Chemical Composition and Attractant Activity of Volatiles from Rhus potaninii to The Spring Aphid Kaburagia rhusicola
Abstract
1. Introduction
2. Materials and Methods
2.1. Plants and Insects
2.2. Chemicals and Instruments
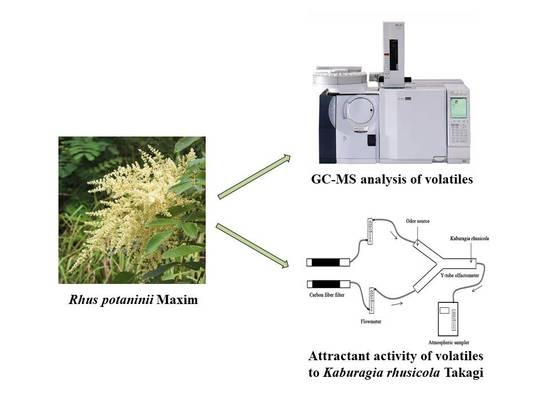
2.3. Collection of Volatiles
2.4. Attractant Activity
2.5. Chemical Composition Identification
3. Results and Discussion
3.1. Attractant Activity
3.2. Characterization of Volatiles
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Min, L.Y.; Longton, R.E. Mosses and the production of Chinese gallnuts. J. Bryol. 1993, 17, 421–430. [Google Scholar] [CrossRef]
- Ren, Z.M.; Su, X.; von Dohlen, C.D.; Wen, J. Nurudea zhengii Ren, A New Species of the Rhus Gall Aphids (Aphididae: Eriosomatinae: Fordini) from Eastern China. Pak. J. Zool. 2018, 50, 2087–2092. [Google Scholar] [CrossRef]
- Gao, J.; Yang, X.; Yin, W.; Li, M. Gallnuts: A Potential Treasure in Anticancer Drug Discovery. Evid. Based Complementary Altern. Med. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Liu, S.; Li, S.; Lin, G.; Markkinen, N.; Yang, H.; Zhu, B.; Zhang, B. Anthocyanin copigmentation and color attributes of bog bilberry syrup wine during bottle aging: Effect of tannic acid and gallic acid extracted from Chinese gallnut. J. Food Process. Pres. 2019, 43, e14041. [Google Scholar] [CrossRef]
- Han, J. Botanical provenance of historical Chinese dye plants. Econ. Bot. 2015, 69, 230–239. [Google Scholar] [CrossRef]
- Deveoglu, O.; Torgan, E.; Karadag, R. Identification by rp-hplc-dad of natural dyestuffs from lake pigments prepared with a mixture of weld and dyer’s oak dye plants. J. Liq. Chromatogr. Relat. Technol. 2012, 35, 331–342. [Google Scholar] [CrossRef]
- Yener, İ.; Ölmez, Ö.T.; Ertas, A.; Yilmaz, M.A.; Firat, M.; Kandemir, S.İ.; Öztürk, M.; Kolak, U.; Temel, H. A detailed study on chemical and biological profile of nine Euphorbia species from Turkey with chemometric approach: Remarkable cytotoxicity of E. fistulasa and promising tannic acid content of E. eriophora. Ind. Crop. Prod. 2018, 123, 442–453. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, Z.; Han, F.; Kang, X.; Gu, H.; Yang, L. Microwave-assisted method for simultaneous hydrolysis and extraction in obtaining ellagic acid, gallic acid and essential oil from Eucalyptus globulus leaves using Brönsted acidic ionic liquid [HO3S(CH2)4mim] HSO4. Ind. Crop. Prod. 2016, 81, 152–161. [Google Scholar] [CrossRef]
- Zhang, C.X.; Tang, X.D.; Cheng, J.A. The utilization and industrialization of insect resources in China. Entomol. Res. 2008, 38, S38–S47. [Google Scholar] [CrossRef]
- Zha, Y.P.; Zhang, Z.Y.; Chen, J.Y.; Yue, J.G.; Wu, B.; Song, D.Y. Phototaxis of the summer migrant Kaburagia rhusicola Takagi. Chin. J. Appl. Entomol. 2019, 56, 982–988. [Google Scholar]
- Ruan, Z.Y.; Chen, X.M.; Yang, P.; Wang, B.Y. Roles played by invertase and gene expression in the development of the horn-shaped gall on leaves of Rhus chinensis. Funct. Plant. Biol. 2017, 44, 1160–1170. [Google Scholar] [CrossRef]
- Wang, S.L.; Yang, Z.X.; Yang, P.; Zhang, C.X. De novo assembled transcriptome of horned gall aphid, Schlechtendalia chinensis Bell, suggest changes in functional gene expression during host alternation. Entomol. Res. 2016, 46, 314–323. [Google Scholar] [CrossRef]
- Tong, S.; Fu, M.; Cao, X.; Firempong, C.K.; Yi, C.; Zhen, Q.; Zhong, H.; Yu, J.; Xu, X. Lipid raft stationary phase chromatography for screening anti-tumor components from Galla chinensis. Chromatographia 2014, 77, 419–429. [Google Scholar] [CrossRef]
