Role of β-Caryophyllene in the Antinociceptive and Anti-Inflammatory Effects of Tagetes lucida Cav. Essential Oil
Abstract
1. Introduction
2. Results
2.1. Phytochemical Analysis
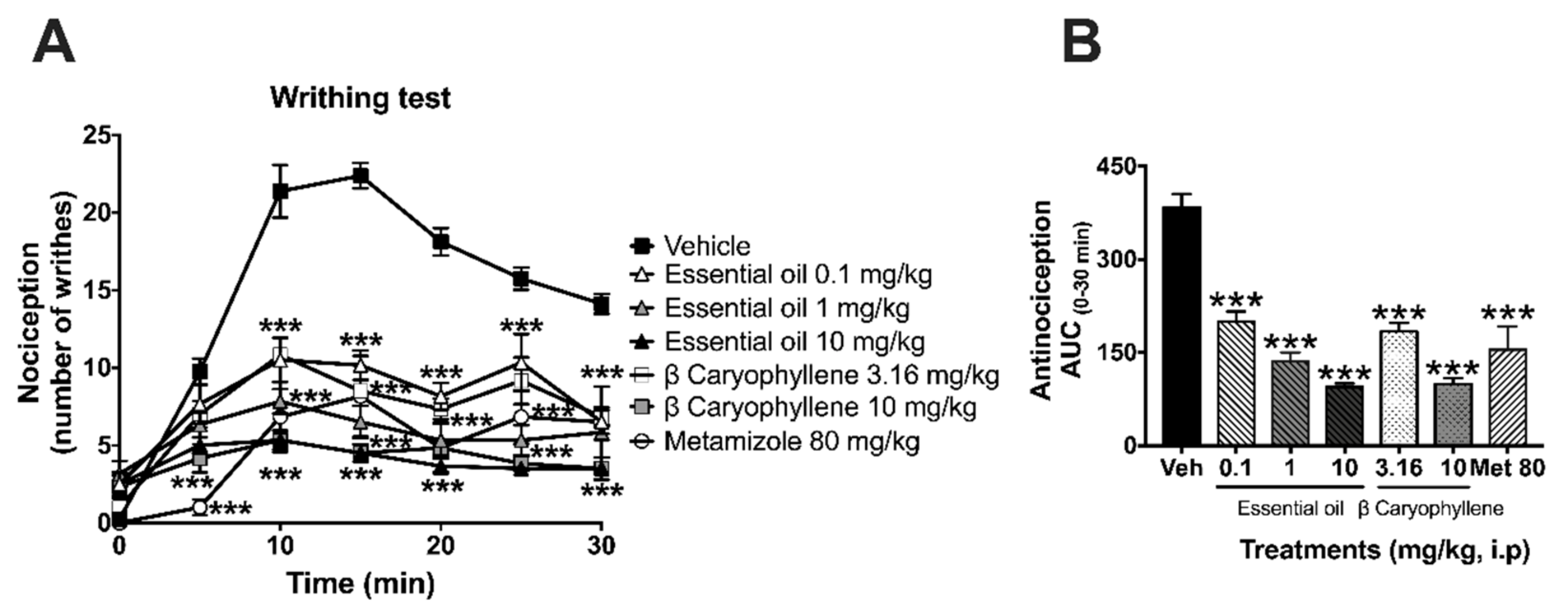
2.2. Antinociceptive Activity in the Writhing Test
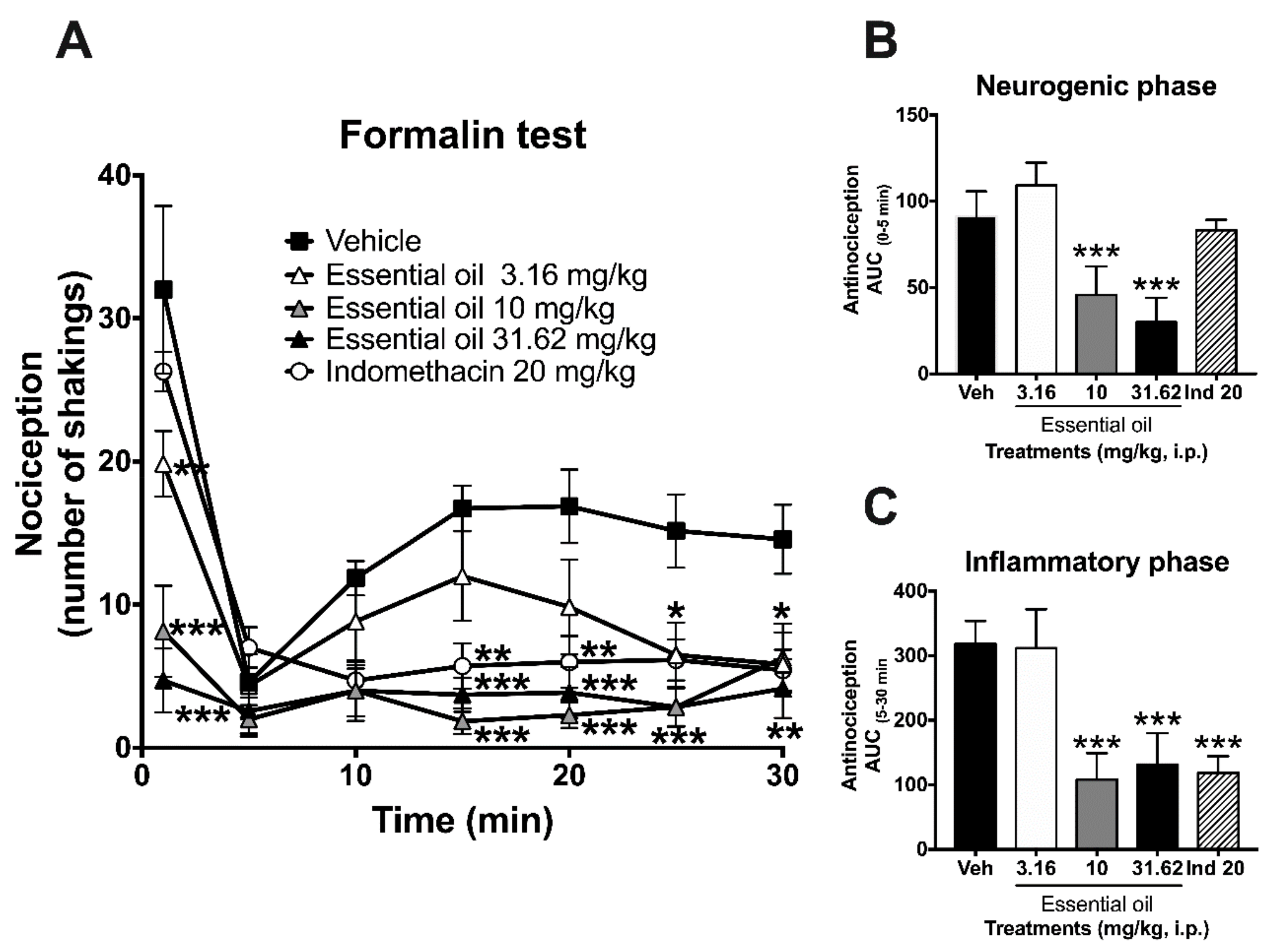
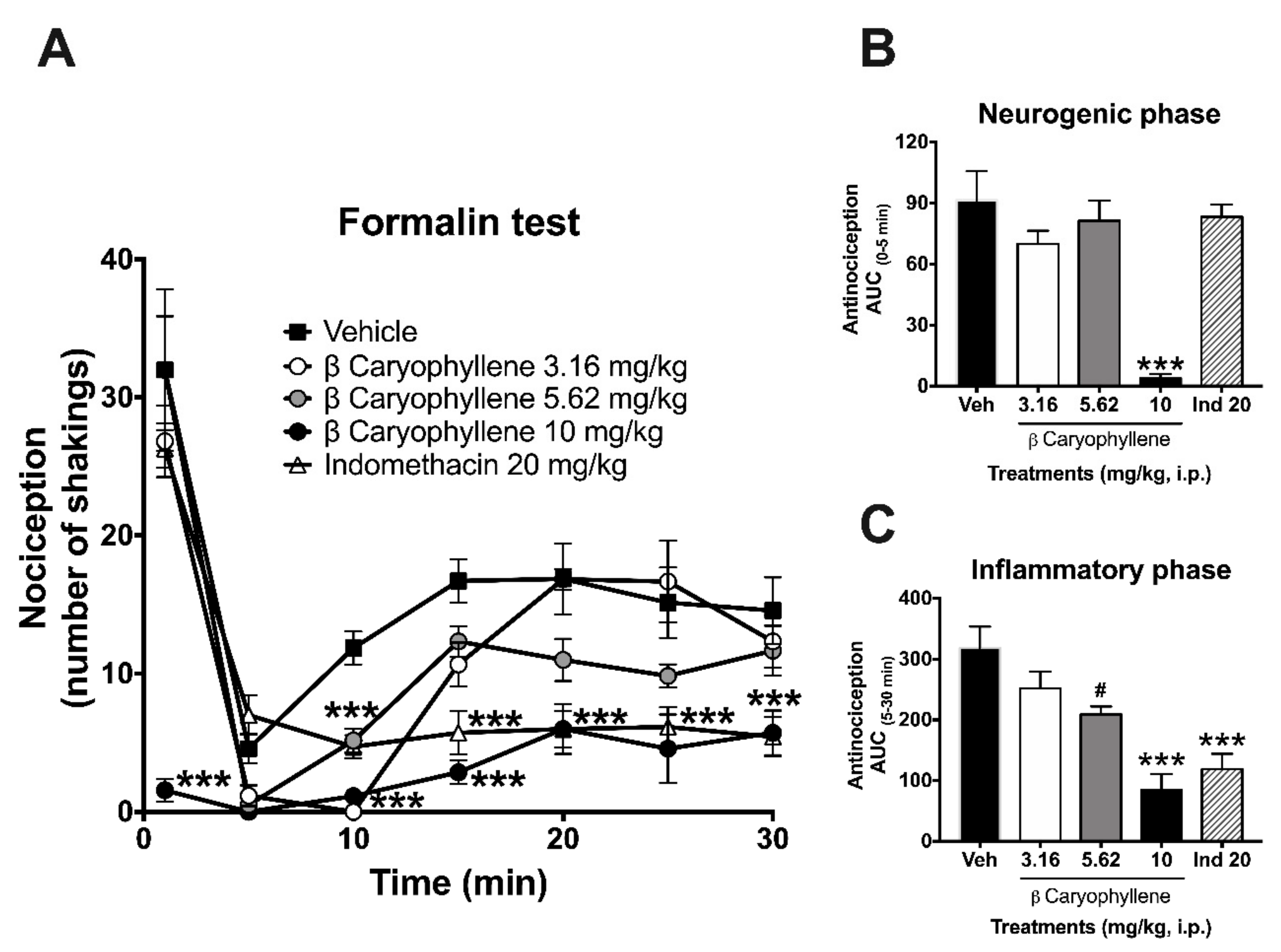
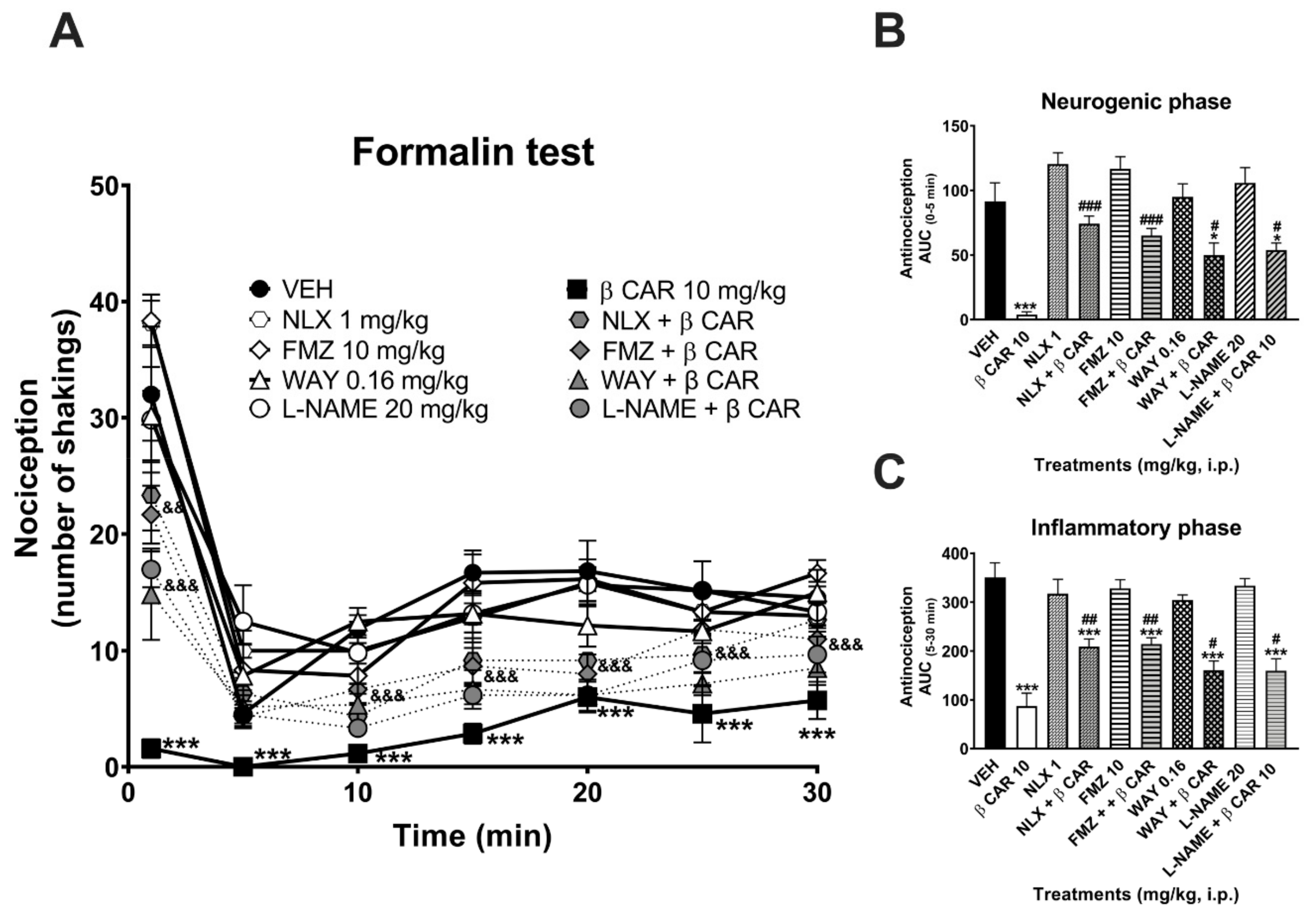
2.3. Antinociceptive Activity in the Formalin Test
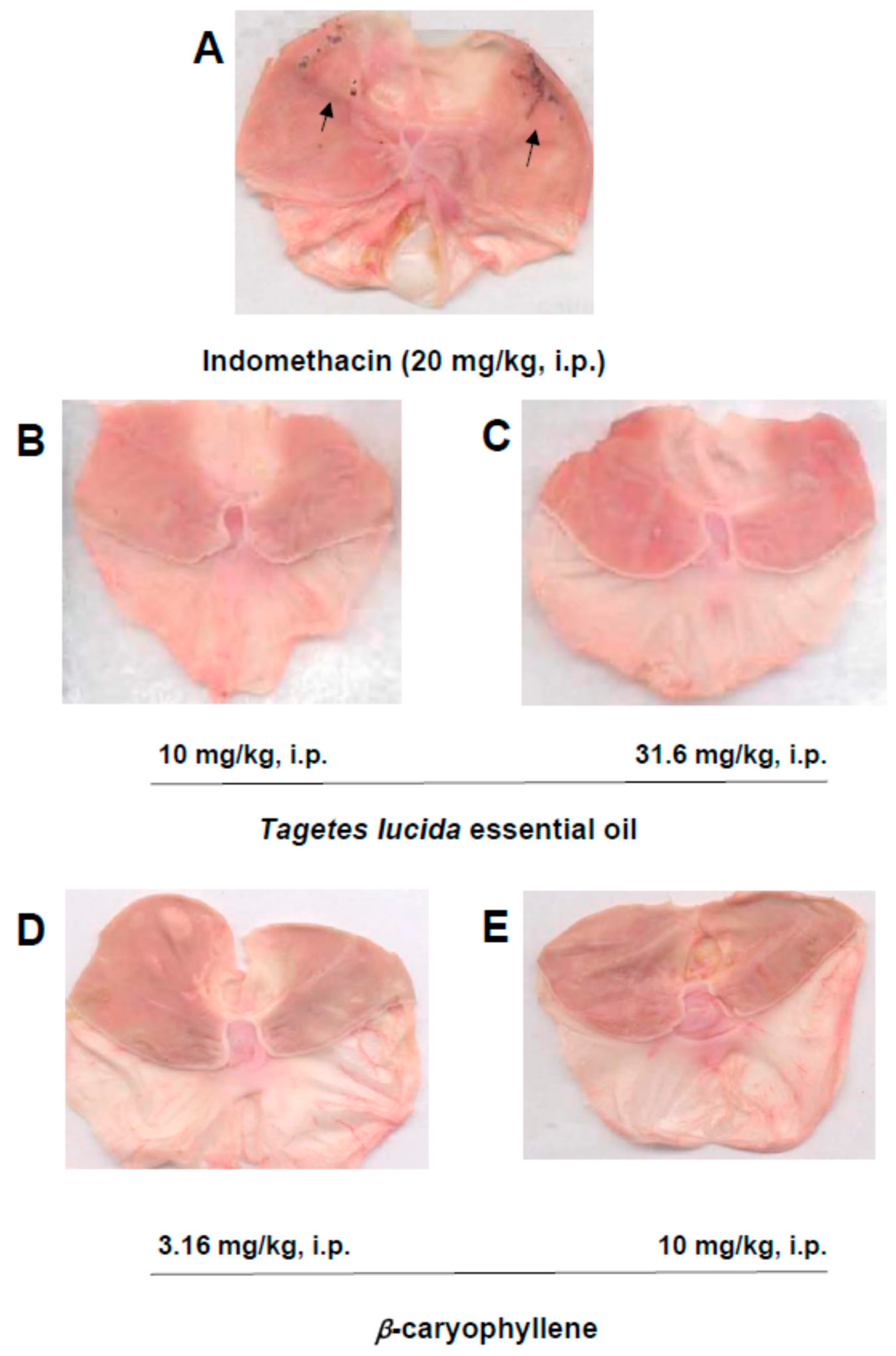
2.4. Gastric Damage
2.5. Median Lethal Dose (LD50)
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Essential Oil Preparation
4.3. Equipment and Chromatographic Conditions
4.4. Pharmacological Study
4.4.1. Animals
4.4.2. Reagents and Drugs
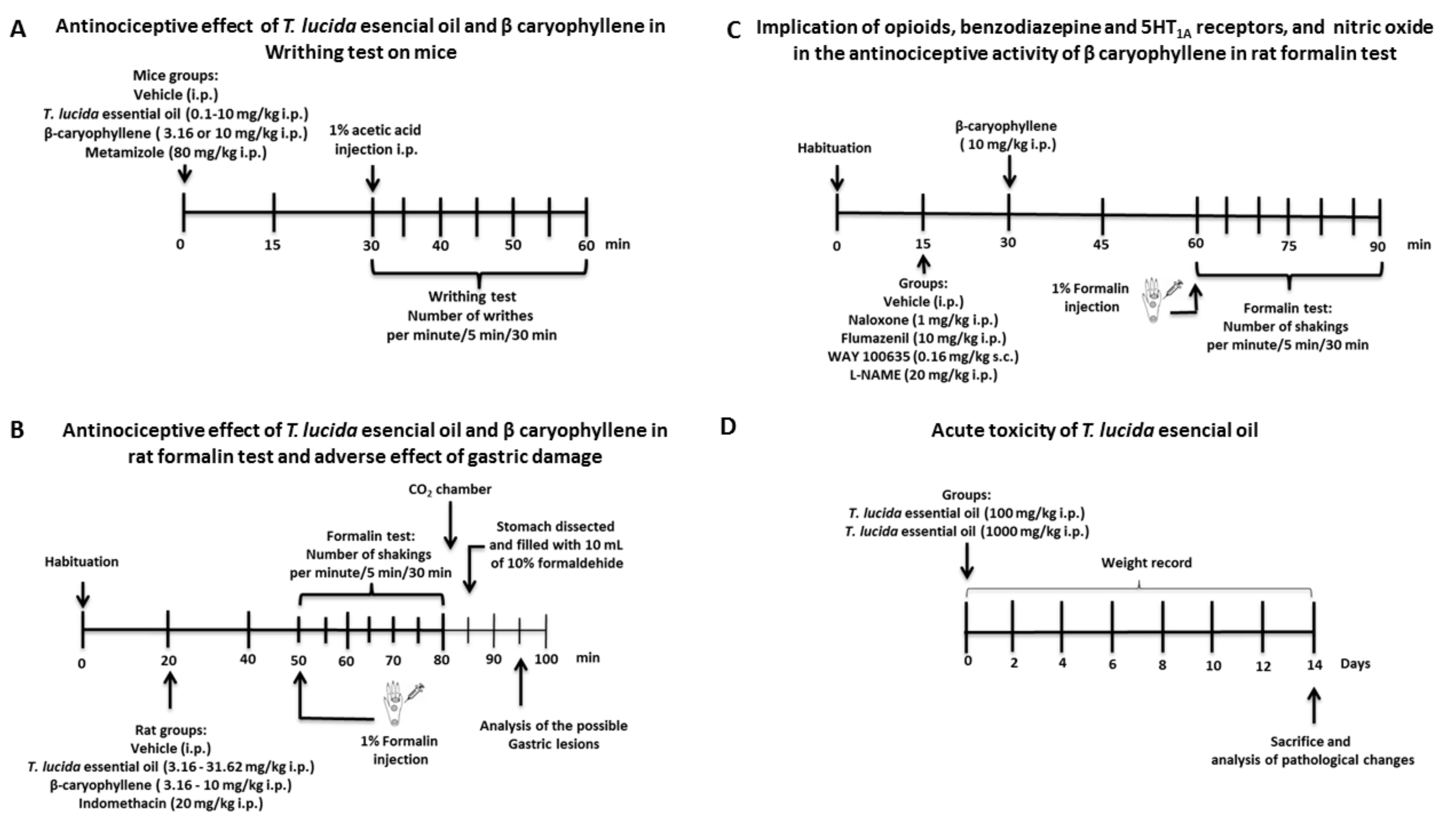
4.4.3. Experimental Design
Antinociceptive Activity
Gastric Damage as an Adverse Effect
Acute Toxicity
Statistical Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Mata-Pinzón, S.; Pérez-Ortega, G.; Reyes-Chilpa, R. Medicinal plants for the treatment of “susto” and “mal de ojo”. Analysis of its possible effects on the central nervous system by transdermal and inhalation. Rev. Ethnobiol. 2018, 16, 30–47. [Google Scholar]
- Firenzuoli, F.; Jaitak, V.; Horvath, G.; Bassolé, I.H.; Setzer, W.N.; Gori, L. Essential oils: New perspectives in human health and wellness. Evid. Based Complement. Altern. Med. 2014, 2014, 467363. [Google Scholar] [CrossRef] [PubMed]
- Elshafie, H.S.; Camele, I. An overview of the biological effects of some mediterranean essential oils on human health. BioMed Res. Int. 2017, 2017, 9268468. [Google Scholar] [CrossRef] [PubMed]
- Neher, R.T. The ethnobotany of Tagetes. Econ. Bot. 1968, 22, 317–325. [Google Scholar] [CrossRef]
