Tuning Surface Morphology of Fluorescent Hydrogels Using a Vortex Fluidic Device
Abstract
1. Introduction
2. Hydrogel Constructs under Thin Film Formation
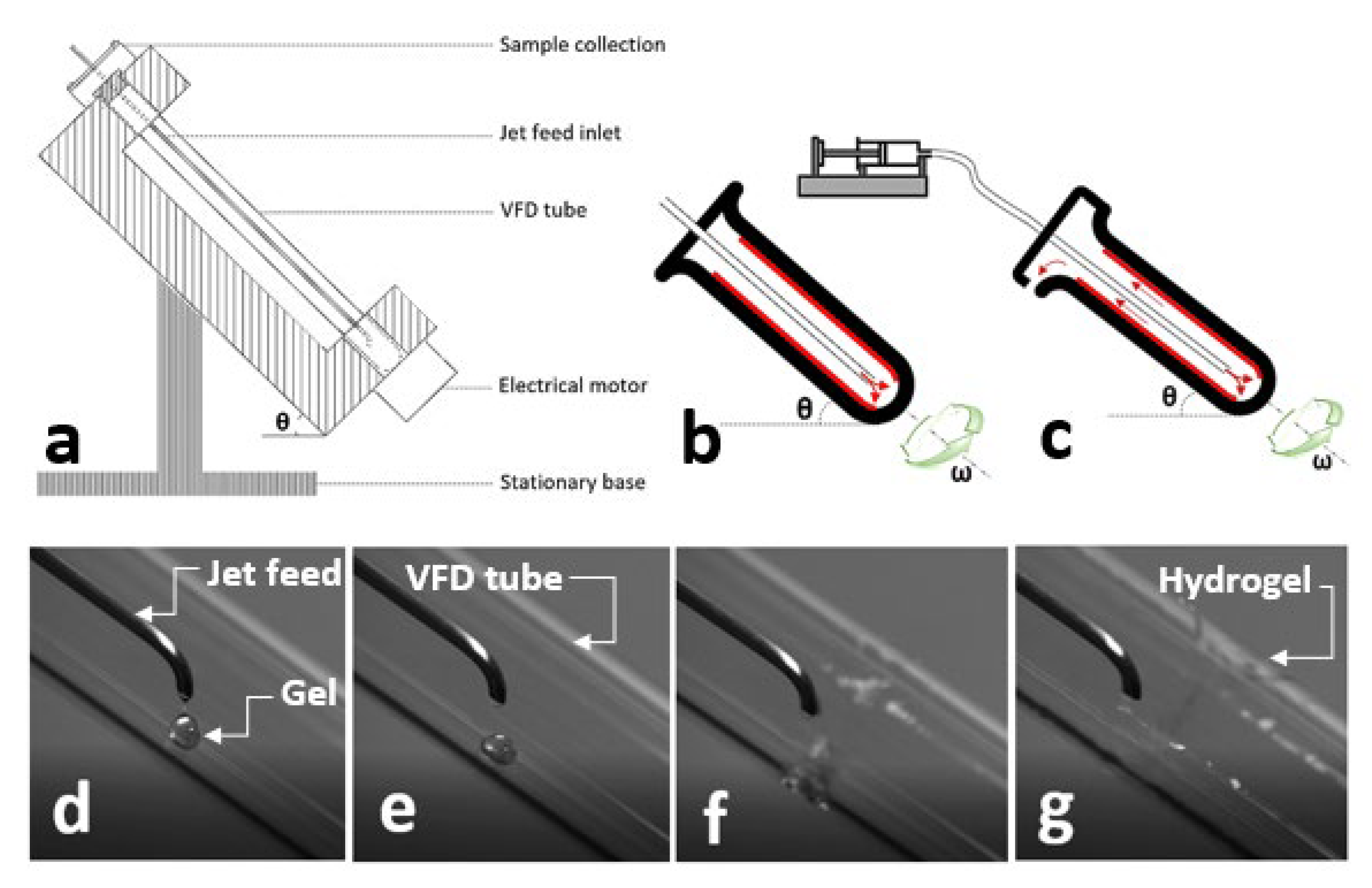
2.1. Vortex Fluidic Device (VFD)
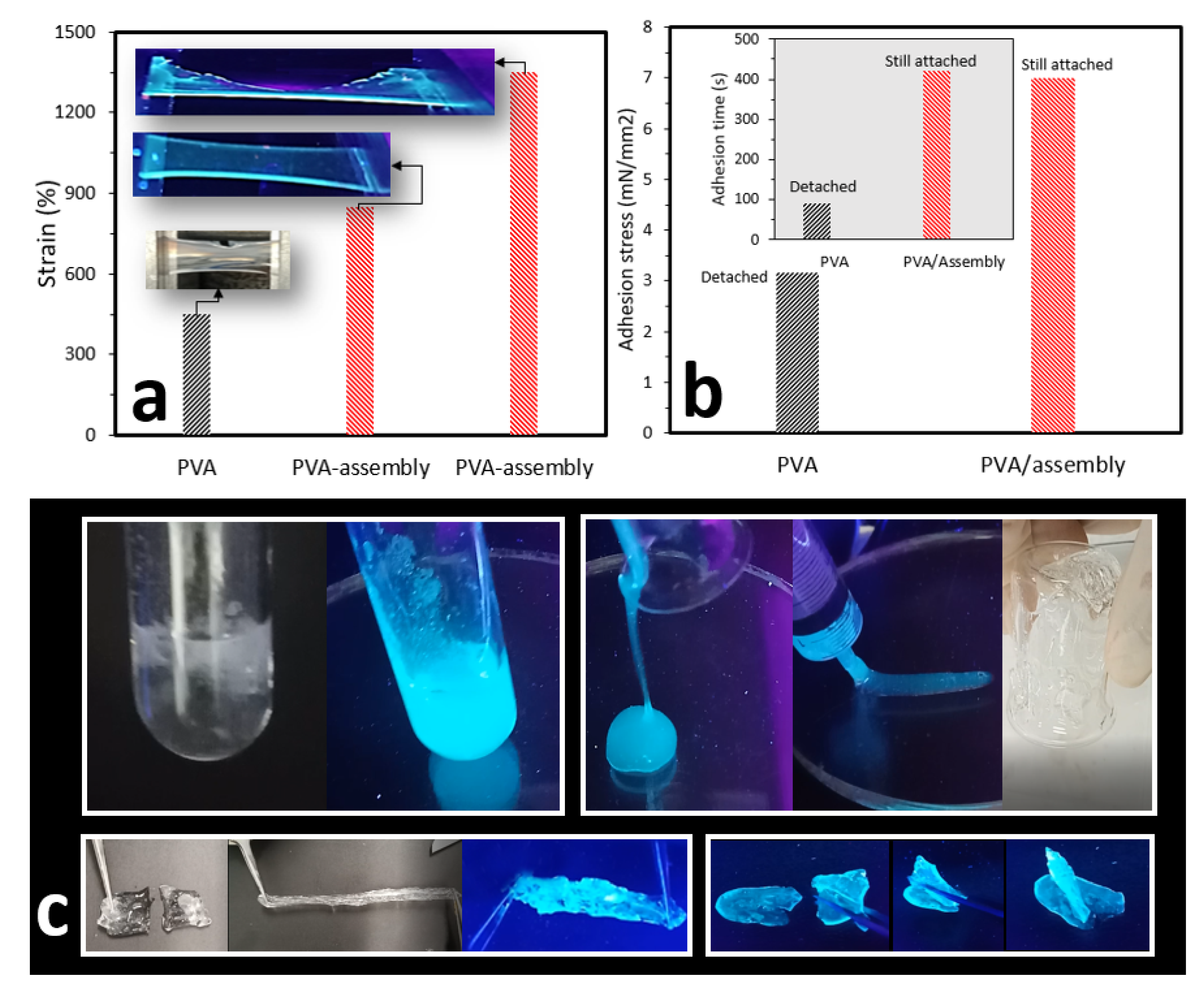
2.2. Bulk Hydrogels
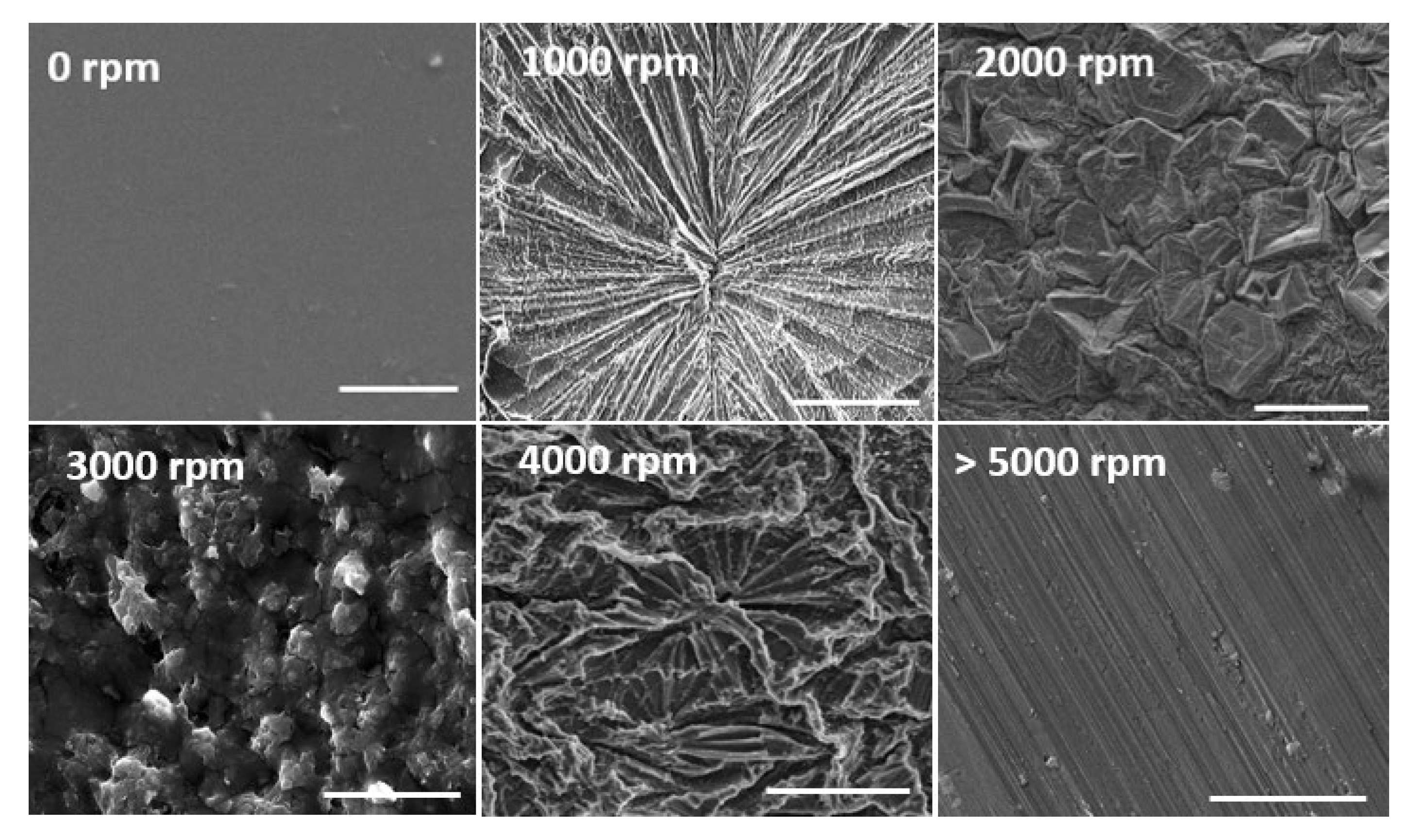
2.3. Surface Morphology
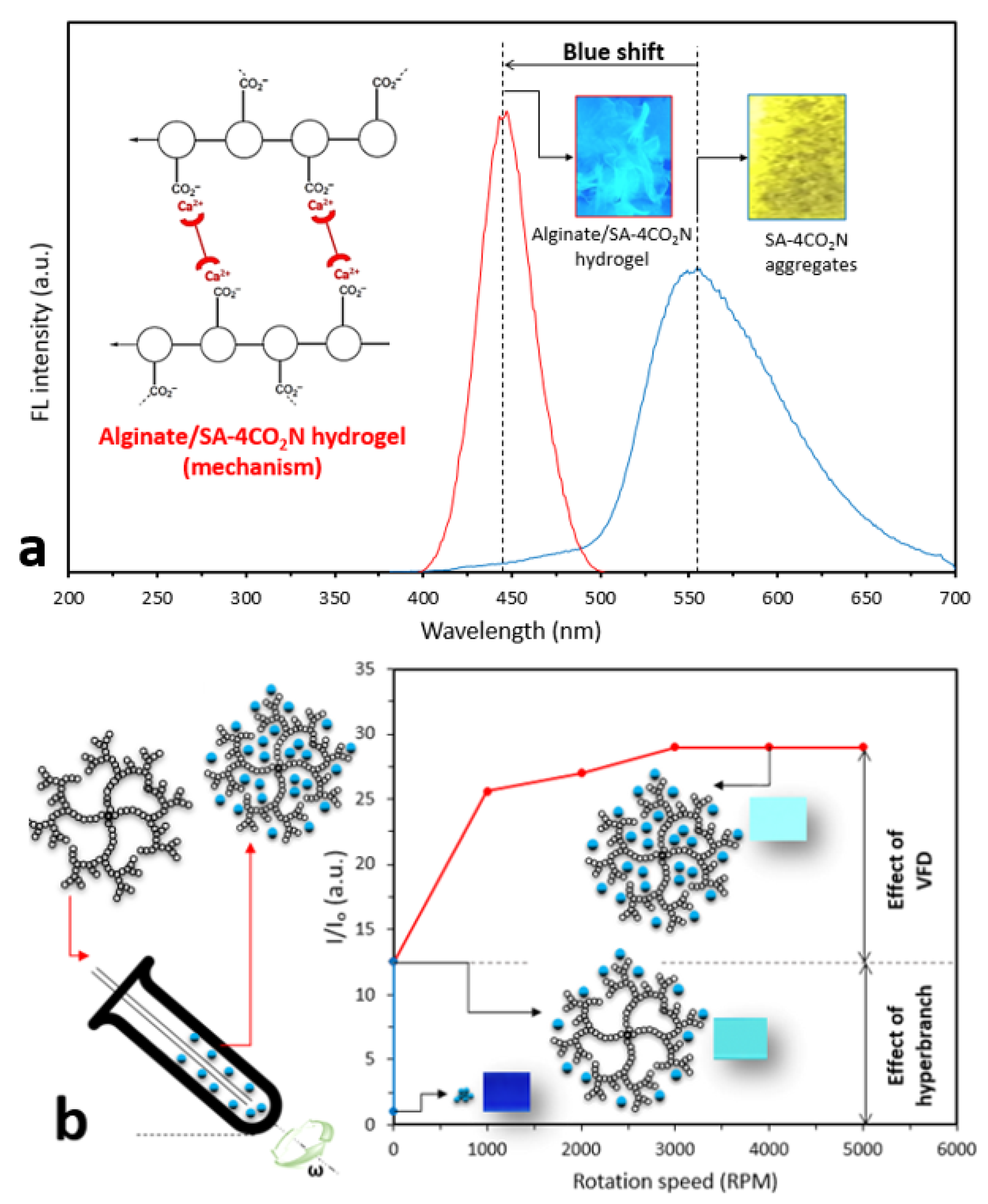
2.4. Fluorescent Hydrogels
3. Future Outlook
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Narayanaswamy, R.; Torchilin, V.P. Hydrogels and their applications in targeted drug delivery. Molecules 2019, 24, 603. [Google Scholar] [CrossRef]
- Auriemma, G.; Russo, P.; Del Gaudio, P.; García-González, C.A.; Landín, M.; Aquino, R.P. Technologies and formulation design of polysaccharide-based hydrogels for drug delivery. Molecules 2020, 25, 3156. [Google Scholar] [CrossRef]
- Fennell, E.; Huyghe, J.M. Chemically responsive hydrogel deformation mechanics: A review. Molecules 2019, 24, 3521. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Wang, Z.; Xiao, Y.; Zhang, S.; Wang, J. Advances in crosslinking strategies of biomedical hydrogels. Biomater. Sci. 2019, 7, 843–855. [Google Scholar] [CrossRef] [PubMed]
- Parhi, R. Cross-linked hydrogel for pharmaceutical applications: A review. Adv. Pharm. Bull. 2017, 7, 515–530. [Google Scholar] [CrossRef]
- Bayer, I.S. Hyaluronic acid and controlled release: A review. Molecules 2020, 25, 2649. [Google Scholar] [CrossRef]
- Huang, H.; Chauhan, S.; Geng, J.; Qin, Y.; Watson, D.F.; Lovell, J.F. Implantable tin porphyrin-PEG hydrogels with pH-responsive fluorescence. Biomacromolecules 2017, 18, 562–567. [Google Scholar] [CrossRef]
- Rizwan, M.; Yahya, R.; Hassan, A.; Yar, M.; Azzahari, A.D.; Selvanathan, V.; Sonsudin, F.; Abouloula, C.N. pH sensitive hydrogels in drug delivery: Brief history, properties, swelling, and release mechanism, material selection and applications. Polymers 2017, 9, 137. [Google Scholar] [CrossRef]
- Rasool, A.; Ata, S.; Islam, A. Stimuli responsive biopolymer (chitosan) based blend hydrogels for wound healing application. Carbohydr. Polym. 2019, 203, 423–429. [Google Scholar] [CrossRef]
- Choi, M.-Y.; Shin, Y.; Lee, H.S.; Kim, S.Y.; Na, J.-H. Multipolar spatial electric field modulation for freeform electroactive hydrogel actuation. Sci. Rep. 2020, 10, 1–8. [Google Scholar]
