Design of Targeted Nanostructured Coordination Polymers (NCPs) for Cancer Therapy
Abstract
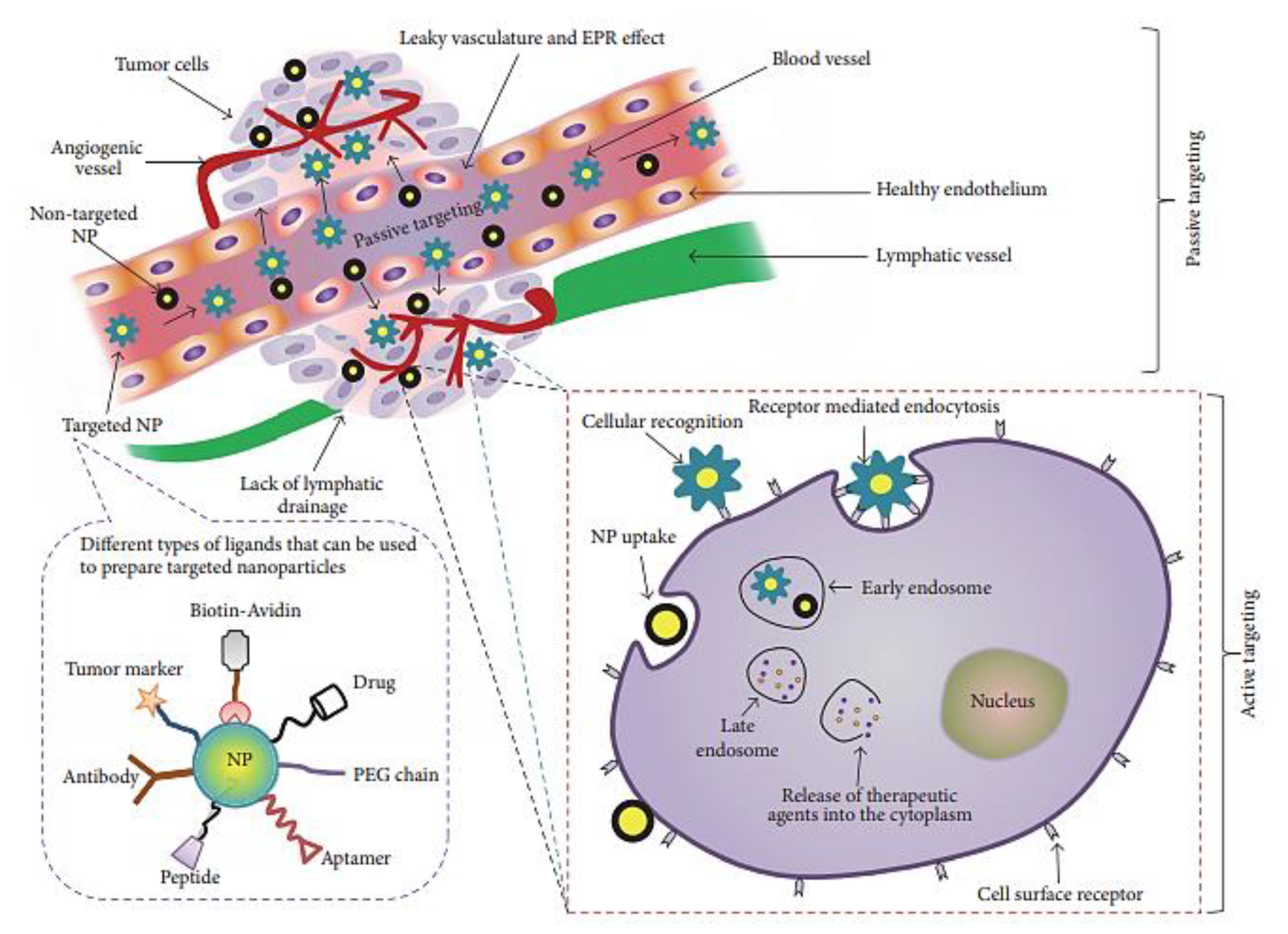
1. Introduction
2. NCPs for Cancer Treatment
3. Design of NCPs Nano-Formulations
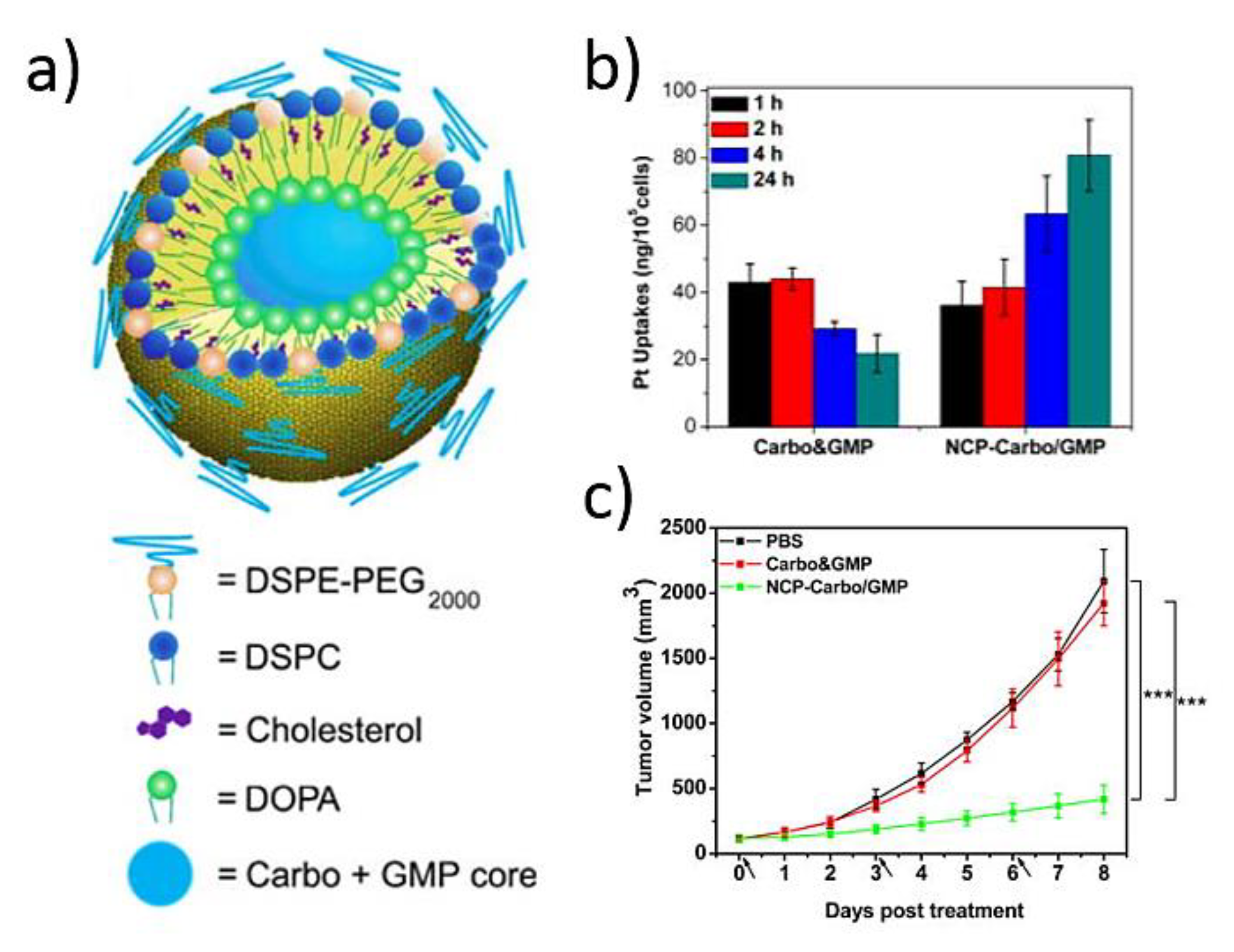
3.1. Platinum-Based NCPs
3.2. NCPs Including Non-Metal Chemotherapeutics
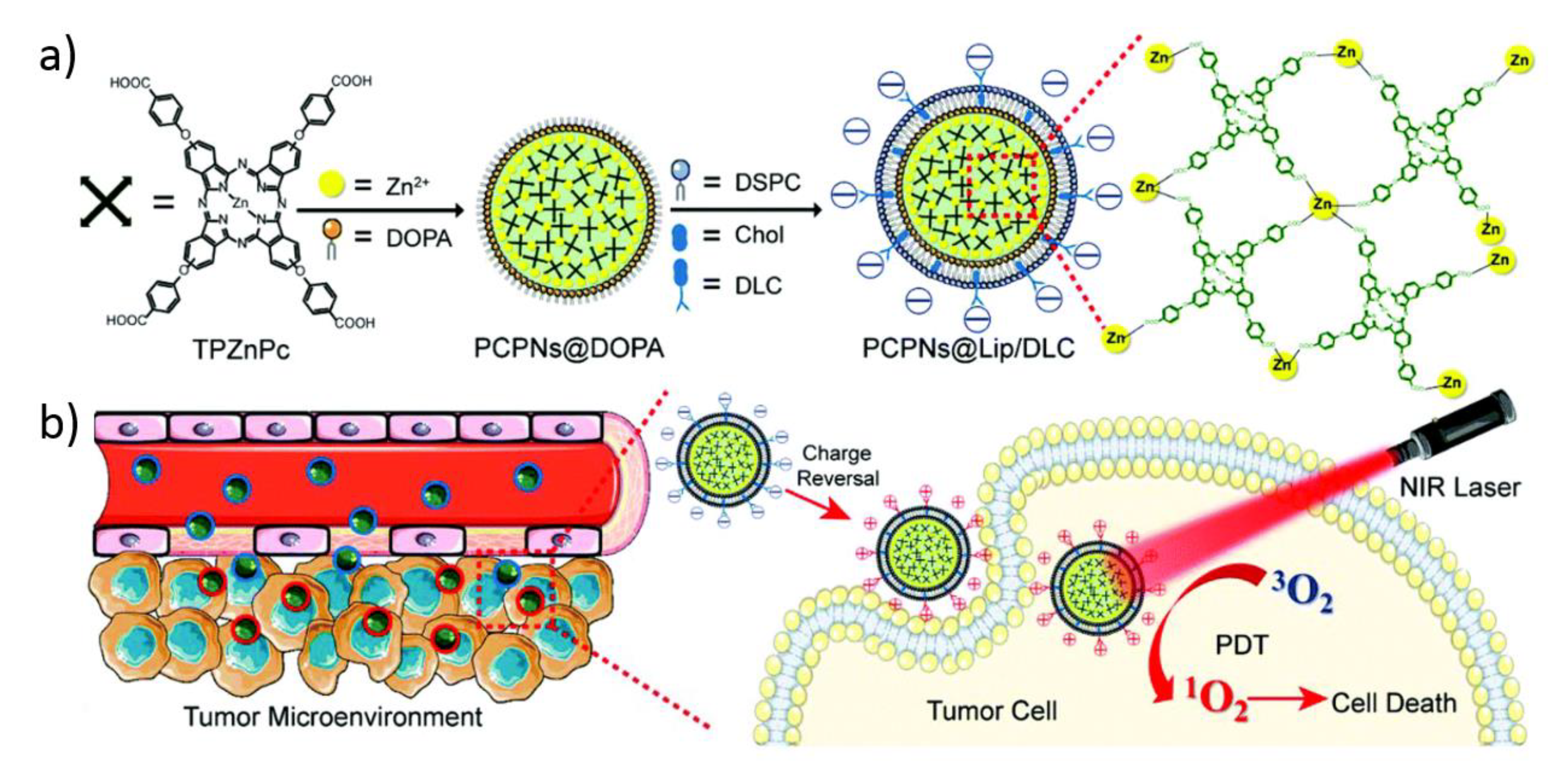
3.3. Development of Targeting NCPs for Photodynamic Therapy
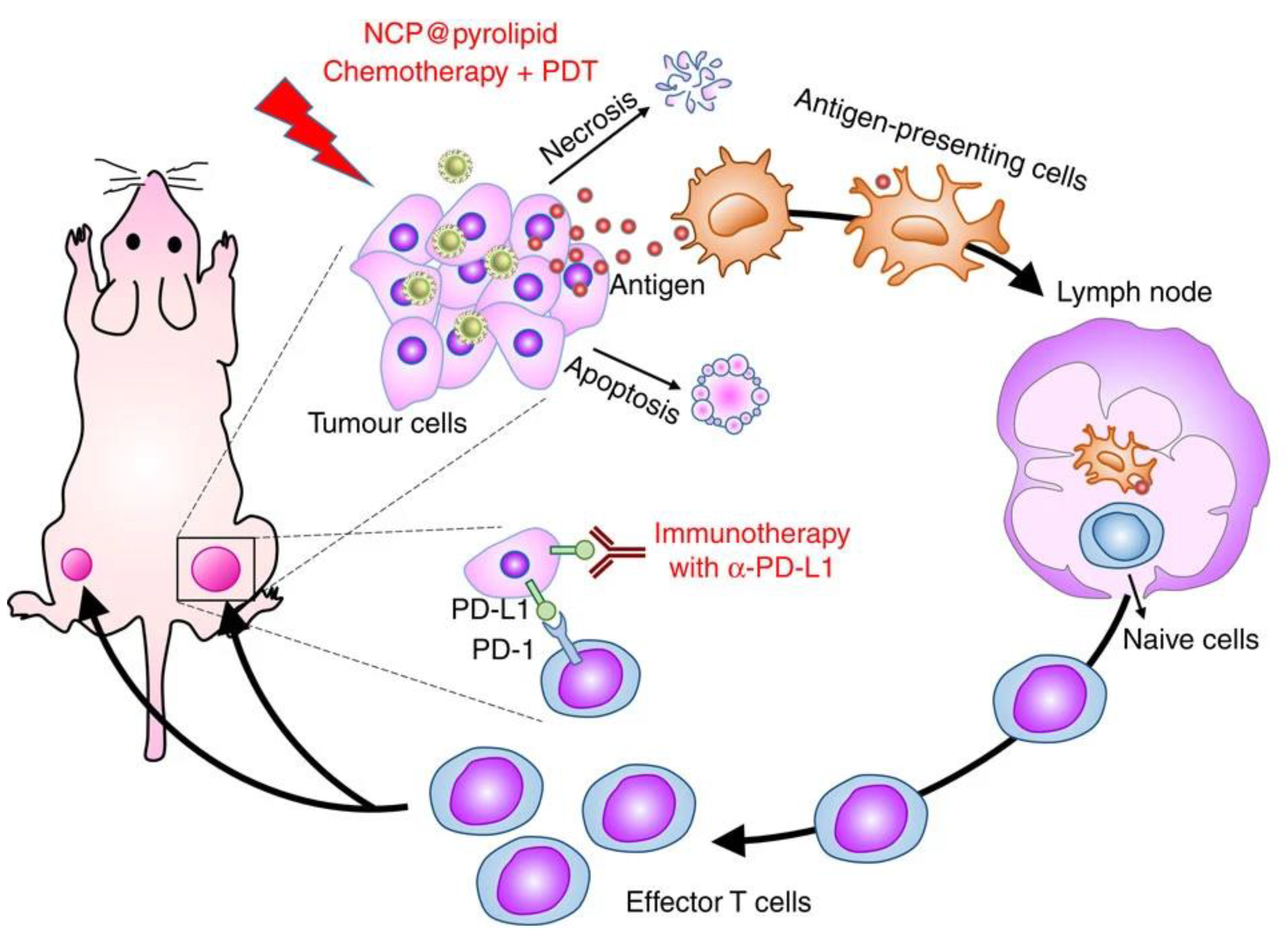
3.4. Other Explored Applications of NCPs in Cancer Treatment
4. Conclusions and Future Perspective
Funding
Conflicts of Interest
References
- Batten, S.R.; Neville, S.M.; Turner, D.R. Coordination Polymers: Design, Analysis and Application, 1st ed.; RSC Publishing: Cambridge, UK, 2009. [Google Scholar]
- Mu, J.; He, L.; Huang, P.; Chen, X. Engineering of nanoscale coordination polymers with biomolecules for advanced applications. Coord. Chem. Rev. 2019, 399, 213039. [Google Scholar] [CrossRef]
- Chen, L.J.; Yang, H.B. Construction of Stimuli-Responsive Functional Materials via Hierarchical Self-Assembly Involving Coordination Interactions. Acc. Chem. Res. 2018, 51, 2699–2710. [Google Scholar] [CrossRef]
- Giménez-Marqués, M.; Hidalgo, T.; Serre, C.; Horcajada, P. Nanostructured metal–organic frameworks and their bio-related applications. Coord. Chem. Rev. 2016, 307, 342–360. [Google Scholar] [CrossRef]
- Dang, S.; Zhu, Q.L.; Xu, Q. Nanomaterials Derived from Metal-Organic Frameworks. Nat. Rev. Mater. 2017, 3, 17075. [Google Scholar] [CrossRef]
- Sun, W.; Li, S.; Tang, G.; Luo, Y.; Ma, S.; Sun, S.; Ren, J.; Gong, Y.; Xie, C. Recent Progress of Nanoscale Metal-Organic Frameworks in Cancer Theranostics and the Challenges of Their Clinical Application. Int. J. Nanomed. 2019, 14, 10195–10207. [Google Scholar] [CrossRef]
- Morsali, A.; Hashemi, L. Chapter Two—Nanoscale coordination polymers: Preparation, function and application. Adv. Inorg. Chem. 2020, 76, 33–72. [Google Scholar]
- Oh, M.; Mirkin, C.A. Chemically Tailorable Colloidal Particles from Infinite Coordination Polymers. Nature 2005, 438, 651–654. [Google Scholar] [CrossRef]
- Sun, X.; Dong, S.; Wang, E. Coordination-Induced Formation of Submicrometer-Scale, Monodisperse, Spherical Colloids of Organic-Inorganic Hybrid Materials at Room Temperature. J. Am. Chem. Soc. 2005, 127, 13102–13103. [Google Scholar] [CrossRef]
- Park, K.H.; Jang, K.; Son, S.U.; Sweigart, D.A. Self-Supported Organometallic Rhodium Quinonoid Nanocatalysts for Stereoselective Polymerization of Phenylacetylene. J. Am. Chem. Soc. 2006, 128, 8740–8741. [Google Scholar] [CrossRef]
- Mohammadikish, M.; Yarahmadi, S. New self-supporting heterogeneous catalyst based on infinite coordination polymer nanoparticles. J. Phys. Chem. Solids 2020, 141, 109434. [Google Scholar] [CrossRef]
- Li, W.; Li, F.; Yang, H.; Wu, X.; Zhang, P.; Shan, Y.; Sun, L. A bio-inspired coordination polymer as outstanding water oxidation catalyst via second coordination sphere engineering. Nat. Commun. 2019, 10, 5074. [Google Scholar] [CrossRef] [PubMed]