- Xu, L.; Chen, J.; Qi, H.; Shi, Y. Phytochemicals and their biological activities of plants in Tagetes L. Chin. Herb. Med. 2012, 4, 103–117. [Google Scholar]
- Pérez-Ortega, G.; González-Trujano, M.E.; Ángeles-López, G.E.; Brindis, F.; Vibrans, H.; Reyes-Chilpa, R. Tagetes lucida Cav.: Ethnobotany, phytochemistry and pharmacology of its tranquilizing properties. J. Ethnopharmacol. 2016, 181, 221–228. [Google Scholar] [CrossRef]
- Igwaran, A.; Iweriebor, B.C.; Okoh, S.O.; Nwodo, U.U.; Obi, L.C.; Okoh, A.I. Chemical constituents, antibacterial and antioxidant properties of the essential oil flower of Tagetes minuta grown in Cala community Eastern Cape, South Africa. BMC Complement. Altern. Med. 2017, 17, 351. [Google Scholar] [CrossRef]
- Karimian, P.; Kavoosi, G.; Amirghofran, Z. Anti-oxidative and anti-inflammatory effects of Tagetes minuta essential oil in activated macrophages. Asian Pac. J. Trop Biomed. 2014, 4, 219–227. [Google Scholar] [CrossRef]
- Madhavan, J.; Chandrasekharan, S.; Priya, M.K.; Godavarthi, A. Modulatory effect of carotenoid supplement constituting lutein and zeaxanthin (10:1) on anti-oxidant enzymes and macular pigments level in rats. Pharmacogn. Mag. 2018, 14, 268–274. [Google Scholar] [CrossRef]
- Regalado, E.L.; Fernãnd, M.D.; Pino, J.A.; Mendiola, J.; Echemendia, O.A. Chemical composition and biological properties of the leaf essential oil of Tagetes lucida Cav. from Cuba. J. Essent. Oil Res. 2011, 23, 63–67. [Google Scholar] [CrossRef]
- Omer, E.; Hendawy, S.F.; El-Deen, A.M.N.; Zaki, F.N.; Abd-Elgawad, M.; Kandeel, A.M.; Ibrahim, A.K.; Ismail, R.F. Some biological activities of Tagetes lucida plant cultivated in Egypt. Adv. Environ. Biol. 2015, 9, 82–88. [Google Scholar]
