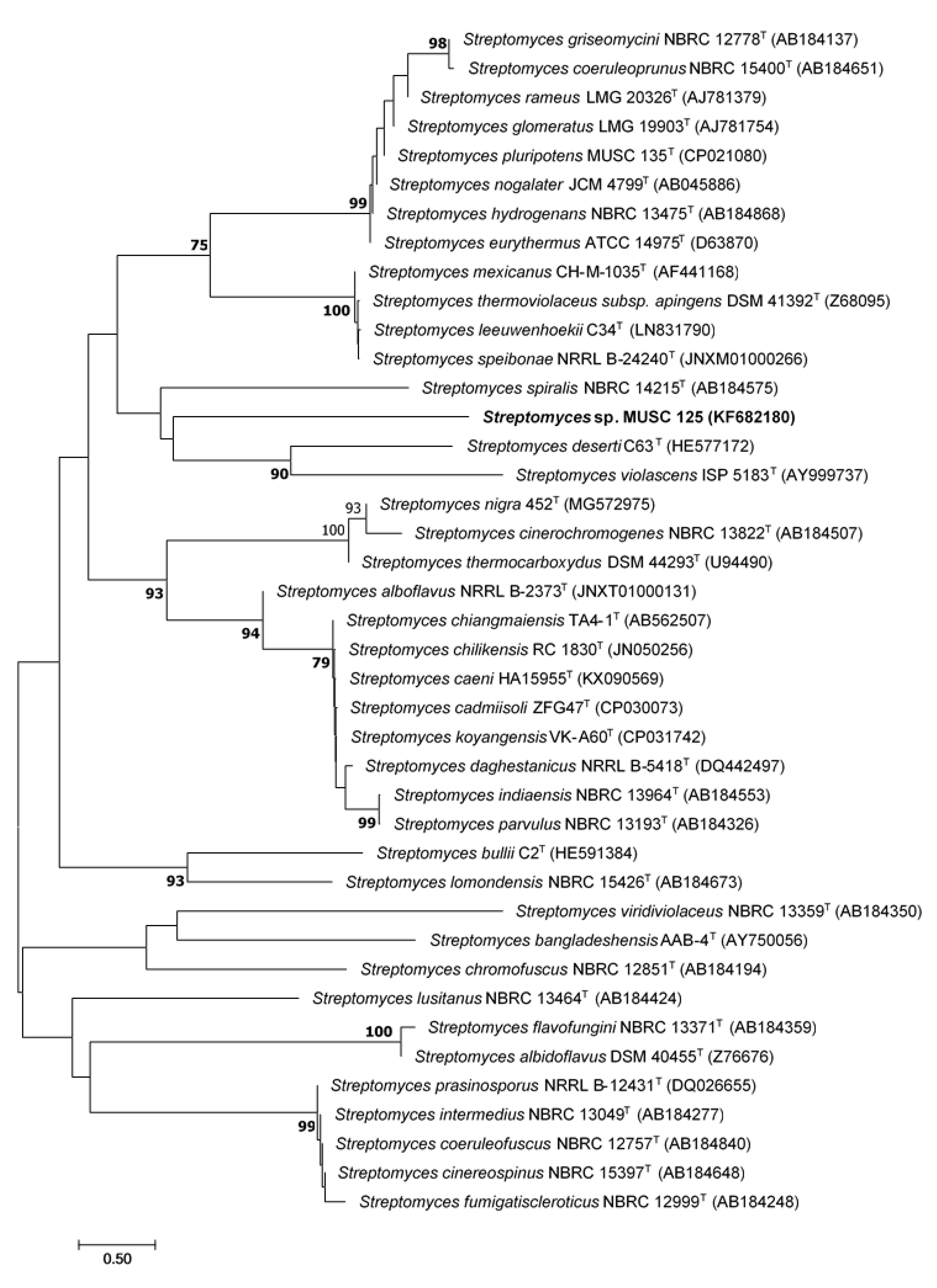
2.1. Phylogenetic Analysis of Strain MUSC 125
2.2. Phenotypic Characterization of Strain MUSC 125
2.3. Anti-MRSA Activity by Agar Well Diffusion Assay
2.4. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of Methanolic Extract MUSC 125
2.5. Anti-Biofilm/Anti-Adherence Activity of Methanolic Extract of Strain MUSC 125
2.6. Antioxidant Assays
2.6.1. 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) and 2,2-diphenyl-1-picrylhydrazyl (DPPH) Antioxidant Assays
2.6.2. Metal Chelating Assay
2.6.3. Ferric Reduction Antioxidant Power (FRAP) Assay
2.7. Total Phenolic Content (TPC) Determination with Folin–Ciocalteu’s Reagent Method
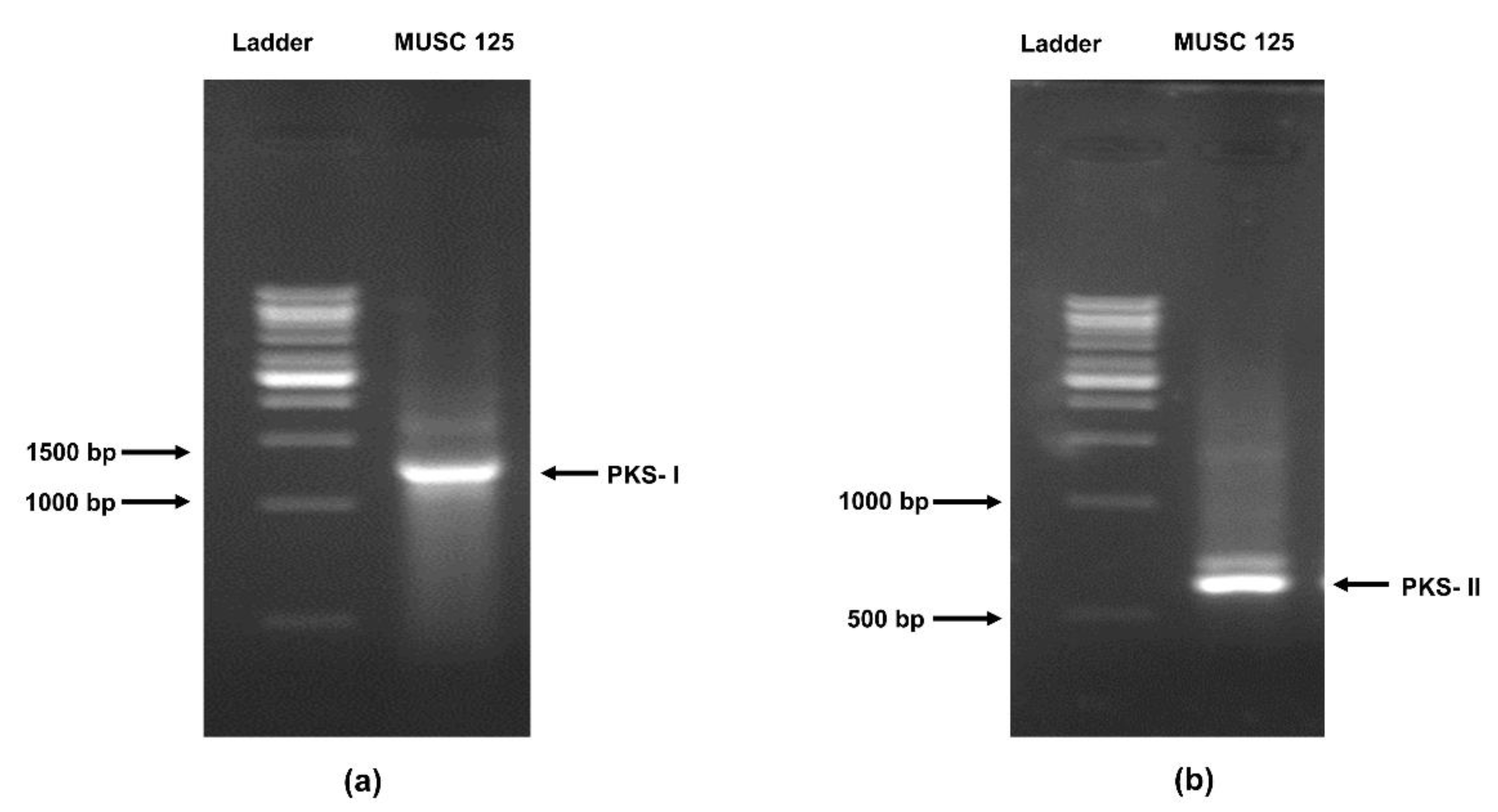
2.8. Detection of the Polyketide Synthase (pks) and Non-Ribosomal Peptides Synthase (nrps) Genes in Strain MUSC 125
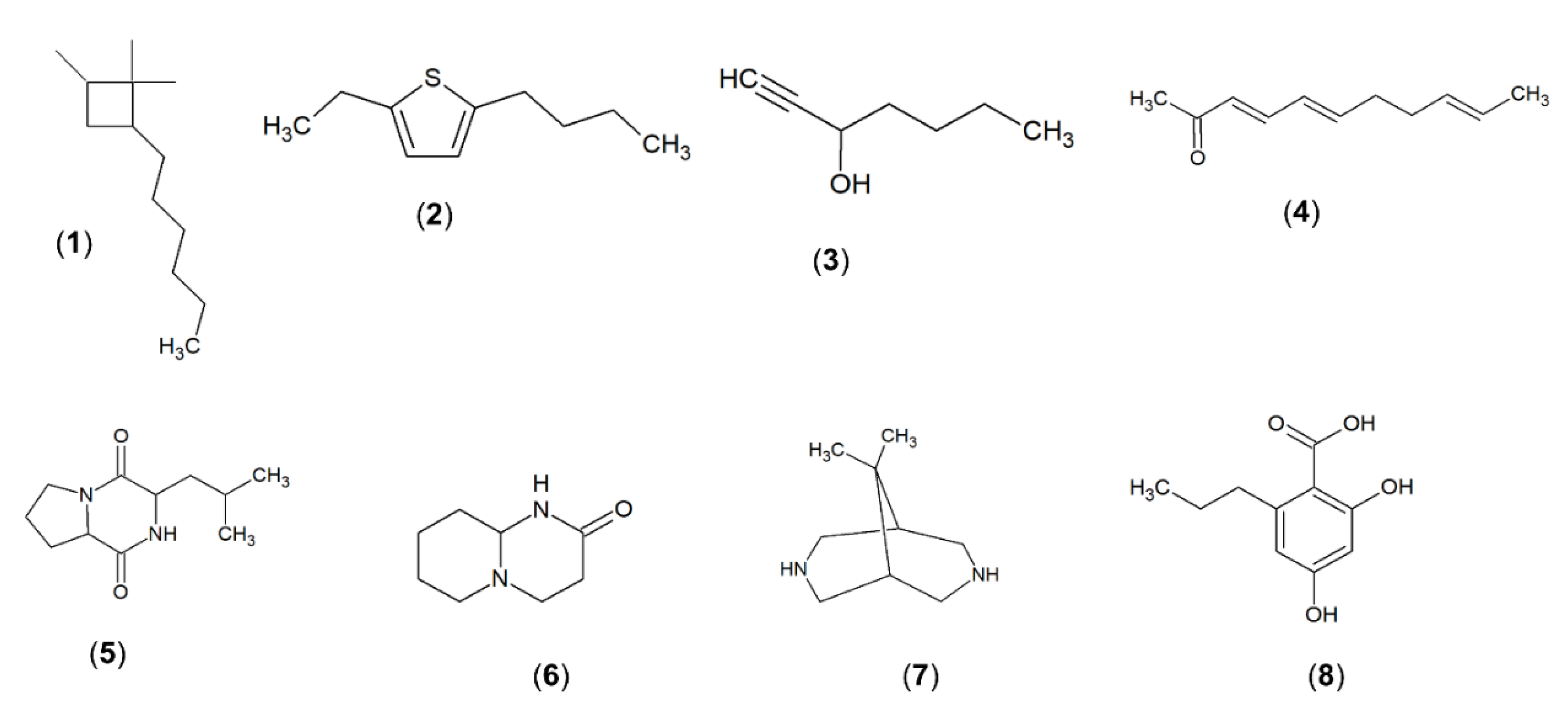
2.9. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis of Methanolic Extract of Strain MUSC 125
3. Discussion
4. Materials and Methods
4.1. Isolation Source and Maintenance of Strain MUSC 125
4.2. Extraction of DNA and 16S rRNA Phylogenetic Analysis
4.3. Phenotypic Characteristics of Strain MUSC 125
4.4. Fermentation and Extract Preparation of Strain MUSC 125
4.5. Anti-MRSA Activity of Methanolic Extract of Strain MUSC 125
4.6. MIC and MBC of the Methanolic Extract MUSC 125
4.7. Anti-Biofilm/Anti-Adherence activity of the Methanolic Extract MUSC 125
4.8. Antioxidant Assays
4.8.1. ABTS Scavenging Antioxidant Assay
4.8.2. DPPH Scavenging Antioxidant Assay
4.8.3. Metal Chelating Assay
4.8.4. Ferric Reduction Antioxidant Power (FRAP) Assay
4.9. TPC by Folin–Ciocalteu’s Reagent Method
