Abstract
Artemisinin combination therapy (ACT) is recommended by the World Health Organization (WHO) as first line treatment for uncomplicated malaria both in adults and children. During pregnancy, ACT is considered safe only in the second and third trimester, since animal studies have demonstrated that artemisinin derivatives can cause foetal death and congenital malformation within a narrow time window in early embryogenesis. During this period, artemisinin derivatives induce defective embryonic erythropoiesis and vasculogenesis/angiogenesis in experimental models. However, clinical data on the safety profile of ACT in pregnant women have not shown an increased risk of miscarriage, stillbirth, or congenital malformation, nor low birth weight, associated with exposure to artemisinins in the first trimester. Although further studies are needed, the evidence collected up to now is prompting the WHO towards a change in the guidelines for the treatment of uncomplicated malaria, allowing the use of ACT also in the first trimester of pregnancy.
1. Introduction
Malaria continues to be a major threat in the developing world, with 228 million clinical episodes and 405,000 deaths in 2018 [1]. Most of disease-associated morbidity and mortality occurs in children and pregnant women in sub-Saharan Africa and Plasmodium (P.) falciparum is the most common cause. Clinical features of P. falciparum infection include asymptomatic parasitemia, uncomplicated malaria and complicated or severe malaria, often lethal. Severe malaria is essentially due to the cytoadherence of infected erythrocytes to the vascular endothelium of vital organs, causing microcirculatory obstruction and the development of a generalized inflammatory response [2]. Malaria during pregnancy can produce harmful effects on both the mother and the foetus, depending on transmission intensity. In high transmission areas the risks of adverse effects are most prominent for women in their first pregnancy. In low transmission areas, where women have low acquired immunity, malaria often develops into severe diseases [3].
Artemisinin derivatives are the mainstay class of drugs for malaria therapy, showing excellent efficacy in both severe and uncomplicated malaria. Artemisinin (Figure 1) is the active principle extracted from Artemisia annua (qinghao), a plant of the Asteraceae family, used for ages in traditional Chinese medicine [4]. It is a sesquiterpene lactone with a peroxide bridge essential for the antimalarial effect. To overcome the poor solubility of artemisinin and, thus, the formulation issues, semisynthetic derivatives of artemisinin with improved pharmacological properties, including artemether, artesunate and dihydroartemisinin (DHA), were developed (Figure 1). Artemisinins are safe and well tolerated drugs, with a very rapid cytotoxic action against all parasite stages, including early ring stage [5,6,7,8]. They are also active against early stage gametocytes, responsible for transmission from humans to mosquitos [9,10]. The large deployment and usage of Artemisinin-based combination therapy (ACT) in endemic settings has contributed significantly to the reduction in mortality and morbidity by malaria over the last decade. ACT is suggested during the second and third trimester. However, based on experimental data, the use of ACT during the first trimester of pregnancy is cautioned for evidences of embryotoxicity [11,12], and WHO guidelines still do not recommend ACT for uncomplicated malaria in the first trimester of pregnancy [13,14].
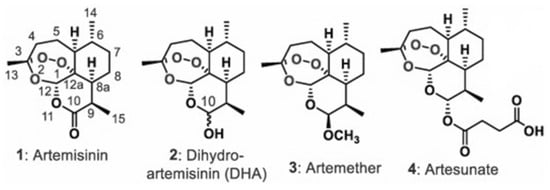
Figure 1.
Artemisinin 1 and its derivatives, dihydroartemisinin (DHA) 2, artemether 3, and artesunate 4.
Several studies recently concluded indicate that artemisinins are safe and could be used also during the first trimester [15]. Therefore, a reexamination of WHO policy is probably necessary.
2. Pathophysiology and Epidemiology of Malaria in Pregnancy
Children and pregnant woman are particularly susceptible to severe malaria caused by P. falciparum infection. In pregnant women, the accumulation of infected erythrocytes in the placenta accounts for low birth weight, infant anaemia and stillbirth [16]. Sequestration of infected erythrocytes in the placenta is due to the expression, on the surface of infected red blood cells, of variant surface antigens (VAR2CSA) able to mediate the adherence to placental chondroitin sulfate A and to other placental receptors [17,18]. This sequestration leads to mononuclear cell recruitment, placental damage, inflammation, clogging and failure to clear parasites through normal immunity [19]. Women during their first pregnancy have increased risk of developing the severe forms of malaria, especially severe anemia, but also acute respiratory distress syndrome, renal failure and cerebral malaria. Malaria during pregnancy is responsible for adverse effects on the fetus, too. Besides stillbirth, whose impact depends on malaria endemicity, low birth weight and preterm delivery are common and are responsible for increased infant mortality [3].
In 2018, about 11 million women were exposed to malaria during pregnancy. Prevalence of exposure to malaria infection during pregnancy was 30% or more in 20 African countries. About 39% of malaria pregnancy cases were in the Democratic Republic of the Congo and Nigeria. WHO analysis showed a positive correlation between maternal anemia and prevalence of exposure to malaria infection during pregnancy [1].
3. Current Therapies of Malaria in Pregnancy
3.1. Therapy of Malaria in Pregnancy
ACTs are recommended by the World Health Organization (WHO) for adults and children as first line treatment for uncomplicated malaria but in pregnant women ACTs are restricted to the second and third trimester (Table 1). The current treatments with artemether/lumefantrine or dihydroartemisinin/piperaquine have demonstrated themselves to be well tolerated and safe during the second and third trimester of pregnancy in controlled trials. However, as stated above, due to their potential embryotoxicity, ACTs have not been considered safe in the first trimester of pregnancy. As a consequence, the WHO recommends quinine plus clindamycin to treat uncomplicated malaria (Table 1) [13,20]. Intravenous artesunate is, however, the treatment of choice for severe malaria in children and adults, including pregnant women in the first trimester [21]. The primary objective of the treatment of severe malaria in pregnancy is indeed to save the life of the mother and randomized clinical trials showed a significant reduction of mortality with parental artesunate compared to quinine [22,23]. Data on animal models indicate that artemisinins are embryotoxic during a limited time window in the first trimester of pregnancy (corresponding to 4–10 weeks of human gestation), probably acting by altering erythropoiesis and vasculogenesis/angiogenesis [24]. Unfortunately, the information regarding the safety profile of most of the licensed antimalarial treatments in humans during pregnancy is limited since pregnant women are not usually involved in clinical trials during drug development. It is indeed unclear if artemisinin-induced embryotoxicity can occur in human embryos, as well. Current data are incomplete and further studies are needed to determine artemisinin’s safety in the first trimester of pregnancy. However, in the recent years, meta-analysis and observational/prospective studies have been performed, which support the decision of the Malaria Policy Advisory Committee of the WHO to endorse the use of ACTs in the first trimester, although this decision has not been converted to a recommendation in the treatment guidelines, yet [13,25,26,27].

Table 1.
Recommended treatment for P.falciparum malaria during pregnancy (WHO, Guidelines for treatment of malaria).
