Pharmacological Potential of Small Molecules for Treating Corneal Neovascularization
Abstract
1. Introduction
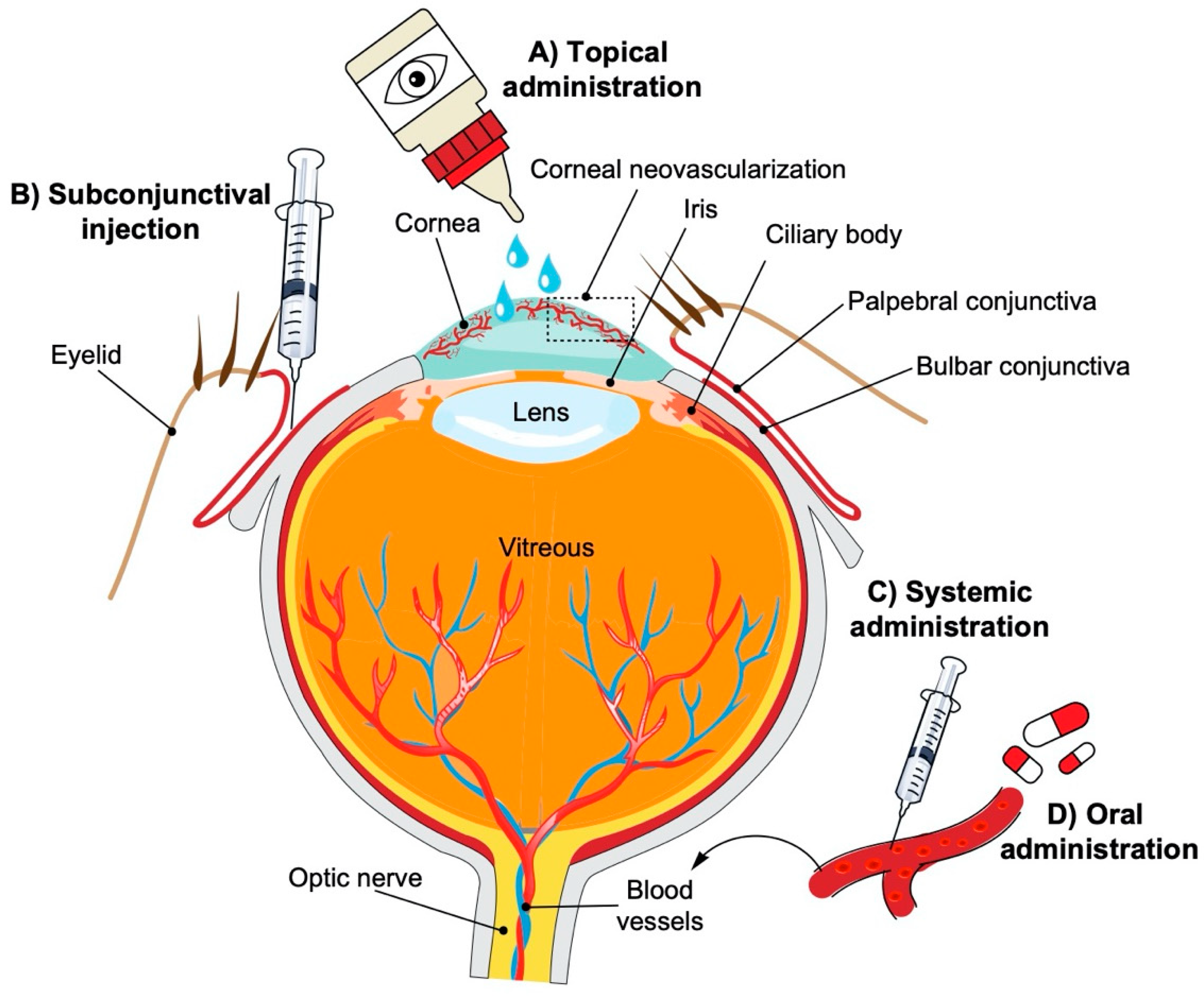
2. Models of CoNV
3. Synthetic Small Molecules
3.1. Tyrosine Kinase Inhibitors
3.2. Repurposed Antimicrobials
3.3. Other Synthetics
4. Natural Products
4.1. Polyphenols: Flavonoids
4.2. Non-Flavonoid Phytochemicals
4.3. Immunosuppressants
Macrolides
4.4. Vitamins and Photoactivatable Compounds
4.5. HDAC Inhibitors
5. Discussion/Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Azar, D.T. Corneal angiogenic privilege: Angiogenic and antiangiogenic factors in corneal avascularity, vasculogenesis, and wound healing (an American Ophthalmological Society thesis). Trans. Am. Ophthalmol. Soc. 2006, 104, 264–302. [Google Scholar]
- Chang, J.H.; Gabison, E.E.; Kato, T.; Azar, D.T. Corneal neovascularization. Curr. Opin. Ophthalmol. 2001, 12, 242–249. [Google Scholar] [CrossRef]
- World Health Organization. Blindness and Vision Impairment Prevention. Available online: https://www.who.int/blindness/causes/priority/en/index8.html (accessed on 29 May 2020).
- Pineda, R. World corneal blindness. In Foundations of Corneal Disease; Springer: New York, NY, USA, 2020; pp. 299–305. [Google Scholar]
- Gupta, D.; Illingworth, C. Treatments for corneal neovascularization: A review. Cornea 2011, 30, 927–938. [Google Scholar] [CrossRef]
- Cursiefen, C.; Wenkel, H.; Martus, P.; Langenbucher, A.; Nguyen, N.X.; Seitz, B.; Kuchle, M.; Naumann, G.O. Impact of short-term versus long-term topical steroids on corneal neovascularization after non-high-risk keratoplasty. Graefes Arch. Clin. Exp. Ophthalmol. 2001, 239, 514–521. [Google Scholar] [CrossRef]
- Al-Torbak, A.; Al-Amri, A.; Wagoner, M.D. Deep corneal neovascularization after implantation with intrastromal corneal ring segments. Am. J. Ophthalmol. 2005, 140, 926–927. [Google Scholar] [CrossRef]
- Krebs, I.; Lie, S.; Stolba, U.; Zeiler, F.; Felke, S.; Binder, S. Efficacy of intravitreal bevacizumab (Avastin) therapy for early and advanced neovascular age-related macular degeneration. Acta Ophthalmol. 2009, 87, 611–617. [Google Scholar] [CrossRef]
- Kim, S.W.; Ha, B.J.; Kim, E.K.; Tchah, H.; Kim, T.I. The effect of topical bevacizumab on corneal neovascularization. Ophthalmology 2008, 115, e33–e38. [Google Scholar] [CrossRef]
- Ozdemir, O.; Altintas, O.; Altintas, L.; Ozkan, B.; Akdag, C.; Yuksel, N. Comparison of the effects of subconjunctival and topical anti-VEGF therapy (bevacizumab) on experimental corneal neovascularization. Arq. Bras. Oftalmol. 2014, 77, 209–213. [Google Scholar] [CrossRef]
- Corson, T.W.; Crews, C.M. Molecular understanding and modern application of traditional medicines: Triumphs and trials. Cell 2007, 130, 769–774. [Google Scholar] [CrossRef]
- Chang, J.-H.; Garg, N.K.; Lunde, E.; Han, K.-Y.; Jain, S.; Azar, D.T. Corneal neovascularization: An anti-VEGF therapy review. Surv. Ophthalmol. 2012, 57, 415–429. [Google Scholar] [CrossRef]
- Liang, C.-C.; Park, A.Y.; Guan, J.-L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329. [Google Scholar] [CrossRef]
- Madri, J.A.; Pratt, B.M.; Tucker, A.M. Phenotypic modulation of endothelial cells by transforming growth factor-beta depends upon the composition and organization of the extracellular matrix. J. Cell Biol. 1988, 106, 1375–1384. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.S.; Birsner, A.E.; D’Amato, R.J. The mouse cornea micropocket angiogenesis assay. Nat. Protoc. 2007, 2, 2545–2550. [Google Scholar] [CrossRef] [PubMed]
- Montezuma, S.R.; Vavvas, D.; Miller, J.W. Review of the ocular angiogenesis animal models. Semin. Ophthalmol. 2009, 24, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Joussen, A.M.; Beecken, W.D.; Moromizato, Y.; Schwartz, A.; Kirchhof, B.; Poulaki, V. Inhibition of inflammatory corneal angiogenesis by TNP-470. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2510–2516. [Google Scholar]
- Mahoney, J.M.; Waterbury, L.D. Drug effects on the neovascularization response to silver nitrate cauterization of the rat cornea. Curr. Eye Res. 1985, 4, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Pal-Ghosh, S.; Tadvalkar, G.; Jurjus, R.A.; Zieske, J.D.; Stepp, M.A. BALB/c and C57BL6 mouse strains vary in their ability to heal corneal epithelial debridement wounds. Exp. Eye Res. 2008, 87, 478–486. [Google Scholar] [CrossRef]
- Gotink, K.J.; Verheul, H.M. Anti-angiogenic tyrosine kinase inhibitors: What is their mechanism of action? Angiogenesis 2010, 13, 1–14. [Google Scholar] [CrossRef]
- Faivre, S.; Demetri, G.; Sargent, W.; Raymond, E. Molecular basis for sunitinib efficacy and future clinical development. Nat. Rev. Drug Discov. 2007, 6, 734–745. [Google Scholar] [CrossRef]
- Lohela, M.; Bry, M.; Tammela, T.; Alitalo, K. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr. Opin Cell Biol. 2009, 21, 154–165. [Google Scholar] [CrossRef]
- Detry, B.; Blacher, S.; Erpicum, C.; Paupert, J.; Maertens, L.; Maillard, C.; Munaut, C.; Sounni, N.E.; Lambert, V.; Foidart, J.M.; et al. Sunitinib inhibits inflammatory corneal lymphangiogenesis. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3082–3093. [Google Scholar] [CrossRef] [PubMed]
- Perez-Santonja, J.J.; Campos-Mollo, E.; Lledo-Riquelme, M.; Javaloy, J.; Alio, J.L. Inhibition of corneal neovascularization by topical bevacizumab (anti-VEGF) and sunitinib (anti-VEGF and anti-PDGF) in an animal model. Am. J. Ophthalmol. 2010, 150, 519–528.e1. [Google Scholar] [CrossRef] [PubMed]
- Ko, B.Y.; Kim, Y.S.; Baek, S.G.; Lee, G.W.; Kim, J.M.; Jean, W.S.; Lee, N.S.; Kang, J. Inhibition of corneal neovascularization by subconjunctival and topical bevacizumab and sunitinib in a rabbit model. Cornea 2013, 32, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Hellstrom, M.; Kalen, M.; Lindahl, P.; Abramsson, A.; Betsholtz, C. Role of PDGF-B and PDGFR-β in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 1999, 126, 3047–3055. [Google Scholar]
- Dell, S.; Peters, S.; Muther, P.; Kociok, N.; Joussen, A.M. The role of PDGF receptor inhibitors and PI3-kinase signaling in the pathogenesis of corneal neovascularization. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1928–1937. [Google Scholar] [CrossRef] [PubMed]
- Hos, D.; Bock, F.; Dietrich, T.; Onderka, J.; Kruse, F.E.; Thierauch, K.H.; Cursiefen, C. Inflammatory corneal (lymph)angiogenesis is blocked by VEGFR-tyrosine kinase inhibitor ZK 261991, resulting in improved graft survival after corneal transplantation. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1836–1842. [Google Scholar] [CrossRef]
- Wilhelm, S.M.; Carter, C.; Tang, L.; Wilkie, D.; McNabola, A.; Rong, H.; Chen, C.; Zhang, X.; Vincent, P.; McHugh, M.; et al. BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004, 64, 7099–7109. [Google Scholar] [CrossRef]
- Kane, R.C.; Farrell, A.T.; Madabushi, R.; Booth, B.; Chattopadhyay, S.; Sridhara, R.; Justice, R.; Pazdur, R. Sorafenib for the treatment of unresectable hepatocellular carcinoma. Oncologist 2009, 14, 95–100. [Google Scholar] [CrossRef]
- Seo, J.W.; Chung, S.H.; Choi, J.S.; Joo, C.K. Inhibition of corneal neovascularization in rats by systemic administration of sorafenib. Cornea 2012, 31, 907–912. [Google Scholar] [CrossRef]
- Fong, T.A.; Shawver, L.K.; Sun, L.; Tang, C.; App, H.; Powell, T.J.; Kim, Y.H.; Schreck, R.; Wang, X.; Risau, W.; et al. SU5416 is a potent and selective inhibitor of the vascular endothelial growth factor receptor (Flk-1/KDR) that inhibits tyrosine kinase catalysis, tumor vascularization, and growth of multiple tumor types. Cancer Res. 1999, 59, 99–106. [Google Scholar]