- Vega-Avila, E.; Espejo-Serna, A.; Alarcón-Aguilar, F.; Velasco-Lezama, R. Cytotoxic activity of four Mexican medicinal plants. Proc. West. Pharmacol. Soc. 2009, 52, 78–82. [Google Scholar] [PubMed]
- Mejia-Barajas, J.A.; Del Rio, R.E.N.; Martinez-Muñoz, R.E.; Flores-Garcia, A.; Martinez-Pacheco, M.M. Cytotoxic activity in Tagetes lucida Cav. Emir. J. Food Agri.c 2012, 24, 142–147. [Google Scholar]
- Guadarrama-Cruz, G.; Alarcon-Aguilar, F.J.; Lezama-Velasco, R.; Vazquez-Palacios, G.; Bonilla-Jaime, H. Antidepressant-like effects of Tagetes lucida Cav. in the forced swimming test. J. Ethnopharmacol. 2008, 120, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Moorlag, H.; Vries de, J.G.; Kaptein, B.; Schoemaker, H.E.; Kamphuis, J.; Kellogg, R.M. Palladium-catalyzed allylation of α-hydroxy acids. Recl. Trav. Chim. Pays-Bas 1992, 111, 129–137. [Google Scholar] [CrossRef]
- Villaseñor, J.L. Diversidad y distribución de la familia Asteraceae en México. Bot. Sci. 2018, 96, 332–358. [Google Scholar] [CrossRef]
- Bicchi, C.; Fresia, M.; Rubiolo, P.; Monti, D.; Franz, C.; Goehler, I. Constituents of Tagetes lucida Cav. ssp. lucida essential oil. Flavour Frag. J. 1997, 12, 47–52. [Google Scholar] [CrossRef]
- Cicció, J.F. A source of almost pure methyl chavicol: Volatile oil from the aerial parts of Tagetes lucida (Asteraceae) cultivated in Costa Rica. Rev. Biol. Trop. 2004, 52, 853–857. [Google Scholar]
- Marotti, M.; Piccaglia, R.; Biavati, B.; Marotti, I. Characterization and yield evaluation of essential oils from different tagetes species. J. Essent. Oil Res. 2004, 16, 440–444. [Google Scholar] [CrossRef]