3.2. Intermittent Preventive Treatment (IPT)
Intermittent preventive treatment during pregnancy (IPTp) consists of the administration of an antimalarial drug to all pregnant women, independently from malaria diagnosis. The WHO recommends IPTp with sulfadoxine–pyrimethamine (IPTp-SP) in all areas with moderate to high malaria transmission in Africa but not in areas of low transmission level [28]. IPT should be administered at each routine prenatal care visit, starting as early as possible in the second trimester, not during the first trimester. The women should receive at least three doses of SP during pregnancy (IPTp3), with each dose administrated at least 1 month apart. Less than three doses is considered sub-optimal. SP can safely be administered until delivery [29]. Based on currently available evidence, IPTp-SP remains effective in preventing the adverse consequences of malaria on maternal and foetal outcomes even in areas where quintuple mutations linked to SP resistance are prevalent in P. falciparum. Therefore, there is a need to continuously monitor the effectiveness of this useful intervention in the light of increasing P. falciparum resistance to SP [30]. Pregnant women are regularly given a folic acid supplementation. However, since high doses of folic acid counteract the effect of sulfadoxine–pyrimetamine, it is preferred that women take only the recommended daily dose of 0.4 mg folic acid. In the countries, where 5 mg of folic acid is used, it is recommended to suspend folic acid supplementation for two weeks after taking IPTp with sulfadoxine–pyrimethamine to ensure optimal efficacy. Between 2010 and 2015, there was a five-fold increase in the percentage of women receiving the recommended three or more doses of IPTp in 20 African countries. To date, 36 African countries have adopted this policy and reported routine health facility data from the public sector on the number of women receiving the first, second, third and fourth doses of IPTp (i.e., IPTp1, IPTp2, IPTp3 and IPTp4). As of 2018, coverage rates of IPTp1, IPTp2 and IPTp3 were 60%, 49% and 31%, respectively [1]. Only Burkina Faso and the United Republic of Tanzania were estimated as having more than half of pregnant women receiving IPTp3 in 2018. The gap between high antenatal care (ANC) attendance and the low proportion of eligible pregnant women receiving IPTp3 largely reflects a failure of the health system to provide IPTp-SP at ANC facilities [31]. This is an economical–political aspect that should be considered and, hopefully, improved since it is demonstrated that IPTp decreases the incidence of low birth weight by 29%, severe maternal anaemia by 38%, and neonatal mortality by 31% [32].
Increasing parasite resistance to SP has led to evaluation of other combination therapies as potential alternatives [33,34]. Different clinical trials have shown the potential of ACTs as IPT, but at present no clear indication emerged to induce a change in policy. A meta-analysis considered 11 published randomised controlled trials or prospective cohort studies on the use of dihydroartemisinin (DHA)–piperaquine (DHA–PPQ). Among these, nine were IPT trials. The authors conclude that DHA–PPQ is safe, well tolerated, and more effective than other treatments, in terms of protection, for IPT [35]. However, the prolonged exposure needed for IPT needs further investigation on potential cardiac toxicity. Another very recent review and meta-analysis of DHA–PPQ versus SP for malaria prevention in pregnancy suggest that DHA–PPQ is more effective than SP in decreasing maternal and placental malaria [36].
4. Embryotoxicity of Artemisinins: An Overview of the In Vivo Studies
To better understand the results on the embryotoxicity of artemisinins in experimental animal models, a description of the morphogenesis of the circulatory system in these models is provided in the following sections.
4.1. Circulatory System Morphogenesis in Mammal Embryos
Heart morphogenesis: Heart progenitor cells are recognizable at the gastrula stage and aggregate to form two pair groups of cells constituting the cardiogenic areas. This cardiac field contains multipotent progenitor cells that will differentiate into hemangioblasts (the precursors of vessels and blood) or in multipotent cardiac precursors. The fusion of the pair heart primordia occurs in humans at 3 weeks of gestation and corresponds to the beginning of pulsation. Contemporaneously, blood vessels form independently [37,38].
Blood vessel formation: The development of blood vessels occurs by two temporally separated processes: vasculogenesis (the formation de novo of a network of blood vessels) and angiogenesis (consisting of the remodeling of the primary formed network to differentiate distinct capillaries, arteries and veins). During the first phase of vasculogenesis, hemangioblasts condense in aggregates called blood islands; in the second phase, the inner cells of blood islands are instructed to differentiate into blood cells, while the outer cells (angioblasts) will differentiate into endothelial elements that interact with smooth muscle-like cells (pericytes); in the third phase, finally, the endothelial cells of different tubes interconnect and form the primary capillary plexus. In amniotes (including mammals), vasculogenesis occurs firstly at the level of the yolk sac (extraembryonic vasculogenesis) and later at the level of the embryo itself (intraembryonic vasculogenesis) in order to form the aorta and the great vessels [39,40].
During angiogenesis, vascular endothelial growth factor (VEGF) instructs some endothelial cells (tip cells) to sprout to form new vessels toward the source of VEGF, distributed in the different organs, allowing the organ-specific capillary formation.
Hematopoiesis: As mentioned above, the primary sites of mammal’s hematopoiesis are the blood islands. Hematopoietic stem cells, originated at the level of yolk sac blood islands, seem to migrate and colonize the liver and the bone marrow to generate the adult hematopoietic stem cells. However, an intraembryonic production of hematopoietic stem cells (from the hemogenic endothelium at the level of the aorta–gonad–mesonephros, or AGM) has been demonstrated, as well.
4.2. Hematopoiesis in Amphibian (Xenopus) Embryos
In amphibians, the intermediate and definitive hematopoiesis is at the level of the hepatic marrow. In the Xenopus embryo, the liver is colonized by hematopoietic stem cells which originate, from the mesoderm of the ventral blood islands (homolog to the mammalian extraembryonic site) and from the dorsal lateral plate mesoderm (homolog to the mammalian intraembryonic sites).
4.3. Hematopoiesis in Fish (Zebrafish) Embryos
Zebrafish are an alternative model to evaluate angiogenesis since fish, like amniotes, develop yolk sac vasculature as the primary hematopoietic source. In Zebrafish, the primitive hematopoietic sites are the bilateral stripes of the lateral mesoderm, which converge medially to form the region where primitive erythrocytes are produced. After the onset of circulation (about 24 h postfertilization), definitive hemopoietic stem cells appear in the AGM region. These cells, 2–6 days postfertilization migrate and colonize the intermediate site of blood development (the caudal hematopoietic tissue, the thymus, and the kidney). In adulthood, the kidney marrow is the definitive primary site of hematopoiesis.
5. Developmental Toxicity Data of Artemisinin Derivatives
5.1. In Vivo Studies
In this section, we will comment on the in vivo results from the most relevant studies describing the embryotoxic effects and malformations induced by artemisinin derivatives in different animal species. To better understand the results of this section, Figure 2 indicates the different phases of hematopoiesis in experimental rodents and humans during embryogenesis. Experimental toxicological data were obtained both after in utero exposure, by treating pregnant rodent females, and after in vitro exposure of embryos, using rodent postimplantation whole embryo culture (WEC) [41]. This model, widely used to study developmental toxicity, consists of culturing for about 48 h the entire embryo in vitro, excluding maternal factors from the system. One advantage is that mechanistic studies, such as drug–drug interactions, are more feasible. Dihydroartemisinin (DHA) was used in many in vivo studies since it is an antimalarial drug on its own and it is the main metabolite of other artemisinin derivatives used in human therapy. For instance, artesunate, the most broadly used artemisinin derivative, is readily hydrolysed to DHA both in animals and humans, as demonstrated also in pregnant women [42].
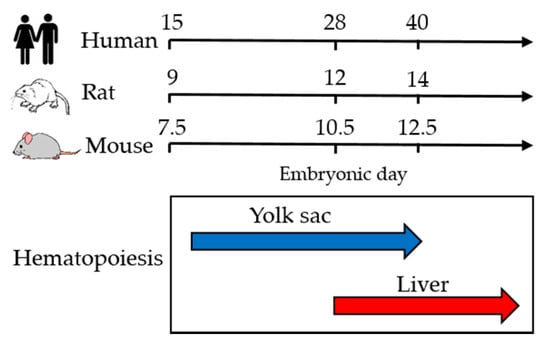
Figure 2.