- Keskin, U.; Totan, Y.; Karadag, R.; Erdurmus, M.; Aydin, B. Inhibitory effects of SU5416, a selective vascular endothelial growth factor receptor tyrosine kinase inhibitor, on experimental corneal neovascularization. Ophthalmic Res. 2012, 47, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Kuenen, B.C.; Rosen, L.; Smit, E.F.; Parson, M.R.; Levi, M.; Ruijter, R.; Huisman, H.; Kedde, M.A.; Noordhuis, P.; Van Der Vijgh, W.J. Dose-finding and pharmacokinetic study of cisplatin, gemcitabine, and SU5416 in patients with solid tumors. J. Clin. Oncol. 2002, 20, 1657–1667. [Google Scholar] [CrossRef] [PubMed]
- Takeda, A.; Hata, Y.; Shiose, S.; Sassa, Y.; Honda, M.; Fujisawa, K.; Sakamoto, T.; Ishibashi, T. Suppression of experimental choroidal neovascularization utilizing KDR selective receptor tyrosine kinase inhibitor. Graefes Arch. Clin. Exp. Ophthalmol. 2003, 241, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.J.; Woo, J.M.; Ji, Y.S.; Yoon, K.C. Comparison of the therapeutic efficacies of topical rivoceranib and topical bevacizumab in a murine model of corneal neovascularization. Medicina (Kaunas) 2019, 55, 729. [Google Scholar] [CrossRef] [PubMed]
- Eisen, T.; Joensuu, H.; Nathan, P.D.; Harper, P.G.; Wojtukiewicz, M.Z.; Nicholson, S.; Bahl, A.; Tomczak, P.; Pyrhonen, S.; Fife, K.; et al. Regorafenib for patients with previously untreated metastatic or unresectable renal-cell carcinoma: A single-group phase 2 trial. Lancet Oncol. 2012, 13, 1055–1062. [Google Scholar] [CrossRef]
- Onder, H.I.; Erdurmus, M.; Bucak, Y.Y.; Simavli, H.; Oktay, M.; Kukner, A.S. Inhibitory effects of regorafenib, a multiple tyrosine kinase inhibitor, on corneal neovascularization. Int. J. Ophthalmol. 2014, 7, 220–225. [Google Scholar] [CrossRef][Green Version]
- Konecny, G.E.; Pegram, M.D.; Venkatesan, N.; Finn, R.; Yang, G.; Rahmeh, M.; Untch, M.; Rusnak, D.W.; Spehar, G.; Mullin, R.J.; et al. Activity of the dual kinase inhibitor lapatinib (GW572016) against HER-2-overexpressing and trastuzumab-treated breast cancer cells. Cancer Res. 2006, 66, 1630–1639. [Google Scholar] [CrossRef]
- Rusnak, D.W.; Lackey, K.; Affleck, K.; Wood, E.R.; Alligood, K.J.; Rhodes, N.; Keith, B.R.; Murray, D.M.; Knight, W.B.; Mullin, R.J.; et al. The effects of the novel, reversible epidermal growth factor receptor/ErbB-2 tyrosine kinase inhibitor, GW2016, on the growth of human normal and tumor-derived cell lines in vitro and in vivo. Mol. Cancer 2001, 1, 85–94. [Google Scholar]
- Kaya, M.K.; Demir, T.; Bulut, H.; Akpolat, N.; Turgut, B. Effects of lapatinib and trastuzumab on vascular endothelial growth factor in experimental corneal neovascularization. Clin. Exp. Ophthalmol. 2015, 43, 449–457. [Google Scholar] [CrossRef]
- Pfizer. Inlyta (Axitinib) Tablets for Oral Administration. Available online: http://labeling.pfizer.com/ShowLabeling.aspx?id=759 (accessed on 29 May 2020).
- Lledo Riquelme, M.; Campos-Mollo, E.; Fernandez-Sanchez, L. Topical axitinib is a potent inhibitor of corneal neovascularization. Clin. Exp. Ophthalmol. 2018, 46, 1063–1074. [Google Scholar] [CrossRef]
- Lee, S.H.; Lopes de Menezes, D.; Vora, J.; Harris, A.; Ye, H.; Nordahl, L.; Garrett, E.; Samara, E.; Aukerman, S.L.; Gelb, A.B.; et al. In vivo target modulation and biological activity of CHIR-258, a multitargeted growth factor receptor kinase inhibitor, in colon cancer models. Clin. Cancer Res. 2005, 11, 3633–3641. [Google Scholar] [CrossRef] [PubMed]
- Sahan, B.; Ciftci, F.; Eyuboglu, S.; Yaba, A.; Yilmaz, B.; Yalvac, B.I. Comparison of the effects of dovitinib and bevacizumab on reducing neovascularization in an experimental rat corneal neovascularization model. Cornea 2019, 38, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Ekim, Y.; Kara, S.; Gencer, B.; Karaca, T. Efficacy of sunitinib, sunitinib-hesperetin, and sunitinib-doxycycline combinations on experimentally-induced corneal neovascularization. Curr. Eye Res. 2019, 44, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, J.J.; Pryszlak, M.; Smith, L.; Yanchus, C.; Kurji, N.; Shahani, V.M.; Molinski, S.V. Giving drugs a second chance: Overcoming regulatory and financial hurdles in repurposing approved drugs as cancer therapeutics. Front. Oncol. 2017, 7, 273. [Google Scholar] [CrossRef] [PubMed]
- Sapadin, A.N.; Fleischmajer, R. Tetracyclines: Nonantibiotic properties and their clinical implications. J. Am. Acad. Derm. 2006, 54, 258–265. [Google Scholar] [CrossRef]
- Dan, L.; Shi-long, Y.; Miao-li, L.; Yong-ping, L.; Hong-jie, M.; Ying, Z.; Xiang-gui, W. Inhibitory effect of oral doxycycline on neovascularization in a rat corneal alkali burn model of angiogenesis. Curr. Eye Res. 2008, 33, 653–660. [Google Scholar] [CrossRef]
- Aydin, E.; Kivilcim, M.; Peyman, G.A.; Esfahani, M.R.; Kazi, A.A.; Sanders, D.R. Inhibition of experimental angiogenesis of cornea by various doses of doxycycline and combination of triamcinolone acetonide with low-molecular-weight heparin and doxycycline. Cornea 2008, 27, 446–453. [Google Scholar] [CrossRef]
- Su, W.; Li, Z.; Li, F.; Chen, X.; Wan, Q.; Liang, D. Doxycycline-mediated inhibition of corneal angiogenesis: An MMP-independent mechanism. Investig. Ophthalmol. Vis. Sci. 2013, 54, 783–788. [Google Scholar] [CrossRef]
- Yao, J.S.; Shen, F.; Young, W.L.; Yang, G.Y. Comparison of doxycycline and minocycline in the inhibition of VEGF-induced smooth muscle cell migration. Neurochem. Int. 2007, 50, 524–530. [Google Scholar] [CrossRef]
- Xiao, O.; Xie, Z.L.; Lin, B.W.; Yin, X.F.; Pi, R.B.; Zhou, S.Y. Minocycline inhibits alkali burn-induced corneal neovascularization in mice. PLoS ONE 2012, 7, e41858. [Google Scholar] [CrossRef]
- Peterson, L.R. A review of tigecycline—The first glycylcycline. Int. J. Antimicrob. Agents 2008, 32 (Suppl. S4), S215–S222. [Google Scholar] [CrossRef]
- Goktas, S.; Erdogan, E.; Sakarya, R.; Sakarya, Y.; Yilmaz, M.; Ozcimen, M.; Unlukal, N.; Alpfidan, I.; Tas, F.; Erdogan, E.; et al. Inhibition of corneal neovascularization by topical and subconjunctival tigecycline. J. Ophthalmol. 2014, 2014, 452685. [Google Scholar] [CrossRef] [PubMed]
- Chong, C.R.; Xu, J.; Lu, J.; Bhat, S.; Sullivan, D.J., Jr.; Liu, J.O. Inhibition of angiogenesis by the antifungal drug itraconazole. ACS Chem. Biol. 2007, 2, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Goktas, S.; Sakarya, R.; Erdogan, E.; Sakarya, Y.; Ozcimen, M.; Dursunoglu, D.; Kocacan, M.; Alpfidan, I.; Erdogan, E.; Bukus, A.; et al. Antiangiogenic effect of itraconazole on corneal neovascularization: A pilot experimental investigation. Ophthalmic Res. 2014, 52, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Klayman, D.L. Qinghaosu (artemisinin): An antimalarial drug from China. Science 1985, 228, 1049–1055. [Google Scholar] [CrossRef]
- Chen, H.H.; Zhou, H.J.; Fang, X. Inhibition of human cancer cell line growth and human umbilical vein endothelial cell angiogenesis by artemisinin derivatives in vitro. Pharm Res. 2003, 48, 231–236. [Google Scholar] [CrossRef]
- Zhong, Y.Y.; Zhang, H.F.; Zhong, J.X.; Bai, L.; Lu, X.H. Topical dihydroartemisinin inhibits suture-induced neovascularization in rat corneas through ERK1/2 and p38 pathways. Int. J. Ophthalmol. 2011, 4, 150–155. [Google Scholar] [CrossRef]
- Francis, S.E.; Goh, K.L.; Hodivala-Dilke, K.; Bader, B.L.; Stark, M.; Davidson, D.; Hynes, R.O. Central roles of α5β1 integrin and fibronectin in vascular development in mouse embryos and embryoid bodies. Arter. Thromb. Vasc. Biol. 2002, 22, 927–933. [Google Scholar] [CrossRef]