- Neves, M.I.; Araujo, M.; Moroni, L.; da Silva, R.M.P.; Barrias, C.C. Glycosaminoglycan-inspired biomaterials for the development of bioactive hydrogel networks. Molecules 2020, 25, 978. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.J.; Li, C.Y.; Zhao, X.Y.; Wu, Z.L.; Zheng, Q. Thermo-and photo-responsive composite hydrogels with programmed deformations. J. Mater. Chem. B 2019, 7, 1674–1678. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Han, G.; Wang, Q.; Zhang, S.; You, R.; Luo, Z.; Xu, A.; Li, X.; Li, M.; Zhang, Q. Directed assembly of robust and biocompatible silk fibroin/hyaluronic acid composite hydrogels. Compos. Part B 2019, 176, 107204. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Li, A.; Ye, Y.; Peng, S.; Deng, M.; Jiang, B. A novel pH- and salt-responsive n-succinyl-chitosan hydrogel via a one-step hydrothermal process. Molecules 2019, 24, 4211. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Chi-Leung Hui, P. Review of stimuli-responsive polymers in drug delivery and textile application. Molecules 2019, 24, 2547. [Google Scholar] [CrossRef]
- Garcia, A.; Kurbasic, M.; Kralj, S.; Melchionna, M.; Marchesan, S. A biocatalytic and thermoreversible hydrogel from a histidine-containing tripeptide. Chem. Commun. 2017, 53, 8110–8113. [Google Scholar] [CrossRef]
- Ding, L.; Cui, X.; Jiang, R.; Zhou, K.; Wen, Y.; Wang, C.; Yue, Z.; Shen, S.; Pan, X. Design, synthesis and characterization of a novel type of thermo-responsible phospholipid microcapsule-alginate composite hydrogel for drug delivery. Molecules 2020, 25, 694. [Google Scholar] [CrossRef]
- Chuah, C.; Wang, J.; Tavakoli, J.; Tang, Y. Novel bacterial cellulose-poly (acrylic acid) hybrid hydrogels with controllable antimicrobial ability as dressings for chronic wounds. Polymers 2018, 10, 1323. [Google Scholar] [CrossRef]
- Guo, Z.; Xia, J.; Mi, S.; Sun, W. Mussel-inspired naturally derived double-network hydrogels and their application in 3D printing: From soft, injectable bioadhesives to mechanically strong hydrogels. ACS Biomater. Sci. Eng. 2020, 6, 1798–1808. [Google Scholar] [CrossRef]
- Erol, O.; Pantula, A.; Liu, W.; Gracias, D.H. Transformer hydrogels: A review. Adv. Mater. Technol. 2019, 4, 1900043. [Google Scholar] [CrossRef]
- Dehbari, N.; Tavakoli, J.; Zhao, J.; Tang, Y. In situ formed internal water channels improving water swelling and mechanical properties of water swellable rubber composites. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Marques, M.P.; Fernandes, P. Microfluidic devices: Useful tools for bioprocess intensification. Molecules 2011, 16, 8368–8401. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Biswas, S.K.; Yano, H.; Abe, K. Fabrication of ultrastiff and strong hydrogels by in situ polymerization in layered cellulose nanofibers. Cellulose 2020, 27, 693–702. [Google Scholar] [CrossRef]