4.10. Detection of pks and nrps Genes in Strain MUSC 125
4.11. Chemical Profiling of Methanolic Extract MUSC 125 with GC-MS
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Data Availability Statement
References
- Teerawattanapong, N.; Panich, P.; Kulpokin, D.; Ranong, S.N.; Kongpakwattana, K.; Saksinanon, A.; Goh, B.-H.; Lee, L.-H.; Apisarnthanarak, A.; Chaiyakunapruk, N. A systematic review of the burden of multidrug-resistant healthcare-associated infections among intensive care unit patients in Southeast Asia: The rise of multidrug-resistant Acinetobacter baumannii. Infect. Control Hosp. Epidemiol. 2018, 39, 525–533. [Google Scholar] [CrossRef]
- Letchumanan, V.; Loo, K.-Y.; Law, J.W.-F.; Wong, S.H.; Goh, B.-H.; Ab Mutalib, N.-S.; Lee, L.-H. Vibrio parahaemolyticus: The protagonist of foodborne diseases. Prog. Microbes Mol. Biol. 2019, 2, a0000029. [Google Scholar] [CrossRef]
- Jevons, M.P. “Celbenin”-resistant staphylococci. Br. Med. J. 1961, 1, 124. [Google Scholar] [CrossRef]
- Mahdiyoun, S.M.; Kazemian, H.; Ahanjan, M.; Houri, H.; Goudarzi, M. Frequency of aminoglycoside-resistance genes in methicillin-resistant Staphylococcus aureus (MRSA) isolates from hospitalized patients. Jundishapur J. Microbiol. 2016, 9, e35052. [Google Scholar] [CrossRef]
- Khan, T.M.; Kok, Y.L.; Bukhsh, A.; Lee, L.-H.; Chan, K.-G.; Goh, B.-H. Incidence of methicillin resistant Staphylococcus aureus (MRSA) in burn intensive care unit: A systematic review. Germs 2018, 8, 113. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S.; de Lencastre, H.; Garau, J.; Kluytmans, J.; Malhotra-Kumar, S.; Peschel, A.; Harbarth, S. Methicillin-resistant Staphylococcus aureus. Nat. Rev. Dis. Primers 2018, 4, 18033. [Google Scholar] [CrossRef] [PubMed]
