Functional Mining of the Crotalus Spp. Venom Protease Repertoire Reveals Potential for Chronic Wound Therapeutics
Abstract
1. Introduction
2. Results
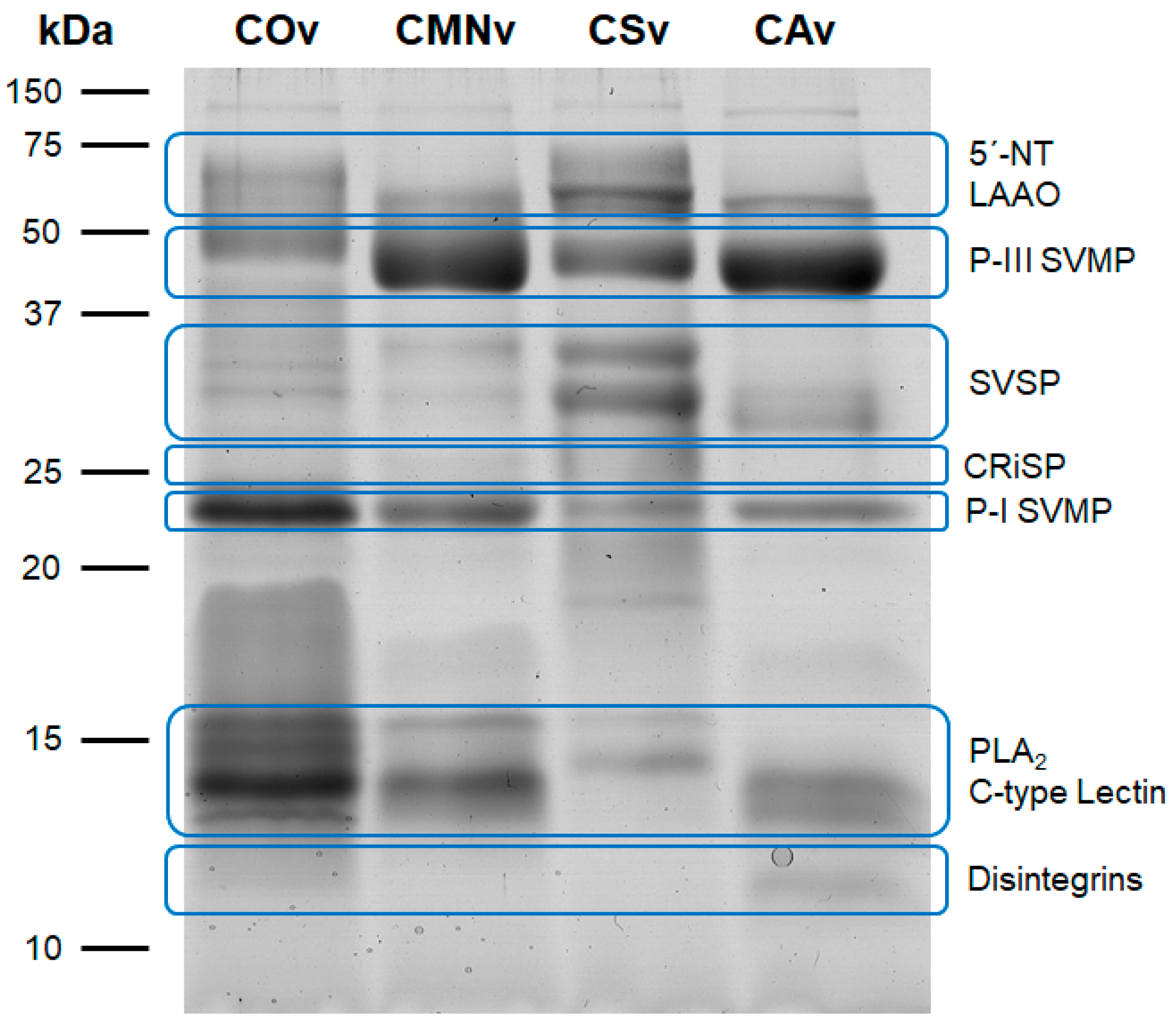
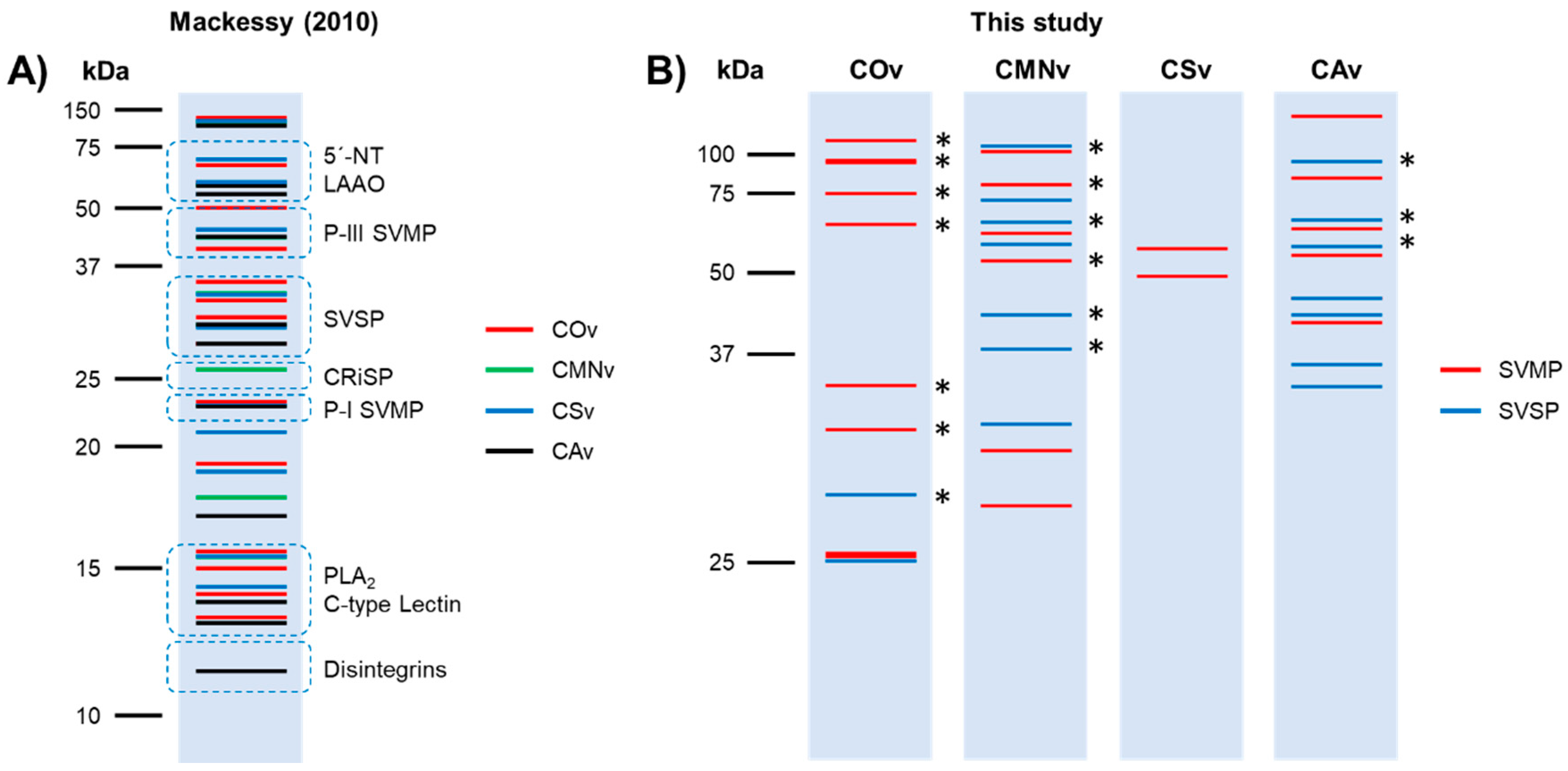
2.1. Crotalus Spp.’ Venom Toxin Families Identification by SDS-PAGE
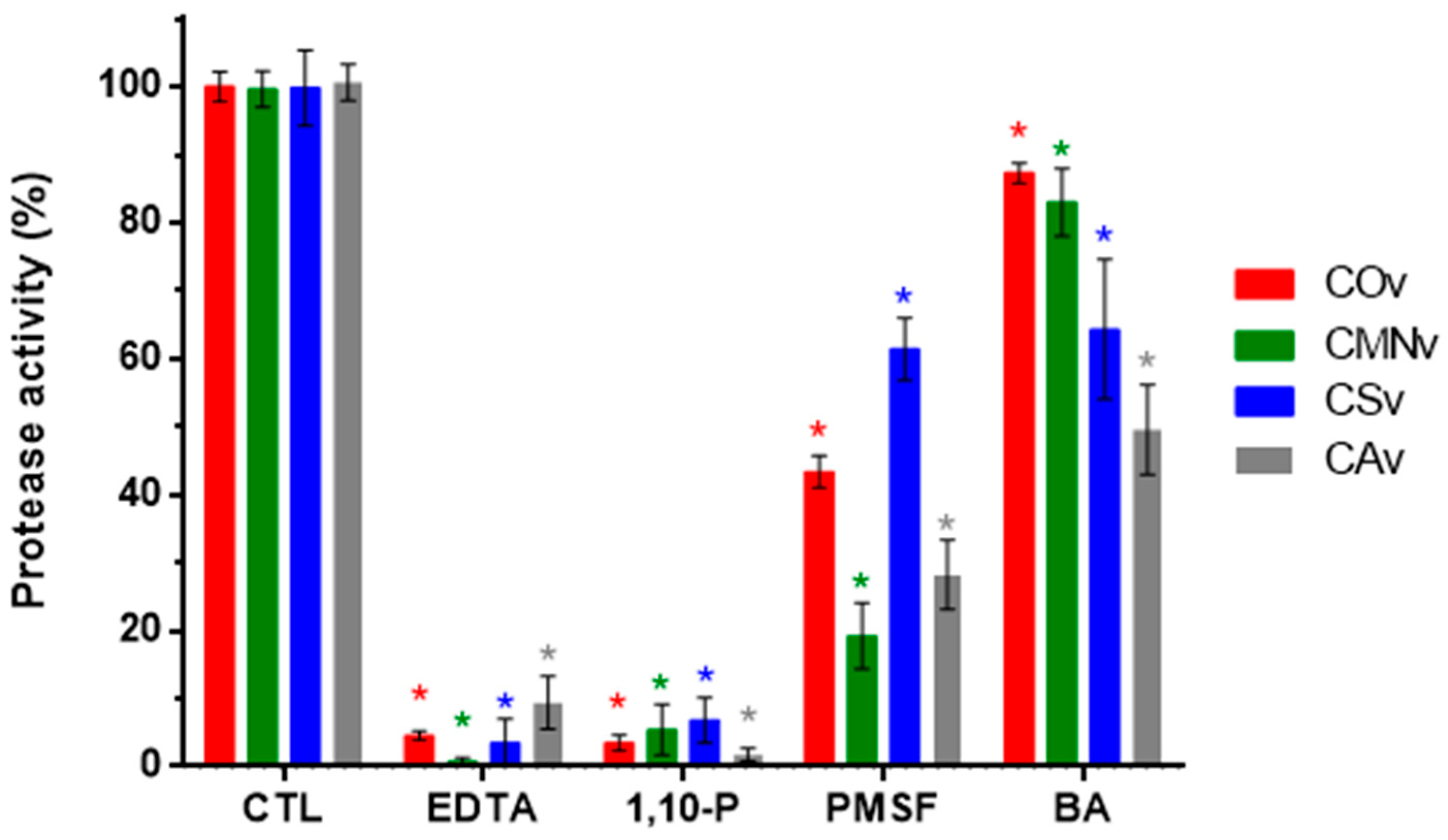
2.2. Protease Inhibitors
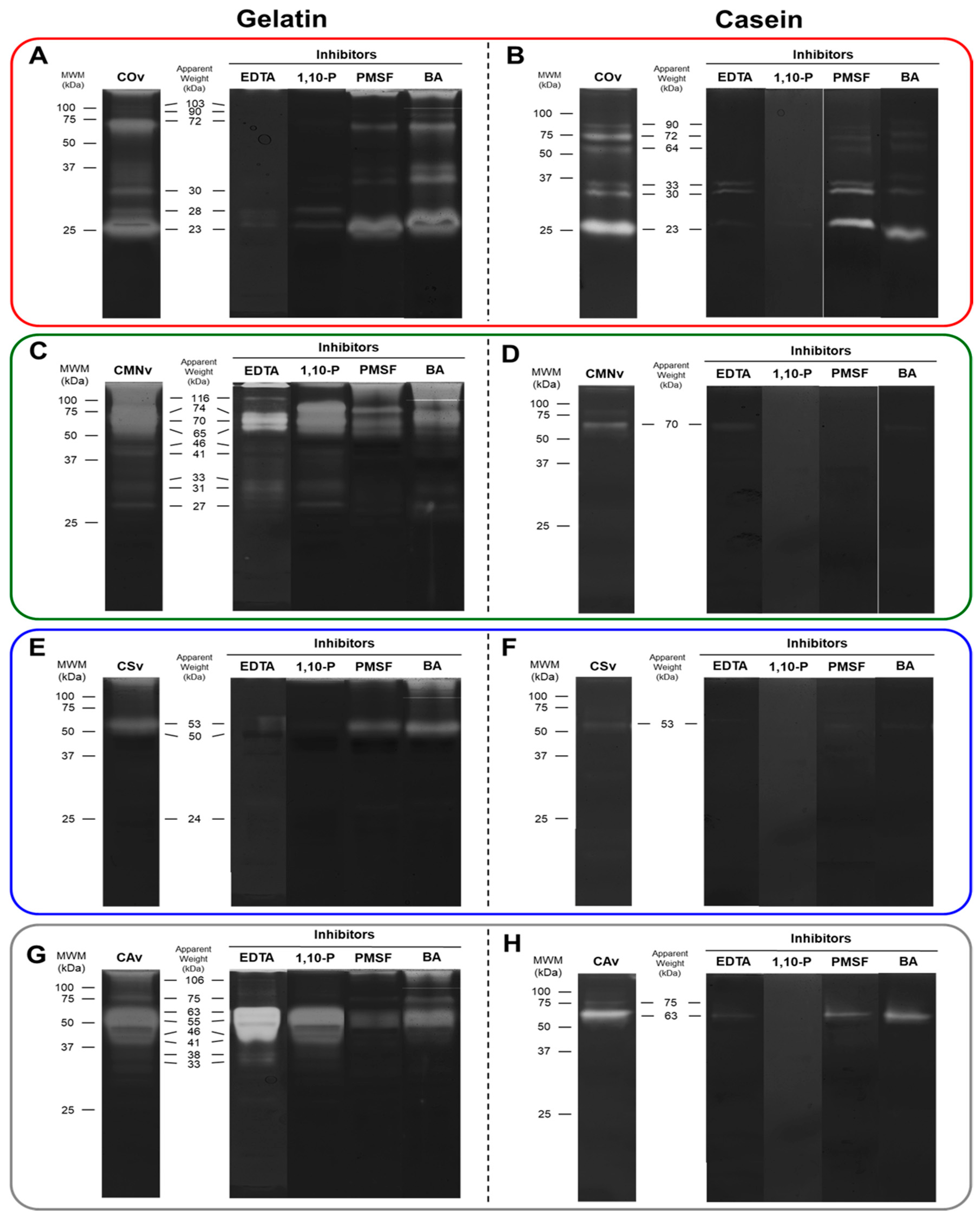
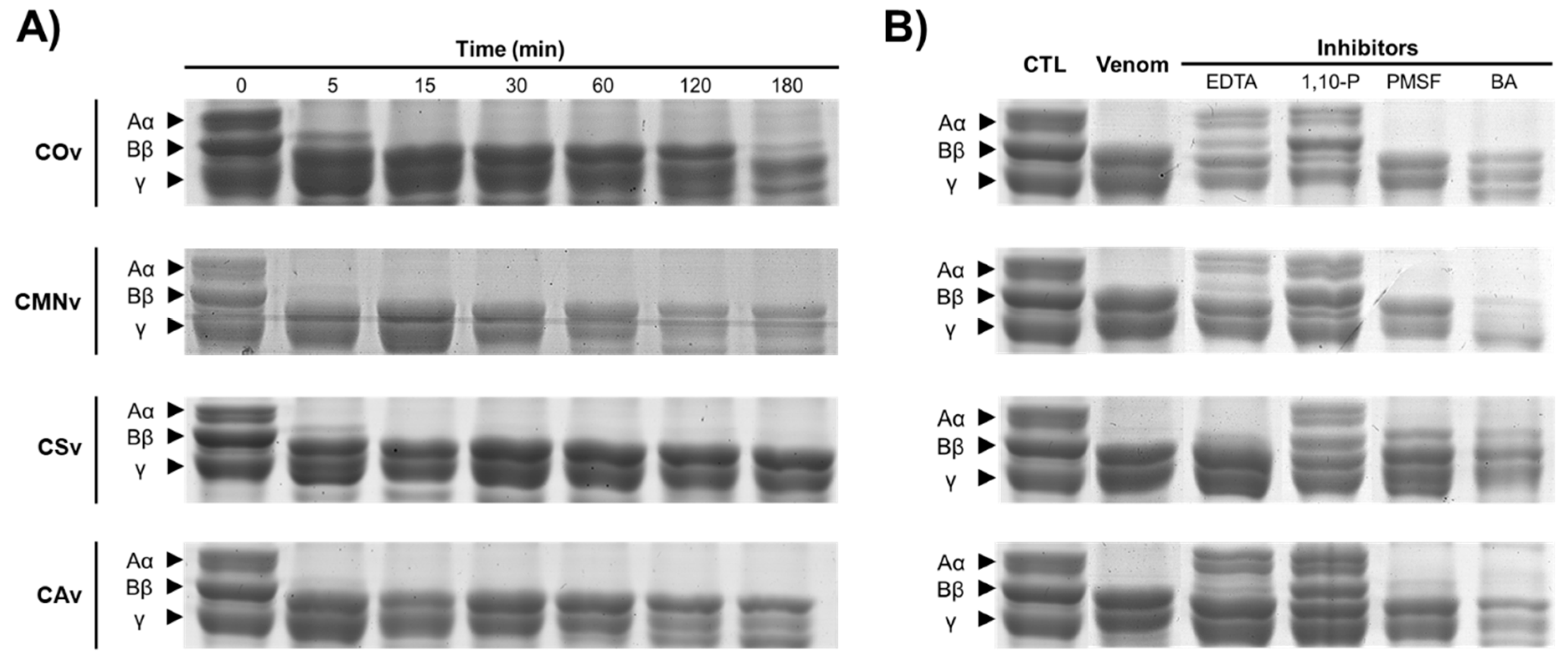
2.3. In-Gel Zymography
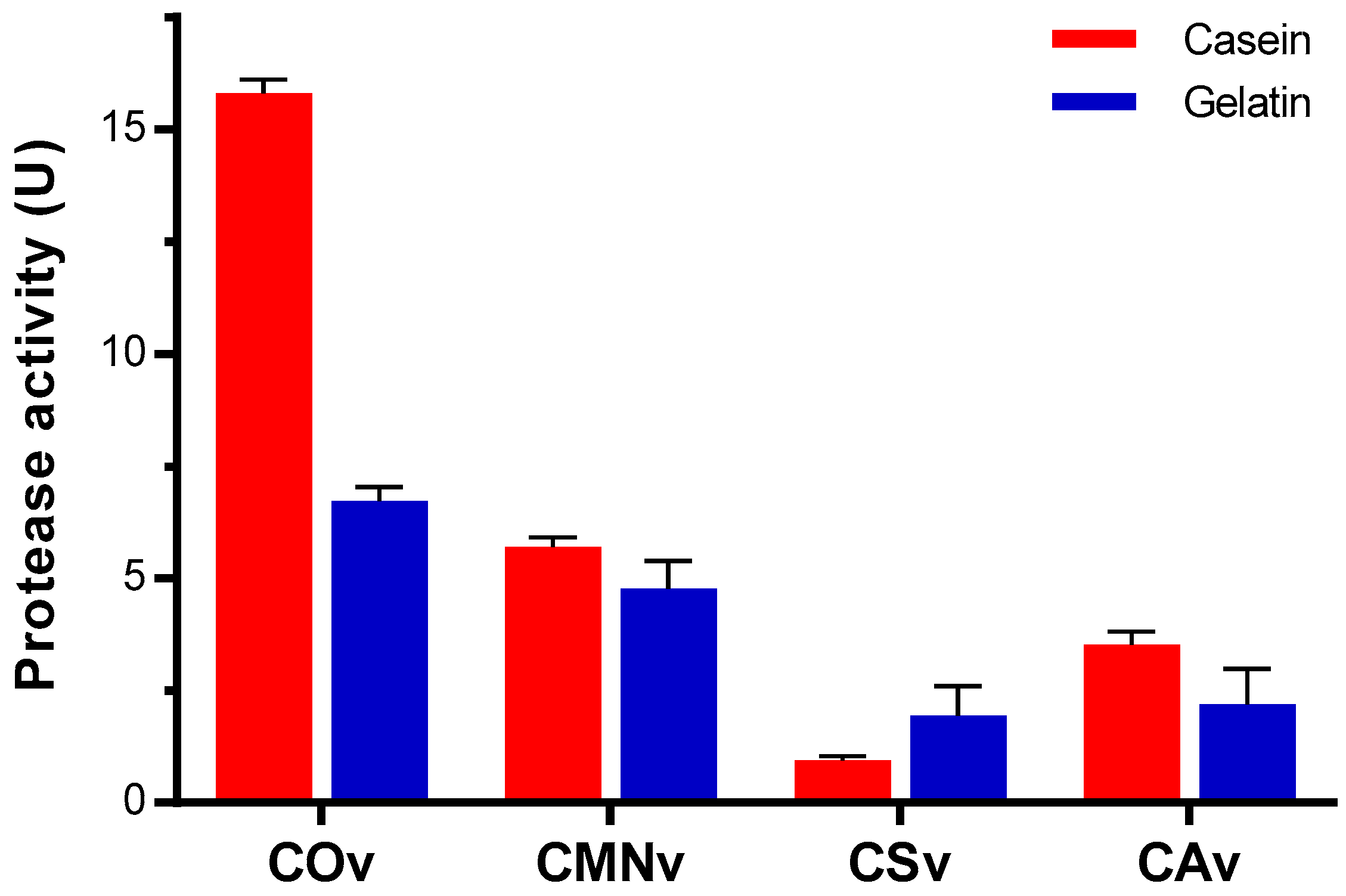
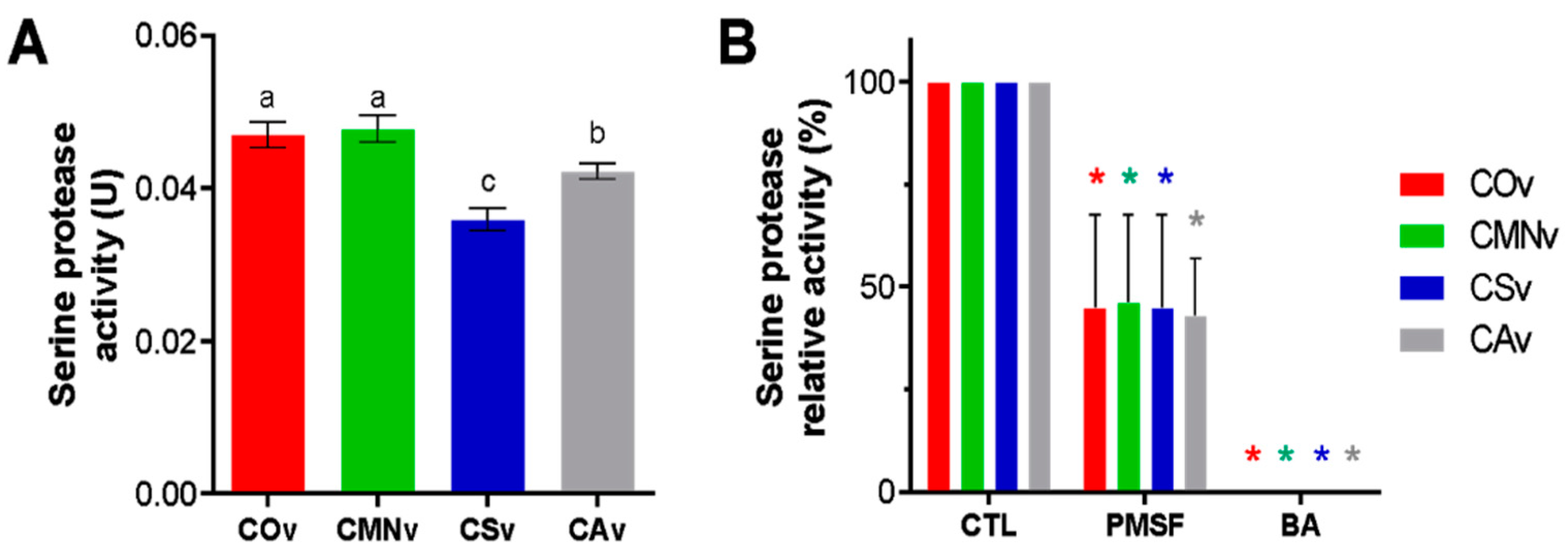
2.4. Crotalus Ornatus Venom Proteases are the Most Active among Crotalus Spp.’ Venoms
2.5. Snake Venom Serine Protease Characterization on Crotalus Spp.’ Venoms
2.6. Snake Venom Metalloproteases and Snake Venom Serine Proteases
2.7. Crotalus Sp.’ Snake Venom Metalloproteases Degrade Fibrin
3. Discussion
3.1. Venom Profiling
3.2. In-Gel Zymography
3.3. Protease Substrate Preference
3.4. Serine Protease Activity
3.5. Fibrinogenolytic Activity
3.6. Fibrinolytic Activity
4. Materials and Methods
4.1. Snake Venom Samples and Quantification
4.2. SDS-PAGE
4.3. Proteolytic Activity
4.4. Serine Protease Activity
4.5. Fibrinogenolytic Activity
4.6. Fibrinolytic Activity
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Izadi, K.; Ganchi, P. Chronic Wounds. Clin. Plast. Surg. 2005, 32, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care (New Rochelle) 2015, 4, 560–582. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.G.; Higham, C.; Broussard, K.; Phillips, T.J. Wound healing and treating wounds: Chronic wound care and management. J. Am. Acad. Dermatol. 2016, 74, 607–625. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.; Hamm, R.L. Factors That Impair Wound Healing. J. Am. Coll. Clin. Wound Spec. 2014, 4, 84–91. [Google Scholar] [CrossRef]