- Puigmartí-Luis, J. Microfluidic Platforms: A Mainstream Technology for the Preparation of Crystals. Chem. Soc. Rev. 2014, 43, 2253–2271. [Google Scholar] [CrossRef]
- Salmon, L.; Catala, L. Spin-crossover nanoparticles and nanocomposite materials. C. R. Chim. 2018, 21, 1230–1269. [Google Scholar] [CrossRef]
- Huang, P.; Mao, J.; Yang, L.; Yu, P.; Mao, L. Bioelectrochemically Active Infinite Coordination Polymer Nanoparticles: One-Pot Synthesis and Biosensing Property. Chem. Eur. J. 2011, 17, 11390–11393. [Google Scholar] [CrossRef]
- Deng, J.; Wu, F.; Yu, P.; Mao, L. On-site sensors based on infinite coordination polymer nanoparticles: Recent progress and future challenge. Appl. Mater. Today 2018, 11, 338–351. [Google Scholar] [CrossRef]
- Tan, H.; Liu, B.; Chen, Y. Lanthanide Coordination Polymer Nanoparticles for Sensing of Mercury(II) by Photoinduced Electron Transfer. ACS Nano 2012, 6, 10505–10511. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.M.; Armatas, G.S.; Heo, J.; Kanatzidis, M.G.; Mirkin, C.A. Amorphous Infinite Coordination Polymer Microparticles: A New Class of Selective Hydrogen Storage Materials. Adv. Mater. 2008, 20, 2105–2110. [Google Scholar] [CrossRef]
- Bennett, T.D.; Horike, S. Liquid, glass and amorphous solid states of coordination polymers and metal–organic frameworks. Nat. Rev. Mater. 2018, 3, 431–440. [Google Scholar] [CrossRef]
- Amorín-Ferré, L.; Busqué, F.; Bourdelande, J.L.; Ruiz-Molina, D.; Hernando, J.; Novio, F. Encapsulation and Release Mechanisms in Coordination Polymer Nanoparticles. Chem. Eur. J. 2013, 19, 17508–17516. [Google Scholar] [CrossRef]
- Novio, F.; Simmchen, J.; Vázquez-Mera, N.; Amorín-Ferré, L.; Ruiz-Molina, D. Coordination Polymer Nanoparticles in Medicine. Coord. Chem. Rev. 2013, 257, 2839–2847. [Google Scholar] [CrossRef]
- Nador, F.; Novio, F.; Ruiz-Molina, D. Coordination Polymer Particles with Ligand-Centred PH-Responses and Spin Transition. Chem. Commun. 2014, 50, 14570–14572. [Google Scholar] [CrossRef] [PubMed]
- Golombek, S.K.; May, J.-N.; Theek, B.; Appold, L.; Drude, N.; Kiessling, F.; Lammers, T. Tumor Targeting via EPR: Strategies to Enhance Patient Responses. Adv. Drug Deliv. Rev. 2018, 130, 17–38. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Kanatani, S.; Tomer, R.; Sahlgren, C.; Kronqvist, P.; Kaczynska, D.; Louhivuori, L.; Kis, L.; Lindh, C.; Mitura, P.; et al. Whole-tissue biopsy phenotyping of three-dimensional tumours reveals patterns of cancer heterogeneity. Nat. Biomed. Eng. 2017, 1, 796–806. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Ahn, S.; Seo, E.; Kim, K.; Lee, S.J. Controlled cellular uptake and drug efficacy of nanotherapeutics. Sci. Rep. 2013, 3, 1997. [Google Scholar] [CrossRef]
- Jahan, S.T.; Sadat, S.M.A.; Walliser, M.; Haddadi, A. Targeted Therapeutic Nanoparticles: An Immense Promise to Fight against Cancer. J. Drug Deliv. 2017, 9090325. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e101413. [Google Scholar] [CrossRef]