- Omidi, M.; Firoozeh, F.; Saffari, M.; Sedaghat, H.; Zibaei, M.; Khaledi, A. Ability of biofilm production and molecular analysis of spa and ica genes among clinical isolates of methicillin-resistant Staphylococcus aureus. BMC Res. Notes 2020, 13, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Choo, E.J.; Chambers, H.F. Treatment of methicillin-resistant Staphylococcus aureus bacteremia. Infect. Chemother. 2016, 48, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Ghahremani, M.; Jazani, N.H.; Sharifi, Y. Emergence of vancomycin-intermediate and-resistant Staphylococcus aureus among methicillin-resistant S. aureus isolated from clinical specimens in the northwest of Iran. J. Glob. Antimicrob. Resist. 2018, 14, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Vamsimohan, A.; Gupta, S.; Muralidharan, S. Daptomycin resistance in methicillin-resistant Staphylococcus aureus: A report from Southern India. Germs 2014, 4, 70. [Google Scholar] [CrossRef]
- World Health Organization WHO Publishes List of Bacteria for Which New Antibiotics are Urgently Needed. Available online: http://www.who.int/mediacentre/news/releases/2017/bacteria-antibiotics-needed/en/ (accessed on 8 June 2020).
- Waksman, S.A.; Henrici, A.T. The nomenclature and classification of the actinomycetes. J. Bacteriol. 1943, 46, 337. [Google Scholar] [CrossRef] [PubMed]
- The List of Prokaryotic Names with Standing in Nomenclature Genus Streptomyces. Available online: https://lpsn.dsmz.de/genus/streptomyces (accessed on 24 April 2020).