- Avila Rodríguez, M.I.; Rodríguez Barroso, L.G.; Sánchez, M.L. Collagen: A review on its sources and potential cosmetic applications. J. Cosmet. Dermatol. 2018, 17, 20–26. [Google Scholar] [CrossRef]
- Fonder, M.A.; Lazarus, G.S.; Cowan, D.A.; Aronson-Cook, B.; Kohli, A.R.; Mamelak, A.J. Treating the chronic wound: A practical approach to the care of nonhealing wounds and wound care dressings. J. Am. Acad. Dermatol. 2008, 58, 185–206. [Google Scholar] [CrossRef]
- Khan, W.; Jones, W. Debridement: Defining something we all do. J. Trauma Orhtopedics 2016, 4, 48–51. [Google Scholar]
- Kwan, S.H.; Ismail, M.N. Identification of the Potential Bio-active Proteins Associated with Wound Healing Properties in Snakehead Fish (Channa striata) Mucus. Curr. Proteomics 2018, 15, 299–312. [Google Scholar] [CrossRef]
- Sun, B.K.; Siprashvili, Z.; Khavari, P.A. Advances in skin grafting and treatment of cutaneous wounds. Science 2014, 346, 941. [Google Scholar] [CrossRef]
- Ladin, D. Becaplermin gel (PDGF-BB) as topical wound therapy. Plastic Surgery Educational Foundation DATA Committee. Plast. Reconstr. Surg. 2000, 105, 1230–1231. [Google Scholar] [CrossRef]
- Clark, R. Wound repair: Overview and general considerations. In The Molecular and Cellular Biology of Wound Repair; Plenum Press: New York, NY, USA, 1995; pp. 3–55. [Google Scholar]