- Li, Y.; Motschman, J.D.; Kelly, S.T.; Yellen, B.B. Injection molded microfluidics for establishing high-density single cell arrays in an open hydrogel format. Anal. Chem. 2020, 92, 2794–2801. [Google Scholar] [CrossRef] [PubMed]
- Meier, Y.A.; Zhang, K.; Spencer, N.D.; Simic, R. Linking friction and surface properties of hydrogels molded against materials of different surface energies. Langmuir 2019, 35, 15805–15812. [Google Scholar] [CrossRef]
- Hahn, M.S.; Miller, J.S.; West, J.L. Laser scanning lithography for surface micropatterning on hydrogels. Adv. Mater. 2005, 17, 2939–2942. [Google Scholar] [CrossRef]
- Howell, S.T.; Grushina, A.; Holzner, F.; Brugger, J. Thermal scanning probe lithography—A review. Microsyst. Nanoeng. 2020, 6, 1–24. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, Y.; Ni, C.; Ma, C.; Yin, J.; Bai, H.; Luo, Y.; Huang, F.; Xie, T.; Zhao, Q. Drilling by light: Ice-templated photo-patterning enabled by a dynamically crosslinked hydrogel. Mater. Horiz. 2019, 6, 1013–1019. [Google Scholar] [CrossRef]
- Dobos, A.; Van Hoorick, J.; Steiger, W.; Gruber, P.; Markovic, M.; Andriotis, O.G.; Rohatschek, A.; Dubruel, P.; Thurner, P.J.; Van Vlierberghe, S. Thiol–gelatin–norbornene bioink for laser-based high-definition bioprinting. Adv. Healthc. Mater. 2019, 1900752. [Google Scholar] [CrossRef]
- Rasappa, S.; Hulkkonen, H.; Schulte, L.; Ndoni, S.; Reuna, J.; Salminen, T.; Niemi, T. High molecular weight block copolymer lithography for nanofabrication of hard mask and photonic nanostructures. J. Colloid Interface Sci. 2019, 534, 420–429. [Google Scholar] [CrossRef]
- Pavel, E.; Prodan, G.; Marinescu, V.; Trusca, R. Recent advances in 3-to 10-nm quantum optical lithography. J. Micro/Nanolithogr. MEMS MOEMS 2019, 18, 020501. [Google Scholar] [CrossRef]
- Cesaria, M.; Taurino, A.; Manera, M.G.; Minunni, M.; Scarano, S.; Rella, R. Gold nanoholes fabricated by colloidal lithography: Novel insights into nanofabrication, short-range correlation and optical properties. Nanoscale 2019, 11, 8416–8432. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Zhao, D.; Liu, D.; Ma, B.; Yao, G.; Li, Q.; Han, A.; Qiu, M. Three-dimensional in situ electron-beam lithography using water ice. Nano Lett. 2018, 18, 5036–5041. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, X.; Zhao, Z.; Pu, M.; Gao, P.; Luo, Y.; Jin, J.; Wang, C.; Luo, X. Batch fabrication of metasurface holograms enabled by plasmonic cavity lithography. Adv. Opt. Mater. 2017, 5, 1700429. [Google Scholar] [CrossRef]
- Hui, L.; Xu, A.; Liu, H. DNA-based nanofabrication for antifouling applications. Langmuir 2019, 35, 12543–12549. [Google Scholar] [CrossRef]