- Tan, L.T.-H.; Lee, L.-H.; Goh, B.-H. The bioprospecting of anti-Vibrio Streptomyces species: Prevalence and applications. Prog. Microbes Mol. Biol. 2019, 2, a0000034. [Google Scholar] [CrossRef]
- Tan, L.T.-H.; Lee, L.-H.; Goh, B.-H. Critical review of fermentation and extraction of anti-Vibrio compounds from Streptomyces. Prog. Microbes Mol. Biol. 2020, 3, a0000051. [Google Scholar] [CrossRef]
- de Lima Procópio, R.E.; da Silva, I.R.; Martins, M.K.; de Azevedo, J.L.; de Araújo, J.M. Antibiotics produced by Streptomyces. Braz. J. Infect. Dis. 2012, 16, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Kemung, H.M.; Tan, L.T.H.; Khan, T.M.; Chan, K.-G.; Pusparajah, P.; Goh, B.H.; Lee, L.-H. Streptomyces as a prominent resource of future anti-MRSA drugs. Front. Microbiol. 2018, 9, 2221. [Google Scholar] [CrossRef]
- Law, J.W.-F.; Pusparajah, P.; Ab Mutalib, N.-S.; Wong, S.H.; Goh, B.-H.; Lee, L.-H. A Review on Mangrove Actinobacterial Diversity: The Roles of Streptomyces and Novel Species Discovery. Prog. Microbes Mol. Biol. 2019, 1, a0000024. [Google Scholar] [CrossRef]
- Alongi, D.M. Mangrove Forests. In Blue Carbon; Springer: Berlin, Germany, 2018; pp. 23–36. [Google Scholar]
- Uddin, S.M.; Hoque, A.R.; Abdullah, S.A. The changing landscape of mangroves in Bangladesh compared to four other countries in tropical regions. J. For. Res. 2014, 25, 605–611. [Google Scholar] [CrossRef]
- Ashton, E.C.; Macintosh, D.J.; Hogarth, P.J. A baseline study of the diversity and community ecology of crab and molluscan macrofauna in the Sematan mangrove forest, Sarawak, Malaysia. J. Trop. Ecol. 2003, 19, 127–142. [Google Scholar] [CrossRef]
- Hamilton, S.E. Botany of Mangroves. In Mangroves and Aquaculture; Springer: Berlin, Germany, 2020; pp. 1–40. [Google Scholar]
- Nabeelah Bibi, S.; Fawzi, M.M.; Gokhan, Z.; Rajesh, J.; Nadeem, N.; RR, R.K.; RDDG, A.; Pandian, S.K. Ethnopharmacology, phytochemistry, and global distribution of mangroves―A comprehensive review. Mar. Drugs 2019, 17, 231. [Google Scholar] [CrossRef]
- Law, J.W.-F.; Chan, K.-G.; He, Y.-W.; Khan, T.M.; Ab Mutalib, N.-S.; Goh, B.-H.; Lee, L.-H. Diversity of Streptomyces spp. from mangrove forest of Sarawak (Malaysia) and screening of their antioxidant and cytotoxic activities. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef]
- Ancheeva, E.; Daletos, G.; Proksch, P. Lead compounds from mangrove-associated microorganisms. Mar. Drugs 2018, 16, 319. [Google Scholar] [CrossRef] [PubMed]
- Kemung, H.M.; Tan, L.T.-H.; Chan, K.-G.; Ser, H.-L.; Law, J.W.-F.; Lee, L.-H.; Goh, B.-H. Investigating the antioxidant potential of Streptomyces sp. MUSC 11 from mangrove soil in Malaysia. Prog. Drug Discov. Biomed. Sci. 2019, 2, a0000033. [Google Scholar] [CrossRef]
- Ser, H.-L.; Tan, L.T.-H.; Palanisamy, U.D.; Abd Malek, S.N.; Yin, W.-F.; Chan, K.-G.; Goh, B.-H.; Lee, L.-H. Streptomyces antioxidans sp. nov. a novel mangrove soil actinobacterium with antioxidative and neuroprotective potentials. Front. Microbiol. 2016, 7, 899. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.T.H.; Mahendra, C.K.; Yow, Y.Y.; Chan, K.G.; Khan, T.M.; Lee, L.H.; Goh, B.H. Streptomyces sp. MUM273b: A mangrove-derived potential source for antioxidant and UVB radiation protectants. MicrobiologyOpen 2019, 8, e859. [Google Scholar] [CrossRef] [PubMed]
- Kemung, H.M.; Tan, L.T.-H.; Chan, K.-G.; Ser, H.-L.; Law, J.W.-F.; Lee, L.-H.; Goh, B.-H. Antioxidant Activities of Streptomyces sp. strain MUSC 14 from Mangrove Forest Soil in Malaysia. BioMed Res. Int. 2020, 2020, 6402607. [Google Scholar] [CrossRef]
- Williams, S.T. Genus Streptomyces Waksman and Henrici 1943; Williams & Wilkins Co.: Philadelphia, PA, USA, 1989; Volume 4, pp. 2452–2492. [Google Scholar]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically—Tenth Edition: Approved Standard M07-A10; C.L.S.I.: Wayne, PA, USA, 2015. [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance standards for Antimicrobial Susceptibility Testing. Available online: https://clsi.org/media/2663/m100ed29_sample.pdf (accessed on 23 July 2020).