- Rieter, W.J.; Pott, K.M.; Taylor, K.M.L.; Lin, W. Nanoscale Coordination Polymers for Platinum-Based Anticancer Drug Delivery. J. Am. Chem. Soc. 2008, 130, 11584–11585. [Google Scholar] [CrossRef]
- Yang, J.; Liu, W.; Sui, M.; Tang, J.; Shen, Y. Platinum (IV)-Coordinate Polymers as Intracellular Reduction-Responsive Backbone-Type Conjugates for Cancer Drug Delivery. Biomaterials 2011, 32, 9136–9143. [Google Scholar] [CrossRef]
- Solórzano, R.; Tort, O.; García-Pardo, J.; Escribà, T.; Lorenzo, J.; Arnedo, M.; Ruiz-Molina, D.; Alibés, R.; Busqué, F.; Novio, F. Versatile Iron-Catechol-Based Nanoscale Coordination Polymers with Antiretroviral Ligand Functionalization and Their Use as Efficient Carriers in HIV/AIDS Therapy. Biomater. Sci. 2019, 7, 178–186. [Google Scholar] [CrossRef]
- Wang, K.; Ma, X.; Shao, D.; Geng, Z.; Zhang, Z.; Wang, Z. Coordination-Induced Assembly of Coordination Polymer Submicrospheres: Promising Antibacterial and in Vitro Anticancer Activities. Cryst. Growth Des. 2012, 12, 3786–3791. [Google Scholar] [CrossRef]
- Imaz, I.; Rubio-Martínez, M.; García-Fernández, L.; García, F.; Ruiz-Molina, D.; Hernando, J.; Puntes, V.; Maspoch, D. Coordination Polymer Particles as Potential Drug Delivery Systems. Chem. Commun. 2010, 46, 4737–4739. [Google Scholar] [CrossRef]
- Novio, F.; Lorenzo, J.; Nador, F.; Wnuk, K.; Ruiz-Molina, D. Carboxyl Group (-CO2H) Functionalized Coordination Polymer Nanoparticles as Efficient Platforms for Drug Delivery. Chem. Eur. J. 2014, 20, 15443–15450. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Zheng, H.; Cao, Y.; Che, S. Coordination Polymer Coated Mesoporous Silica Nanoparticles for pH-Responsive Drug Release. Adv. Mater. 2012, 24, 6433–6437. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Zheng, H.; Che, S. A pH-responsive cleavage route based on a meta-organic coordination bond. Chem. Eur. J. 2011, 17, 7271–7275. [Google Scholar] [CrossRef]
- Xing, L.; Cao, Y.; Che, S. Synthesis of core–shell coordination polymernanoparticles (CPNs) for pH-responsive controlled drug release. Chem. Commun. 2012, 48, 5995–5997. [Google Scholar] [CrossRef]
- Green, J.R. Bisphosphonates: Preclinical Review. Oncologist 2004, 9, 3–13. [Google Scholar] [CrossRef]
- Wang, S.; Della Rocca, J.; Lin, W.; Huxford-Phillips, R.C.; Kramer, S.A.; Liu, D. Coercing Bisphosphonates to Kill Cancer Cells with Nanoscale Coordination Polymers. Chem. Commun. 2012, 48, 2668–2670. [Google Scholar]
- Etaiw, S.E.H.; Fayed, T.A.; El-bendary, M.M.; Marie, H. Three-dimensional coordination polymers based on trimethyltin cation with nicotinic and isonicotinic acids as anticancer agents. Appl. Organomet. Chem. 2018, 32, 4066. [Google Scholar] [CrossRef]
- Shen, S.; Wu, Y.; Li, K.; Wang, Y.; Wu, J.; Zeng, Y.; Wu, D. Versatile Hyaluronic Acid Modified AQ4N-Cu(II)-Gossypol Infinite Coordination Polymer Nanoparticles: Multiple Tumor Targeting, Highly Efficient Synergistic Chemotherapy, and Real-Time Self-Monitoring. Biomaterials 2018, 154, 197–212. [Google Scholar] [CrossRef]