- Sepúlveda-Arias, J.C.; Veloza, L.A.; Escobar, L.M.; Orozco, L.M.; Lopera, I.A. Anti-inflammatory effects of the main constituents and epoxides derived from the essential oils obtained from Tagetes lucida, Cymbopogon citratus, Lippia alba and Eucalyptus citriodora. J. Essent. Oil Res. 2013, 25, 186–193. [Google Scholar] [CrossRef]
- Salehi, B.; Valussi, M.; Morais-Braga, M.F.B.; Carneiro, J.N.P.; Leal, A.L.A.B.; Coutinho, H.D.M.; Vitalini, S.; Kregiel, D.; Antolak, H.; Sharifi-Rad, M.; et al. Tagetes spp. Essential oils and other extracts: Chemical characterization and biological activity. Molecules 2018, 23, 2847. [Google Scholar]
- De Vincenzi, M.; Silano, M.; Maialetti, F.; Scazzocchio, B. Constituents of aromatic plants: II. Estragole. Fitoterapia 2000, 71, 725–729. [Google Scholar] [CrossRef]
- EMA. Public statement on the use of herbal medicinal products containing estragole. Comm. Herb. Med. Prod. 2014, 2005, 1–19. [Google Scholar]
- Krishna, A.; Mallavarapu, G.R.; Ramesh, S. Composition of the essential oils of the leaves and flowers of Tagetes erecta L. J. Essent. Oil Res. 2004, 16, 520–522. [Google Scholar] [CrossRef]
- Ogunwande, I.A.; Olawore, N.O. The essential oil from the leaves and flowers of “African Marigold,” Tagetes erecta L. J. Essent. Oil Res. 2006, 18, 366–368. [Google Scholar] [CrossRef]
- Pérez-Ortega, G.; Angeles-López, G.E.; Argueta-Villamar, A.; González-Trujano, M.E. Preclinical evidence of the anxiolytic and sedative-like activities of Tagetes erecta L. reinforces its ethnobotanical approach. Biomed. Pharmacother. 2017, 93, 383–390. [Google Scholar] [CrossRef]
- Sagar, D.V.; Naik, S.N.; Rout, P.K.; Rao, Y.R. Composition of essential oils of Tagetes patula l. growing in Northern India. J. Essent. Oil Res. 2005, 17, 446–448. [Google Scholar] [CrossRef]