- Figueiredo Azevedo, F.; Santanna, L.P.; Bobbo, V.C.; Libert, E.A.; Araujo, E.P.; Abdalla Saad, M.; Lima, M.H.M. Evaluating the Effect of 3% Papain Gel Application in Cutaneous Wound Healing in Mice. Wounds 2017, 29, 96–101. [Google Scholar] [PubMed]
- Giudice, G.; Filoni, A.; Maggio, G.; Bonamonte, D.; Vestita, M. Cost Analysis of a Novel Enzymatic Debriding Agent for Management of Burn Wounds. BioMed Res. Int. 2017, 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Klasen, H.J. A review on the nonoperative removal of necrotic tissue from burn wounds. Burns 2000, 26, 207–222. [Google Scholar] [CrossRef]
- Cazander, G.; Pritchard, D.I.; Nigam, Y.; Jung, W.; Nibbering, P.H. Multiple actions of Lucilia sericata larvae in hard-to-heal wounds. BioEssays 2013, 35, 1083–1092. [Google Scholar] [CrossRef]
- Jordan, A.; Khiyani, N.; Bowers, S.; Lukaszczyk, J.; Stawicki, S. Maggot debridement therapy: A practical review. Int. J. Acad. Med. 2018, 4, 21–34. [Google Scholar] [CrossRef]
- Akunne, T.; Okafor, S.; Okechukwu, D.C.; Nwankwor, S.S.; Emene, J.O.; Okoro, B.N. Catfish (Clarias gariepinus) Slime Coat Possesses Antimicrobial and Wound Healing Activities. UK J. Pharmacol. Biosci. 2016, 4. [Google Scholar] [CrossRef]
- Costa-Neto, E.M. Implications and applications of folk zootherapy in the state of Bahia, Northeastern Brazil. Sustain. Dev. 2004, 12, 161–174. [Google Scholar] [CrossRef]
- Manan Mat Jais, A. Pharmacognosy and pharmacology of Haruan (Channa striatus), a medicinal fish with wound healing properties. Boletín Latinoam. y del Caribe de Plantas Med. y Aromáticas 2007, 6, 52–60. [Google Scholar]