- Adarsh, N.N.; Frias, C.; Ponnoth Lohidakshan, T.M.; Lorenzo, J.; Novio, F.; Garcia-Pardo, J.; Ruiz-Molina, D. Pt(IV)-based nanoscale coordination polymers: Antitumor activity, cellular uptake and interactions with nuclear DNA. Chem. Eng. J. 2018, 340, 94–102. [Google Scholar] [CrossRef]
- Poon, C.; He, C.; Liu, D.; Lu, K.; Lin, W. Self-Assembled Nanoscale Coordination Polymers Carrying Oxaliplatin and Gemcitabine for Synergistic Combination Therapy of Pancreatic Cancer. J. Control. Release 2015, 201, 90–99. [Google Scholar] [CrossRef]
- Poon, C.; Duan, X.; Chan, C.; Han, W.; Lin, W. Nanoscale Coordination Polymers Codeliver Carboplatin and Gemcitabine for Highly Effective Treatment of Platinum-Resistant Ovarian Cancer. Mol. Pharm. 2016, 13, 3665–3675. [Google Scholar] [CrossRef]
- Li, J.; Murakami, T.; Higuchi, M. Metallo-Supramolecular Polymers: Versatile DNA Binding and Their Cytotoxicity. J. Inorg. Organomet. Polym. Mater. 2013, 23, 119–125. [Google Scholar] [CrossRef]
- Lakshmipraba, J.; Arunachalam, S.; Riyasdeen, A.; Dhivya, R.; Vignesh, S.; Akbarsha, M.A.; James, R.A. DNA/RNA Binding and Anticancer/Antimicrobial Activities of Polymer-Copper(II) Complexes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 109, 23–31. [Google Scholar] [CrossRef]
- Nehru, S.; Arunachalam, S.; Arun, R.; Premkumar, K. Polymer-Cobalt(III) Complexes: Structural Analysis of Metal Chelates on DNA Interaction and Comparative Cytotoxic Activity. J. Biomol. Struct. Dyn. 2014, 32, 1876–1888. [Google Scholar] [CrossRef]
- Raja, D.S.; Bhuvanesh, N.S.P.; Natarajan, K. Novel Water Soluble Ligand Bridged Cobalt(II) Coordination Polymer of 2-Oxo-1,2-Dihydroquinoline-3-Carbaldehyde (Isonicotinic) Hydrazone: Evaluation of the DNA Binding, Protein Interaction, Radical Scavenging and Anticancer Activity. Dalt. Trans. 2012, 41, 4365–4377. [Google Scholar] [CrossRef]
- Shu, D.; Chen, W. Synthesis, Structure, and in Vitro Anti-Lung Cancer Activity on an In-Based Nanoscale Coordination Polymer. Main Group Met. Chem. 2018, 41, 129–133. [Google Scholar] [CrossRef]
- Bai, L.; Song, F.; Wang, X.H.; Cao, J.Y.Q.; Han, X.; Wang, X.L.; Wang, Y.Z. Ligand-Metal-Drug Coordination Based Micelles for Efficient Intracellular Doxorubicin Delivery. RSC Adv. 2015, 5, 47629–47639. [Google Scholar] [CrossRef]
- Han, K.; Zhang, W.Y.; Zhang, J.; Ma, Z.Y.; Han, H.Y. pH-Responsive Nanoscale Coordination Polymer for Efficient Drug Delivery and Real-Time Release Monitoring. Adv. Healthc. Mater. 2017, 6, 1–9. [Google Scholar] [CrossRef]
- Xu, S.; Liu, J.; Li, D.; Wang, L.; Guo, J.; Wang, C.; Chen, C. Fe-Salphen Complexes from Intracellular pH-Triggered Degradation Of Fe3O4@Salphen-InIII CPPs for Selectively Killing Cancer Cells. Biomaterials 2014, 35, 1676–1685. [Google Scholar] [CrossRef]