- Faustino, V.; Catarino, S.O.; Lima, R.; Minas, G. Biomedical microfluidic devices by using low-cost fabrication techniques: A review. J. Biomech. 2016, 49, 2280–2292. [Google Scholar] [CrossRef]
- Fox, K.E.; Tran, N.L.; Nguyen, T.A.; Nguyen, T.T.; Tran, P.A. Surface modification of medical devices at nanoscale—Recent development and translational perspectives. In Biomaterials in Translational Medicine; Elsevier: Cambridge, MA, USA, 2019; pp. 163–189. [Google Scholar]
- Ainur, S.; Florencio, P., Jr.; Naganand, R.; Heribert, H.; Nunes, S.P. Nanofabrication of isoporous membranes for cell fractionation. Sci. Rep. 2020, 10, 1–9. [Google Scholar]
- Kang, K.; Cho, Y.; Yu, K.J. Novel nano-materials and nano-fabrication techniques for flexible electronic systems. Micromachines 2018, 9, 263. [Google Scholar] [CrossRef]
- Kim, M.-G.; Brown, D.K.; Brand, O. Nanofabrication for all-soft and high-density electronic devices based on liquid metal. Nat. Commun. 2020, 11, 1–11. [Google Scholar]
- Yao, Y.; Zhang, L.; Orgiu, E.; Samorì, P. Unconventional nanofabrication for supramolecular electronics. Adv. Mater. 2019, 31, 1900599. [Google Scholar] [CrossRef]
- Rangelow, I.W.; Lenk, C.; Hofmann, M.; Ivanov, T.; Lenk, S.; Guliyev, E.; Kaestner, M.; Aydogan, C.; Bicer, M.; Alaca, B.E. Single nano-digit and closed-loop scanning probe lithography for manufacturing of electronic and optical nanodevices. In Proceedings of the Nanophotonics Australasia, Melbourne, Australia, 10 December 2017; p. 1045621. [Google Scholar]
- Ha, D.; Hong, J.; Shin, H.; Kim, T. Unconventional micro-/nanofabrication technologies for hybrid-scale lab-on-a-chip. Lab Chip 2016, 16, 4296–4312. [Google Scholar] [CrossRef] [PubMed]
- Scholten, K.; Meng, E. Electron-beam lithography for polymer bioMEMS with submicron features. Microsyst. Nanoeng. 2016, 2, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cong, Y.; Fu, J. Stretchable and tough conductive hydrogels for flexible pressure and strain sensors. J. Mater. Chem. B 2020, 8, 3437–3459. [Google Scholar] [CrossRef]
- Feng, B.; Deng, J.; Lu, B.; Xu, C.; Wang, Y.; Wan, J.; Chen, Y. Nanofabrication of silicon nanowires with high aspect ratio for photo-electron sensing. Microelectron. Eng. 2018, 195, 139–144. [Google Scholar] [CrossRef]
- Jiang, Y.; Tang, N.; Zhou, C.; Han, Z.; Qu, H.; Duan, X. A chemiresistive sensor array from conductive polymer nanowires fabricated by nanoscale soft lithography. Nanoscale 2018, 10, 20578–20586. [Google Scholar] [PubMed]
- Chen, C.-Y.; Wang, C.-M.; Liao, W.-S. A special connection between nanofabrication and analytical devices: Chemical lift-off lithography. Bull. Chem. Soc. Jpn. 2019, 92, 600–607. [Google Scholar] [CrossRef]