- Wu, J.; Jahncke, M.L.; Eifert, J.D.; O’Keefe, S.F.; Welbaum, G.E. Pomegranate peel (Punica granatum L.) extract and Chinese gall (Galla chinensis) extract inhibit Vibrio parahaemolyticus and Listeria monocytogenes on cooked shrimp and raw tuna. Food Control. 2016, 59, 695–699. [Google Scholar] [CrossRef]
- Tan, D.; Yan, Q.; Ma, X. Chemical constituents of Rhus chinensis. Chem. Nat. Compd. 2015, 51, 542–543. [Google Scholar] [CrossRef]
- Wool, D. Galling aphids: Specialization, biological complexity, and variation. Annu. Rev. Entomol. 2004, 49, 175–192. [Google Scholar] [CrossRef]
- Yang, Y.; Su, Q.; Shi, L.; Chen, G.; Zeng, Y.; Shi, C.; Zhang, Y. Electrophysiological and behavioral responses of Bradysia odoriphaga (Diptera: Sciaridae) to volatiles from its Host Plant, Chinese Chives (Allium tuberosum Rottler ex Spreng). J. Econ. Entomol. 2019, 112, 1638–1644. [Google Scholar] [CrossRef]
- Su, Q.; Zhou, Z.; Zhang, J.; Shi, C.; Zhang, G.; Jin, Z.; Wang, W.; Li, C. Effect of plant secondary metabolites on common cutworm, Spodoptera litura (Lepidoptera: Noctuidae). Entomol. Res. 2018, 48, 18–26. [Google Scholar] [CrossRef]
- Liu, X.F.; Chen, H.H.; Li, J.K.; Zhang, R.; Turlings, T.C.; Chen, L. Volatiles released by Chinese liquorice roots mediate host location behaviour by neonate Porphyrophora sophorae (Hemiptera: Margarodidae). Pest. Manag. Sci. 2016, 72, 1959–1964. [Google Scholar] [CrossRef]
- Cha, D.H.; Powell, T.H.; Feder, J.L.; Linn, C.E. Identification of a New Blend of Host Fruit Volatiles from Red Downy Hawthorn, Crataegus mollis, Attractive to Rhagoletis pomonella Flies from the Northeastern United States. J. Chem. Ecol. 2018, 44, 671–680. [Google Scholar] [CrossRef]
- Herrmann, A. The Chemistry and Biology of Volatiles; John Wiley & Sons, Ltd: Hoboken, NJ, USA, 2010. [Google Scholar] [CrossRef]
- Chen, L.; Ochieng, S.A.; He, X.; Fadamiro, H.Y. Comparing electroantennogram and behavioral responses of two Pseudacteon phorid fly species to body extracts of Black, Red and Hybrid imported fire ants, Solenopsis spp. J. Insect Physiol. 2012, 58, 1360–1367. [Google Scholar] [CrossRef]
- Xiu, C.; Zhang, W.; Xu, B.; Wyckhuys, K.A.; Cai, X.; Su, H.; Lu, Y. Volatiles from aphid-infested plants attract adults of the multicolored Asian lady beetle Harmonia axyridis. Biol. Control. 2019, 129, 1–11. [Google Scholar] [CrossRef]
- Visser, J.H.; Fu-shun, Y. Electroantennogram responses of the grain aphids Sitobion avenae (F.) and Metopolophium dirhodum (Walk.) (Hom., Aphididae) to plant odour components. J. Appl. Entomol. 1995, 119, 539–542. [Google Scholar] [CrossRef]
- Zhu, J.; Park, K.C. Methyl salicylate, a soybean aphid-induced plant volatile attractive to the predator Coccinella septempunctata. J. Chem. Ecol. 2005, 31, 1733–1746. [Google Scholar] [CrossRef]
- Visser, J.H.; Piron, P.G.M. Olfactory antennal responses to plant volatiles in apterous virginoparae of the vetch aphid Megoura viciae. Entomol. Exp. Appl. 1995, 77, 37–46. [Google Scholar] [CrossRef]
- Breer, H. Olfactory receptors: Molecular basis for recognition and discrimination of odors. Anal. Bioanal. Chem. 2003, 377, 427–433. [Google Scholar] [CrossRef]
- Fan, J.; Francis, F.; Liu, Y.; Chen, J.L.; Cheng, D.F. An overview of odorant-binding protein functions in insect peripheral olfactory reception. Genet. Mol. Res. 2011, 10, 3056–3069. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