- Lubis, R.R.; Marlisa, D.D.W. Antibacterial activity of betle leaf (Piper betle l.) extract on inhibiting Staphylococcus aureus in conjunctivitis patient. Am. J. Clin. Exp. Immunol. 2020, 9, 1. [Google Scholar]
- Biswas, K.; Sinha, S.N. Evaluation of Phytoconstituents and Antibacterial Activity of Vanda tessellata Using In Vitro Model. In Orchid Biology: Recent Trends & Challenges; Springer: Berlin, Germany, 2020; pp. 473–480. [Google Scholar]
- Ikram, M.; Beshbishy, A.M.; Kifayatullah, M.; Olukanni, A.; Zahoor, M.; Naeem, M.; Amin, M.; Shah, M.; Abdelaziz, A.S.; Ullah, R. Chemotherapeutic Potential of Carthamus Oxycantha Root Extract as Antidiarrheal and In Vitro Antibacterial Activities. Antibiotics 2020, 9, 226. [Google Scholar] [CrossRef]
- Saleem, H.; Htar, T.T.; Naidu, R.; Nawawi, N.S.; Ahmad, I.; Ashraf, M.; Ahemad, N. Biological, chemical and toxicological perspectives on aerial and roots of Filago germanica (L.) huds: Functional approaches for novel phyto-pharmaceuticals. Food Chem. Toxicol. 2019, 123, 363–373. [Google Scholar] [CrossRef]
- Ser, H.-L.; Tan, W.-S.; Ab Mutalib, N.-S.; Yin, W.-F.; Chan, K.-G.; Goh, B.-H.; Lee, L.-H. Draft genome sequence of mangrove-derived Streptomyces sp. MUSC 125 with antioxidant potential. Front. Microbiol. 2016, 7, 1470. [Google Scholar] [CrossRef]
- Tan, L.T.-H.; Chan, K.-G.; Khan, T.M.; Bukhari, S.I.; Saokaew, S.; Duangjai, A.; Pusparajah, P.; Lee, L.-H.; Goh, B.-H. Streptomyces sp. MUM212 as a source of antioxidants with radical scavenging and metal chelating properties. Front. Pharmacol. 2017, 8, 276. [Google Scholar] [CrossRef]
- Nett, M.; Ikeda, H.; Moore, B.S. Genomic basis for natural product biosynthetic diversity in the actinomycetes. Nat. Prod. Rep. 2009, 26, 1362–1384. [Google Scholar] [CrossRef] [PubMed]
- Thatcher, L.F.; Myers, C.A.; O’Sullivan, C.A.; Roper, M.M. Draft Genome Sequences of Streptomyces sp. Strains MH60 and 111WW2. Genome Announc. 2018, 6, e00356-18. [Google Scholar] [CrossRef] [PubMed]
- Ser, H.-L.; Tan, W.-S.; Ab Mutalib, N.-S.; Yin, W.-F.; Chan, K.-G.; Goh, B.-H.; Lee, L.-H. Genome sequence of Streptomyces mangrovisoli MUSC 149T isolated from intertidal sediments. Braz. J. Microbiol. 2018, 49, 13–15. [Google Scholar] [CrossRef] [PubMed]
- Ser, H.-L.; Tan, W.-S.; Ab Mutalib, N.-S.; Cheng, H.-J.; Yin, W.-F.; Chan, K.-G.; Lee, L.-H. Genome sequence of Streptomyces pluripotens MUSC 135T exhibiting antibacterial and antioxidant activity. Mar. Genomics 2015, 24, 281–283. [Google Scholar] [CrossRef] [PubMed]
- Ser, H.-L.; Ab Mutalib, N.-S.; Yin, W.-F.; Chan, K.-G.; Goh, B.-H.; Lee, L.-H. Evaluation of antioxidative and cytotoxic activities of Streptomyces pluripotens MUSC 137 isolated from mangrove soil in Malaysia. Front. Microbiol. 2015, 6, 1398. [Google Scholar] [CrossRef]
- Tan, L.T.-H.; Chan, K.-G.; Chan, C.K.; Khan, T.M.; Lee, L.-H.; Goh, B.-H. Antioxidative potential of a Streptomyces sp. MUM292 isolated from mangrove soil. BioMed Res. Int. 2018, 2018, 4823126. [Google Scholar] [CrossRef]
- Tan, L.T.-H.; Ser, H.-L.; Yin, W.-F.; Chan, K.-G.; Lee, L.-H.; Goh, B.-H. Investigation of antioxidative and anticancer potentials of Streptomyces sp. MUM256 isolated from Malaysia mangrove soil. Front. Microbiol. 2015, 6, 1316. [Google Scholar] [CrossRef]
- Tan, L.T.-H.; Chan, K.-G.; Pusparajah, P.; Yin, W.-F.; Khan, T.M.; Lee, L.-H.; Goh, B.-H. Mangrove derived Streptomyces sp. MUM265 as a potential source of antioxidant and anticolon-cancer agents. BMC Microbiol. 2019, 19, 38. [Google Scholar] [CrossRef]
- Rautela, I.; Dheer, P.; Thapliyal, P.; Joshi, T.; Sharma, N.; Sharma, M.D. GC-MS analysis of plant leaf extract of Datura stramonium in different solvent system. European J. Biomed. Pharm. Sci. 2018, 5, 236–245. [Google Scholar]
- Al-Qudah, M.A. Chemical composition and antimicrobial activity of the essential oil of Linum pubescens growing wild in Jordan. Nat. Prod. Res. 2013, 27, 1141–1144. [Google Scholar] [CrossRef]
- Kedia, A.; Prakash, B.; Mishra, P.K.; Dwivedy, A.K.; Dubey, N. Trachyspermum ammi L. essential oil as plant based preservative in food system. Ind. Crop. Prod. 2015, 69, 104–109. [Google Scholar] [CrossRef]
- Wang, B.; Ge, L.; Mo, J.; Su, L.; Li, Y.; Yang, K. Essential oils and ethanol extract from Camellia nitidissima and evaluation of their biological activity. J. Food Sci. Technol. 2018, 55, 5075–5081. [Google Scholar] [CrossRef] [PubMed]
- Rigano, D.; Marrelli, M.; Formisano, C.; Menichini, F.; Senatore, F.; Bruno, M.; Conforti, F. Phytochemical profile of three Ballota species essential oils and evaluation of the effects on human cancer cells. Nat. Prod. Res. 2017, 31, 436–444. [Google Scholar] [CrossRef]