- Vanderpoorten, O.; Peter, Q.; Challa, P.K.; Keyser, U.F.; Baumberg, J.; Kaminski, C.F.; Knowles, T.P. Scalable integration of nano-, and microfluidics with hybrid two-photon lithography. Microsyst. Nanoeng. 2019, 5, 1–9. [Google Scholar]
- Raoufi, M.A.; Bazaz, S.R.; Niazmand, H.; Rouhi, O.; Asadnia, M.; Razmjou, A.; Warkiani, M.E. Fabrication of unconventional inertial microfluidic channels using wax 3D printing. Soft Matter 2020, 16, 2448–2459. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, T.; Zhang, Y.; Qu, G.; Wei, S.; Liu, Z.; Kong, T. Ultrastretchable and wireless bioelectronics based on all-hydrogel microfluidics. Adv. Mater. 2019, 31, 1902783. [Google Scholar] [CrossRef]
- Goy, C.B.; Chaile, R.E.; Madrid, R.E. Microfluidics and hydrogel: A powerful combination. React. Funct. Polym. 2019, 145, 104314. [Google Scholar] [CrossRef]
- Nie, J.; Gao, Q.; Wang, Y.; Zeng, J.; Zhao, H.; Sun, Y.; Shen, J.; Ramezani, H.; Fu, Z.; Liu, Z. Vessel-on-a-chip with hydrogel-based microfluidics. Small 2018, 14, 1802368. [Google Scholar] [CrossRef] [PubMed]
- Na, W.; Nam, D.; Lee, H.; Shin, S. Rapid molecular diagnosis of infectious viruses in microfluidics using DNA hydrogel formation. Biosens. Bioelectron. 2018, 108, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; van Zee, M.; Goda, K.; Di Carlo, D. Size-based sorting of hydrogel droplets using inertial microfluidics. Lab Chip 2018, 18, 2575–2582. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Yang, C.J. Hydrogel droplet microfluidics for high-throughput single molecule/cell analysis. Acc. Chem. Res. 2017, 50, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Warkiani, M.E.; Khoo, B.L.; Wu, L.; Tay, A.K.P.; Bhagat, A.A.S.; Han, J.; Lim, C.T. Ultra-fast, label-free isolation of circulating tumor cells from blood using spiral microfluidics. Nat. Protoc. 2016, 11, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.; Prentice, H.; De Oliveira, J.; Madziva, N.; Warkiani, M.E.; Hamel, J.-F.P.; Han, J. Microfluidic cell retention device for perfusion of mammalian suspension culture. Sci. Rep. 2017, 7, 1–11. [Google Scholar]
- Zhang, J.; Yan, S.; Yuan, D.; Alici, G.; Nguyen, N.-T.; Warkiani, M.E.; Li, W. Fundamentals and applications of inertial microfluidics: A review. Lab Chip 2016, 16, 10–34. [Google Scholar] [CrossRef]
- Kulasinghe, A.; Tran, T.H.P.; Blick, T.; O’Byrne, K.; Thompson, E.W.; Warkiani, M.E.; Nelson, C.; Kenny, L.; Punyadeera, C. Enrichment of circulating head and neck tumour cells using spiral microfluidic technology. Sci. Rep. 2017, 7, 42517. [Google Scholar] [CrossRef]