Phases of hematopoiesis in experimental rodents and humans during embryogenesis.
Developmental in vivo toxicity studies demonstrated that artemisinins can induce foetal death and congenital malformations in rodents only after embryo exposure within a narrow window in the early embryogenesis. Embryo deaths and malformations have been, in fact, observed in rats following oral administration of artesunate on single days between gestation day (GD) 10 and 14, while the same dose of the drug administered on GD 9 or a higher dose on GD 16 or 17 did not induce embryotoxicity. GDs 10–14 were consequently identified as the developmental period more sensitive to the artemisinins, with GD10 the most sensitive day to induced malformations (mostly cardiovascular defects and bone anomalies) and GD11 the most sensitive to induced embryolethality [43]. In other studies, the same pattern of teratogenicity was seen when different artemisinin derivatives (DHA, artesunate, arteether and artemether) were given at GD10, indicating that embryolethality and malformations were probably associated with the endoperoxidic bridge which characterizes the drug class and is responsible for the antimalarial activity [44]. This would exclude the possibility of developing artemisinin derivatives which maintain the antimalarial activity while reducing embryotoxic effects. However, endoperoxide artefenomel (OZ439) showed an improved safety margin compared to artesunate [45]. The authors hypothesized that artemisinins’ embryotoxicity is due to the reduction of heme biosynthesis and that artefenomel, due to its structure, is less active at this level. Another approach to decrease embryotoxicity of artemisinins is the association with other treatments which protect from toxicity. For example, folic acid administration in mice prevents the damages induced by artesunate in heart development, measured as septal defects, and the thickness of ventricular and atrial septa [46]. Drug conversion between the animal studies and human treatment indicate that the drug concentrations tested in these animal studies were within the range of the exposure doses during human treatment [47].
To better evaluate the pathogenetic mechanisms related to the artemisinins’ embryotoxic effects, Longo and colleagues [48], using the WEC method, showed that the yolk sac is the primary site highly susceptible to artemisinin compounds: a severe depletion of circulating erythroblasts was observed in rat embryos exposed in vitro during the whole culture period (GD9.5–11.5) to DHA. Similar results were observed when the embryos were exposed for 1.5 h at the beginning of the culture (GD9.5), while only effects on red blood cell morphology were seen after exposure for 1.5 h at the end of the culture period (GD11.5). The tested concentrations (0.01–2 µg/mL) were those in the range of maximal concentration detected in the plasma of patients after administration of the drug. It is relevant to consider that the WEC method covers a significant period of yolk sac hematopoiesis (primary hematopoiesis), which occurs from GD 9 to GD14 in rats and corresponds to the gestational period from GD 15 to week 6 in humans. After a single in utero exposure to DHA (treatment of a pregnant rat with 7.5 or 15 mg/kg), the same research group showed that, at GD 9.5 and 10.5, primitive erythrocytes from yolk sac hematopoiesis were affected, causing anemia and consequent hypoxia. Cell damage was evident in the embryo at GD 11.5 and, later, embryo–fetal death occurred [49]. In another study, the WEC model was used to analyse the mechanisms inducing depletion of the embryonic erythroblast. Abnormal cell division and apoptosis of the embryonic erythroblast after DHA treatment of embryos were demonstrated by the presence of increased symmetric and asymmetric binuclear cells, Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)- and Caspase-3-positive cells and embryonic erythroblasts with fragmented nuclei [50].
Although the majority of the studies on artemisinins’ developmental effects are in rodents, alternative animal models such as the FETAX (Frog Embryo Teratogenesis Assay-Xenopus) were also used. A reduction of the primitive red blood cells were seen after the exposure of Xenopus laevis embryos to DHA (0.01–0.5 µg/mL) during the early developmental period (from 24 h post fertilization), whereas the red blood cells of older larvae were only minimally affected [51]. In this study, embryolethality did not occur and congenital anomalies were induced only at the highest dose level (0.1–0.5 µg/mL).
The zebrafish model was also used to test the potential embryotoxicity of DHA [52]. DHA exposure (1–10 µg/mL) during primitive hematopoiesis and hematopoietic stem cell specification caused an abnormal embryonic phenotype (pericardial edema, abnormal trunk axis, abnormal pigmentation), developmental delays or death, similarly to the other animal models. However, differently from the other animal models, DHA in zebrafish increased vasculogenesis and angiogenesis. This may be due to differences in culture conditions and drug delivery route in zebrafish compared to mammals, but it may also suggest a different mode of action. The fact that the hematopoietic sites are different in zebrafish vs. mammals must be taken in account.
Only one study was performed in primates. Artesunate showed a dose- and time-dependent developmental toxicity after treatment of 15 pregnant cynomolgus monkeys on GD 20–50 for different days intervals. The authors concluded that artesunate caused embryo-lethality at doses ≥12 mg/kg/day administered for more than 12 days at the beginning of organogenesis, but not when administered for shorter treatment period [53]. It is interesting to note that although ACT and not artemisinin monotherapy, is the recommended treatment against uncomplicated malaria, the developmental toxicity of ACTs, compared to artemisinin derivatives alone, has been scarcely studied in vitro [54,55].
5.2. In Vitro Studies
In vitro studies were performed from our group to characterize the target erythroid population in humans. As a model, human CD34+ stem cells from peripheral blood were chosen. DHA caused inhibition of cell proliferation and a delay of erythroid differentiation when it was added to the pro- and basophilic erythroblasts but not on mature erythroid stages (Figure 3). It seems indeed that DHA specifically target the pro-erythroblast and basophilic erythroblast stage when human CD34+ stem cells differentiate toward the erythroid lineage [56]. These data are in line with the animal studies described above, which suggest that embryotoxicity depends on the GD when artemisinins are administered, both for in utero and for the WEC studies. The experimental data from our group suggest that a window of susceptibility to artemisinins could occur also in human erythropoiesis.
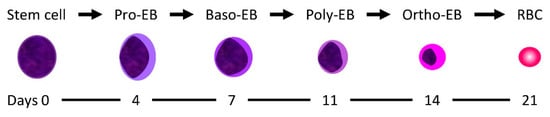
Figure 3.
Erythroid development from the hematopoietic stem cell to mature erythrocytes (EB: erythroblast; baso: basophilic; poly: polychromatic; ortho: orthochromatic.
As mentioned above, all the artemisinin compounds possess a distinctive and essential 1,2,4-trioxane pharmacophore. When artemisinin toxicity was studied in vitro against the K562 leukaemia cell line (a model for differentiating early human erythroblasts), it was found that the peroxide bridge was responsible for the inhibition of erythroid differentiation. All artemisinin derivatives used in the study were able to inhibit erythroid differentiation and, among different artemisinins, DHA was the most toxic. By contrast, the non-peroxidic deoxyartemisinin failed to inhibit the process [57]. Moreover, a different substituent in position C10 of the molecule can modulate the drug toxicity. Another in vitro study in fact indicated that artemisone, a novel 10-alkylamino derivative highly potent on P.falciparum [58], was less antiangiogenic than dihydroartemisinin, suggesting that it could be safer during pregnancy [59].
Taken together, in vitro human and in vivo animal models encourage the search for new artemisinin derivatives which maintain a fast antimalarial activity but show lower developmental effects in pre-clinical models.
6. Human Data on Artemisinin Derivatives: Embryotoxicity or Safety?
Pregnant women, especially in the first trimester of pregnancy, are frequently excluded from pharmaceutical trials for drug development because of the possible harm to the woman and/or the embryo/fetus. There are indeed limited available data regarding safety and efficacy profiles of most licensed antimalarials, including artemisinin derivatives, in pregnancy. Consequently, limited information is also available on the changes in ACT pharmacokinetics during pregnancy, especially in the first trimester.