- Muether, P.S.; Dell, S.; Kociok, N.; Zahn, G.; Stragies, R.; Vossmeyer, D.; Joussen, A.M. The role of integrin α5β1 in the regulation of corneal neovascularization. Exp. Eye Res. 2007, 85, 356–365. [Google Scholar] [CrossRef]
- Semenza, G.L. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu. Rev. Pathol. 2014, 9, 47–71. [Google Scholar] [CrossRef]
- Kim, B.H.; Lee, J.; Choi, J.S.; Park, D.Y.; Song, H.Y.; Park, T.K.; Cho, C.H.; Ye, S.K.; Joo, C.K.; Koh, G.Y.; et al. Imidazole-based alkaloid derivative LCB54–0009 suppresses ocular angiogenesis and lymphangiogenesis in models of experimental retinopathy and corneal neovascularization. Br. J. Pharm. 2015, 172, 3875–3889. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, A.S., Jr. The NF-κB and IκB proteins: New discoveries and insights. Annu. Rev. Immunol. 1996, 14, 649–683. [Google Scholar] [CrossRef] [PubMed]
- Kubota, M.; Shimmura, S.; Kubota, S.; Miyashita, H.; Kato, N.; Noda, K.; Ozawa, Y.; Usui, T.; Ishida, S.; Umezawa, K.; et al. Hydrogen and N-acetyl-L-cysteine rescue oxidative stress-induced angiogenesis in a mouse corneal alkali-burn model. Investig. Ophthalmol. Vis. Sci. 2011, 52, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Lennikov, A.; Hiraoka, M.; Abe, A.; Ohno, S.; Fujikawa, T.; Itai, A.; Ohguro, H. IκB kinase-β inhibitor IMD-0354 beneficially suppresses retinal vascular permeability in streptozotocin-induced diabetic mice. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6365–6373. [Google Scholar] [CrossRef]
- Lennikov, A.; Mirabelli, P.; Mukwaya, A.; Schaupper, M.; Thangavelu, M.; Lachota, M.; Ali, Z.; Jensen, L.; Lagali, N. Selective IKK2 inhibitor IMD0354 disrupts NF-κB signaling to suppress corneal inflammation and angiogenesis. Angiogenesis 2018, 21, 267–285. [Google Scholar] [CrossRef]
- Garcia-Recio, S.; Gascon, P. Biological and pharmacological aspects of the NK1-receptor. Biomed. Res. Int. 2015, 2015, 495704. [Google Scholar] [CrossRef]
- Son, Y.; Hong, H.; Kim, J. Identification of substance-P as an early inductive cytokine of corneal wound and its possible role in the mobilization of mesenchymal stem cell and corneal wound healing. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1423. [Google Scholar]
- Bignami, F.; Giacomini, C.; Lorusso, A.; Aramini, A.; Rama, P.; Ferrari, G. NK1 receptor antagonists as a new treatment for corneal neovascularization. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6783–6794. [Google Scholar] [CrossRef]
- Crane, I.J.; Wallace, C.A.; McKillop-Smith, S.; Forrester, J.V. CXCR4 receptor expression on human retinal pigment epithelial cells from the blood-retina barrier leads to chemokine secretion and migration in response to stromal cell-derived factor 1 alpha. J. Immunol. 2000, 165, 4372–4378. [Google Scholar] [CrossRef]
- Takeda, A.; Baffi, J.Z.; Kleinman, M.E.; Cho, W.G.; Nozaki, M.; Yamada, K.; Kaneko, H.; Albuquerque, R.J.; Dridi, S.; Saito, K.; et al. CCR3 is a target for age-related macular degeneration diagnosis and therapy. Nature 2009, 460, 225–230. [Google Scholar] [CrossRef]
- Zhou, W.J.; Liu, G.Q.; Li, L.B.; Zhang, X.G.; Lu, P.R. Inhibitory effect of CCR3 signal on alkali-induced corneal neovascularization. Int. J. Ophthalmol. 2012, 5, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Asahara, T.; Murohara, T.; Sullivan, A.; Silver, M.; van der Zee, R.; Li, T.; Witzenbichler, B.; Schatteman, G.; Isner, J.M. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997, 275, 964–967. [Google Scholar] [CrossRef] [PubMed]
- Petit, I.; Jin, D.; Rafii, S. The SDF-1-CXCR4 signaling pathway: A molecular hub modulating neo-angiogenesis. Trends Immunol. 2007, 28, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.H.; Shen, W.; Yong, W.; Lu, L.; Liu, L. Effects of AMD3100 subconjunctival injection on alkali burn induced corneal neovascularization in mice. Int. J. Ophthalmol. 2011, 4, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Markowska, A.I.; Jefferies, K.C.; Panjwani, N. Galectin-3 protein modulates cell surface expression and activation of vascular endothelial growth factor receptor 2 in human endothelial cells. J. Biol. Chem. 2011, 286, 29913–29921. [Google Scholar] [CrossRef]
- Chen, W.S.; Cao, Z.; Leffler, H.; Nilsson, U.J.; Panjwani, N. Galectin-3 inhibition by a small-molecule inhibitor reduces both pathological corneal neovascularization and fibrosis. Investig. Ophthalmol. Vis. Sci. 2017, 58, 9–20. [Google Scholar] [CrossRef]
- Ingber, D.; Fujita, T.; Kishimoto, S.; Sudo, K.; Kanamaru, T.; Brem, H.; Folkman, J. Synthetic analogues of fumagillin that inhibit angiogenesis and suppress tumour growth. Nature 1990, 348, 555–557. [Google Scholar] [CrossRef]
- Sin, N.; Meng, L.; Wang, M.Q.; Wen, J.J.; Bornmann, W.G.; Crews, C.M. The anti-angiogenic agent fumagillin covalently binds and inhibits the methionine aminopeptidase, MetAP-2. Proc. Natl. Acad. Sci. USA 1997, 94, 6099–6103. [Google Scholar] [CrossRef]
- Zhang, Y.; Griffith, E.C.; Sage, J.; Jacks, T.; Liu, J.O. Cell cycle inhibition by the anti-angiogenic agent TNP-470 is mediated by p53 and p21WAF1/CIP1. Proc. Natl. Acad. Sci. USA 2000, 97, 6427–6432. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Dona, M.; Dell’Aica, I.; Calabrese, F.; Benelli, R.; Morini, M.; Albini, A.; Garbisa, S. Neutrophil restraint by green tea: Inhibition of inflammation, associated angiogenesis, and pulmonary fibrosis. J. Immunol. 2003, 170, 4335–4341. [Google Scholar] [CrossRef] [PubMed]
- Kojima-Yuasa, A.; Hua, J.J.; Kennedy, D.O.; Matsui-Yuasa, I. Green tea extract inhibits angiogenesis of human umbilical vein endothelial cells through reduction of expression of VEGF receptors. Life Sci. 2003, 73, 1299–1313. [Google Scholar] [CrossRef]
- Shimizu, M.; Shirakami, Y.; Moriwaki, H. Targeting receptor tyrosine kinases for chemoprevention by green tea catechin, EGCG. Int. J. Mol. Sci. 2008, 9, 1034–1049. [Google Scholar] [CrossRef] [PubMed]
- Valcic, S.; Muders, A.; Jacobsen, N.E.; Liebler, D.C.; Timmermann, B.N. Antioxidant chemistry of green tea catechins. Identification of products of the reaction of (-)-epigallocatechin gallate with peroxyl radicals. Chem. Res. Toxicol. 1999, 12, 382–386. [Google Scholar] [CrossRef]
- Koh, C.H.; Lee, H.S.; Chung, S.K. Effect of topical epigallocatechin gallate on corneal neovascularization in rabbits. Cornea 2014, 33, 527–532. [Google Scholar] [CrossRef]
- Castro, M.R.; Lutz, D.; Edelman, J.L. Effect of COX inhibitors on VEGF-induced retinal vascular leakage and experimental corneal and choroidal neovascularization. Exp. Eye Res. 2004, 79, 275–285. [Google Scholar] [CrossRef]
- Chang, C.Y.; Wang, M.C.; Miyagawa, T.; Chen, Z.Y.; Lin, F.H.; Chen, K.H.; Liu, G.S.; Tseng, C.L. Preparation of arginine-glycine-aspartic acid-modified biopolymeric nanoparticles containing epigalloccatechin-3-gallate for targeting vascular endothelial cells to inhibit corneal neovascularization. Int. J. Nanomed. 2017, 12, 279–294. [Google Scholar] [CrossRef]
- Chen, A.Y.; Chen, Y.C. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013, 138, 2099–2107. [Google Scholar] [CrossRef]
- Luo, H.; Rankin, G.O.; Liu, L.; Daddysman, M.K.; Jiang, B.-H.; Chen, Y.C. Kaempferol inhibits angiogenesis and VEGF expression through both HIF dependent and independent pathways in human ovarian cancer cells. Nutr. Cancer 2009, 61, 554–563. [Google Scholar] [CrossRef]