- Almeida, F.; Pimenta, A.; Cristina Oliveira, M.; Lima, M. Venoms, toxins and derivatives from the Brazilian fauna: Valuable sources for drug discovery. Acta Physiol. Sin. 2015, 67, 261–270. [Google Scholar]
- Kumar, V.A.; Wickremasinghe, N.C.; Shi, S.; Hartgerink, J.D. Nanofibrous Snake Venom Hemostat. ACS Biomater. Sci. Eng. 2015, 1, 1300–1305. [Google Scholar] [CrossRef] [PubMed]
- Sant’Ana, E.M.C.; Gouvêa, C.M.C.P.; Durigan, J.L.Q.; Cominetti, M.R.; Pimentel, E.R.; Selistre-de-Araújo, H.S. Rat skin wound healing induced by alternagin-C, a disintegrin-like, Cys-rich protein from Bothrops alternatus venom. Int. Wound J. 2011, 8, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Sant′Ana, E.M.C.; Gouvêa, C.M.C.P.; Nakaie, C.R.; Selistre-de-Araújo, H.S. Angiogenesis and growth factor modulation induced by alternagin C, a snake venom disintegrin-like, cysteine-rich protein on a rat skin wound model. Arch. Biochem. Biophys. 2008, 479, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Soto, J.G.; Perez, J.C.; Minton, S.A. Proteolytic, hemorrhagic and hemolytic activities of snake venoms. Toxicon 1988, 26, 875–882. [Google Scholar] [CrossRef]
- Estevão-Costa, M.-I.; Sanz-Soler, R.; Johanningmeier, B.; Eble, J.A. Snake venom components in medicine: From the symbolic rod of Asclepius to tangible medical research and application. Int. J. Biochem. Cell Biol. 2018, 104, 94–113. [Google Scholar] [CrossRef]
- Uetz, P.; Freed, P.; Hošek, J. (Eds.) The Reptile Database. Available online: http://www.reptile-database.org (accessed on 2 February 2020).