Clinical studies evaluating the safety of ACTs treatments in the second and third trimester of pregnancy demonstrated that ACTs are safe for women and children [20]. Regarding the use of artemisinins in the first trimester of pregnancy, only observational study and not randomized controlled trials have been done to date.
Table 2 shows observational studies published from 1998 to 2020 reporting data on the association between the use of artemisinin derivatives in the first trimester and pregnancy outcome, including miscarriage, neonatal mortality, low birth weight (LBW), congenital malformations. The total number of women treated with artemisinin derivatives in these studies is 1045 (753 in Africa and 292 in Asia). Artemether/lumefantrine (AL) is the most used ACT in the studies on artemisinin safety in pregnancy. Few studies were conducted with monotherapy or other ACTs. In some studies, the specific type of ACT is not indicated.

Table 2.
Observational studies on the use of artemisinin derivatives in the first trimester of pregnancy.
As shown in Table 2, data on the safety of AL in the first trimester of pregnancy are reported in four studies conducted in Africa. The most recent study is by Augusto et al. in 2020 [60]. This paper analyzed the data coming from a multi-center prospective observational cohort study involving women at health and demographic surveillance sites in three countries in Africa: Burkina Faso, Kenya and Mozambique [74]. The study included 26 and 92 women exposed in first trimester to AL or quinine, respectively, and aimed to determine the association between drug exposure and low birth weight (LBW) and small for gestational age (SGA). The results indicate that AL exposure in the first trimester is not associated with an increased occurrence of LBW or SGA compared to non-exposed women. However, a higher prevalence of LBW and SGA was observed for children born to quinine-exposed pregnancies. The LBW prevalence among newborns was 10.9% and 26.9% among women exposed to ACT or quinine, respectively, compared to 9.5% among women unexposed to antimalarials. Quinine, but not AL exposure, was associated with adverse pregnancy outcome in another study in Tanzania that considered 319 women who assumed antimalarials in the first trimester [66]. The majority of these women, 172 (53%) used AL, the others used quinine (24.4%), sulfadoxine–pyrimethamine (20.7%) or amodiaquine (3.4%). Exposure to AL in the first trimester seemed indeed more common than other antimalarials. Increased risk of miscarriage and stillbirth was associated with the use of quinine and not with the other treatment including AL that resulted safer than quinine. The use of AL as a common practice in women in the first trimester of pregnancy was also reported in Uganda. Even if this study did not report the safety of the treatment, 98.6% of all 500 pregnancies (in all trimester) ended in a live birth and stillbirth occurred in seven cases [75].
Another observational study in Zambia [63] focused on first trimester exposures to AL considering data from a previous analysis of the full cohort of pregnant women in all trimesters of pregnancy, where it was observed that exposure to AL was not associated with perinatal mortality, malformations, or developmental impairment [76]. When focused on first trimester, 156 and 138 women exposed to AL or SP, respectively, were considered. Women in both groups had rates of preterm deliveries or LBW similar to those of women who did not receive treatments. Moreover, infant neurodevelopment up to 12 months was similar between the arms.
No significant increase in congenital defects was also observed in a prospective observational study in Rwanda. However, in this study, an increased frequency of obstetric adverse outcomes (abortion, peri-natal mortality, stillbirth and premature delivery) was observed after AL treatment during all trimesters of pregnancy [68]. In another observational study, in the first trimester of pregnancy, the risk of abortion was over 60% in women receiving an ACT (7/11) compared to 1% (1/38) in women treated with quinine. None of the 10 women who received intravenous artesunate miscarried. The authors assumed that the higher rates of abortion in women exposed to dihydroartemisinin–piperaquine (DHA–PQ) may reflect intrinsic biases within the non-comparative observational study. In this study prescription of DHA–PQ in the first trimester was reserved for those women who were more unwell, and this may explain the poor outcome [65].
Taken together, the ten studies conducted in Africa show that AL is the most common ACT treatment in the first trimester of pregnancy. The treatment is not teratogenic, nor does it increase the number of babies with low birth weight or small for gestational age, nor the stillbirths or the impairment in infant neurodevelopment and it seems safer than quinine.
The use of ACT other than AL is reported in six other studies in Africa (Table 2). In an observational study in central eastern Sudan, 62 pregnant women who had received artemisinin derivatives (48 received artemether injections, 11 artesunate plus sulfadoxine–pyrimethamine, 3 artemether plus lumefantrine) during the first trimester were followed-up until delivery and babies were followed-up until the first birthday. Two of the women treated with artemether injections miscarried while receiving quinine for a second malaria attack. The other 60 women who had received artemisinins delivered healthy infants at full term without congenital malformations and all infants survived in their first year of life. Moreover, no maternal deaths were recorded during the follow-up [69].
Although with limitations for the very low number of women involved, similar conclusions came from a recent prospective observational study in which thirteen women were identified who received artesunate–amodiaquine during the first trimester of pregnancy. Twelve women had experienced deliveries of live newborns with no congenital malformations. One woman had experienced a spontaneous abortion with a birth defect which was judged not to be related to ACT treatment, but rather to placental malaria [61].
Artesunate treatment in the first trimester of pregnancy is not as common and most of the studies have been done in Thailand [77,78]. The rates for abortion, stillbirth, congenital abnormality, and mean gestation at delivery did not differ from the rate of their communities.
Saito and colleagues performed a systematic literature review and meta-analysis considering 48 eligible efficacy studies, both observational and interventional cohort studies, including 7279 treated P.falciparum episodes of uncomplicated malaria in all trimesters of pregnancy [79]. Artemisinin based treatment showed higher efficacy than quinine treatment. However, the authors emphasize a high inhomogeneity in the design of studies, suggesting a standard framework for efficacy studies in order to obtain more comparable and reliable results [80]. More recently, 1242 papers with 5510 participants were included in a meta-analysis of safety of anti-malarial treatment in pregnant women. The results indicated significantly lower risks of abortion with quinine or AL compared to dihydroartemisinin–piperaquine, artesunate–mefloquine and artesunate–amodiaquine. However, no significant differences in the risk of stillbirths or neonatal deaths were observed with any of the drugs [81].
Studies on the use of antimalarial drugs during pregnancy and lactation, including the artemisinin derivatives, have been also reviewed by Gomes in 2016 [54] and by Saito and colleagues [11] in 2018. From these reports and the data shown in Table 2, it appears that ACT is more efficacious than monotherapy and well-tolerated in pregnancy and should be considered first-line for treating all pregnant and lactating women with uncomplicated falciparum malaria. However, the authors recommend that systematic monitoring for adverse effects is continued.
The Centers for Disease Control and Prevention (CDC) is more prudent in proposing the ACTs artemether–lumefantrine during the first trimester of pregnancy only when other treatment options (mefloquine or quinine plus clindamycin) are unavailable. Ballard and colleagues updated CDC recommendations after a literature review of 21 articles, which included one meta-analysis and five randomized open label controlled trials about the efficacy of ACT in uncomplicated malaria cases in pregnant women [82]. The authors analysed the results of sixteen studies on ACT safety in the second and third trimester, whereas for the first trimester they only considered the meta-analysis by Dellicour and colleagues that is described above [15].
All the studies reported above seem to indicate that artemisinins in different combinations or artesunate as monotherapy are safe even when administered in the first trimester of pregnancy. However, in the absence of randomized clinical trials, it is still difficult to draw definitive conclusions.
7. Differences between Animal Studies and Human Data
The differences observed between animal studies and human data about the safety of artemisinin derivatives in pregnancy are difficult to reconcile and can be attributed to different causes.