- Kadhim, M. In Vitro antifungal potential of Acinetobacter baumannii and determination of its chemical composition by gas chromatography-mass spectrometry. Der Pharma Chem. 2016, 8, 657–665. [Google Scholar]
- Devi, N.N.; Singh, M.S. GC-MS Analysis of metabolites from endophytic fungus Colletotrichum gloeosporioides isolated from Phlogacanthus thyrsiflorus Nees. Int. J. Pharm. Sci. Rev. Res. 2013, 23, 392–395. [Google Scholar]
- Sisodia, R.; Verma, S.; Rani, A.; Dureja, P. Antibacterial and antioxidant activity of lichen species Ramalina roesleri. Nat. Prod. Res. 2013, 27, 2235–2239. [Google Scholar] [CrossRef]
- Yuan, C.; Zhang, X.J.; Du, Y.D.; Guo, Y.H.; Sun, L.Y.; Ren, Q.; Zhao, Z.T. Antibacterial compounds and other constituents of Evernia divaricata (L.) Ach. J. Chem. Soc. Pakistan 2010, 32, 189–193. [Google Scholar]
- Law, J.W.-F.; Letchumanan, V.; Tan, L.T.-H.; Ser, H.-L.; Goh, B.-H.; Lee, L.-H. The Rising of “Modern Actinobacteria” Era. Prog. Microbes Mol. Biol. 2020, 3, a0000064. [Google Scholar] [CrossRef][Green Version]
- Tan, L.T.-H.; Chan, C.-K.; Chan, K.-G.; Pusparajah, P.; Khan, T.M.; Ser, H.-L.; Lee, L.-H.; Goh, B.-H. Streptomyces sp. MUM256: A source for apoptosis inducing and cell cycle-arresting bioactive compounds against colon cancer cells. Cancers (Basel) 2019, 11, 1742. [Google Scholar] [CrossRef]
- Lee, L.-H.; Zainal, N.; Azman, A.-S.; Eng, S.-K.; Ab Mutalib, N.-S.; Yin, W.-F.; Chan, K.-G. Streptomyces pluripotens sp. nov. a bacteriocin-producing streptomycete that inhibits meticillin-resistant Staphylococcus aureus. Int. J. Syst. Evol. Microbiol. 2014, 64, 3297–3306. [Google Scholar] [CrossRef]
- Waksman, S.A.; Reilly, H.C.; Harris, D.A. Streptomyces griseus (Krainsky) Waksman and Henrici. J. Bacteriol. 1948, 56, 259. [Google Scholar] [CrossRef] [PubMed]
- Martinet, L.; Naômé, A.; Baiwir, D.; De Pauw, E.; Mazzucchelli, G.; Rigali, S. On the Risks of Phylogeny-Based Strain Prioritization for Drug Discovery: Streptomyces lunaelactis as a Case Study. Biomolecules 2020, 10, 1027. [Google Scholar] [CrossRef]
- Carvajal, F. Biologic strains of Streptomyces griseus. Mycologia 1946, 38, 596–607. [Google Scholar] [CrossRef] [PubMed]
- Ser, H.-L.; Law, J.W.-F.; Chaiyakunapruk, N.; Jacob, S.A.; Palanisamy, U.D.; Chan, K.-G.; Goh, B.-H.; Lee, L.-H. Fermentation conditions that affect clavulanic acid production in Streptomyces clavuligerus: A systematic review. Front. Microbiol. 2016, 7, 522. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, A.; Sengupta, S.; Bhattacharyya, M. Microbial Diversity and Community Analysis of the Sundarbans Mangrove, a World Heritage Site. In Microbial Diversity in the Genomic Era; Elsevier: Amsterdam, The Netherlands, 2019; pp. 65–76. [Google Scholar]
- Dholakiya, R.N.; Kumar, R.; Mishra, A.; Mody, K.H.; Jha, B. Antibacterial and antioxidant activities of novel actinobacteria strain isolated from Gulf of Khambhat, Gujarat. Front. Microbiol. 2017, 8, 2420. [Google Scholar] [CrossRef] [PubMed]
- Axenov-Gribanov, D.V.; Voytsekhovskaya, I.V.; Rebets, Y.V.; Tokovenko, B.T.; Penzina, T.A.; Gornostay, T.G.; Adelshin, R.V.; Protasov, E.S.; Luzhetskyy, A.N.; Timofeyev, M.A. Actinobacteria possessing antimicrobial and antioxidant activities isolated from the pollen of scots pine (Pinus sylvestris) grown on the Baikal shore. Antonie Van Leeuwenhoek 2016, 109, 1307–1322. [Google Scholar] [CrossRef] [PubMed]
- Cherdtrakulkiat, R.; Boonpangrak, S.; Sinthupoom, N.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Derivatives (halogen, nitro and amino) of 8-hydroxyquinoline with highly potent antimicrobial and antioxidant activities. Biochem. Biophys. Rep. 2016, 6, 135–141. [Google Scholar] [CrossRef]
- Cherdtrakulkiat, R.; Worachartcheewan, A.; Tantimavanich, S.; Lawung, R.; Sinthupoom, N.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Discovery of novel halogenated 8-hydroxyquinoline-based anti-MRSA agents: In vitro and QSAR studies. Drug Dev. Res. 2020, 81, 127–135. [Google Scholar] [CrossRef]
- Ding, X.; Ouyang, M.-A.; Shen, Y.-S. Evaluation of Anti-MRSA and Xanthine Oxidase Inhibition Activities of Phenolic Constituents from Plumula nelumbinis. J. Chem. 2015, 2015, 825792. [Google Scholar] [CrossRef]
- Noreen, H.; Semmar, N.; Farman, M.; McCullagh, J.S. Measurement of total phenolic content and antioxidant activity of aerial parts of medicinal plant Coronopus didymus. Asian Pac. J. Trop. Med. 2017, 10, 792–801. [Google Scholar] [CrossRef]