- Chuang, Y.-L.; Fang, H.-W.; Ajitsaria, A.; Chen, K.-H.; Su, C.-Y.; Liu, G.-S.; Tseng, C.-L. Development of kaempferol-loaded gelatin nanoparticles for the treatment of corneal neovascularization in mice. Pharmaceutics 2019, 11, 635. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Du, Q.; Peng, C.; Wang, N.; Tang, H.; Xie, X.; Shen, J.; Chen, J. A review: The pharmacology of isoliquiritigenin. Phytother. Res. 2015, 29, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Jhanji, V.; Liu, H.; Law, K.; Lee, V.Y.; Huang, S.F.; Pang, C.P.; Yam, G.H. Isoliquiritigenin from licorice root suppressed neovascularisation in experimental ocular angiogenesis models. Br. J. Ophthalmol. 2011, 95, 1309–1315. [Google Scholar] [CrossRef] [PubMed]
- Joussen, A.M.; Rohrschneider, K.; Reichling, J.; Kirchhof, B.; Kruse, F.E. Treatment of corneal neovascularization with dietary isoflavonoids and flavonoids. Exp. Eye Res. 2000, 71, 483–487. [Google Scholar] [CrossRef]
- Shao, Z.M.; Wu, J.; Shen, Z.Z.; Barsky, S.H. Genistein exerts multiple suppressive effects on human breast carcinoma cells. Cancer Res. 1998, 58, 4851–4857. [Google Scholar]
- Martinez, R.M.; Pinho-Ribeiro, F.A.; Steffen, V.S.; Caviglione, C.V.; Vignoli, J.A.; Barbosa, D.S.; Baracat, M.M.; Georgetti, S.R.; Verri, W.A., Jr.; Casagrande, R. Naringenin inhibits UVB irradiation-induced inflammation and oxidative stress in the skin of hairless mice. J. Nat. Prod. 2015, 78, 1647–1655. [Google Scholar] [CrossRef]
- Pinho-Ribeiro, F.A.; Zarpelon, A.C.; Mizokami, S.S.; Borghi, S.M.; Bordignon, J.; Silva, R.L.; Cunha, T.M.; Alves-Filho, J.C.; Cunha, F.Q.; Casagrande, R.; et al. The citrus flavonone naringenin reduces lipopolysaccharide-induced inflammatory pain and leukocyte recruitment by inhibiting NF-κB activation. J. Nutr. Biochem. 2016, 33, 8–14. [Google Scholar] [CrossRef]
- Xu, X.R.; Yu, H.T.; Hang, L.; Shao, Y.; Ding, S.H.; Yang, X.W. Preparation of naringenin/β-cyclodextrin complex and its more potent alleviative effect on choroidal neovascularization in rats. Biomed. Res. Int. 2014, 2014, 623509. [Google Scholar] [CrossRef]
- Oguido, A.; Hohmann, M.S.N.; Pinho-Ribeiro, F.A.; Crespigio, J.; Domiciano, T.P.; Verri, W.A., Jr.; Casella, A.M.B. Naringenin eye drops inhibit corneal neovascularization by anti-inflammatory and antioxidant mechanisms. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5764–5776. [Google Scholar] [CrossRef]
- Das, U.N. Arachidonic acid in health and disease with focus on hypertension and diabetes mellitus: A review. J. Adv. Res. 2018, 11, 43–55. [Google Scholar] [CrossRef]
- Haynes, W.L.; Proia, A.D.; Klintworth, G.K. Effect of inhibitors of arachidonic acid metabolism on corneal neovascularization in the rat. Investig. Ophthalmol. Vis. Sci. 1989, 30, 1588–1593. [Google Scholar]
- Hope, W.C.; Welton, A.F.; Fiedler-Nagy, C.; Batula-Bernardo, C.; Coffey, J.W. In vitro inhibition of the biosynthesis of slow reacting substance of anaphylaxis (SRS-A) and lipoxygenase activity by quercetin. Biochem. Pharm. 1983, 32, 367–371. [Google Scholar] [CrossRef]
- Neichi, T.; Koshihara, Y.; Murota, S. Inhibitory effect of esculetin on 5-lipoxygenase and leukotriene biosynthesis. Biochim. Biophys. Acta 1983, 753, 130–132. [Google Scholar] [PubMed]
- Araujo, C.C.; Leon, L.L. Biological activities of Curcuma longa L. Mem. Inst. Oswaldo Cruz 2001, 96, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Menon, L.G.; Kuttan, R.; Kuttan, G. Inhibition of lung metastasis in mice induced by B16F10 melanoma cells by polyphenolic compounds. Cancer Lett. 1995, 95, 221–225. [Google Scholar] [CrossRef]
- Phan, T.T.; See, P.; Lee, S.T.; Chan, S.Y. Protective effects of curcumin against oxidative damage on skin cells in vitro: Its implication for wound healing. J. Trauma 2001, 51, 927–931. [Google Scholar] [CrossRef]
- Kim, J.S.; Choi, J.S.; Chung, S.K. The effect of curcumin on corneal neovascularization in rabbit eyes. Curr. Eye Res. 2010, 35, 274–280. [Google Scholar] [CrossRef]
- Bian, F.; Zhang, M.C.; Zhu, Y. Inhibitory effect of curcumin on corneal neovascularization in vitro and in vivo. Ophthalmologica 2008, 222, 178–186. [Google Scholar] [CrossRef]
- Pradhan, N.; Guha, R.; Chowdhury, S.; Nandi, S.; Konar, A.; Hazra, S. Curcumin nanoparticles inhibit corneal neovascularization. J. Mol. Med. (Berl.) 2015, 93, 1095–1106. [Google Scholar] [CrossRef]
- Burgos-Morón, E.; Calderón-Montaño, J.M.; Salvador, J.; Robles, A.; López-Lázaro, M. The dark side of curcumin. Int. J. Cancer 2010, 126, 1771–1775. [Google Scholar] [CrossRef]
- Bhat, K.P.; Pezzuto, J.M. Cancer chemopreventive activity of resveratrol. Ann. N. Y. Acad. Sci. 2002, 957, 210–229. [Google Scholar] [CrossRef] [PubMed]
- Brakenhielm, E.; Cao, R.; Cao, Y. Suppression of angiogenesis, tumor growth, and wound healing by resveratrol, a natural compound in red wine and grapes. FASEB J. 2001, 15, 1798–1800. [Google Scholar] [CrossRef] [PubMed]
- Doganay, S.; Firat, P.G.; Cankaya, C.; Kirimlioglu, H. Evaluation of the effects of resveratrol and bevacizumab on experimental corneal alkali burn. Burns 2013, 39, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Mohan, R.; Hammers, H.J.; Bargagna-Mohan, P.; Zhan, X.H.; Herbstritt, C.J.; Ruiz, A.; Zhang, L.; Hanson, A.D.; Conner, B.P.; Rougas, J.; et al. Withaferin A is a potent inhibitor of angiogenesis. Angiogenesis 2004, 7, 115–122. [Google Scholar] [CrossRef]
- Bargagna-Mohan, P.; Hamza, A.; Kim, Y.E.; Ho, Y.K.A.; Mor-Vaknin, N.; Wendschlag, N.; Liu, J.; Evans, R.M.; Markovitz, D.M.; Zhan, C.G.; et al. The tumor inhibitor and antiangiogenic agent withaferin A targets the intermediate filament protein vimentin. Chem. Biol. 2007, 14, 623–634. [Google Scholar] [CrossRef]
- Chandel, S.; Bagai, U.; Vashishat, N. Antiplasmodial activity of Xanthium strumarium against Plasmodium berghei-infected BALB/c mice. Parasitol. Res. 2012, 110, 1179–1183. [Google Scholar] [CrossRef]
- Li, W.D.; Wu, Y.; Zhang, L.; Yan, L.G.; Yin, F.Z.; Ruan, J.S.; Chen, Z.P.; Yang, G.M.; Yan, C.P.; Zhao, D.; et al. Characterization of xanthatin: Anticancer properties and mechanisms of inhibited murine melanoma in vitro and in vivo. Phytomedicine 2013, 20, 865–873. [Google Scholar] [CrossRef]
- Pinel, B.; Landreau, A.; Seraphin, D.; Larcher, G.; Bouchara, J.P.; Richomme, P. Synthesis of reduced xanthatin derivatives and in vitro evaluation of their antifungal activity. J. Enzym. Inhib. Med. Chem. 2005, 20, 575–579. [Google Scholar] [CrossRef]
- Yu, Y.; Yu, J.; Pei, C.G.; Li, Y.Y.; Tu, P.; Gao, G.P.; Shao, Y. Xanthatin, a novel potent inhibitor of VEGFR2 signaling, inhibits angiogenesis and tumor growth in breast cancer cells. Int. J. Clin. Exp. Pathol. 2015, 8, 10355–10364. [Google Scholar]
- Shen, M.; Zhou, X.Z.; Ye, L.; Yuan, Q.; Shi, C.; Zhu, P.W.; Jiang, N.; Ma, M.Y.; Yang, Q.C.; Shao, Y. Xanthatin inhibits corneal neovascularization by inhibiting the VEGFR2 mediated STAT3/PI3K/Akt signaling pathway. Int. J. Mol. Med. 2018, 42, 769–778. [Google Scholar] [CrossRef]
- Kiviharju, T.M.; Lecane, P.S.; Sellers, R.G.; Peehl, D.M. Antiproliferative and proapoptotic activities of triptolide (PG490), a natural product entering clinical trials, on primary cultures of human prostatic epithelial cells. Clin. Cancer Res. 2002, 8, 2666–2674. [Google Scholar] [PubMed]
- Qiu, D.; Kao, P.N. Immunosuppressive and anti-inflammatory mechanisms of triptolide, the principal active diterpenoid from the Chinese medicinal herb Tripterygium wilfordii Hook. f. Drugs R D 2003, 4, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, N.; Lv, X.; Ahmed, N.; Li, X.; Liu, H.; Ma, J.; Zhang, Y. Triptolide suppresses alkali burn-induced corneal angiogenesis along with a downregulation of VEGFA and VEGFC expression. Anat. Rec. 2017, 300, 1348–1355. [Google Scholar] [CrossRef] [PubMed]
- Houghton, P.J.; Zarka, R.; de las Heras, B.; Hoult, J. Fixed oil of Nigella sativa and derived thymoquinone inhibit eicosanoid generation in leukocytes and membrane lipid peroxidation. Planta Med. 1995, 61, 33–36. [Google Scholar] [CrossRef]