- Tiwari, A.; Goswami, P.; Bisht, B.S.; Chauhan, A.; Verma, R.S.; Padalia, R.C. Essential oil composition of African marigold (Tagetes minuta L.) harvested at different growth stages in foothills agroclimatic conditions of North India. Am. J. Essent. Oils Nat. Prod. 2016, 4, 4–7. [Google Scholar]
- Quintans-Junior, L.; Moreira, J.C.; Pasquali, M.A.; Rabie, S.M.; Pires, A.S.; Schöder, R.; Rabelo, T.K.; Santos, J.P.; Lima, P.S.; Cavalcanti, S.C.; et al. Antinociceptive activity and redox profile of the monoterpenes (+)-Camphene, p-Cymene, and Geranyl Acetate in experimental models. ISRN Toxicol. 2013, 2013, 1–11. [Google Scholar] [CrossRef]
- La Rocca, V.; da Fonsêca, D.V.; Silva-Alves, K.S.; Ferreira-da-Silva, F.W.; de Sousa, D.P.; Santos, P.L.; Quintans-Júnior, L.J.; Leal-Cardoso, J.H.; de Almeida, R.N. Geraniol induces antinociceptive effect in mice evaluated in behavioural and electrophysiological models. Basic Clin. Pharmacol. Toxicol. 2017, 20, 22–29. [Google Scholar] [CrossRef]
- Lv, Y.; Zhang, L.; Li, N.; Mai, N.; Zhang, Y.; Pan, S. Geraniol promotes functional recovery and attenuates neuropathic pain in rats with spinal cord injury. Can. J. Physiol. Pharmacol. 2017, 95, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
- Segat, G.C.; Manjavachi, M.N.; Matias, D.O.; Passos, G.F.; Freitas, C.S.; Costa, R.; Calixto, J.B. Antiallodynic effect of β-caryophyllene on paclitaxel-induced peripheral neuropathy in mice. Neuropharmacology 2017, 125, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Machado, K.C.; Islam, M.T.; Ali, E.S.; Rouf, R.; Uddin, S.J.; Dev, S.; Shilpi, J.A.; Shill, M.C.; Reza, H.M.; Das, A.K.; et al. A systematic review on the neuroprotective perspectives of beta-caryophyllene. Phytother. Res. 2018, 32, 2376–2388. [Google Scholar] [CrossRef] [PubMed]