- Moshksayan, K.; Kashaninejad, N.; Warkiani, M.E.; Lock, J.G.; Moghadas, H.; Firoozabadi, B.; Saidi, M.S.; Nguyen, N.-T. Spheroids-on-a-chip: Recent advances and design considerations in microfluidic platforms for spheroid formation and culture. Sens. Actuators B 2018, 263, 151–176. [Google Scholar] [CrossRef]
- Britton, J.; Stubbs, K.A.; Weiss, G.A.; Raston, C.L. Vortex fluidic chemical transformations. Chem. Europ. J. 2017, 23, 13270–13278. [Google Scholar] [CrossRef]
- Al-Antaki, A.H.M.; Luo, X.; Alharbi, T.M.; Harvey, D.P.; Pye, S.; Zou, J.; Lawrance, W.; Raston, C.L. Inverted vortex fluidic exfoliation and scrolling of hexagonal-boron nitride. RSC Adv. 2019, 9, 22074–22079. [Google Scholar] [CrossRef]
- He, S.; Joseph, N.; Luo, X.; Raston, C.L. Vortex fluidic mediated food processing. PLoS ONE 2019, 14, e0216816. [Google Scholar] [CrossRef] [PubMed]
- Sitepu, E.K.; Jones, D.B.; Zhang, Z.; Tang, Y.; Leterme, S.C.; Heimann, K.; Raston, C.L.; Zhang, W. Turbo thin film continuous flow production of biodiesel from fungal biomass. Bioresour. Technol. 2019, 273, 431–438. [Google Scholar] [CrossRef]
- Tavakoli, J.; Raston, C.L.; Ma, Y.; Tang, Y. Vortex fluidic mediated one-step fabrication of polyvinyl alcohol hydrogel films with tunable surface morphologies and enhanced self-healing properties. Sci. China Mater. 2020. [Google Scholar] [CrossRef]
- Tavakoli, J.; Joseph, N.; Raston, C.L.; Tang, Y. A hyper-branched polymer tunes the size and enhances the fluorescent properties of aggregation-induced emission nanoparticles. Nanoscale Adv. 2020, 2, 633–641. [Google Scholar] [CrossRef]
- Tong, C.L.; Stroeher, U.H.; Brown, M.H.; Raston, C.L. Continuous flow vortex fluidic synthesis of silica xerogel as a delivery vehicle for curcumin. RSC Adv. 2015, 5, 7953–7958. [Google Scholar] [CrossRef]
- Kumari, H.; Kline, S.R.; Kennedy, S.R.; Garvey, C.; Raston, C.L.; Atwood, J.L.; Steed, J.W. Manipulating three-dimensional gel network entanglement by thin film shearing. Chem. Commun. 2016, 52, 4513–4516. [Google Scholar] [CrossRef]
- Liu, C.; Lei, F.; Li, P.; Jiang, J.; Wang, K. Borax crosslinked fenugreek galactomannan hydrogel as potential water-retaining agent in agriculture. Carbohydr. Polym. 2020, 116100. [Google Scholar] [CrossRef]
- Takeno, H.; Inoguchi, H.; Hsieh, W.-C. Mechanical and structural properties of cellulose nanofiber/poly (vinyl alcohol) hydrogels cross-linked by a freezing/thawing method and borax. Cellulose 2020, 1–15. [Google Scholar] [CrossRef]
- Li, Y.; Young, D.J.; Loh, X.J. Fluorescent gels: A review of synthesis, properties, applications and challenges. Mater. Chem. Front. 2019, 3, 1489–1502. [Google Scholar] [CrossRef]