- SEMARNAT. Programa de Acción para la Conservación de las Especies: Serpientes de Cascabel (Crotalus spp.); SEMARNAT/CONANP Press: Mexico city, Mexico, 2018; Available online: https://www.gob.mx/conanp/documentos/programa-de-accion-para-la-conservacion-de-la-especie-pace-serpientes-de-cascabel-crotalus-spp?state=published (accessed on 2 July 2020).
- Tasoulis, T.; Isbister, G.K. A Review and Database of Snake Venom Proteomes. Toxins 2017, 9, 290. [Google Scholar] [CrossRef]
- Marsh, N.A. Diagnostic Uses of Snake Venom. Pathophysiol. Haemost. Thromb. 2001, 31, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Toombs, C.F. Alfimeprase: Pharmacology of a Novel Fibrinolytic Metalloproteinase for Thrombolysis. Pathophysiol. Haemost. Thromb. 2001, 31, 141–147. [Google Scholar] [CrossRef]
- Koh, D.C.I.; Armugam, A.; Jeyaseelan, K. Snake venom components and their applications in biomedicine. Cell. Mol. Life Sci. CMLS 2006, 63, 3030–3041. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Escalante, T.; Rucavado, A.; Herrera, C.; Fox, J.W. A Comprehensive View of the Structural and Functional Alterations of Extracellular Matrix by Snake Venom Metalloproteinases (SVMPs): Novel Perspectives on the Pathophysiology of Envenoming. Toxins (Basel) 2016, 8, 304. [Google Scholar] [CrossRef]
- Kini, R.M.; Koh, C.Y. Metalloproteases Affecting Blood Coagulation, Fibrinolysis and Platelet Aggregation from Snake Venoms: Definition and Nomenclature of Interaction Sites. Toxins (Basel) 2016, 8, 284. [Google Scholar] [CrossRef]
- Waheed, H.; Moin, S.F.; Choudhary, M.I. Snake Venom: From Deadly Toxins to Life-saving Therapeutics. Curr. Med. Chem. 2017, 24, 1874–1891. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, C.d.F.P.; Fernandes, C.M.; Zuliani, J.P.; Zamuner, S.F. Inflammatory effects of snake venom metalloproteinases. Mem. do Inst. Oswaldo Cruz 2005, 100, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.K.; Joshi, M.B.; Ullah, A.; Masood, R.; Biligiri, S.G.; Arni, R.K.; Satyamoorthy, K. Serine proteinases from Bothrops snake venom activates PI3K/Akt mediated angiogenesis. Toxicon 2016, 124, 63–72. [Google Scholar] [CrossRef]
- Choudhury, M.; Suvilesh, K.N.; Vishwanath, B.S.; Velmurugan, D. EC-PIII, a novel non-hemorrhagic procoagulant metalloproteinase: Purification and characterization from Indian Echis carinatus venom. Int. J. Biol. Macromol. 2018, 106, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.S.; de Barros, L.C.; Abbade, L.P.F.; Barraviera, S.R.C.S.; Silvares, M.R.C.; de Pontes, L.G.; dos Santos, L.D.; Barraviera, B. Heterologous fibrin sealant derived from snake venom: From bench to bedside—an overview. J. Venom. Anim. Toxins incl. Trop. Dis. 2017, 23, 21. [Google Scholar] [CrossRef]
- Howes, J.-M.; Kamiguti, A.S.; Theakston, R.D.G.; Wilkinson, M.C.; Laing, G.D. Effects of three novel metalloproteinases from the venom of the West African saw-scaled viper, Echis ocellatus on blood coagulation and platelets. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2005, 1724, 194–202. [Google Scholar] [CrossRef]
- Silva, M.B.; Schattner, M.; Ramos, C.R.R.; Junqueira-de-Azevedo, I.L.M.; Guarnieri, M.C.; Lazzari, M.A.; Sampaio, C.A.M.; Pozner, R.G.; Ventura, J.S.; Ho, P.L.; et al. A prothrombin activator from Bothrops erythromelas (jararaca-da-seca) snake venom: Characterization and molecular cloning. Biochem. J. 2003, 369, 129–139. [Google Scholar] [CrossRef]
- Fernandes, C.M.; Zamuner, S.R.; Zuliani, J.P.; Rucavado, A.; Gutiérrez, J.M.; Teixeira, C.d.F.P. Inflammatory effects of BaP1 a metalloproteinase isolated from Bothrops asper snake venom: Leukocyte recruitment and release of cytokines. Toxicon 2006, 47, 549–559. [Google Scholar] [CrossRef]
- Ferreira, B.A.; Deconte, S.R.; de Moura, F.B.R.; Tomiosso, T.C.; Clissa, P.B.; Andrade, S.P.; de Assis Araújo, F. Inflammation, angiogenesis and fibrogenesis are differentially modulated by distinct domains of the snake venom metalloproteinase jararhagin. Int. J. Biol. Macromol. 2018, 119, 1179–1187. [Google Scholar] [CrossRef]
- Silva, C.A.; Zuliani, J.P.; Assakura, M.T.; Mentele, R.; Camargo, A.C.M.; Teixeira, C.F.P.; Serrano, S.M.T. Activation of αMβ2-mediated phagocytosis by HF3, a P-III class metalloproteinase isolated from the venom of Bothrops jararaca. Biochem. Biophys. Res. Commun. 2004, 322, 950–956. [Google Scholar] [CrossRef]
- Tseng, Y.-L.; Lee, C.-J.; Huang, T.-F. Effects of a snake venom metalloproteinase, triflamp, on platelet aggregation, platelet-neutrophil and neutrophil-neutrophil interactions: Involvement of platelet GPIbalpha and neutrophil PSGL-1. Thromb. Haemost. 2004, 91, 315–324. [Google Scholar] [CrossRef]
- Bernardes, C.P.; Menaldo, D.L.; Camacho, E.; Rosa, J.C.; Escalante, T.; Rucavado, A.; Lomonte, B.; Gutiérrez, J.M.; Sampaio, S.V. Proteomic analysis of Bothrops pirajai snake venom and characterization of BpirMP, a new P-I metalloproteinase. J. Proteomics 2013, 80, 250–267. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, E.F.; Richardson, M.; Gremski, L.H.; Veiga, S.S.; Yarleque, A.; Niland, S.; Lima, A.M.; Estevao-Costa, M.I.; Eble, J.A. Data for a direct fibrinolytic metalloproteinase, barnettlysin-I from Bothrops barnetti (barnett(,)s pitviper) snake venom with anti-thrombotic effect. Data Br. 2016, 7, 1609–1613. [Google Scholar] [CrossRef] [PubMed]
- Zigrino, P.; Kamiguti, A.S.; Eble, J.; Drescher, C.; Nischt, R.; Fox, J.W.; Mauch, C. The Reprolysin Jararhagin, a Snake Venom Metalloproteinase, Functions as a Fibrillar Collagen Agonist Involved in Fibroblast Cell Adhesion and Signaling. J. Biol. Chem. 2002, 277, 40528–40535. [Google Scholar] [CrossRef] [PubMed]
- Costa, É.P.; Santos, M.F. Jararhagin, a snake venom metalloproteinase-disintegrin, stimulates epithelial cell migration in an in vitro restitution model. Toxicon 2004, 44, 861–870. [Google Scholar] [CrossRef]