- Klauke, A.L.; Racz, I.; Pradier, B.; Markert, A.; Zimmer, A.M.; Gertsch, J.; Zimmer, A. The cannabinoid CB2 receptor-selective phytocannabinoid beta-caryophyllene exerts analgesic effects in mouse models of inflammatory and neuropathic pain. Eur. Neuropsychopharmacol. 2014, 24, 608–620. [Google Scholar] [CrossRef]
- González-Trujano, M.E.; Gutiérrez-Valentino, C.; Hernández-Arámburo, M.Y.; Díaz-Reval, M.I.; Pellicer, F. Identification of some bioactive metabolites and inhibitory receptors in the antinociceptive activity of Tagetes lucida Cav. Life Sci. 2019, 231, 1–9. [Google Scholar] [CrossRef]
- Martínez, R.; Díaz, B.; Vásquez, L.; Compagnone, R.S.; Tillett, S.; Canelón, D.J.; Torrico, F.; Suárez, A.I. Chemical composition of essential oils and toxicological evaluation of Tagetes erecta and Tagetes patula from Venezuela. J. Essent. Oil-Bearing Plants 2009, 12, 476–481. [Google Scholar] [CrossRef]
- Wallace, J.L.; McKnight, W.; Reuter, B.K.; Vergnolle, N. NSAID-Induced gastric damage in rats: Requirement for inhibition of both cyclooxygenase 1 and 2. Gastroenterology 2000, 119, 706–714. [Google Scholar] [CrossRef]
- Khizhazi, A.A. The therapeutic and prophylactic anti-ulcerogenic action of marigold (Tagetes patula L.) and sea buckthorn (Hippophae) oils in neurogenic ulcerative lesions caused by immobilization, noise and vibration. Lik. Sprav. 1998, 1, 172–176. [Google Scholar]