- Wang, T.; Liu, X.; Zhu, Y.; Cui, Z.D.; Yang, X.Z.; Pan, H.; Yeung, K.W.K.; Wu, S. Metal Ion Coordination Polymer-Capped pH-Triggered Drug Release System on Titania Nanotubes for Enhancing Self-Antibacterial Capability of Ti Implants. ACS Biomater. Sci. Eng. 2017, 3, 816–825. [Google Scholar] [CrossRef]
- Ju, Y.; Cui, J.; Sun, H.; Müllner, M.; Dai, Y.; Guo, J.; Bertleff-Zieschang, N.; Suma, T.; Richardson, J.J.; Caruso, F. Engineered Metal-Phenolic Capsules Show Tunable Targeted Delivery to Cancer Cells. Biomacromolecules 2016, 17, 2268–2276. [Google Scholar] [CrossRef]
- Zein, R.; Sharrouf, W.; Selting, K. Physical properties of nanoparticles that result in improved cancer targeting. J. Oncol. 2020, 5194780. [Google Scholar]
- Kang, H.; Rho, S.; Stiles, W.R.; Hu, S.; Baek, Y.; Hwang, D.W.; Kashiwagi, S.; Kim, M.S.; Choi, H.S. Size-dependent EPR effect of polymeric nanoparticles on Tumor targeting. Adv. Healthc. Mater. 2020, 9, 1901223. [Google Scholar] [CrossRef]
- Thanou, M.; Duncan, R. Polymer-protein and polymer-drug conjugates in cancer therapy. Curr. Opin. Investig. Drugs 2003, 4, 701–709. [Google Scholar]
- Evans, W.E.; Relling, M.V. Moving towards individualized medicine with pharmacogenomics. Nature 2004, 429, 464–468. [Google Scholar] [CrossRef]
- Jhonstone, T.C.; Suntharalingam, K.; Lippard, S.J. The next generation of platinum drugs: Targeted Pt(II) agents, nanoparticles delivary, and Pt(IV) prodrugs. Chem. Rev. 2016, 116, 3436–3486. [Google Scholar] [CrossRef]
- Jeong, Y.H.; Shin, H.W.; Kwon, J.Y.; Lee, S.M. Cisplatin-Encapsulated Polymeric Nanoparticles with Molecular Geometry-Regulated Colloidal Properties and Controlled Drug Release. ACS Appl. Mater. Interfaces 2018, 10, 23617–23629. [Google Scholar] [CrossRef]
- Avan, A.; Postma, T.J.; Ceresa, C.; Avan, A.; Cavaletti, G.; Giovannetti, E.; Peters, G.J. Platinum-Induced Neurotoxicity and Preventive Strategies: Past, Present, and Future. Oncologist 2015, 20, 411–432. [Google Scholar] [CrossRef]
- Huxford-Phillips, R.C.; Russell, S.R.; Liu, D.; Lin, W. Lipid-Coated Nanoscale Coordination Polymers for Targeted Cisplatin Delivery. RSC Adv. 2013, 3, 14438–14443. [Google Scholar] [CrossRef]
- Liu, D.; Poon, C.; Lu, K.; He, C.; Lin, W. Self-Assembled Nanoscale Coordination Polymers with Trigger Release Properties for Effective Anticancer Therapy. Nat. Commun. 2014, 5, 4182. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Poon, C.; Chan, C.; Yamada, S.D.; Lin, W. Nanoscale Coordination Polymers Codeliver Chemotherapeutics and siRNAs to Eradicate Tumors of Cisplatin-Resistant Ovarian Cancer. J. Am. Chem. Soc. 2016, 138, 6010–6019. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Liu, D.; Lin, W. Self-Assembled Nanoscale Coordination Polymers Carrying SiRNAs and Cisplatin for Effective Treatment of Resistant Ovarian Cancer. Biomaterials 2015, 36, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Lv, T.; Ma, Y.; Xu, J.; Zhang, Y.; Hou, Y.; Huang, Z.; Ding, Y. Nanoscale Coordination Polymers for Synergistic NO and Chemodynamic Therapy of Liver Cancer. Nano Lett. 2019, 19, 2731–2738. [Google Scholar] [CrossRef]