- Schattner, M.; Fritzen, M.; de Souza Ventura, J.; de Albuquerque Modesto, J.C.; Pozner, R.G.; Moura-da-Silva, A.M.; Chudzinski-Tavassi, A.M. The snake venom metalloproteases berythractivase and jararhagin activate endothelial cells. Biol. Chem. 2005, 386, 369–374. [Google Scholar] [CrossRef]
- Lopes, D.S.; Baldo, C.; de Freitas Oliveira, C.; Machado de Alcântara, T.; Dias Oliveira, J.D.; Gourlart, L.R.; Hamaguchi, A.; Homsi-Brandeburgo, M.I.; Moura-da-Silva, A.M.; Clissa, P.B.; et al. Characterization of inflammatory reaction induced by neuwiedase, a P-I metalloproteinase isolated from Bothrops neuwiedi venom. Toxicon 2009, 54, 42–49. [Google Scholar] [CrossRef]
- Tachoua, W.; Boukhalfa-Abib, H.; Laraba-Djebari, F. Hemorrhagic metalloproteinase, Cc HSM-III, isolated from Cerastes cerastes venom: Purification and biochemical characterization. J. Biochem. Mol. Toxicol. 2017, 31, e21899. [Google Scholar] [CrossRef]
- Wu, W.-B.; Huang, T.-F. Activation of MMP-2, cleavage of matrix proteins, and adherens junctions during a snake venom metalloproteinase-induced endothelial cell apoptosis. Exp. Cell Res. 2003, 288, 143–157. [Google Scholar] [CrossRef]
- Mackessy, S.P. The field of reptile toxinology: Snakes, lizards, and their venoms. In Handbook of Venoms and Toxins of Reptiles; CRC Press: Boca Raton, FL, USA, 2010; pp. 3–23. [Google Scholar]
- Harvey, A.L. Toxins and drug discovery. Toxicon 2014, 92, 193–200. [Google Scholar] [CrossRef]
- Peigneur, S.; Tytgat, J. Toxins in Drug Discovery and Pharmacology. Toxins 2018, 10, 126. [Google Scholar] [CrossRef]
- Meléndez-Martínez, D.; Macias-Rodríguez, E.; Vargas-Caraveo, A.; Martínez-Martínez, A.; Gatica-Colima, A.; Plenge-Tellechea, L.F. Capillary damage in the area postrema by venom of the northern black-tailed rattlesnake (Crotalus molossus molossus). J. Venom Res. 2014, 5, 1–5. [Google Scholar]
- Hardy, D.L.; Jeter, M.; Corrigan, J.J. Envenomation by the northern blacktail rattlesnake (Crotalus molossus molossus): Report of two cases and the vitro effects of the venom on fibrinolysis and platelet aggregation. Toxicon 1982, 20, 487–493. [Google Scholar] [CrossRef]
- Sánchez, E.E.; Soliz, L.A.; Ramírez, M.S.; Pérez, J.C. Partial characterization of a basic protein from Crotalus molossus molossus (northern blacktail rattlesnake) venom and production of a monoclonal antibody. Toxicon 2001, 39, 523–537. [Google Scholar] [CrossRef]
- Chen, T.; Rael, E.D. Purification of M5, a fibrinolytic proteinase from Crotalus molossus molossus venom that attacks complement. Int. J. Biochem. Cell Biol. 1997, 29, 789–799. [Google Scholar] [CrossRef]
- Rael, E.D.; Martinez, M.; Molina, O. Isolation of a fibrinolytic protease, M4, from venom of Crotalus molossus molossus (northern blacktail rattlesnake). Haemostasis 1992, 22, 41–49. [Google Scholar] [CrossRef]
- Tsai, I.-H.; Wang, Y.-M.; Chen, Y.-H.; Tu, A.T. Geographic variations, cloning, and functional analyses of the venom acidic phospholipases A2 of Crotalus viridis viridis. Arch. Biochem. Biophys. 2003, 411, 289–296. [Google Scholar] [CrossRef]
- Meléndez-Martínez, D.; Macías-Rodríguez, E.; Vázquez-Briones, R.; López-Vera, E.; Sandra Cruz-Pérez, M.; Vargas-Caraveo, A.; Gatica-Colima, A.; Fernando Plenge-Tellechea, L. In vitro hemotoxic, α-neurotoxic and vasculotoxic effects of the Mexican black-tailed rattlesnake (Crotalus molossus nigrescens) venom. J. Venom Res. 2017, 8, 1–8. [Google Scholar]
- Ramírez, G.A.; Fletcher, P.L.; Possani, L.D. Characterization of the venom from Crotalus molossus nigrescens Gloyd (black tail rattlesnake): Isolation of two proteases. Toxicon 1990, 28, 285–297. [Google Scholar] [CrossRef]
- Borja, M.; Neri-Castro, E.; Pérez-Morales, R.; Strickland, J.L.; Ponce-López, R.; Parkinson, C.L.; Espinosa-Fematt, J.; Sáenz-Mata, J.; Flores-Martínez, E.; Alagón, A.; et al. Ontogenetic Change in the Venom of Mexican Black-Tailed Rattlesnakes (Crotalus molossus nigrescens). Toxins (Basel) 2018, 10, 501. [Google Scholar] [CrossRef]
- Massey, D.J.; Calvete, J.J.; Sánchez, E.E.; Sanz, L.; Richards, K.; Curtis, R.; Boesen, K. Venom variability and envenoming severity outcomes of the Crotalus scutulatus scutulatus (Mojave rattlesnake) from Southern Arizona. J. Proteomics 2012, 75, 2576–2587. [Google Scholar] [CrossRef] [PubMed]
- Dobson, J.; Yang, D.C.; op den Brouw, B.; Cochran, C.; Huynh, T.; Kurrupu, S.; Sánchez, E.E.; Massey, D.J.; Baumann, K.; Jackson, T.N.W.; et al. Rattling the border wall: Pathophysiological implications of functional and proteomic venom variation between Mexican and US subspecies of the desert rattlesnake Crotalus scutulatus. Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol. 2018, 205, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Zepeda, H.; Rael, E.D.; Alec Knight, R. Isolation of two phospholipases A2 from mojave rattlesnake (Crotalus scutulatus scutulatus) venom and variation of immunologically related venom proteins in different populations. Comp. Biochem. Physiol. Part B: Comp. Biochem. 1985, 81, 319–324. [Google Scholar] [CrossRef]
- Martinez, M.; Rael, E.D.; Maddux, N.L. Isolation of a hemorrhagic toxin from Mojave rattlesnake (Crotalus scutulatus scutulatus) venom. Toxicon 1990, 28, 685–694. [Google Scholar] [CrossRef]
- Calvete, J.J.; Fasoli, E.; Sanz, L.; Boschetti, E.; Righetti, P.G. Exploring the Venom Proteome of the Western Diamondback Rattlesnake, Crotalus atrox, via Snake Venomics and Combinatorial Peptide Ligand Library Approaches. J. Proteome Res. 2009, 8, 3055–3067. [Google Scholar] [CrossRef]
- Bjarnason, J.B.; Barish, A.; Direnzo, G.S.; Campbell, R.; Fox, J.W. Kallikrein-like enzymes from Crotalus atrox venom. J. Biol. Chem. 1983, 258, 12566–12573. [Google Scholar]