- Al-Ghamdi, M. The anti-inflammatory, analgesic and antipyretic activity of Nigella sativa. J. Ethnopharmacol. 2001, 76, 45–48. [Google Scholar] [CrossRef]
- Burits, M.; Bucar, F. Antioxidant activity of Nigella sativa essential oil. Phytother. Res. 2000, 14, 323–328. [Google Scholar] [CrossRef]
- Gali-Muhtasib, H.; Roessner, A.; Schneider-Stock, R. Thymoquinone: A promising anti-cancer drug from natural sources. Int. J. Biochem. Cell Biol. 2006, 38, 1249–1253. [Google Scholar] [CrossRef]
- Erdurmus, M.; Yagci, R.; Yilmaz, B.; Hepsen, I.F.; Turkmen, C.; Aydin, B.; Karadag, R. Inhibitory effects of topical thymoquinone on corneal neovascularization. Cornea 2007, 26, 715–719. [Google Scholar] [CrossRef]
- Calò, L.A.; Zaghetto, F.; Pagnin, E.; Davis, P.A.; De Mozzi, P.; Sartorato, P.; Martire, G.; Fiore, C.; Armanini, D. Effect of aldosterone and glycyrrhetinic acid on the protein expression of PAI-1 and p22phox in human mononuclear leukocytes. J. Clin. Endocrinol. Metab. 2004, 89, 1973–1976. [Google Scholar] [CrossRef]
- Shah, S.L.; Wahid, F.; Khan, N.; Farooq, U.; Shah, A.J.; Tareen, S.; Ahmad, F.; Khan, T. Inhibitory effects of Glycyrrhiza glabra and its major constituent glycyrrhizin on inflammation-associated corneal neovascularization. Evid.-Based Complementary Altern. Med. 2018, 2018, 8438101. [Google Scholar] [CrossRef]
- Cai, X.; Chen, Z.; Pan, X.; Xia, L.; Chen, P.; Yang, Y.; Hu, H.; Zhang, J.; Li, K.; Ge, J. Inhibition of angiogenesis, fibrosis and thrombosis by tetramethylpyrazine: Mechanisms contributing to the SDF-1/CXCR4 axis. PLoS ONE 2014, 9, e88176. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-g.; Jiang, C. Ligustrazine as a salvage agent for patients with relapsed or refractory non-Hodgkin’s lymphoma. Chin. Med. J. 2010, 123, 3206–3211. [Google Scholar] [PubMed]
- Yu, K.; Zhuang, J.; Kaminski, J.M.; Ambati, B.; Gao, Q.; Ma, P.; Liao, D.; Li, F.; Liu, X.; Ge, J. CXCR4 down-regulation by small interfering RNA inhibits invasion and tubule formation of human retinal microvascular endothelial cells. Biochem. Biophys. Res. Comm. 2007, 358, 990–996. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, Z.; Yang, Y.; Qiu, J.; Yang, M.; Wu, C.; Lai, Z.; Tang, M.; Ge, J.; Yu, K. Tetramethylpyrazine (TMP) ameliorates corneal neovascularization via regulating cell infiltration into cornea after alkali burn. Biomed. Pharm. 2019, 109, 1041–1051. [Google Scholar] [CrossRef]
- Tian, H.; Cronstein, B.N. Understanding the mechanisms of action of methotrexate. Bull. NYU Hosp. Jt. Dis. 2007, 65, 168–173. [Google Scholar]
- Mizusawa, S.; Kondoh, Y.; Murakami, M.; Nakamichi, H.; Sasaki, H.; Komatsu, K.; Takahashi, A.; Kudoh, Y.; Watanabe, K.; Ono, Y.; et al. Effect of methotrexate on local cerebral blood flow in conscious rats. Jpn. J. Pharm. 1988, 48, 499–501. [Google Scholar] [CrossRef]
- Byun, Y.S.; Chung, S.K. The effect of methotrexate on corneal neovascularization in rabbits. Cornea 2011, 30, 442–446. [Google Scholar] [CrossRef]
- Joussen, A.M.; Kruse, F.E.; Volcker, H.E.; Kirchhof, B. Topical application of methotrexate for inhibition of corneal angiogenesis. Graefes Arch. Clin. Exp. Ophthalmol. 1999, 237, 920–927. [Google Scholar] [CrossRef]
- Blaschke, G.; Kraft, H.P.; Fickentscher, K.; Kohler, F. Chromatographic separation of racemic thalidomide and teratogenic activity of its enantiomers (author’s transl). Arzneimittelforschung 1979, 29, 1640–1642. [Google Scholar]
- Joussen, A.M.; Germann, T.; Kirchhof, B. Effect of thalidomide and structurally related compounds on corneal angiogenesis is comparable to their teratological potency. Graefes Arch. Clin. Exp. Ophthalmol. 1999, 237, 952–961. [Google Scholar] [CrossRef]
- Kruse, F.E.; Joussen, A.M.; Rohrschneider, K.; Becker, M.D.; Volcker, H.E. Thalidomide inhibits corneal angiogenesis induced by vascular endothelial growth factor. Graefes Arch. Clin. Exp. Ophthalmol. 1998, 236, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, E.; Yamamoto, T.; Ito, E.; Shibata, N. Understanding the thalidomide chirality in biological processes by the self-disproportionation of enantiomers. Sci. Rep. 2018, 8, 17131. [Google Scholar] [CrossRef]
- Marriott, J.B.; Westby, M.; Cookson, S.; Guckian, M.; Goodbourn, S.; Muller, G.; Shire, M.G.; Stirling, D.; Dalgleish, A.G. CC-3052: A water-soluble analog of thalidomide and potent inhibitor of activation-induced TNF-α production. J. Immunol. 1998, 161, 4236–4243. [Google Scholar]
- Lee, Y.K.; Chung, S.K. The inhibitory effect of thalidomide analogue on corneal neovascularization in rabbits. Cornea 2013, 32, 1142–1148. [Google Scholar] [CrossRef]
- Li, P.-K.; Pandit, B.; Sackett, D.L.; Hu, Z.; Zink, J.; Zhi, J.; Freeman, D.; Robey, R.W.; Werbovetz, K.; Lewis, A. A thalidomide analogue with in vitro antiproliferative, antimitotic, and microtubule-stabilizing activities. Mol. Cancer 2006, 5, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, P.-K.; Ma, J.-X.; Chen, D. Therapeutic effects of a novel phenylphthalimide analog for corneal neovascularization and retinal vascular leakage. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3630–3642. [Google Scholar] [CrossRef]
- Lima, L.d.M.; Castro, P.; Machado, A.L.; Fraga, C.A.M.; Lugnier, C.; de Moraes, V.L.G.; Barreiro, E.J. Synthesis and anti-inflammatory activity of phthalimide derivatives, designed as new thalidomide analogues. Bioorg. Med. Chem. 2002, 10, 3067–3073. [Google Scholar] [CrossRef]
- Rocco, P.; Momesso, D.; Figueira, R.; Ferreira, H.; Cadete, R.; Légora-Machado, A.; Koatz, V.; Lima, L.; Barreiro, E.; Zin, W. Therapeutic potential of a new phosphodiesterase inhibitor in acute lung injury. Eur. Respir. J. 2003, 22, 20–27. [Google Scholar] [CrossRef]
- Ribeiro, J.C.M.; Fechine, F.V.; Ribeiro, M.Z.M.; Barreiro, E.J.; Lima, L.M.; Ricardo, N.M.; De Moraes, M.E.A.; De Moraes, M.O. Potential inhibitory effect of LASSBio-596, a new thalidomide hybrid, on inflammatory corneal angiogenesis in rabbits. Ophthalmic Res. 2012, 48, 177–185. [Google Scholar] [CrossRef]
- Benelli, U.; Ross, J.R.; Nardi, M.; Klintworth, G.K. Corneal neovascularization induced by xenografts or chemical cautery. Inhibition by cyclosporin A. Investig. Ophthalmol. Vis. Sci. 1997, 38, 274–282. [Google Scholar]
- Bucak, Y.Y.; Erdurmus, M.; Terzi, E.H.; Kükner, A.; Çelebi, S. Inhibitory effects of topical cyclosporine A 0.05% on immune-mediated corneal neovascularization in rabbits. Graefes Arch. Clin. Exp. Ophthalmol. 2013, 251, 2555–2561. [Google Scholar] [CrossRef]
- Durrani, K.; Zakka, F.R.; Ahmed, M.; Memon, M.; Siddique, S.S.; Foster, C.S. Systemic therapy with conventional and novel immunomodulatory agents for ocular inflammatory disease. Surv. Ophthalmol. 2011, 56, 474–510. [Google Scholar] [CrossRef]
- Cejkova, J.; Cejka, C.; Trosan, P.; Zajicova, A.; Sykova, E.; Holan, V. Treatment of alkali-injured cornea by cyclosporine A-loaded electrospun nanofibers—An alternative mode of therapy. Exp. Eye Res. 2016, 147, 128–137. [Google Scholar] [CrossRef]
- Groth, C.G.; Bäckman, L.; Morales, J.-M.; Calne, R.; Kreis, H.; Lang, P.; Touraine, J.-L.; Claesson, K.; Campistol, J.M.; Durand, D. Sirolimus (rapamycin)-based therapy in human renal transplantation: Similar efficacy and different toxicity compared with cyclosporine 1, 2. Transplantation 1999, 67, 1036–1042. [Google Scholar] [CrossRef]
- Sonpavde, G.; Choueiri, T.K. Biomarkers: The next therapeutic hurdle in metastatic renal cell carcinoma. Br. J. Cancer 2012, 107, 1009–1016. [Google Scholar] [CrossRef][Green Version]