First of all, the animal studies are conducted in healthy animals, whereas the drug exposed women are infected with malaria parasites. Situation, pregnancy and malaria are known to influence the pharmacokinetic properties of several drugs, including antimalarials [83]. After oral administration of AS, the plasma concentrations of the parent drug AS and its main metabolite, DHA were higher in pregnant women with malaria compared with those in the same women in a healthy state namely in the postpartum period [42]. AS absorption and its conversion to DHA is different in malaria patients and in healthy volunteers [84]. Moreover, the stability of endoperoxide drugs can be different in different physiological situations. We demonstrated that the degradation of DHA in vitro is influenced by different assay conditions (pH, temperature, haemolysis) indicating that clinical disorders such as fever, haemolysis or acidosis associated with malaria severity may contribute to artemisinin instability [85]. Pregnancy may add further variability.
In malaria patients, artemisinins concentrate mainly on infected erythrocytes [86]. It has been proposed that, during malaria infection, artemisinins are less available to pass to the embryo since higher quantities are activated, and thus degraded, to form carbon-centered radicals which bind parasite proteins [54].
In rat and monkey embryos, the target of artemisinin toxicity was the circulating primitive erythroblasts [43,49,53]. Thus, considering that the primitive erythroblasts are predominant in the circulation of the human embryo between weeks 6 and12, it has been estimated that this is the most sensitive period in humans [53]. However, the treatment in humans with ACT lasts for a very short period (3–7 days) in the time window of greater susceptibility to artemisinin, when the proliferation of erythroblasts is very intense. Therefore, even if erythroblasts are damaged by DHA, they could be easily replaced by others of new formation and the toxic effects become marginal and with no clinical consequences [54,87]. The length of the exposure period of treatment appears thus, to be critical. Both data on safety in humans and data on embryotoxicity in animal models may be biased due to the time of exposure.
8. Conclusions
Are artemisinins safe in the first trimester of pregnancy? The lack of clinical trials specifically designed to answer to this question is a problem that cannot be easily solved due to ethical concerns. However, in the last few years, the subject was examined in more detail in clinical settings. As summarized in this review, several observational/comparative, prospective studies, and meta-analysis were conducted which have assessed the safety of using ACTs in the first trimester of pregnancy. The endorsement of the use of ACTs in the first trimester by the Malaria Policy Advisory Committee of WHO in 2015 was a first and important step towards the revision of WHO guidelines for the treatment of malaria in pregnancy that is still waiting to be implemented.
Author Contributions
S.D. and N.D. conceived the review and wrote the first draft of the manuscript. All authors listed made a direct, substantial and intellectual contribution to the work. All authors have read and agreed to the published version of the manuscript.
Funding
Work in the laboratory of N.B., D.T. and S.D. was founded by MAECI-ISARP grant PGR00948, by the MIUR, PRIN2015, project 20154JRJPP and by Fondazione Cariplo (grant number 2017–0846).
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization (WHO). World Malaria Report 2019; World Health Organization: Geneva, Switzerland, 2019; Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- Autino, B.; Corbett, Y.; Castelli, F.; Taramelli, D. Pathogenesis of malaria in tissues and blood. Mediterr. J. Hematol. Infect. Dis. 2012, 4, e2012061. [Google Scholar] [CrossRef]
- Fried, M.; Duffy, P.E. Malaria during Pregnancy. Cold Spring Harb. Perspect. Med. 2017, 7. [Google Scholar] [CrossRef]
- Wright, C.W. Artemisia; Taylor & Francis: London, UK, 2002. [Google Scholar]
- Tu, Y. The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat. Med. 2011, 17, 1217–1220. [Google Scholar] [CrossRef]
- de Vries, P.J.; Dien, T.K. Clinical pharmacology and therapeutic potential of artemisinin and its derivatives in the treatment of malaria. Drugs 1996, 52, 818–836. [Google Scholar] [CrossRef]
- Meshnick, S.R.; Taylor, T.E.; Kamchonwongpaisan, S. Artemisinin and the antimalarial endoperoxides: From herbal remedy to targeted chemotherapy. Microbiol. Rev. 1996, 60, 301–315. [Google Scholar] [CrossRef] [PubMed]
- Woodrow, C.J.; Haynes, R.K.; Krishna, S. Artemisinins. Postgrad. Med. J. 2005, 81, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.N.; Padín-Irizarry, V.; van der Watt, M.E.; Reader, J.; Liebenberg, W.; Wiesner, L.; Smith, P.; Eribez, K.; Winzeler, E.A.; Kyle, D.E.; et al. Optimal 10-Aminoartemisinins With Potent Transmission-Blocking Capabilities for New Artemisinin Combination Therapies-Activities Against Blood Stage. Front. Chem. 2019, 7, 901. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Zheng, H. Stage-specific gametocytocidal effect in vitro of the antimalaria drug qinghaosu on Plasmodium falciparum. Parasitol. Res. 1990, 76, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Gilder, M.E.; McGready, R.; Nosten, F. Antimalarial drugs for treating and preventing malaria in pregnant and lactating women. Expert Opin. Drug Saf. 2018, 17, 1129–1144. [Google Scholar] [CrossRef] [PubMed]
- Nambozi, M.; Tinto, H.; Mwapasa, V.; Tagbor, H.; Kabuya, J.B.; Hachizovu, S.; Traoré, M.; Valea, I.; Tahita, M.C.; Ampofo, G.; et al. Artemisinin-based combination therapy during pregnancy: Outcome of pregnancy and infant mortality: A cohort study. Malar. J. 2019, 18, 105. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Guidelines for the Treatment of Malaria 2015; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- World Health Organization (WHO). Intermittent Preventive Treatment in Pregnancy. Available online: https://www.who.int/malaria/areas/preventive_therapies/pregnancy/en/ (accessed on 29 May 2020).