| Treatment | Set 1 | Set 2 | Set 3 | Selection Rate (%) |
|---|---|---|---|---|
| Volatiles | 37 | 34 | 33 | 69.3 ± 2.4 a a |
| Solvent | 6 | 8 | 7 | 14.0 ± 1.2 b |
| No. | Retention Time (min) | Compound | Mol. Weight | Molecular Formula | Class of Compound | Relative Content (%) |
|---|---|---|---|---|---|---|
| 1 | 9.088 | Ethylbenzene | 106 | C8H10 | Arene | 1.75 ± 0.28 a |
| 2 | 9.292 | p-Xylene | 106 | C8H10 | Arene | 0.84 ± 0.10 |
| 3 | 12.915 | Styrene | 104 | C8H8 | Alkene | 1.26 ± 0.18 |
| 4 | 13.883 | Octanal | 128 | C8H16O | Aldehyde | 2.02 ± 0.12 |
| 5 | 16.798 | Tetradecane | 198 | C14H30 | Alkane | 0.26 ± 0.05 |
| 6 | 16.910 | Nonanal | 142 | C9H18O | Aldehyde | 1.14 ± 0.12 |
| 7 | 18.169 | 1-Ethyl-3-vinylbenzene | 132 | C10H12 | Alkene | 0.55 ± 0.10 |
| 8 | 18.461 | 1-Ethyl-4-vinylbenzene | 132 | C10H12 | Alkene | 0.99 ± 0.16 |
| 9 | 19.546 | Pentadecane | 212 | C15H32 | Alkane | 0.66 ± 0.15 |
| 10 | 19.627 | 2-Ethylhexan-1-ol | 130 | C8H18O | Alcohol | 3.35 ± 0.19 |
| 11 | 20.526 | Benzaldehyde | 106 | C7H6O | Aldehyde | 0.42 ± 0.03 |
| 12 | 20.663 | 1,2,3,4-Tetrahydronaphthalene | 132 | C10H12 | Arene | 0.35 ± 0.15 |
| 13 | 21.798 | Longifolene | 204 | C15H24 | Tricyclic sesquiterpenes | 2.53 ± 0.17 |
| 14 | 23.845 | Acetophenone | 120 | C8H8O | Ketone | 0.50 ± 0.04 |
| 15 | 25.265 | 3-Ethylbenzaldehyde | 134 | C9H10O | Aldehyde | 3.32 ± 0.28 |
| 16 | 26.003 | Benzaldehyde, 4-ethyl- | 134 | C9H10O | aldehyde | 2.07 ± 0.26 |
| 17 | 26.010 | Isophthalaldehyde | 134 | C8H6O2 | Aldehyde | 1.32 ± 0.27 |
| 18 | 26.078 | Naphthalene | 128 | C10H8 | Arene | 0.38 ± 0.04 |
| 19 | 28.181 | 1-(4-Ethylphenyl)ethanone | 148 | C10H12O | Ketone | 11.84 ± 1.79 |
| 20 | 28.937 | 4-Vinylbenzoic acid | 148 | C9H8O2 | Acid | 0.50 ± 0.11 |
| 21 | 29.167 | 5-Allyl-1,3-benzodioxole | 162 | C10H10O2 | Alkene | 0.34 ± 0.05 |
| 22 | 31.319 | p-Cymen-7-ol | 150 | C10H14O | Aromatic alcohol | 7.08 ± 1.23 |
| 23 | 31.698 | 1-(4-hydroxy-3-methylphenyl)ethanone | 150 | C9H10O2 | Ketone | 11.20 ± 1.39 |
| 24 | 36.040 | 2,3-dimethylphenol | 122 | C8H10O | Phenol | 0.29 ± 0.08 |
| 25 | 38.757 | 1,1′-(1,4-phenylene)diethanone | 162 | C10H10O2 | Ketone | 0.40 ± 0.06 |
| Total | 55.33 ± 4.64 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; Li, L.; Hsiang, T.; Zha, Y.; Zhou, Z.; Chen, R.; Wang, X.; Wu, Q.; Li, J. Chemical Composition and Attractant Activity of Volatiles from Rhus potaninii to The Spring Aphid Kaburagia rhusicola. Molecules 2020, 25, 3412. https://doi.org/10.3390/molecules25153412
Zhu X, Li L, Hsiang T, Zha Y, Zhou Z, Chen R, Wang X, Wu Q, Li J. Chemical Composition and Attractant Activity of Volatiles from Rhus potaninii to The Spring Aphid Kaburagia rhusicola. Molecules. 2020; 25(15):3412. https://doi.org/10.3390/molecules25153412
Chicago/Turabian StyleZhu, Xiang, Li Li, Tom Hsiang, Yuping Zha, Zhixiong Zhou, Ran Chen, Xian Wang, Qinglai Wu, and Junkai Li. 2020. "Chemical Composition and Attractant Activity of Volatiles from Rhus potaninii to The Spring Aphid Kaburagia rhusicola" Molecules 25, no. 15: 3412. https://doi.org/10.3390/molecules25153412
APA StyleZhu, X., Li, L., Hsiang, T., Zha, Y., Zhou, Z., Chen, R., Wang, X., Wu, Q., & Li, J. (2020). Chemical Composition and Attractant Activity of Volatiles from Rhus potaninii to The Spring Aphid Kaburagia rhusicola. Molecules, 25(15), 3412. https://doi.org/10.3390/molecules25153412