- Kwon, Y.S.; Hong, H.S.; Kim, J.C.; Shin, J.S.; Son, Y. Inhibitory effect of rapamycin on corneal neovascularization in vitro and in vivo. Investig. Ophthalmol. Vis. Sci. 2005, 46, 454–460. [Google Scholar] [CrossRef]
- Shin, Y.J.; Hyon, J.Y.; Choi, W.S.; Yi, K.; Chung, E.S.; Chung, T.Y.; Wee, W.R. Chemical injury-induced corneal opacity and neovascularization reduced by rapamycin via TGF-β1/ERK pathways regulation. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4452–4458. [Google Scholar] [CrossRef]
- Çakmak, H.; Ergin, K.; Bozkurt, G.; Kocatürk, T.; Evliçoğlu, G.E. The effects of topical everolimus and sunitinib on corneal neovascularization. Cutan. Ocul. Toxicol. 2016, 35, 97–103. [Google Scholar] [CrossRef]
- Kino, T.; Hatanaka, H.; Hashimoto, M.; Nishiyama, M.; Goto, T.; Okuhara, M.; Kohsaka, M.; Aoki, H.; Imanaka, H. FK-506, a novel immunosuppressant isolated from a Streptomyces. I. Fermentation, isolation, and physico-chemical and biological characteristics. J. Antibiot. (Tokyo) 1987, 40, 1249–1255. [Google Scholar] [CrossRef]
- Turgut, B.; Guler, M.; Akpolat, N.; Demir, T.; Celiker, U. The impact of tacrolimus on vascular endothelial growth factor in experimental corneal neovascularization. Curr. Eye Res. 2011, 36, 34–40. [Google Scholar] [CrossRef]
- Park, J.H.; Joo, C.K.; Chung, S.K. Comparative study of tacrolimus and bevacizumab on corneal neovascularization in rabbits. Cornea 2015, 34, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Roomi, M.W.; Ivanov, V.; Kalinovsky, T.; Niedzwiecki, A.; Rath, M. In vitro and in vivo antitumorigenic activity of a mixture of lysine, proline, ascorbic acid, and green tea extract on human breast cancer lines MDA-MB-231 and MCF-7. Med. Oncol. 2005, 22, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Peyman, G.A.; Kivilcim, M.; Morales, A.M.; DellaCroce, J.T.; Conway, M.D. Inhibition of corneal angiogenesis by ascorbic acid in the rat model. Graefes Arch. Clin. Exp. Ophthalmol. 2007, 245, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.Y.; Chung, S.K. Treatment of corneal neovascularization by topical application of ascorbic acid in the rabbit model. Cornea 2012, 31, 1165–1169. [Google Scholar] [CrossRef] [PubMed]
- Kohlhaas, M.; Spoerl, E.; Speck, A.; Schilde, T.; Sandner, D.; Pillunat, L. A new treatment of keratectasia after LASIK by using collagen with riboflavin/UVA light cross-linking. Klin. Mon. Augenheilkd. 2005, 222, 430. [Google Scholar] [CrossRef] [PubMed]
- Wollensak, G.; Spoerl, E.; Seiler, T. Riboflavin/ultraviolet-A–induced collagen crosslinking for the treatment of keratoconus. Am. J. Ophthalmol. 2003, 135, 620–627. [Google Scholar] [CrossRef]
- Spoerl, E.; Mrochen, M.; Sliney, D.; Trokel, S.; Seiler, T. Safety of UVA-riboflavin cross-linking of the cornea. Cornea 2007, 26, 385–389. [Google Scholar] [CrossRef]
- Hou, Y.; Le, V.N.H.; Toth, G.; Siebelmann, S.; Horstmann, J.; Gabriel, T.; Bock, F.; Cursiefen, C. UV light crosslinking regresses mature corneal blood and lymphatic vessels and promotes subsequent high-risk corneal transplant survival. Am. J. Transpl. 2018, 18, 2873–2884. [Google Scholar] [CrossRef]
- Blinder, K.J.; Blumenkranz, M.S.; Bressler, N.M.; Bressler, S.B.; Donato, G.; Lewis, H.; Lim, J.I.; Menchini, U.; Miller, J.W.; Mones, J.M. Verteporfin therapy of subfoveal choroidal neovascularization in pathologic myopia: 2-year results of a randomized clinical trial—VIP report no. 3. Ophthalmology 2003, 110, 667–673. [Google Scholar]
- Weiss, A.; van den Bergh, H.; Griffioen, A.W.; Nowak-Sliwinska, P. Angiogenesis inhibition for the improvement of photodynamic therapy: The revival of a promising idea. Biochim. Biophys. Acta-Rev. Cancer 2012, 1826, 53–70. [Google Scholar] [CrossRef]
- Hou, Y.; Le, V.N.H.; Clahsen, T.; Schneider, A.C.; Bock, F.; Cursiefen, C. Photodynamic therapy leads to time-dependent regression of pathologic corneal (lymph) angiogenesis and promotes high-risk corneal allograft survival. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5862–5869. [Google Scholar] [CrossRef] [PubMed]
- Shokravi, M.T.; Marcus, D.M.; Alroy, J.; Egan, K.; Saornil, M.A.; Albert, D.M. Vitamin D inhibits angiogenesis in transgenic murine retinoblastoma. Investig. Ophthalmol. Vis. Sci. 1995, 36, 83–87. [Google Scholar]
- Merrigan, S.L.; Park, B.; Ali, Z.; Jensen, L.D.; Corson, T.W.; Kennedy, B.N. Calcitriol and non-calcemic vitamin D analogue, 22-oxacalcitriol, attenuate developmental and pathological choroidal vasculature angiogenesis ex vivo and in vivo. Oncotarget 2020, 11, 493. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Sano, Y.; Kinoshita, S. Effects of 1α, 25-dihydroxyvitamin D3 on Langerhans cell migration and corneal neovascularization in mice. Investig. Ophthalmol. Vis. Sci. 2000, 41, 154–158. [Google Scholar]
- Ha, C.H.; Jhun, B.S.; Kao, H.Y.; Jin, Z.G. VEGF stimulates HDAC7 phosphorylation and cytoplasmic accumulation modulating matrix metalloproteinase expression and angiogenesis. Arter. Thromb. Vasc. Biol. 2008, 28, 1782–1788. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Luesch, H. Largazole: From discovery to broad-spectrum therapy. Nat. Prod. Rep. 2012, 29, 449–456. [Google Scholar] [CrossRef]
- Zhou, H.; Jiang, S.; Chen, J.; Ren, X.; Jin, J.; Su, S.B. Largazole, an inhibitor of class I histone deacetylases, attenuates inflammatory corneal neovascularization. Eur. J. Pharm. 2014, 740, 619–626. [Google Scholar] [CrossRef]
- Zhou, H.; Jiang, S.; Chen, J.; Su, S.B. Suberoylanilide hydroxamic acid suppresses inflammation-induced neovascularization. Can. J. Physiol. Pharm. 2014, 92, 879–885. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Q.; Hanus, J.; Anderson, C.; Zhang, H.; Dellinger, M.; Brekken, R.; Wang, S. Inhibition of multiple pathogenic pathways by histone deacetylase inhibitor SAHA in a corneal alkali-burn injury model. Mol. Pharm. 2013, 10, 307–318. [Google Scholar] [CrossRef][Green Version]
- Ngo, H.X.; Garneau-Tsodikova, S. What are the drugs of the future? MedChemComm 2018, 9, 757–758. [Google Scholar] [CrossRef]
- Berkowitz, S.A.; Engen, J.R.; Mazzeo, J.R.; Jones, G.B. Analytical tools for characterizing biopharmaceuticals and the implications for biosimilars. Nat. Rev. Drug Discov. 2012, 11, 527–540. [Google Scholar] [CrossRef]
- Sugiki, T.; Furuita, K.; Fujiwara, T.; Kojima, C. Current NMR Techniques for structure-based drug discovery. Molecules 2018, 23, 148. [Google Scholar] [CrossRef] [PubMed]
- Jitendra; Sharma, P.K.; Bansal, S.; Banik, A. Noninvasive routes of proteins and peptides drug delivery. Indian J. Pharm. Sci. 2011, 73, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Gaudana, R.; Ananthula, H.K.; Parenky, A.; Mitra, A.K. Ocular drug delivery. AAPS J. 2010, 12, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Cholkar, K.; Patel, S.P.; Vadlapudi, A.D.; Mitra, A.K. Novel strategies for anterior segment ocular drug delivery. J. Ocul. Pharm. 2013, 29, 106–123. [Google Scholar] [CrossRef] [PubMed]
- Lallemand, F.; Felt-Baeyens, O.; Besseghir, K.; Behar-Cohen, F.; Gurny, R. Cyclosporine A delivery to the eye: A pharmaceutical challenge. Eur. J. Pharm. Biopharm. 2003, 56, 307–318. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Multitargeted therapy of cancer by green tea polyphenols. Cancer Lett. 2008, 269, 269–280. [Google Scholar] [CrossRef]
- EL-Meghawry, E.; Rahman, H.; Abdelkarim, G.; Najda, A. Natural products against cancer angiogenesis. Tumor Biol. 2016, 37, 14513–14536. [Google Scholar]
- Sulaiman, R.S.; Basavarajappa, H.D.; Corson, T.W. Natural product inhibitors of ocular angiogenesis. Exp. Eye Res. 2014, 129, 161–171. [Google Scholar] [CrossRef]