- Johari, M.A.; Khong, H.Y. Total phenolic content and antioxidant and antibacterial activities of Pereskia bleo. Adv. Pharmacol. Pharm. Sci. 2019, 2019, 7428593. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, K.; Veeresh, V.; Vipan, K.; Sudheer, M.; Priyadarsini, K.; Satish, R.; Unnikrishnan, M. Bioactivity-guided fractionation of Coronopus didymus: A free radical scavenging perspective. Phytomedicine 2006, 13, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Chamy, M.C.; Gambaro, V.; Garbarino, J.A.; Quilhot, W. Studies on Chilean Lichens, VII. The phenolic constituents of Protusnea malacea. J. Nat. Prod. 1985, 48, 307–309. [Google Scholar] [CrossRef]
- Sroka, Z.; Cisowski, W. Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids. Food Chem. Toxicol. 2003, 41, 753–758. [Google Scholar] [CrossRef]
- Alves, M.J.; Ferreira, I.C.; Froufe, H.J.; Abreu, R.; Martins, A.; Pintado, M. Antimicrobial activity of phenolic compounds identified in wild mushrooms, SAR analysis and docking studies. J. Appl. Microbiol. 2013, 115, 346–357. [Google Scholar] [CrossRef]
- Kajiya, K.; Hojo, H.; Suzuki, M.; Nanjo, F.; Kumazawa, S.; Nakayama, T. Relationship between antibacterial activity of (+)-catechin derivatives and their interaction with a model membrane. J. Agric. Food Chem. 2004, 52, 1514–1519. [Google Scholar] [CrossRef] [PubMed]
- Shibata, H.; Kondo, K.; Katsuyama, R.; Kawazoe, K.; Sato, Y.; Murakami, K.; Takaishi, Y.; Arakaki, N.; Higuti, T. Alkyl gallates, intensifiers of β-lactam susceptibility in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2005, 49, 549–555. [Google Scholar] [CrossRef]
- Ahire, J.J.; Dicks, L.M. Nisin incorporated with 2, 3-dihydroxybenzoic acid in nanofibers inhibits biofilm formation by a methicillin-resistant strain of Staphylococcus aureus. Probiotics Antimicrob. Proteins 2015, 7, 52–59. [Google Scholar] [CrossRef]
- Kang, S.S.; Kim, J.-G.; Lee, T.-H.; Oh, K.-B. Flavonols inhibit sortases and sortase-mediated Staphylococcus aureus clumping to fibrinogen. Biol. Pharm. Bull. 2006, 29, 1751–1755. [Google Scholar] [CrossRef]
- Brambilla, L.Z.; Endo, E.H.; Cortez, D.A.; Dias Filho, B.P. Anti-biofilm activity against Staphylococcus aureus MRSA and MSSA of neolignans and extract of Piper regnellii. Rev. Bras. Farmacogn. 2017, 27, 112–117. [Google Scholar] [CrossRef]
- Rane, R.A.; Sahu, N.U.; Shah, C.P. Synthesis and antibiofilm activity of marine natural product-based 4-thiazolidinones derivatives. Bioorg. Med. Chem. Lett. 2012, 22, 7131–7134. [Google Scholar] [CrossRef] [PubMed]
- Beeton, M.L.; Aldrich-Wright, J.R.; Bolhuis, A. The antimicrobial and antibiofilm activities of copper (II) complexes. J. Inorg. Biochem. 2014, 140, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Montero, K.; Lamilla, C.; Abanto, M.; Maruyama, F.; Jorquera, M.A.; Santos, A.; Martinez-Urtaza, J.; Barrientos, L. Antarctic Streptomyces fildesensis So13.3 strain as a promising source for antimicrobials discovery. Sci. Rep. 2019, 9, 7488. [Google Scholar] [CrossRef] [PubMed]
- Maglangit, F.; Fang, Q.; Leman, V.; Soldatou, S.; Ebel, R.; Kyeremeh, K.; Deng, H. Accramycin A, a New Aromatic Polyketide, from the Soil Bacterium, Streptomyces sp. MA37. Molecules 2019, 24, 3384. [Google Scholar] [CrossRef]
- Sun, W.; Wu, W.; Liu, X.; Zaleta-Pinet, D.A.; Clark, B.R. Bioactive compounds isolated from marine-derived microbes in China: 2009–2018. Mar. Drugs 2019, 17, 339. [Google Scholar] [CrossRef] [PubMed]
- Risdian, C.; Mozef, T.; Wink, J. Biosynthesis of polyketides in streptomyces. Microorganisms 2019, 7, 124. [Google Scholar] [CrossRef]
- Ser, H.-L.; Palanisamy, U.D.; Yin, W.-F.; Abd Malek, S.N.; Chan, K.-G.; Goh, B.-H.; Lee, L.-H. Presence of antioxidative agent, Pyrrolo [1,2-a] pyrazine-1, 4-dione, hexahydro-in newly isolated Streptomyces mangrovisoli sp. nov. Front. Microbiol. 2015, 6, 854. [Google Scholar] [CrossRef]
- Kiran, G.S.; Priyadharsini, S.; Sajayan, A.; Ravindran, A.; Selvin, J. An antibiotic agent pyrrolo [1,2-a] pyrazine-1, 4-dione, hexahydro isolated from a marine bacteria Bacillus tequilensis MSI45 effectively controls multi-drug resistant Staphylococcus aureus. RSC Adv. 2018, 8, 17837–17846. [Google Scholar] [CrossRef]
- Lee, L.-H.; Zainal, N.; Azman, A.-S.; Eng, S.-K.; Goh, B.-H.; Yin, W.-F.; Ab Mutalib, N.-S.; Chan, K.-G. Diversity and antimicrobial activities of actinobacteria isolated from tropical mangrove sediments in Malaysia. Sci. World J. 2014, 2014, 698178. [Google Scholar] [CrossRef]
- Hong, K.; Gao, A.-H.; Xie, Q.-Y.; Gao, H.G.; Zhuang, L.; Lin, H.-P.; Yu, H.-P.; Li, J.; Yao, X.-S.; Goodfellow, M. Actinomycetes for marine drug discovery isolated from mangrove soils and plants in China. Mar. Drugs 2009, 7, 24–44. [Google Scholar] [CrossRef]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.-S.; Cho, Y.-J.; Lee, K.; Yoon, S.-H.; Kim, M.; Na, H.; Park, S.-C.; Jeon, Y.S.; Lee, J.-H.; Yi, H. Introducing EzTaxon-e: A prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int. J. Syst. Evol. Microbiol. 2012, 62, 716–721. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K. Color-Name Charts Illustrated With Centroid Colors. In Inter-Society Color Council-National Bureau of Standards; US Government Printing Office Chicago: Washington, DC, USA, 1964. [Google Scholar]