- Chan, C.; Guo, N.; Duan, X.; Han, W.; Xue, L.; Bryan, D.; Wightman, S.C.; Khodarev, N.N.; Weichselbaum, R.R.; Lin, W. Systemic MiRNA Delivery by Nontoxic Nanoscale Coordination Polymers Limits Epithelial-to-Mesenchymal Transition and Suppresses Liver Metastases of Colorectal Cancer. Biomaterials 2019, 210, 94–104. [Google Scholar] [CrossRef]
- He, Y.; Huang, Y.; Huang, Z.; Jiang, Y.; Sun, X.; Shen, Y.; Chu, W.; Zhao, C. Bisphosphonate-Functionalized Coordination Polymer Nanoparticles for the Treatment of Bone Metastatic Breast Cancer. J. Control Release 2017, 264, 76–88. [Google Scholar] [CrossRef]
- Velpurisiva, P.; Gad, A.; Piel, B.; Jadia, R.; Rai, P. Nanoparticle Design Strategies for Effective Cancer Immunotherapy. J. Biomed. 2017, 2, 64–77. [Google Scholar] [CrossRef]
- Duan, X.; Chan, C.; Han, W.; Guo, N.; Weichselbaum, R.R.; Lin, W. Immunostimulatory Nanomedicines Synergize with Checkpoint Blockade Immunotherapy to Eradicate Colorectal Tumors. Nat. Commun. 2019, 10, 1899. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Y.; Jiang, N.; Zhao, X.; Sang, X.; Yang, N.; Feng, Y.; Chen, R.; Chen, Q. Dihydroartemisinin regulates the immune system by promotion of CD8+ T lymphocytes and suppression of B cell responses. Sci. China Life Sci. 2020, 63, 737–749. [Google Scholar] [CrossRef]
- Kim, S.H.; Kang, S.H.; Kang, B.S. Therapeutic effects of dihydroartemisinin and transferrin against glioblastoma. Nutr. Res. Pract. 2016, 10, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Wang, F.; Wang, H. Immunomodulation of artemisinin and its derivatives. Sci. Bull. 2016, 61, 1399–1406. [Google Scholar] [CrossRef]
- Imaz, I.; Hernando, J.; Ruiz-Molina, D.; Maspoch, D. Metal-Organic Spheres as Functional Systems for Guest Encapsulation. Angew. Chem. Int. Ed. 2009, 48, 2325–2329. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Wang, D.A. Novel Gelatin-Based Nano-Gels with Coordination-Induced Drug Loading for Intracellular Delivery. J. Mater. Sci. Technol. 2016, 32, 840–844. [Google Scholar] [CrossRef]
- Rezaei, M.; Abbasi, A.; Dinarvand, R.; Jeddi-Tehrani, M.; Janczak, J. Design and Synthesis of a Biocompatible 1D Coordination Polymer as Anti-Breast Cancer Drug Carrier, 5-Fu: In Vitro and in Vivo Studies. ACS Appl Mater. Interfaces 2018, 10, 17594–17604. [Google Scholar] [CrossRef]
- Alvarez, E.; Marquez, A.G.; Devic, T.; Steunou, N.; Serre, C.; Bonhomme, C.; Gervais, C.; Izquierdo-Barba, I.; Vallet-Regi, M.; Laurencin, D.; et al. A Biocompatible Calcium Bisphosphonate Coordination Polymer: Towards a Metal-Linker Synergistic Therapeutic Effect? CrystEngComm 2013, 15, 9899–9905. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, F.; Wang, K.; Luo, P.; Wei, Y.; Liu, S. Activatable Fluorescence Imaging and Targeted Drug Delivery via Extracellular Vesicle-Like Porous Coordination Polymer Nanoparticles. Anal. Chem. 2019, 91, 14036–14042. [Google Scholar] [CrossRef]