- Dellicour, S.; Sevene, E.; McGready, R.; Tinto, H.; Mosha, D.; Manyando, C.; Rulisa, S.; Desai, M.; Ouma, P.; Oneko, M.; et al. First-trimester artemisinin derivatives and quinine treatments and the risk of adverse pregnancy outcomes in Africa and Asia: A meta-analysis of observational studies. PLoS Med. 2017, 14, e1002290. [Google Scholar] [CrossRef] [PubMed]
- Bauserman, M.; Conroy, A.L.; North, K.; Patterson, J.; Bose, C.; Meshnick, S. An overview of malaria in pregnancy. Semin. Perinatol. 2019, 43, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Ayres Pereira, M.; Mandel Clausen, T.; Pehrson, C.; Mao, Y.; Resende, M.; Daugaard, M.; Riis Kristensen, A.; Spliid, C.; Mathiesen, L.; E Knudsen, L.; et al. Placental Sequestration of Plasmodium falciparum Malaria Parasites Is Mediated by the Interaction Between VAR2CSA and Chondroitin Sulfate A on Syndecan-1. PLoS Pathog. 2016, 12, e1005831. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.L. Genesis of placental sequestration in malaria and possible targets for drugs for placental malaria. Birth Defects Res. 2019, 111, 569–583. [Google Scholar] [CrossRef]
- Rogerson, S.J.; Hviid, L.; Duffy, P.E.; Leke, R.F.; Taylor, D.W. Malaria in pregnancy: Pathogenesis and immunity. Lancet Infect. Dis. 2007, 7, 105–117. [Google Scholar] [CrossRef]
- Kovacs, S.D.; van Eijk, A.M.; Sevene, E.; Dellicour, S.; Weiss, N.S.; Emerson, S.; Steketee, R.; Ter Kuile, F.O.; Stergachis, A. The Safety of Artemisinin Derivatives for the Treatment of Malaria in the 2nd or 3rd Trimester of Pregnancy: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0164963. [Google Scholar] [CrossRef]
- Kovacs, S.D.; Rijken, M.J.; Stergachis, A. Treating severe malaria in pregnancy: A review of the evidence. Drug Saf. 2015, 38, 165–181. [Google Scholar] [CrossRef]
- Dondorp, A.; Nosten, F.; Stepniewska, K.; Day, N.; White, N.; South East Asian Quinine Artesunate Malaria Trial (SEAQUAMAT) group. Artesunate versus quinine for treatment of severe falciparum malaria: A randomised trial. Lancet 2005, 366, 717–725. [Google Scholar] [CrossRef]
- Dondorp, A.M.; Fanello, C.I.; Hendriksen, I.C.; Gomes, E.; Seni, A.; Chhaganlal, K.D.; Bojang, K.; Olaosebikan, R.; Anunobi, N.; Maitland, K.; et al. Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): An open-label, randomised trial. Lancet 2010, 376, 1647–1657. [Google Scholar] [CrossRef]
- White, T.E.; Clark, R.L. Sensitive periods for developmental toxicity of orally administered artesunate in the rat. Birth Defects Res. B Dev. Reprod. Toxicol. 2008, 83, 407–417. [Google Scholar] [CrossRef]
- D’Alessandro, U.; Hill, J.; Tarning, J.; Pell, C.; Webster, J.; Gutman, J.; Sevene, E. Treatment of uncomplicated and severe malaria during pregnancy. Lancet Infect. Dis. 2018, 18, e133–e146. [Google Scholar] [CrossRef]
- Rogerson, S.J. Management of malaria in pregnancy. Indian J. Med. Res. 2017, 146, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Malaria Policy Advisory Committee to the WHO: Conclusions and recommendations of eighth biannual meeting (September 2015). Malar. J. 2016, 15, 117. [CrossRef] [PubMed]
- World Health Organization (WHO). WHO Policy Brief for the Implementation of Intermittent Preventive Treatment of Malaria in Pregnancy Using Sulfadoxine-pyrimethamine (IPTp-SP); World Health Organization: Geneva, Switzerland, April 2013; (rev January 2014). [Google Scholar]
- Kayentao, K.; Garner, P.; van Eijk, A.M.; Naidoo, I.; Roper, C.; Mulokozi, A.; MacArthur, J.R.; Luntamo, M.; Ashorn, P.; Doumbo, O.K.; et al. Intermittent preventive therapy for malaria during pregnancy using 2 vs 3 or more doses of sulfadoxine-pyrimethamine and risk of low birth weight in Africa: Systematic review and meta-analysis. JAMA 2013, 309, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Mlugu, E.M.; Minzi, O.; Asghar, M.; Färnert, A.; Kamuhabwa, A.A.R.; Aklillu, E. Effectiveness of Sulfadoxine-Pyrimethamine for Intermittent Preventive Treatment of Malaria and Adverse Birth Outcomes in Pregnant Women. Pathogens 2020, 9, 207. [Google Scholar] [CrossRef] [PubMed]
- Roman, E.; Andrejko, K.; Wolf, K.; Henry, M.; Youll, S.; Florey, L.; Ferenchick, E.; Gutman, J.R. Determinants of uptake of intermittent preventive treatment during pregnancy: A review. Malar. J. 2019, 18, 372. [Google Scholar] [CrossRef] [PubMed]
- Bhutta, Z.A.; Das, J.K.; Bahl, R.; Lawn, J.E.; Salam, R.A.; Paul, V.K.; Sankar, M.J.; Sankar, J.M.; Blencowe, H.; Rizvi, A.; et al. Can available interventions end preventable deaths in mothers, newborn babies, and stillbirths, and at what cost? Lancet 2014, 384, 347–370. [Google Scholar] [CrossRef]
- Baraka, V.; Ishengoma, D.S.; Fransis, F.; Minja, D.T.; Madebe, R.A.; Ngatunga, D.; Van Geertruyden, J.P. High-level Plasmodium falciparum sulfadoxine-pyrimethamine resistance with the concomitant occurrence of septuple haplotype in Tanzania. Malar. J. 2015, 14, 439. [Google Scholar] [CrossRef]
- Desai, M.; Gutman, J.; L’lanziva, A.; Otieno, K.; Juma, E.; Kariuki, S.; Ouma, P.; Were, V.; Laserson, K.; Katana, A.; et al. Intermittent screening and treatment or intermittent preventive treatment with dihydroartemisinin-piperaquine versus intermittent preventive treatment with sulfadoxine-pyrimethamine for the control of malaria during pregnancy in western Kenya: An open-label, three-group, randomised controlled superiority trial. Lancet 2015, 386, 2507–2519. [Google Scholar] [CrossRef]
- Gutman, J.; Kovacs, S.; Dorsey, G.; Stergachis, A.; Ter Kuile, F.O. Safety, tolerability, and efficacy of repeated doses of dihydroartemisinin-piperaquine for prevention and treatment of malaria: A systematic review and meta-analysis. Lancet Infect. Dis 2017, 17, 184–193. [Google Scholar] [CrossRef]
- Olaleye, A.; Okusanya, B.O.; Oduwole, O.; Esu, E.; Meremikwu, M. A systematic review and meta-analysis of dihydroartemisinin-piperaquine versus sulphadoxine-pyrimethamine for malaria prevention in pregnancy. Int. J. Gynaecol. Obstet. 2019, 146, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Barateiro, A.; Pereira, M.L.M.; Epiphanio, S.; Marinho, C.R.F. Contribution of Murine Models to the Study of Malaria During Pregnancy. Front. Microbiol. 2019, 10, 1369. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, S.F.; Barresi, M.J. Intermediate and lateral plate mesoderm: Blood, heart, and kidneys. In Developmental Biology, 11th ed.; Sinauer Associated inch. Oxford: Sunderland, MA, USA, 2016. [Google Scholar]
- Coultas, L.; Chawengsaksophak, K.; Rossant, J. Endothelial cells and VEGF in vascular development. Nature 2005, 438, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.H.; Alitalo, K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat. Rev. Mol. Cell Biol. 2007, 8, 464–478. [Google Scholar] [CrossRef]