- Lee, L.-H.; Zainal, N.; Azman, A.-S.; Ab Mutalib, N.-S.; Hong, K.; Chan, K.-G. Mumia flava gen. nov. sp. nov. an actinobacterium of the family Nocardioidaceae. Int. J. Syst. Evol. Microbiol. 2014, 64, 1461–1467. [Google Scholar] [CrossRef]
- Carrillo, P.; Mardaraz, C.; Pitta-Alvarez, S.; Giulietti, A. Isolation and selection of biosurfactant-producing bacteria. World J. Microb. Biot. 1996, 12, 82–84. [Google Scholar] [CrossRef]
- Meena, B.; Rajan, L.A.; Vinithkumar, N.V.; Kirubagaran, R. Novel marine actinobacteria from emerald Andaman & Nicobar Islands: A prospective source for industrial and pharmaceutical byproducts. BMC Microbiol. 2013, 13, 145. [Google Scholar]
- Endo, E.H.; Costa, G.M.; Makimori, R.Y.; Ueda-Nakamura, T.; Nakamura, C.V.; Dias Filho, B.P. Anti-biofilm activity of Rosmarinus officinalis, Punica granatum and Tetradenia riparia against methicillin-resistant Staphylococcus aureus (MRSA) and synergic interaction with penicillin. J. Herb. Med. 2018, 14, 48–54. [Google Scholar] [CrossRef]
- Kemung, H.M.; Tan, L.T.-H.; Khaw, K.Y.; Ong, Y.S.; Chan, C.K.; Low, D.Y.S.; Tang, S.Y.; Goh, B.-H. An Optimized Anti-adherence and Anti-biofilm Assay: Case Study of Zinc Oxide Nanoparticles versus MRSA Biofilm. Prog. Microbes Mol. Biol. 2020, 3, a0000091. [Google Scholar] [CrossRef]
- Adjimani, J.P.; Asare, P. Antioxidant and free radical scavenging activity of iron chelators. Toxicol. Rep. 2015, 2, 721–728. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Sample of the extract is available from the authors. |
| Concentration (mg/mL) | Antioxidant Activities (%) | ||
|---|---|---|---|
| ABTS Radical Scavenging Activity | DPPH Radical Scavenging Activity | Metal Chelating Activity | |
| 0.125 | 10.44 ± 2.68 * | 3.47 ± 2.49 | 26.00 ± 3.29 * |
| 0.25 | 13.32 ± 1.25 * | 4.23 ± 1.37 * | 28.56 ± 2.62 * |
| 0.5 | 18.71 ± 1.47 * | 9.55 ± 1.01 * | 32.49 ± 0.63 * |
| 1 | 27.27 ± 1.17 * | 16.23 ± 3.60 * | 39.07 ± 2.61 * |
| 2 | 41.50 ± 1.06 * | 55.00 ± 3.10 * | 52.75 ± 1.76 * |
| 4 | 67.85 ± 2.16 * | 64.77 ± 1.31 * | 61.10 ± 0.64 * |
| Gallic Acid a | 42.50 ± 0.60 * | - | - |
| Gallic Acid b | - | 53.99 ± 4.06 * | - |
| EDTA c | - | - | 68.49 ± 7.68 * |
| Number | Constituents | Retention Time (min) | Molecular Formula | Molecular Weight | Similarity (%) | Biological Activities of Extract | Reference |
|---|---|---|---|---|---|---|---|
| 1 | Cyclobutane,2-hexyl-1,1,4-trimethyl | 61.569 | C13H26 | 182 | 80.4 | - | [47] |
| 2 | Thiophene, 2-butyl-5-ethyl | 61.97 | C10H16S | 168 | 92.5 | Antimicrobial | [48] |
| 3 | 1-Heptyn-3-ol | 62.013 | C7H120 | 112 | 95.1 | Antifungal, antioxidant | [49] |
| 4 | 8-[N-Aziridylethylamino]-2-6,dimethyloctene-2 | 62.089 | C14H28N2 | 224 | 86.7 | Antioxidant, antibacterial and anticancer | [50,51] |
| 5 | Pyrrolo[1,2-a]pyrazine-1,4-dion,hexahydro | 62.815 | C7H10N2O2 | 154 | 95.3 | Antioxidant, antimicrobial and algicidal | [44,52] |
| 6 | Octahydro-2H-pyrido(1,2-a)pyrimidin-2-one | 64.939 | C8H14N2O | 154 | 97.7 | - | [53] |
| 7 | 9,9-Dimethyl-3,7-diazabicyclo[3.3.1]nonane | 66.304 | C9H18N2 | 154 | 95.2 | Antifungal | [52] |
| 8 | 2,4-Dihydroxy-6-propylbenzoic acid | 66.543 | C10H12O4 | 196 | 96.1 | Antibacterial, antioxidant | [54,55] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mangzira Kemung, H.; Tan, L.T.-H.; Chan, K.-G.; Ser, H.-L.; Law, J.W.-F.; Lee, L.-H.; Goh, B.-H. Streptomyces sp. Strain MUSC 125 from Mangrove Soil in Malaysia with Anti-MRSA, Anti-Biofilm and Antioxidant Activities. Molecules 2020, 25, 3545. https://doi.org/10.3390/molecules25153545
Mangzira Kemung H, Tan LT-H, Chan K-G, Ser H-L, Law JW-F, Lee L-H, Goh B-H. Streptomyces sp. Strain MUSC 125 from Mangrove Soil in Malaysia with Anti-MRSA, Anti-Biofilm and Antioxidant Activities. Molecules. 2020; 25(15):3545. https://doi.org/10.3390/molecules25153545
Chicago/Turabian StyleMangzira Kemung, Hefa, Loh Teng-Hern Tan, Kok-Gan Chan, Hooi-Leng Ser, Jodi Woan-Fei Law, Learn-Han Lee, and Bey-Hing Goh. 2020. "Streptomyces sp. Strain MUSC 125 from Mangrove Soil in Malaysia with Anti-MRSA, Anti-Biofilm and Antioxidant Activities" Molecules 25, no. 15: 3545. https://doi.org/10.3390/molecules25153545
APA StyleMangzira Kemung, H., Tan, L. T.-H., Chan, K.-G., Ser, H.-L., Law, J. W.-F., Lee, L.-H., & Goh, B.-H. (2020). Streptomyces sp. Strain MUSC 125 from Mangrove Soil in Malaysia with Anti-MRSA, Anti-Biofilm and Antioxidant Activities. Molecules, 25(15), 3545. https://doi.org/10.3390/molecules25153545