- Gao, P.F.; Zheng, L.L.; Liang, L.J.; Yang, X.X.; Li, Y.F.; Huang, C.Z. A New Type of pH-Responsive Coordination Polymer Sphere as a Vehicle for Targeted Anticancer Drug Delivery and Sustained Release. J. Mater. Chem. B 2013, 1, 3202–3208. [Google Scholar] [CrossRef]
- Huxford, R.C.; Dekrafft, K.E.; Boyle, W.S.; Liu, D.; Lin, W. Lipid-Coated Nanoscale Coordination Polymers for Targeted Delivery of Antifolates to Cancer Cells. Chem. Sci. 2012, 3, 198–204. [Google Scholar] [CrossRef]
- He, C.; Liu, D.; Lin, W. Self-Assembled Core-Shell Nanoparticles for Combined Chemotherapy and Photodynamic Therapy of Resistant Head and Neck Cancers. ACS Nano 2015, 9, 991–1003. [Google Scholar] [CrossRef]
- He, C.; Duan, X.; Guo, N.; Chan, C.; Poon, C.; Weichselbaum, R.R.; Lin, W. Core-Shell Nanoscale Coordination Polymers Combine Chemotherapy and Photodynamic Therapy to Potentiate Checkpoint Blockade Cancer Immunotherapy. Nat. Commun. 2016, 7, 1–12. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, M.; Cai, Z.; Liao, N.; Ke, K.; Liu, H.; Li, M.; Liu, G.; Yang, H.; Liu, X.; et al. Chemotherapeutic Drug Based Metal–Organic Particles for Microvesicle-Mediated Deep Penetration and Programmable pH/NIR/Hypoxia Activated Cancer Photochemotherapy. Adv. Sci. 2018, 5, 1700648. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; He, C.; Lin, W. A Chlorin-Based Nanoscale Metal-Organic Framework for Photodynamic Therapy of Colon Cancers. J. Am. Chem. Soc. 2015, 137, 7600–7603. [Google Scholar] [CrossRef]
- Guan, Q.; Li, Y.A.; Li, W.Y.; Dong, Y.B. Photodynamic Therapy Based on Nanoscale Metal–Organic Frameworks: From Material Design to Cancer Nanotherapeutics. Chem. Asian J. 2018, 13, 3122–3149. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic Therapy of Cancer: An Update. CA. Cancer J. Clin. 2017, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Ash, C.; Dubec, M.; Donne, K.; Bashford, T. Effect of wavelength and beam width on penetration in light-tissue interaction using computational methods Lasers. Med. Sci. 2017, 32, 1909–1918. [Google Scholar]
- Sandell, J.L.; Zhu, T.C. A review of in-vivo optical properties of human tissues and its impact on PDT. J. Biophotonics. 2011, 4, 773–787. [Google Scholar] [CrossRef] [PubMed]
- Mallidi, S.; Anbil, S.; Bulin, A.L.; Obaid, G.; Ichikawa, M.; Hasan, T. Beyond the Barriers of Light Penetration: Strategies, Perspectives and Possibilities for Photodynamic Therapy. Theranostics 2016, 6, 2458–2487. [Google Scholar] [CrossRef]
- Huang, Z.; Huang, L.; Huang, Y.; He, Y.; Sun, X.; Fu, X.; Xu, X.; Wei, G.; Chen, D.; Zhao, C. Phthalocyanine-Based Coordination Polymer Nanoparticles for Enhanced Photodynamic Therapy. Nanoscale 2017, 9, 15883–15894. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, W.; Feng, L.; Chao, Y.; Yi, X.; Dong, Z.; Yang, K.; Tan, W.; Liu, Z.; Chen, M. G-Quadruplex-Based Nanoscale Coordination Polymers to Modulate Tumor Hypoxia and Achieve Nuclear-Targeted Drug Delivery for Enhanced Photodynamic Therapy. Nano Lett. 2018, 18, 6867–6875. [Google Scholar] [CrossRef]