- Basavarajappa, H.D.; Lee, B.; Lee, H.; Sulaiman, R.S.; An, H.; Magaña, C.; Shadmand, M.; Vayl, A.; Rajashekhar, G.; Kim, E.-Y. Synthesis and biological evaluation of novel homoisoflavonoids for retinal neovascularization. J. Med. Chem. 2015, 58, 5015–5027. [Google Scholar]
- Kim, J.H.; Kim, J.H.; Yu, Y.S.; Jun, H.-O.; Kwon, H.J.; Park, K.H.; Kim, K.-W. Inhibition of choroidal neovascularization by homoisoflavanone, a new angiogenesis inhibitor. Mol. Vis. 2008, 14, 556. [Google Scholar]
- Kim, J.H.; Kim, K.H.; Kim, J.H.; Yu, Y.S.; Kim, Y.-M.; Kim, K.-W.; Kwon, H.J. Homoisoflavanone inhibits retinal neovascularization through cell cycle arrest with decrease of cdc2 expression. Biochem. Biophys. Res. Comm. 2007, 362, 848–852. [Google Scholar] [CrossRef]
- Lee, B.; Basavarajappa, H.D.; Sulaiman, R.S.; Fei, X.; Seo, S.-Y.; Corson, T.W. The first synthesis of the antiangiogenic homoisoflavanone, cremastranone. Org. Biomol. Chem. 2014, 12, 7673–7677. [Google Scholar] [CrossRef]
- Sulaiman, R.S.; Merrigan, S.; Quigley, J.; Qi, X.; Lee, B.; Boulton, M.E.; Kennedy, B.; Seo, S.-Y.; Corson, T.W. A novel small molecule ameliorates ocular neovascularisation and synergises with anti-VEGF therapy. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Basavarajappa, H.D.; Sulaiman, R.S.; Qi, X.; Shetty, T.; Sheik Pran Babu, S.; Sishtla, K.L.; Lee, B.; Quigley, J.; Alkhairy, S.; Briggs, C.M.; et al. Ferrochelatase is a therapeutic target for ocular neovascularization. EMBO Mol. Med. 2017, 9, 786–801. [Google Scholar] [CrossRef]
- Pran Babu, S.P.S.; White, D.; Corson, T.W. Ferrochelatase regulates retinal neovascularization. FASEB J. 2020. [Google Scholar] [CrossRef]
- Shetty, T.; Corson, T.W. Mitochondrial heme synthesis enzymes as therapeutic targets in vascular diseases. Front. Pharm. 2020. [Google Scholar] [CrossRef]
- Sulaiman, R.S.; Park, B.; Sheik Pran Babu, S.P.; Si, Y.; Kharwadkar, R.; Mitter, S.K.; Lee, B.; Sun, W.; Qi, X.; Boulton, M.E. Chemical proteomics reveals soluble epoxide hydrolase as a therapeutic target for ocular neovascularization. ACS Chem. Biol. 2018, 13, 45–52. [Google Scholar] [CrossRef]
- Park, B.; Corson, T.W. Soluble epoxide hydrolase inhibition for ocular diseases: Vision for the future. Front. Pharm. 2019, 10, 95. [Google Scholar] [CrossRef]
- Chaoran, Z.; Zhirong, L.; Gezhi, X. Combination of vascular endothelial growth factor receptor/platelet-derived growth factor receptor inhibition markedly improves the antiangiogenic efficacy for advanced stage mouse corneal neovascularization. Graefes Arch. Clin. Exp. Ophthalmol. 2011, 249, 1493–1501. [Google Scholar] [CrossRef]
| Tyrosine Kinase Inhibitor | Source | Mechanism | Routes | Dose | Model | Ref |
|---|---|---|---|---|---|---|
Sunitinib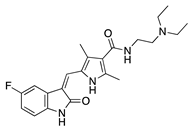 | Synthetic | Inhibition of the VEGFR and PDGFR pathways | Oral | 40 mg/kg | Murine thermal cauterization | [23] |
| Topical | 0.5 mg/mL | Rabbit suture model | [24] | |||
| Subconjunctival Topical | 0.25 mg/0.1 mL 0.5 mg/mL | Rabbit suture model | [25] | |||
AG 1296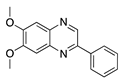 | Synthetic | PI3K-RTK inhibition | Systemic via osmotic pump implantation | 10 ng/mL | Murine alkali burn model | [27] |
Vatalanib succinate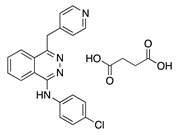 | Synthetic | VEGFR inhibition | Oral | 75 mg/kg; 2x/day | Murine suture model | [28] |
ZK261991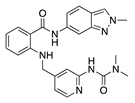 | Synthetic | VEGFR inhibition | Oral | 50 mg/kg; 2x/day | Murine suture model | [28] |
Sorafenib | Synthetic | Inhibition of ERK and VEGFR2 phosphorylation | Oral | 30 mg/kg; 60 mg/kg | Rat silver-nitrate burn model | [31] |
Semaxanib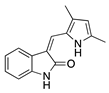 | Synthetic | Selective VEGFR2 inhibition | Intraperitoneal | 25 mg/kg | Rat silver-nitrate burn model | [33] |
Rivoceranib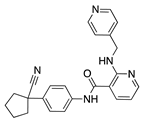 | Synthetic | Selective VEGFR2 inhibition | Topical | 0.1%; 0.5% | Murine alkali burn model | [36] |
Regorafenib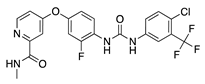 | Synthetic | Decreases epithelial and endothelial VEGF levels | Topical | 1 mg/mL | Rat alkali burn model | [38] |
Lapatinib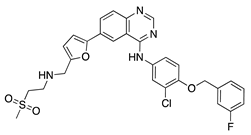 | Synthetic | Decreases corneal epithelial and stromal VEGF expression | Oral | 50 mg/kg | Rat silver-nitrate burn model | [41] |
Axitinib | Synthetic | Inhibition of VEGFR2 and PDGFR | Topical | 0.02, 0.35, 0.5 mg/mL | Rabbit suture model | [43] |
Dovitinib | Synthetic | Inhibition of VEGFRs, PDGFR, FGFR-1 and -3, | Topical | 5 mg/mL; 2x/day | Rat silver-nitrate burn model | [45] |
| Antimicrobial | Source | Mechanism | Routes | Dose | Model | Ref |
|---|---|---|---|---|---|---|
Doxycycline | Semisynthetic | MMP inhibition, and modulation of the MMP-independent PI3K/Akt-eNOS pathway | Oral | 40 mg/kg | Murine alkali burn model | [49] |
| Topical | 0.5 mg/mL | Murine silver-nitrate model | [50] | |||
Minocycline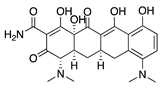 | Semisynthetic | Inhibition of MMP and downregulation of the ERK1/2 and Akt pathways | Intraperitoneal | 30 mg/kg; 60 mg/kg; 2x/day | Murine alkali burn model | [53] |
Tigecycline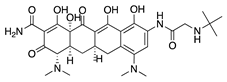 | Synthetic derived from minocycline | Unknown | Topical Subconjunctival | 1 mg/mL 1 mg/mL | Rat silver-nitrate model | [55] |
Itraconazole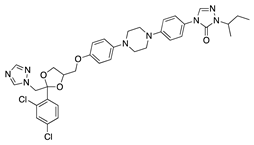 | Synthetic | Inhibition of cholesterol biosynthesis | Topical Subconjunctival Intraperitoneal | 10 mg/mL 10 mg/mL 19 mg/mL | Rat silver-nitrate model | [57] |
Dihydroartemisinin | Semisynthetic derivative of artemisinin | Modulation of the ERK1/2 and p38 pathways | Topical | 5 mg/L, 10 mg/L, 20 mg/L | Rat suture model | [60] |
| Molecule | Source | Mechanism | Routes | Dose | Model | Ref |
|---|---|---|---|---|---|---|
| JSM5562 (Exact structure not reported) | Synthetic | Impairing EC migration, adhesion, and tube formation. Exact mechanism unknown | Systemic via osmotic pump implantation | 0.1 mg/mL, 0.5 mg/mL, 2.5 mg/mL | Murine alkali burn model | [62] |
LCB54–0009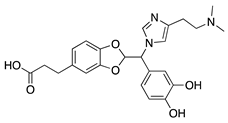 | Imidazole-based alkaloid derivative | Regulation of HIF-1α protein stability and HIF-1α/NF-κB redox sensitivity. Inhibits Ang expression and VEGF signaling cascade | Subconjunctival | 50 µg/ 20 µL | Rat silver-nitrate burn model | [64] |
N-acetyl-l-cysteine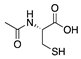 | Synthetic | Antioxidant; downregulates VEGF | Intraperitoneal | 200 mg/kg | Murine alkali burn model | [66] |
IMD0354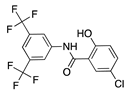 | Synthetic | Inhibition of NF-κB through selective blockage of IKK complex, IKK2 | Systemic | 30 mg/kg | Rat suture model | [68] |
Lanepitant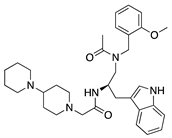 | Synthetic | NK1R antagonist | Topical Subconjunctival | 0.4, 1.6, 6.4 mg/mL 12.8 mg/mL | Murine alkali burn and suture models | [71] |
SB-328437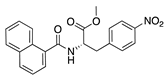 | Synthetic | CCR3 antagonist; reduces MCP-1 and -3. Exact mechanism unknown | Topical | 125 µg/mL, 250 µg/mL, 500 µg/mL | Murine alkali burn model | [74] |
AMD3100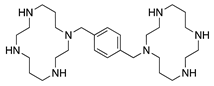 | Synthetic | CXCR4 antagonist; Downregulates VEGF expression and inflammation | Subconjunctival Intraperitoneal | 5 µL 2.5 mg/kg | Murine alkali burn model | [77] |
33-DFTG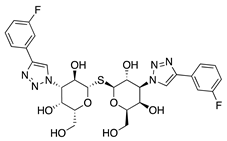 | Synthetic | Downregulates VEGF through unknown mechanism | Subconjunctival | 50 mM | Murine alkali burn and murine silver-nitrate models | [79] |
TNP-470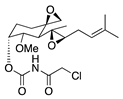 | Synthetic analogue of fumagillin | Targets MetAP2 | Topical Subconjunctival injection | 5 ng/nL; 3x/day 30 mg/kg | Murine alkali burn model | [17] |