- Xu, H.-X.; Tan, Y.; Wang, D.; Wang, X.-L.; An, W.-L.; Xu, P.-P.; Xu, S.; Wang, Y.-Z. Autofluorescence of hydrogels without a fluorophore. Soft Matter 2019, 15, 3588–3594. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.; Parshi, N.; Prodhan, C.; Chaudhuri, K.; Ganguly, J. Characterization of a fluorescent hydrogel synthesized using chitosan, polyvinyl alcohol and 9-anthraldehyde for the selective detection and discrimination of trace Fe3+ and Fe2+ in water for live-cell imaging. Carbohydr. Polym. 2018, 193, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Cringoli, M.C.; Kralj, S.; Kurbasic, M.; Urban, M.; Marchesan, S. Luminescent supramolecular hydrogels from a tripeptide and nitrogen-doped carbon nanodots. Beilstein J. Nanotechnol. 2017, 8, 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Zhong, J.; Lu, C.; Duan, X. Hydroxyl-triggered fluorescence for location of inorganic materials in polymer-matrix composites. Chem. Sci. 2018, 9, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Hong, Y.; Lam, J.W.; Qin, A.; Tang, Y.; Tang, B.Z. Aggregation-induced emission: The whole is more brilliant than the parts. Adv. Mater. 2014, 26, 5429–5479. [Google Scholar] [CrossRef] [PubMed]
- Qin, A.; Jim, C.K.; Tang, Y.; Lam, J.W.; Liu, J.; Mahtab, F.; Gao, P.; Tang, B.Z. Aggregation-enhanced emissions of intramolecular excimers in disubstituted polyacetylenes. J. Phys. Chem. B 2008, 112, 9281–9288. [Google Scholar] [CrossRef]
- Li, Z.; Dong, Y.; Mi, B.; Tang, Y.; Häussler, M.; Tong, H.; Dong, Y.; Lam, J.W.; Ren, Y.; Sung, H.H. Structural control of the photoluminescence of silole regioisomers and their utility as sensitive regiodiscriminating chemosensors and efficient electroluminescent materials. J. Phys. Chem. B 2005, 109, 10061–10066. [Google Scholar] [CrossRef]
- Tavakoli, J.; Gascooke, J.; Xie, N.; Tang, B.Z.; Tang, Y. Enlightening freeze–thaw process of physically cross-linked poly (vinyl alcohol) hydrogels by aggregation-induced emission fluorogens. ACS Appl. Polym. Mater. 2019, 1, 1390–1398. [Google Scholar] [CrossRef]
- Tavakoli, J.; Laisak, E.; Gao, M.; Tang, Y. AIEgen quantitatively monitoring the release of Ca2+ during swelling and degradation process in alginate hydrogels. Mater. Sci. Eng. 2019, 104, 109951. [Google Scholar] [CrossRef]
- Tavakoli, J.; Zhang, H.-P.; Tang, B.Z.; Tang, Y. Aggregation-induced emission lights up the swelling process: A new technique for swelling characterisation of hydrogels. Mater Chem. Front. 2019, 3, 664–667. [Google Scholar] [CrossRef]