- Lee, H.Y.; Inselman, A.L.; Kanungo, J.; Hansen, D.K. Alternative models in developmental toxicology. Syst. Biol. Reprod. Med. 2012, 58, 10–22. [Google Scholar] [CrossRef]
- McGready, R.; Phyo, A.P.; Rijken, M.J.; Tarning, J.; Lindegardh, N.; Hanpithakpon, W.; Than, H.H.; Hlaing, N.; Zin, N.T.; Singhasivanon, P.; et al. Artesunate/dihydroartemisinin pharmacokinetics in acute falciparum malaria in pregnancy: Absorption, bioavailability, disposition and disease effects. Br. J. Clin. Pharmacol. 2012, 73, 467–477. [Google Scholar] [CrossRef]
- White, T.E.; Bushdid, P.B.; Ritter, S.; Laffan, S.B.; Clark, R.L. Artesunate-induced depletion of embryonic erythroblasts precedes embryolethality and teratogenicity in vivo. Birth Defects Res. B Dev. Reprod. Toxicol. 2006, 77, 413–429. [Google Scholar] [CrossRef]
- Clark, R.L.; Lerman, S.A.; Cox, E.M.; Gristwood, W.E.; White, T.E. Developmental toxicity of artesunate in the rat: Comparison to other artemisinins, comparison of embryotoxicity and kinetics by oral and intravenous routes, and relationship to maternal reticulocyte count. Birth Defects Res. B Dev. Reprod. Toxicol. 2008, 83, 397–406. [Google Scholar] [CrossRef]
- Clark, R.L.; Edwards, T.L.; Longo, M.; Kinney, J.; Walker, D.K.; Rhodes, J.; Clode, S.A.; Rückle, T.; Wells, T.; Andenmatten, N.; et al. Improved safety margin for embryotoxicity in rats for the new endoperoxide artefenomel (OZ439) as compared to artesunate. Birth Defects Res. 2018, 110, 553–578. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Tahir, M. Folic Acid Effect on Artemether-Induced Cardiac Anomalies in Mice Embryos. J. Coll. Physicians Surg. Pak. 2017, 27, 532–535. [Google Scholar]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Longo, M.; Zanoncelli, S.; Manera, D.; Brughera, M.; Colombo, P.; Lansen, J.; Mazué, G.; Gomes, M.; Taylor, W.R.; Olliaro, P. Effects of the antimalarial drug dihydroartemisinin (DHA) on rat embryos in vitro. Reprod. Toxicol. 2006, 21, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Longo, M.; Zanoncelli, S.; Torre, P.D.; Riflettuto, M.; Cocco, F.; Pesenti, M.; Giusti, A.; Colombo, P.; Brughera, M.; Mazué, G.; et al. In vivo and in vitro investigations of the effects of the antimalarial drug dihydroartemisinin (DHA) on rat embryos. Reprod. Toxicol. 2006, 22, 797–810. [Google Scholar] [CrossRef] [PubMed]
- Posobiec, L.M.; Clark, R.L.; Bushdid, P.B.; Laffan, S.B.; Wang, K.F.; White, T.E. Dihydroartemisinin (DHA) treatment causes an arrest of cell division and apoptosis in rat embryonic erythroblasts in whole embryo culture. Birth Defects Res. B Dev. Reprod. Toxicol. 2013, 98, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Longo, M.; Zanoncelli, S.; Della Torre, P.; Rosa, F.; Giusti, A.; Colombo, P.; Brughera, M.; Mazué, G.; Olliaro, P. Investigations of the effects of the antimalarial drug dihydroartemisinin (DHA) using the Frog Embryo Teratogenesis Assay-Xenopus (FETAX). Reprod. Toxicol. 2008, 25, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Ba, Q.; Duan, J.; Tian, J.Q.; Wang, Z.L.; Chen, T.; Li, X.G.; Chen, P.Z.; Wu, S.J.; Xiang, L.; Li, J.Q.; et al. Dihydroartemisinin promotes angiogenesis during the early embryonic development of zebrafish. Acta Pharmacol. Sin. 2013, 34, 1101–1107. [Google Scholar] [CrossRef]
- Clark, R.L.; Arima, A.; Makori, N.; Nakata, Y.; Bernard, F.; Gristwood, W.; Harrell, A.; White, T.E.; Wier, P.J. Artesunate: Developmental toxicity and toxicokinetics in monkeys. Birth Defects Res. B Dev. Reprod. Toxicol. 2008, 83, 418–434. [Google Scholar] [CrossRef]
- Gomes, C.; Boareto, A.C.; Dalsenter, P.R. Clinical and non-clinical safety of artemisinin derivatives in pregnancy. Reprod. Toxicol. 2016, 65, 194–203. [Google Scholar] [CrossRef]
- Clark, R.L.; Youreneff, M.; DeLise, A.M. Developmental toxicity studies of lumefantrine and artemether in rats and rabbits. Birth Defects Res. B Dev. Reprod. Toxicol. 2016, 107, 243–257. [Google Scholar] [CrossRef]
- Finaurini, S.; Ronzoni, L.; Colancecco, A.; Cattaneo, A.; Cappellini, M.D.; Ward, S.A.; Taramelli, D. Selective toxicity of dihydroartemisinin on human CD34+ erythroid cell differentiation. Toxicology 2010, 276, 128–134. [Google Scholar] [CrossRef]
- Finaurini, S.; Basilico, N.; Corbett, Y.; D’Alessandro, S.; Parapini, S.; Olliaro, P.; Haynes, R.K.; Taramelli, D. Dihydroartemisinin inhibits the human erythroid cell differentiation by altering the cell cycle. Toxicology 2012, 300, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Haynes, R.K.; Fugmann, B.; Stetter, J.; Rieckmann, K.; Heilmann, H.-D.; Chan, H.-W.; Cheung, M.-K.; Lam, W.-L.; Wong, H.-N.; Croft, S.L.; et al. Artemisone—A Highly Active Antimalarial Drug of the Artemisinin Class. Angew. Chem. Int. Ed. Engl. 2006, 45, 2082–2088. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, S.; Gelati, M.; Basilico, N.; Parati, E.A.; Haynes, R.K.; Taramelli, D. Differential effects on angiogenesis of two antimalarial compounds, dihydroartemisinin and artemisone: Implications for embryotoxicity. Toxicology 2007, 241, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Augusto, O.; Stergachis, A.; Dellicour, S.; Tinto, H.; Valá, A.; Ruperez, M.; Macete, E.; Nakanabo-Diallo, S.; Kazienga, A.; Valéa, I.; et al. First trimester use of artemisinin-based combination therapy and the risk of low birth weight and small for gestational age. Malar. J. 2020, 19, 144. [Google Scholar] [CrossRef] [PubMed]
- Rouamba, T.; Sondo, P.; Derra, K.; Nakanabo-Diallo, S.; Bihoun, B.; Rouamba, E.; Tarnagda, Z.; Kazienga, A.; Valea, I.; Sorgho, H.; et al. Optimal Approach and Strategies to Strengthen Pharmacovigilance in Sub-Saharan Africa: A Cohort Study of Patients Treated with First-Line Artemisinin-Based Combination Therapies in the Nanoro Health and Demographic Surveillance System, Burkina Faso. Drug Des. Dev. Ther. 2020, 14, 1507–1521. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.A.; Simpson, J.A.; Paw, M.K.; Pimanpanarak, M.; Wiladphaingern, J.; Rijken, M.J.; Jittamala, P.; White, N.J.; Fowkes, F.J.I.; Nosten, F.; et al. Safety of artemisinins in first trimester of prospectively followed pregnancies: An observational study. Lancet Infect. Dis. 2016, 16, 576–583. [Google Scholar] [CrossRef]
- Manyando, C.; Njunju, E.M.; Virtanen, M.; Hamed, K.; Gomes, M.; Van Geertruyden, J.P. Exposure to artemether-lumefantrine (Coartem) in first trimester pregnancy in an observational study in Zambia. Malar. J. 2015, 14, 77. [Google Scholar] [CrossRef]
- Dellicour, S.; Desai, M.; Aol, G.; Oneko, M.; Ouma, P.; Bigogo, G.; Burton, D.C.; Breiman, R.F.; Hamel, M.J.; Slutsker, L.; et al. Risks of miscarriage and inadvertent exposure to artemisinin derivatives in the first trimester of pregnancy: A prospective cohort study in western Kenya. Malar. J. 2015, 14, 461. [Google Scholar] [CrossRef]