- Bjarnason, J.B.; Fox, J.W. Characterization of two hemorrhagic zinc proteinases, toxin c and toxin d, from western diamondback rattlesnake (Crotalus atrox) venom. Biochim. Biophys. Acta (BBA)-Protein Struct. Mol. Enzymol. 1987, 911, 356–363. [Google Scholar] [CrossRef]
- Vandooren, J.; Geurts, N.; Martens, E.; Van den Steen, P.E.; Opdenakker, G. Zymography methods for visualizing hydrolytic enzymes. Nat. Methods 2013, 10, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, P.V.; Burdygina, G.I. The structure and properties of solid gelatin and the principles of their modification. Polymer 1983, 24, 651–666. [Google Scholar] [CrossRef]
- Every, D. Quantitative measurement of protease activities in slab polyacrylamide gel electrophoretograms. Anal. Biochem. 1981, 116, 519–523. [Google Scholar] [CrossRef]
- Heussen, C.; Dowdle, E.B. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal. Biochem. 1980, 102, 196–202. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Stetlerstevenson, W.G. Quantitative Zymography: Detection of Picogram Quantities of Gelatinases. Anal. Biochem. 1994, 218, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Croall, D.E.; Moffett, K.; Hatch, H. Casein Zymography of Calpains Using a 4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid–Imidazole Buffer. Anal. Biochem. 2002, 304, 129–132. [Google Scholar] [CrossRef]
- Pan, D.; Hill, A.P.; Kashou, A.; Wilson, K.A.; Tan-Wilson, A. Electrophoretic transfer protein zymography. Anal. Biochem. 2011, 411, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Schonermark, M.P.; Heitefuss, P.; Isernhagen, B.; Lenarz, T. Ultrathin Horizontal Sodium Dodecyl Sulfate Zymography. Anal. Biochem. 1995, 229, 144–145. [Google Scholar] [CrossRef] [PubMed]
- Prezotto-Neto, J.P.; Kimura, L.F.; Alves, A.F.; Gutiérrez, J.M.; Otero, R.; Suárez, A.M.; Santoro, M.L.; Barbaro, K.C. Biochemical and biological characterization of Bothriechis schlegelii snake venoms from Colombia and Costa Rica. Exp. Biol. Med. (Maywood) 2016, 241, 2075–2085. [Google Scholar] [CrossRef]
- Lira, M.S.; Furtado, M.F.; Martins, L.M.P.; Lopes-Ferreira, M.; Santoro, M.L.; Barbaro, K.C. Enzymatic and immunochemical characterization of Bothrops insularis venom and its neutralization by polyspecific Bothrops antivenom. Toxicon 2007, 49, 982–994. [Google Scholar] [CrossRef]
- Torres-Bonilla, K.A.; Andrade-Silva, D.; Serrano, S.M.T.; Hyslop, S. Biochemical characterization of venom from Pseudoboa neuwiedii (Neuwied’s false boa; Xenodontinae; Pseudoboini). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2018, 213, 27–38. [Google Scholar] [CrossRef]
- Fox, J.W.; Serrano, S.M.T. Insights into and speculations about snake venom metalloproteinase (SVMP) synthesis, folding and disulfide bond formation and their contribution to venom complexity. FEBS J. 2008, 275, 3016–3030. [Google Scholar] [CrossRef]
- Dyballa, N.; Metzger, S. Fast and sensitive colloidal coomassie G-250 staining for proteins in polyacrylamide gels. J. Vis. Exp. 2009, 1431. [Google Scholar] [CrossRef] [PubMed]
- Baramova, E.N.; Shannon, J.D.; Bjarnason, J.B.; Fox, J.W. Degradation of extracellular matrix proteins by hemorrhagic metalloproteinases. Arch. Biochem. Biophys. 1989, 275, 63–71. [Google Scholar] [CrossRef]
- Shannon, J.D.; Baramova, E.N.; Bjarnason, J.B.; Fox, J.W. Amino acid sequence of a Crotalus atrox venom metalloproteinase which cleaves type IV collagen and gelatin. J. Biol. Chem. 1989, 264, 11575–11583. [Google Scholar]
- Escalante, T.; Rucavado, A.; Fox, J.W.; Gutiérrez, J.M. Key events in microvascular damage induced by snake venom hemorrhagic metalloproteinases. J. Proteomics 2011, 74, 1781–1794. [Google Scholar] [CrossRef] [PubMed]
- Soto, J.G.; Perez, J.C.; Lopez, M.M.; Martinez, M.; Quintanilla-Hernandez, T.B.; Santa-Hernandez, M.S.; Turner, K.; Glenn, J.L.; Straight, R.C.; Minton, S.A. Comparative enzymatic study of HPLC-fractionated Crotalus venoms. Comp. Biochem. Physiol. Part B Comp. Biochem. 1989, 93, 847–855. [Google Scholar] [CrossRef]
- Roldán-Padrón, O.; Castro-Guillén, J.L.; García-Arredondo, J.A.; Cruz-Pérez, M.S.; Díaz-Peña, L.F.; Saldaña, C.; Blanco-Labra, A.; García-Gasca, T. Snake Venom Hemotoxic Enzymes: Biochemical Comparison between Crotalus Species from Central Mexico. Molecules 2019, 24, 1489. [Google Scholar] [CrossRef] [PubMed]
- Caley, M.P.; Martins, V.L.C.; O’Toole, E.A. Metalloproteinases and Wound Healing. Adv. Wound Care 2015, 4, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Rohani, M.G.; Parks, W.C. Matrix remodeling by MMPs during wound repair. Matrix Biol. 2015, 44–46, 113–121. [Google Scholar] [CrossRef]
- Costa, F.L.S.; Rodrigues, R.S.; Izidoro, L.F.M.; Menaldo, D.L.; Hamaguchi, A.; Homsi-Brandeburgo, M.I.; Fuly, A.L.; Soares, S.G.; Selistre-de-Araújo, H.S.; Barraviera, B.; et al. Biochemical and functional properties of a thrombin-like enzyme isolated from Bothrops pauloensis snake venom. Toxicon 2009, 54, 725–735. [Google Scholar] [CrossRef]
- Lôbo de Araújo, A.; Donato, J.L.; Bon, C. Purification from Bothrops lanceolatus (fer de lance) venom of a fibrino(geno)lytic enzyme with esterolytic activity. Toxicon 1998, 36, 745–758. [Google Scholar] [CrossRef]
- Dutta, S.; Chanda, A.; Kalita, B.; Islam, T.; Patra, A.; Mukherjee, A.K. Proteomic analysis to unravel the complex venom proteome of eastern India Naja naja: Correlation of venom composition with its biochemical and pharmacological properties. J. Proteomics 2017, 156, 29–39. [Google Scholar] [CrossRef]