- Qin, A.; Lam, J.W.; Tang, B.Z. Luminogenic polymers with aggregation-induced emission characteristics. Prog. Polym. Sci. 2012, 37, 182–209. [Google Scholar] [CrossRef]
- Deng, H.; Hu, R.; Zhao, E.; Chan, C.Y.; Lam, J.W.; Tang, B.Z. One-pot three-component tandem polymerization toward functional poly (arylene thiophenylene) with aggregation-enhanced emission characteristics. Macromolecules 2014, 47, 4920–4929. [Google Scholar] [CrossRef]
- Qiu, Z.; Liu, X.; Lam, J.W.; Tang, B.Z. The marriage of aggregation-induced emission with polymer science. Macromol. Rapid Commun. 2019, 40, 1800568. [Google Scholar]
- Xia, Y.; Xue, B.; Qin, M.; Cao, Y.; Li, Y.; Wang, W. Printable fluorescent hydrogels based on self-assembling peptides. Sci. Rep. 2017, 7, 9691. [Google Scholar] [CrossRef]
- Zheng, W.; Yang, G.; Jiang, S.-T.; Shao, N.; Yin, G.-Q.; Xu, L.; Li, X.; Chen, G.; Yang, H.-B. A tetraphenylethylene (TPE)-based supra-amphiphilic organoplatinum (ii) metallacycle and its self-assembly behaviour. Mater. Chem. Front. 2017, 1, 1823–1828. [Google Scholar] [CrossRef]
- Sakurai, T.; Orito, N.; Nagano, S.; Kato, K.; Takata, M.; Seki, S. Electron-transporting foldable alternating copolymers of perylenediimide and flexible macromolecular chains. Mater. Chem. Front. 2018, 2, 718–729. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, P.; Wei, B.; Hu, R.; Tang, B.Z. Aggregation-induced emission-active hyperbranched poly (tetrahydropyrimidine) s synthesized from multicomponent tandem polymerization. Chin. J. Polym. Sci. 2019, 37, 428–436. [Google Scholar]
- Tavakoli, J.; Pye, S.; Reza, A.M.; Xie, N.; Qin, J.; Raston, C.L.; Tang, B.Z.; Tang, Y. Tuning aggregation-induced emission nanoparticle properties under thin film formation. Mater. Chem. Front. 2020, 4, 537–545. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavakoli, J.; Raston, C.L.; Tang, Y. Tuning Surface Morphology of Fluorescent Hydrogels Using a Vortex Fluidic Device. Molecules 2020, 25, 3445. https://doi.org/10.3390/molecules25153445
Tavakoli J, Raston CL, Tang Y. Tuning Surface Morphology of Fluorescent Hydrogels Using a Vortex Fluidic Device. Molecules. 2020; 25(15):3445. https://doi.org/10.3390/molecules25153445
Chicago/Turabian StyleTavakoli, Javad, Colin L. Raston, and Youhong Tang. 2020. "Tuning Surface Morphology of Fluorescent Hydrogels Using a Vortex Fluidic Device" Molecules 25, no. 15: 3445. https://doi.org/10.3390/molecules25153445
APA StyleTavakoli, J., Raston, C. L., & Tang, Y. (2020). Tuning Surface Morphology of Fluorescent Hydrogels Using a Vortex Fluidic Device. Molecules, 25(15), 3445. https://doi.org/10.3390/molecules25153445