- Collier, H.O.J.; Dinneen, L.C.; Johnson, C.A.; Schneider, C. The abdominal constriction response and its supression by analgesic drugsin the mouse. Br. J. Pharm. Chemother. 1968, 32, 295–310. [Google Scholar] [CrossRef]
- Wheeler-Aceto, H.; Porreca, F.; Cowan, A. The rat paw formalin test: Comparison of noxious agents. Pain 1990, 40, 229–238. [Google Scholar] [CrossRef]
- González-Trujano, M.E.; Navarrete, A. Effect of zinc on the cadmium acute intoxication in the gastric injury induced in rats. Rev. Latinoam. Quím. 2011, 39, 45–54. [Google Scholar]
- Lorke, D. A new approach to practical acute toxicity testing. Arch. Toxicol. 1983, 54, 275–287. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds β-caryophyllene and Tagetes lucida essential oil are available from the authors. |
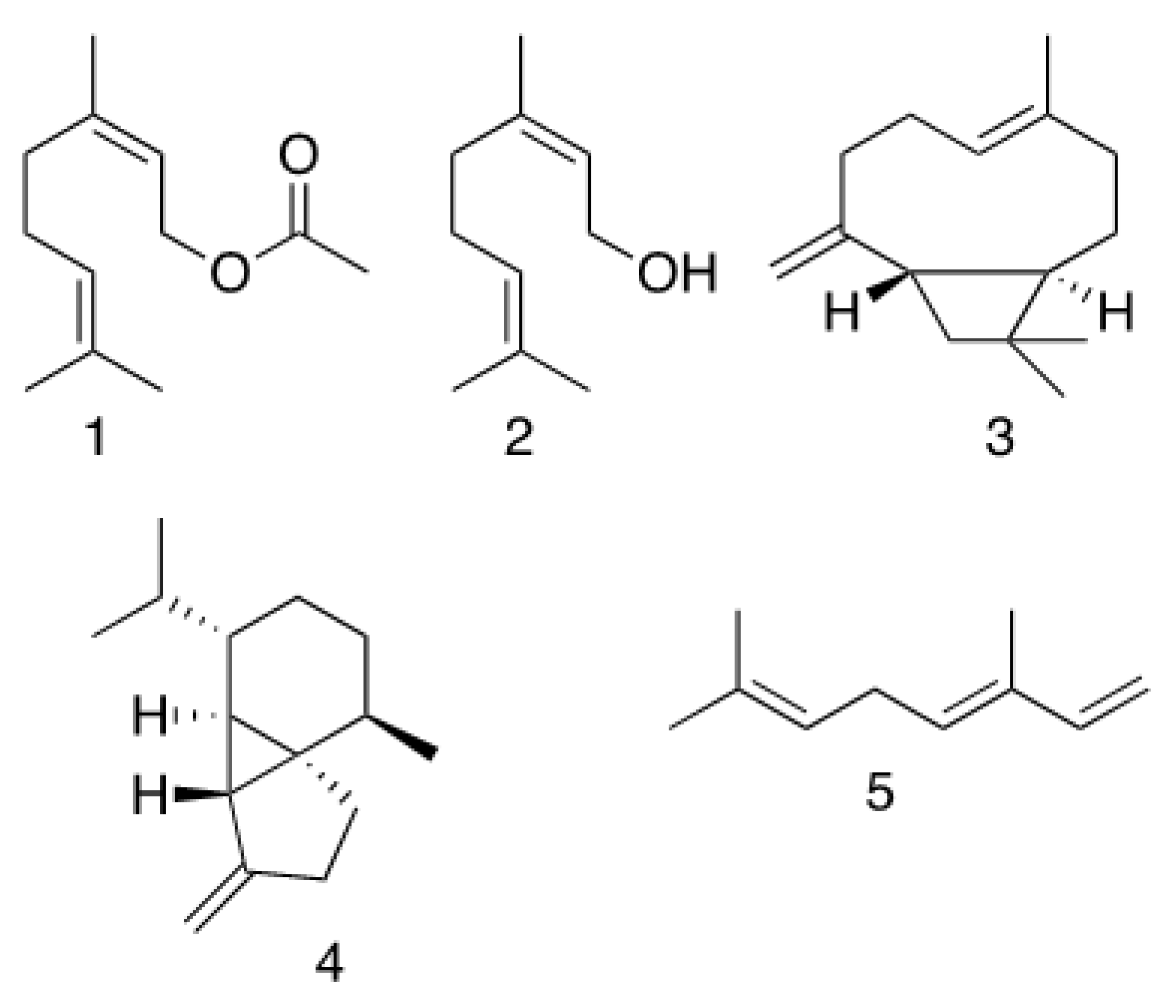
| Compound a | Area (%) | RI b | Chemical Formula | RT (min) | Method of Identification c |
|---|---|---|---|---|---|
| β-Pinene | 2.16 | 979 | C10H16 | 5.67 | a, b |
| cis-β-Ocimene (5) | 5.60 | 1042 | C10H16 | 7.29 | a, b |
| Linalool | 3.61 | 1096 | C10H18O | 8.63 | a, b |
| Geraniol (2) | 7.92 | 1252 | C10H18O | 11.85 | a, b |
| Geranyl acetate (1) | 49.89 | 1383 | C12H20O2 | 14.19 | a, b, c |
| β-Bourbonene | 0.66 | 1388 | C15H24 | 14.40 | a, b |
| β-(E)-Caryophyllene (3) | 6.27 | 1455 | C15H24 | 14.83 | a, b |
| (E,E)-α-Farnesene | 1.93 | 1505 | C15H24 | 15.26 | a, b |
| 7-epi-α-Selinene | 0.69 | 1522 | C15H24 | 15.35 | a, b |
| (−)-β-Cubebene (4) | 7.72 | 1523 | C15H24 | 15.80 | a, b |
| (E)-iso-γ-Bisabolene | 3.01 | 1529 | C15H24 | 16.00 | a, b |
| α-Cadinene | 0.81 | 1538 | C15H24 | 16.14 | a, b |
| α-Muurolol | 1.20 | 1546 | C15H26O | 16.31 | a, b |
| Elemol | 4.90 | 1549 | C15H26O | 17.00 | a, b |
| Germacrene B | 0.91 | 1561 | C15H24 | 17.26 | a, b |