- Poespoprodjo, J.R.; Fobia, W.; Kenangalem, E.; Lampah, D.A.; Sugiarto, P.; Tjitra, E.; Anstey, N.M.; Price, R.N. Dihydroartemisinin-piperaquine treatment of multidrug resistant falciparum and vivax malaria in pregnancy. PLoS ONE 2014, 9, e84976. [Google Scholar] [CrossRef]
- Mosha, D.; Mazuguni, F.; Mrema, S.; Sevene, E.; Abdulla, S.; Genton, B. Safety of artemether-lumefantrine exposure in first trimester of pregnancy: An observational cohort. Malar. J. 2014, 13, 197. [Google Scholar] [CrossRef]
- Dellicour, S.; Brasseur, P.; Thorn, P.; Gaye, O.; Olliaro, P.; Badiane, M.; Stergachis, A.; ter Kuile, F.O. Probabilistic record linkage for monitoring the safety of artemisinin-based combination therapy in the first trimester of pregnancy in Senegal. Drug Saf. 2013, 36, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Rulisa, S.; Kaligirwa, N.; Agaba, S.; Karema, C.; Mens, P.F.; de Vries, P.J. Pharmacovigilance of artemether-lumefantrine in pregnant women followed until delivery in Rwanda. Malar. J. 2012, 11, 225. [Google Scholar] [CrossRef] [PubMed]
- Adam, I.; Elhassan, E.M.; Omer, E.M.; Abdulla, M.A.; Mahgoub, H.M.; Adam, G.K. Safety of artemisinins during early pregnancy, assessed in 62 Sudanese women. Ann. Trop. Med. Parasitol. 2009, 103, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Adam, I.; Elwasila, E.; Mohammed Ali, D.A.; Elansari, E.; Elbashir, M.I. Artemether in the treatment of falciparum malaria during pregnancy in eastern Sudan. Trans. R Soc. Trop. Med. Hyg. 2004, 98, 509–513. [Google Scholar] [CrossRef]
- McGready, R.; Cho, T.; Samuel; Villegas, L.; Brockman, A.; van Vugt, M.; Looareesuwan, S.; White, N.J.; Nosten, F. Randomized comparison of quinine-clindamycin versus artesunate in the treatment of falciparum malaria in pregnancy. Trans. R Soc. Trop. Med. Hyg. 2001, 95, 651–656. [Google Scholar] [CrossRef]
- Deen, J.L.; von Seidlein, L.; Pinder, M.; Walraven, G.E.; Greenwood, B.M. The safety of the combination artesunate and pyrimethamine-sulfadoxine given during pregnancy. Trans. R Soc. Trop. Med. Hyg. 2001, 95, 424–428. [Google Scholar] [CrossRef]
- McGready, R.; Cho, T.; Cho, J.J.; Simpson, J.A.; Luxemburger, C.; Dubowitz, L.; Looareesuwan, S.; White, N.J.; Nosten, F. Artemisinin derivatives in the treatment of falciparum malaria in pregnancy. Trans. R Soc. Trop. Med. Hyg. 1998, 92, 430–433. [Google Scholar] [CrossRef]
- Tinto, H.; Sevene, E.; Dellicour, S.; Calip, G.S.; d’Alessandro, U.; Macete, E.; Nakanabo-Diallo, S.; Kazienga, A.; Valea, I.; Sorgho, H.; et al. Assessment of the safety of antimalarial drug use during early pregnancy (ASAP): Protocol for a multicenter prospective cohort study in Burkina Faso, Kenya and Mozambique. Reprod. Health 2015, 12, 112. [Google Scholar] [CrossRef][Green Version]
- Sangaré, L.R.; Weiss, N.S.; Brentlinger, P.E.; Richardson, B.A.; Staedke, S.G.; Kiwuwa, M.S.; Stergachis, A. Patterns of anti-malarial drug treatment among pregnant women in Uganda. Malar. J. 2011, 10, 152. [Google Scholar] [CrossRef]
- Manyando, C.; Mkandawire, R.; Puma, L.; Sinkala, M.; Mpabalwani, E.; Njunju, E.; Gomes, M.; Ribeiro, I.; Walter, V.; Virtanen, M.; et al. Safety of artemether-lumefantrine in pregnant women with malaria: Results of a prospective cohort study in Zambia. Malar. J. 2010, 9, 249. [Google Scholar] [CrossRef]
- McGready, R.; Cho, T.; Keo, N.K.; Thwai, K.L.; Villegas, L.; Looareesuwan, S.; White, N.J.; Nosten, F. Artemisinin antimalarials in pregnancy: A prospective treatment study of 539 episodes of multidrug-resistant Plasmodium falciparum. Clin. Infect. Dis. 2001, 33, 2009–2016. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McGready, R.; Lee, S.J.; Wiladphaingern, J.; Ashley, E.A.; Rijken, M.J.; Boel, M.; Simpson, J.A.; Paw, M.K.; Pimanpanarak, M.; Mu, O.; et al. Adverse effects of falciparum and vivax malaria and the safety of antimalarial treatment in early pregnancy: A population-based study. Lancet Infect. Dis. 2012, 12, 388–396. [Google Scholar] [CrossRef]
- Saito, M.; Gilder, M.E.; Nosten, F.; McGready, R.; Guérin, P.J. Systematic literature review and meta-analysis of the efficacy of artemisinin-based and quinine-based treatments for uncomplicated falciparum malaria in pregnancy: Methodological challenges. Malar. J. 2017, 16, 488. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Gilder, M.E.; Nosten, F.; Guérin, P.J.; McGready, R. Methodology of assessment and reporting of safety in anti-malarial treatment efficacy studies of uncomplicated falciparum malaria in pregnancy: A systematic literature review. Malar. J. 2017, 16, 491. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, K.; Sivaramakrishnan, G.; Kanters, S. Adverse pregnancy outcomes between the anti-malarial drugs: Is there a difference between the drugs recommended by World Health Organization? Results of a mixed treatment comparison analysis of randomized clinical trials and cohort studies. Int. J. Risk Saf. Med. 2019, 30, 73–89. [Google Scholar] [CrossRef]
- Ballard, S.B.; Salinger, A.; Arguin, P.M.; Desai, M.; Tan, K.R.; MPHc. Updated CDC Recommendations for Using Artemether-Lumefantrine for the Treatment of Uncomplicated Malaria in Pregnant Women in the United States. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 424–431. [Google Scholar] [CrossRef]
- Kloprogge, F.; McGready, R.; Phyo, A.P.; Rijken, M.J.; Hanpithakpon, W.; Than, H.H.; Hlaing, N.; Zin, N.T.; Day, N.P.; White, N.J.; et al. Opposite malaria and pregnancy effect on oral bioavailability of artesunate—A population pharmacokinetic evaluation. Br. J. Clin. Pharmacol. 2015, 80, 642–653. [Google Scholar] [CrossRef]
- Teja-Isavadharm, P.; Watt, G.; Eamsila, C.; Jongsakul, K.; Li, Q.; Keeratithakul, G.; Sirisopana, N.; Luesutthiviboon, L.; Brewer, T.G.; Kyle, D.E. Comparative pharmacokinetics and effect kinetics of orally administered artesunate in healthy volunteers and patients with uncomplicated falciparum malaria. Am. J. Trop. Med. Hyg. 2001, 65, 717–721. [Google Scholar] [CrossRef][Green Version]
- Parapini, S.; Olliaro, P.; Navaratnam, V.; Taramelli, D.; Basilico, N. Stability of the antimalarial drug dihydroartemisinin in under physiologically-relevant conditions: Implications for clinical treatment, pharmacokinetic and in vitro assays. Antimicrob. Agents Chemother. 2015. [Google Scholar] [CrossRef]
- Gu, H.M.; Warhurst, D.C.; Peters, W. Uptake of [3H] dihydroartemisinine by erythrocytes infected with Plasmodium falciparum in vitro. Trans. R Soc. Trop. Med. Hyg. 1984, 78, 265–270. [Google Scholar] [CrossRef]
- Clark, R.L. Embryotoxicity of the artemisinin antimalarials and potential consequences for use in women in the first trimester. Reprod. Toxicol. 2009, 28, 285–296. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).