| Flavonoid | Source | Mechanism | Routes | Dose | Model | Ref |
|---|---|---|---|---|---|---|
Epigallocatechin gallate (EGCG)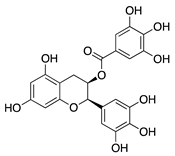 | Green tea (Camellia sinensis) | Unknown; downregulation of VEGF and COX-2 | Topical | 0.01 µg/mL 0.1 µg/mL | Rabbit suture model | [89] |
| Nanoparticle-mediated delivery via eye drops | 30 mg/mL | Murine alkali burn model | [91] | |||
Kaempferol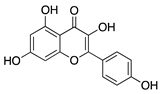 | Fruits and vegetables | Unknown; downregulation of MMP and VEGF | Nanoparticle- mediated delivery via eye drops | 7.5 µg/mL | Murine silver-nitrate/ potassium model | [94] |
Isoliquiritigenin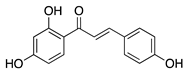 | Licorice root (Glycyrrhiza uralensis) | Unknown; downregulates VEGF and upregulates PEDF | Topical | 0.5, 1, 5, 10, 50 µM | Murine silver-nitrate model | [96] |
Fisetin | Fruits and vegetables | Unknown | Topical | 1.0 mg/mL; 4x/day | Rabbit corneal micropocket b-FGF model | [97] |
Luteolin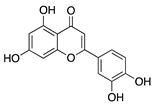 | Fruits and vegetables | Unknown | Topical | 0.5 mg/mL; 4x/day | Rabbit corneal micropocket b-FGF model | [97] |
Genistein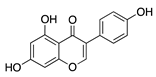 | Soybeans | Unknown; downregulates VEGF and TGF-β | Topical | 0.5 mg/mL; 4x/day | Rabbit corneal micropocket b-FGF model | [97] |
Naringenin | Citrus fruits and vegetables | Unknown; Downregulates NF-κB activity, proangiogenic factors, and reduces production of cytokines IL-1β and IL-6 | Topical | 0.08, 0.8, 8, 80 µg | Rat alkali burn model | [102] |
| Non-Flavonoid Phytochemical | Source | Mechanism | Routes | Dose | Model | Ref |
|---|---|---|---|---|---|---|
Curcumin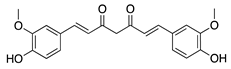 | Turmeric (Curcuma longa) | Unknown; inhibition of several signal transduction pathways, including NF-κB activation | Topical | 40 µM; 2x/day | Rat alkali burn model | [111] |
| Topical | 40, 80, and 160 µM | Rabbit suture model | [110] | |||
| Nanoparticle-mediated delivery via eye drops | 80 mg | Rat silver-nitrate model | [112] | |||
Resveratrol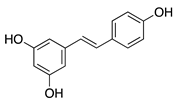 | Grapes and other fruits | Unknown; Downregulates FGF-2 and VEGF | Oral | 48 mg/kg | Murine FGF-2 and VEGF-micropocket model | [115] |
| Subconjunctival | 10 mg/mL | Rabbit alkali burn model | [116] | |||
Withaferin A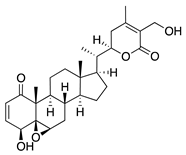 | Steroidal lactone (Withania somnifera) | Targets and downregulates vimentin | Intraperitoneal | 2 mg/kg | Murine de-epithelializ-ation model using wild type and vimentin-null mice | [118] |
Xanthatin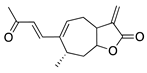 | Sesquiterpene lactone (Xanthium sibiricum) | Inhibition of the VEGFR2-mediated STAT3/PI3K/Akt signaling pathways | Topical | 10 µM; 4x/day | Rat alkali burn model | [123] |
Triptolide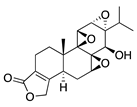 | Tripterygium wilfordii Hook F | Unknown; downregulates VEGF | Topical | 100 nM; 3x/day | Murine alkali burn model | [126] |
Thymoquinone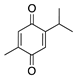 | Volatile oil of black seed (Nigella sativa) | Unknown; Likely related to antioxidant and anti-inflammatory properties | Topical | 0.1%, 0.4% | Rat silver-nitrate model | [131] |
Glycyrrhizin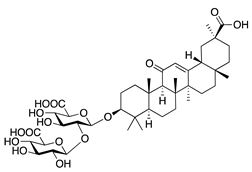 | Saponin from licorice root (Glycyrrhiza glabra) | Unknown | Topical | 1% | Rabbit alkali burn model | [133] |
| Immunosuppressant | Source | Mechanism | Routes | Dose | Model | Ref |
|---|---|---|---|---|---|---|
Tetramethylpyrazine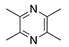 | Bioactive component of chuanxiong (Ligusticum striatum) | Unknown; Downregulates CXCR-4 | Topical | 1.5 mg/mL 4x/day | Murine alkali burn model | [137] |
Methotrexate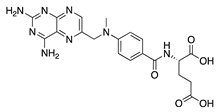 | Synthetic | Unknown; Downregulates VEGF and IL-6 | Topical Subconjunctival | 2 mg/mL, 4 mg/mL 2 mg/mL | Rabbit suture model | [140] |
CC-3052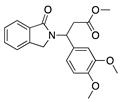 | Thalidomide analogue | Unknown; Downregulates VEGF and TNF-α | Topical Subconjunctival | 0.25%, 0.5%, and 1% 0.5% | Rabbit suture model | [147] |
DAID | Thalidomide analogue | Unknown; Downregulates VEGF | Topical | 0.25% | Murine alkali burn model | [149] |
LASSBio-596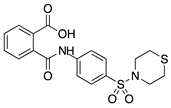 | Thalidomide and arylsulfonamide derivative | Unknown | Topical | 1%; 3x/day | Rabbit alkali burn model | [152] |
Cyclosporine A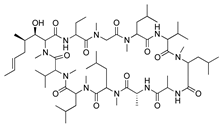 | Secondary metabolite of fungal genus Tolypocladium | Calcineurin inhibition; downregulates MMP-9, VEGF, and iNOS | Topical Subconjunctival | 4% 5 mg/kg | Rat silver-nitrate model | [153] |
| Topical | 0.05% | Rabbit immune-mediated CoNV model | [154] | |||
| Nanofibers | 0.25 mg/mm2 | Rabbit alkali burn model | [156] | |||
Rapamycin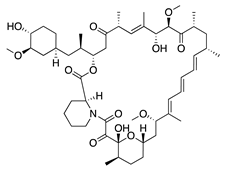 | Product of Streptomyces hygroscopicus | mTOR inhibition; downregulates VEGF, TNF-α, TGF-β, IL-6, and Substance P | Topical Intraperitoneal | 1 mg/mL 0.2 mg/kg | Murine alkali burn model | [160] |
| Intraperitoneal | 2 mg/kg; 1x/day | Murine alkali burn model | [159] | |||
Tacrolimus | Product of Streptomyces tsukubaensis | Calcineurin inhibition; downregulates VEGF, TNF-α, IL-1β, and MCP-1 | Topical Subconjunctival | 5 mg/5 mL 0.25 mg/ 0.05 mL | Rabbit suture model | [164] |
| Vitamin/Photoactivatable Compound | Source | Mechanism | Routes | Dose | Model | Ref |
|---|---|---|---|---|---|---|
Ascorbic acid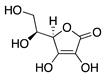 | Diet | Unknown; Downregulation of VEGF and MMP-9 | Topical | 0.5, 1, 10 mg/mL | Rabbit suture model | [167] |
Riboflavin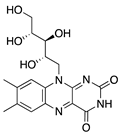 | Diet | Induction of apoptosis in vascular ECs; downregulation of macrophages and CD45+ cells | Topical riboflavin followed by UVA exposure | 0.1% | Murine suture model | [171] |
Verteporfin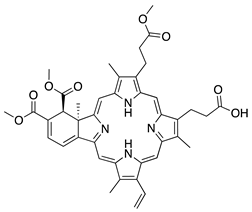 | Synthetic | Suppressed blood vessels and lymphatic vessels | Intravenous followed by light exposure | 6 mg/m2 | Murine suture model | [174] |
1α,25-dihydroxyvitamin D3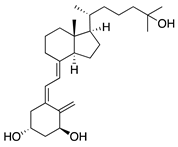 | Diet | Inhibited migration of Langerhans cells into cornea | Topical | 10−7 M, 10−8 M, and 10−9 M | Murine suture model | [177] |
| HDAC Inhibitor | Source | Mechanism | Routes | Dose | Model | Ref |
|---|---|---|---|---|---|---|
Largazole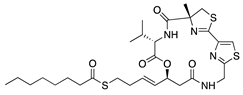 | Macrocyclic depsipeptide from marine cyanobacterium Symploca species | Class I HDAC inhibition; downregulates VEGF, b-FGF, TGF-β1, and EGF; Upregulates Tsp-1, Tsp-2, and ADAMTS-1 | Topical | 5 µL; 2x/day | Murine alkali burn model | [180] |
Vorinostat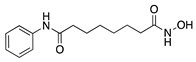 | Synthetic | HDAC inhibition; targets unknown | Topical | 10 µM; 3x/day | Murine alkali burn model | [182] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barry, Z.; Park, B.; Corson, T.W. Pharmacological Potential of Small Molecules for Treating Corneal Neovascularization. Molecules 2020, 25, 3468. https://doi.org/10.3390/molecules25153468
Barry Z, Park B, Corson TW. Pharmacological Potential of Small Molecules for Treating Corneal Neovascularization. Molecules. 2020; 25(15):3468. https://doi.org/10.3390/molecules25153468
Chicago/Turabian StyleBarry, Zachary, Bomina Park, and Timothy W. Corson. 2020. "Pharmacological Potential of Small Molecules for Treating Corneal Neovascularization" Molecules 25, no. 15: 3468. https://doi.org/10.3390/molecules25153468
APA StyleBarry, Z., Park, B., & Corson, T. W. (2020). Pharmacological Potential of Small Molecules for Treating Corneal Neovascularization. Molecules, 25(15), 3468. https://doi.org/10.3390/molecules25153468