| E-Farnesene epoxide | 0.18 | 1624 | C15H24O | 17.66 | a, b |
| n.i. | 0.36 | - | - | 17.76 | - |
| α-Cadinol | 0.40 | 1656 | C15H26O | 17.94 | a, b |
| (−)-Spathulenol | 0.34 | 1578 | C15H24O | 18.05 | a, b |
| α-Muurolol | 0.73 | 1646 | C15H26O | 18.13 | a, b |
| α-Cadinol | 0.42 | 1655 | C15H26O | 18.38 | a, b |
| 14-Hydroxy-9-epi-(E)-caryophyllene | 0.29 | 1670 | C15H24O | 18.59 | a, b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernandez-Leon, A.; González-Trujano, M.E.; Narváez-González, F.; Pérez-Ortega, G.; Rivero-Cruz, F.; Aguilar, M.I. Role of β-Caryophyllene in the Antinociceptive and Anti-Inflammatory Effects of Tagetes lucida Cav. Essential Oil. Molecules 2020, 25, 675. https://doi.org/10.3390/molecules25030675
Hernandez-Leon A, González-Trujano ME, Narváez-González F, Pérez-Ortega G, Rivero-Cruz F, Aguilar MI. Role of β-Caryophyllene in the Antinociceptive and Anti-Inflammatory Effects of Tagetes lucida Cav. Essential Oil. Molecules. 2020; 25(3):675. https://doi.org/10.3390/molecules25030675
Chicago/Turabian StyleHernandez-Leon, Alberto, María Eva González-Trujano, Fernando Narváez-González, Gimena Pérez-Ortega, Fausto Rivero-Cruz, and María Isabel Aguilar. 2020. "Role of β-Caryophyllene in the Antinociceptive and Anti-Inflammatory Effects of Tagetes lucida Cav. Essential Oil" Molecules 25, no. 3: 675. https://doi.org/10.3390/molecules25030675
APA StyleHernandez-Leon, A., González-Trujano, M. E., Narváez-González, F., Pérez-Ortega, G., Rivero-Cruz, F., & Aguilar, M. I. (2020). Role of β-Caryophyllene in the Antinociceptive and Anti-Inflammatory Effects of Tagetes lucida Cav. Essential Oil. Molecules, 25(3), 675. https://doi.org/10.3390/molecules25030675