- Liu, J.; Tian, L.; Zhang, R.; Dong, Z.; Wang, H.; Liu, Z. Collagenase-Encapsulated pH-Responsive Nanoscale Coordination Polymers for Tumor Microenvironment Modulation and Enhanced Photodynamic Nanomedicine. ACS Appl. Mater. Interfaces 2018, 10, 43493–43502. [Google Scholar] [CrossRef]
- Calabrese, C.M.; Merkel, T.J.; Briley, W.E.; Randeria, P.S.; Narayan, S.P.; Rouge, J.L.; Walker, D.A.; Scott, A.W.; Mirkin, C.A. Biocompatible Infinite-Coordination-Polymer Nanoparticle-Nucleic-Acid Conjugates for Antisense Gene Regulation. Angew. Chem. Int. Ed. 2015, 54, 476–480. [Google Scholar] [CrossRef]
- Chao, Y.; Liang, C.; Yang, Y.; Wang, G.; Maiti, D.; Tian, L.; Wang, F.; Pan, W.; Wu, S.; Yang, K.; et al. Highly Effective Radioisotope Cancer Therapy With a Non-Therapeutic Isotope Delivered and Sensitized by Nanoscale Coordination Polymers. ACS Nano 2018, 12, 7519–7528. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liang, J.; Zhang, C.; Ma, G.; Wang, C.; Kong, D. Coordination Microparticle Vaccines Engineered from Tumor Cell Templates. Chem. Commun. 2019, 55, 1568–1571. [Google Scholar] [CrossRef]
- Yang, Q.; Zhou, Z.; Cui, L.; Yang, H.; Yan, C.; Zhou, X.; Yang, S.; Pan, L.; Zhang, X. Coordination Polymer Hybridized Au Nanocages: A Nanoplatform for Dual-Modality Imaging Guided near-Infrared Driven Photothermal Therapy in Vivo. J. Mater. Chem. B 2017, 5, 8761–8769. [Google Scholar]
- Li, M.; Wang, C.; Di, Z.; Li, H.; Zhang, J.; Xue, W.; Zhao, M.; Zhang, K.; Zhao, Y.; Li, L. Engineering Multifunctional DNA Hybrid Nanospheres through Coordination-Driven Self-Assembly. Angew. Chem. Int. Ed. 2019, 58, 1350–1354. [Google Scholar] [CrossRef]
- Wei, Y.; Xia, H.; Zhang, F.; Wang, K.; Luo, P.; Wu, Y.; Liu, S. Theranostic Nanoprobe Mediated Simultaneous Monitoring and Inhibition of P-Glycoprotein Potentiating Multidrug-Resistant Cancer Therapy. Anal. Chem. 2019, 91, 11200–11208. [Google Scholar] [CrossRef]
- Liu, S.; Pan, J.; Liu, J.; Ma, Y.; Qiu, F.; Mei, L.; Zeng, X.; Pan, G. Dynamically PEGylated and Borate-Coordination-Polymer-Coated Polydopamine Nanoparticles for Synergetic Tumor-Targeted, Chemo-Photothermal Combination Therapy. Small 2018, 14, 1703968. [Google Scholar] [CrossRef]

© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novio, F. Design of Targeted Nanostructured Coordination Polymers (NCPs) for Cancer Therapy. Molecules 2020, 25, 3449. https://doi.org/10.3390/molecules25153449
Novio F. Design of Targeted Nanostructured Coordination Polymers (NCPs) for Cancer Therapy. Molecules. 2020; 25(15):3449. https://doi.org/10.3390/molecules25153449
Chicago/Turabian StyleNovio, Fernando. 2020. "Design of Targeted Nanostructured Coordination Polymers (NCPs) for Cancer Therapy" Molecules 25, no. 15: 3449. https://doi.org/10.3390/molecules25153449
APA StyleNovio, F. (2020). Design of Targeted Nanostructured Coordination Polymers (NCPs) for Cancer Therapy. Molecules, 25(15), 3449. https://doi.org/10.3390/molecules25153449