- Gowda, C.D.R.; Nataraju, A.; Rajesh, R.; Dhananjaya, B.L.; Sharath, B.K.; Vishwanath, B.S. Differential action of proteases from Trimeresurus malabaricus, Naja naja and Daboia russellii venoms on hemostasis. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2006, 143, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Prasad, N.B.; Uma, B.; Bhatt, S.K.G.; Gowda, V.T. Comparative characterisation of Russell’s viper (Daboia/Vipera russelli) venoms from different regions of the Indian peninsula. Biochim. Biophys. Acta (BBA)-Gen. Subj. 1999, 1428, 121–136. [Google Scholar] [CrossRef]
- Koh, C.Y.; Modahl, C.M.; Kulkarni, N.; Kini, R.M. Toxins Are an Excellent Source of Therapeutic Agents against Cardiovascular Diseases. Semin. Thromb. Hemost. 2018, 44, 691–706. [Google Scholar] [CrossRef] [PubMed]
- Budzynski, A.Z.; Pandya, B.V.; Rubin, R.N.; Brizuela, B.S.; Soszka, T.; Stewart, G.J. Fibrinogenolytic afibrinogenemia after envenomation by western diamondback rattlesnake (Crotalus atrox). Blood 1984, 63, 1–14. [Google Scholar] [CrossRef]
- Corrigan, J.J.J.; Jeter, M.A. Mojave rattlesnake (Crotalus scutulatus scutulatus) venom: In vitro effect on platelets, fibrinolysis, and fibrinogen clotting. Vet. Hum. Toxicol. 1990, 32, 439–441. [Google Scholar]
- Sapru, Z.Z.; Tu, A.T.; Bailey, G.S. Purification and characterization of a fibrinogenase from the venom of Western Diamondback rattlesnake (Crotalus atrox). Biochim. Biophys. Acta (BBA)-Protein Struct. Mol. Enzymol. 1983, 747, 225–231. [Google Scholar] [CrossRef]
- Bhagat, R.; Sharma, K.; Sarode, R.; Shen, Y.-M. Delayed massive pulmonary thromboembolic phenomenon following envenomation by Mojave rattlesnake (Crotalus scutulatus). Thromb. Haemost. 2017, 104, 186–188. [Google Scholar] [CrossRef]
- Martin, P. Wound Healing--Aiming for Perfect Skin Regeneration. Science 1997, 276, 75. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, V.G.; Frank, N.; Afshar, S. De Novo Assessment and Review of Pan-American Pit Viper Anticoagulant and Procoagulant Venom Activities via Kinetomic Analyses. Toxins 2019, 11, 94. [Google Scholar] [CrossRef] [PubMed]
- Dagda, R.K.; Gasanov, S.; De La OIII, Y.; Rael, E.D.; Lieb, C.S. Genetic Basis for Variation of Metalloproteinase-Associated Biochemical Activity in Venom of the Mojave Rattlesnake (Crotalus scutulatus scutulatus). Biochem. Res. Int. 2013, 2013, 251474. [Google Scholar] [CrossRef]
- Nikai, T.; Mori, N.; Kishida, M.; Tsuboi, M.; Sugihara, H. Isolation and Characterization of Hemorrhagic Toxin g from the Venom of Crotalus Atrox (Western Diamondback Rattlesnake). Am. J. Trop. Med. Hyg. 1985, 34, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Toriseva, M.; Kähäri, V.-M. Proteinases in cutaneous wound healing. Cell. Mol. Life Sci. 2009, 66, 203–224. [Google Scholar] [CrossRef] [PubMed]
- Rømer, J.; Bugge, T.H.; Fyke, C.; Lund, L.R.; Flick, M.J.; Degen, J.L.; Danø, K. Impaired wound healing in mice with a disrupted plasminogen gene. Nat. Med. 1996, 2, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Sambrook, J. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory Press: Woodbury, NY, USA, 2001. [Google Scholar]
- Das, D.; Urs, N.; Hiremath, V.; Vishwanath, B.S.; Doley, R. Biochemical and biological characterization of Naja kaouthia venom from North-East India and its neutralization by polyvalent antivenom. J. Venom Res. 2013, 4, 31–38. [Google Scholar]
- Subramanian, S.; MacKinnon, S.L.; Ross, N.W. A comparative study on innate immune parameters in the epidermal mucus of various fish species. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2007, 148, 256–263. [Google Scholar] [CrossRef]
- Abdel-Aty, A.M.; Wahby, A.F. Purification and characterization of five snake venom metalloproteinases from Egyptian Echis pyramidum pyramidum venom. J. Toxicol. Sci. 2014, 39, 523–536. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ye, F.-P.; Wang, J.; Liao, G.-Y.; Zhang, Y.; Fan, Q.-S.; Lee, W.-H. Purification, characterization and gene cloning of Da-36, a novel serine protease from Deinagkistrodon acutus venom. Toxicon 2013, 67, 1–11. [Google Scholar] [CrossRef]
- Koludarov, I.; Jackson, T.N.; op den Brouw, B.; Dobson, J.; Dashevsky, D.; Arbuckle, K.; Clemente, C.J.; Stockdale, E.J.; Cochran, C.; Debono, J.; et al. Enter the Dragon: The Dynamic and Multifunctional Evolution of Anguimorpha Lizard Venoms. Toxins 2017, 9, 242. [Google Scholar] [CrossRef]
- Noren, I.; Ramstrom, G.; Wallen, P. Fibrin plate method with reagents purified by affinity chromatography and its use for determination of fibrinolytic and other proteolytic activity in saliva, bile and plasma. Haemostasis 1975, 4, 110–124. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meléndez-Martínez, D.; Plenge-Tellechea, L.F.; Gatica-Colima, A.; Cruz-Pérez, M.S.; Aguilar-Yáñez, J.M.; Licona-Cassani, C. Functional Mining of the Crotalus Spp. Venom Protease Repertoire Reveals Potential for Chronic Wound Therapeutics. Molecules 2020, 25, 3401. https://doi.org/10.3390/molecules25153401
Meléndez-Martínez D, Plenge-Tellechea LF, Gatica-Colima A, Cruz-Pérez MS, Aguilar-Yáñez JM, Licona-Cassani C. Functional Mining of the Crotalus Spp. Venom Protease Repertoire Reveals Potential for Chronic Wound Therapeutics. Molecules. 2020; 25(15):3401. https://doi.org/10.3390/molecules25153401
Chicago/Turabian StyleMeléndez-Martínez, David, Luis Fernando Plenge-Tellechea, Ana Gatica-Colima, Martha Sandra Cruz-Pérez, José Manuel Aguilar-Yáñez, and Cuauhtémoc Licona-Cassani. 2020. "Functional Mining of the Crotalus Spp. Venom Protease Repertoire Reveals Potential for Chronic Wound Therapeutics" Molecules 25, no. 15: 3401. https://doi.org/10.3390/molecules25153401
APA StyleMeléndez-Martínez, D., Plenge-Tellechea, L. F., Gatica-Colima, A., Cruz-Pérez, M. S., Aguilar-Yáñez, J. M., & Licona-Cassani, C. (2020). Functional Mining of the Crotalus Spp. Venom Protease Repertoire Reveals Potential for Chronic Wound Therapeutics. Molecules, 25(15), 3401. https://doi.org/10.3390/molecules25153401





