Hydrogels as Durable Anti-Icing Coatings Inhibit and Delay Ice Nucleation
Abstract
1. Introduction
2. Results
2.1. Synthesis of and Characterizations of the SA-g-DA
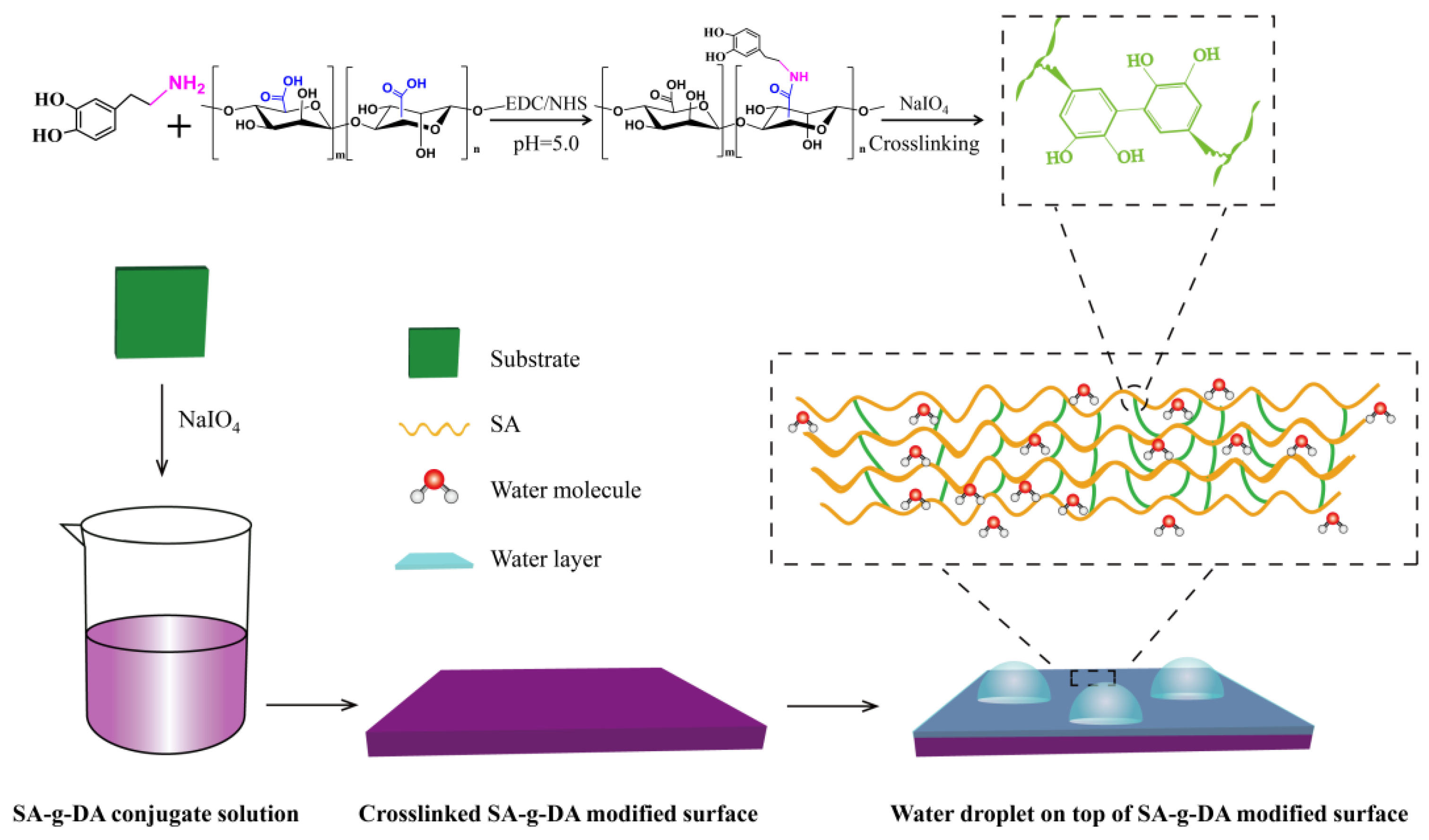
2.2. Synthesis of the Durable Anti-Icing Coating
2.3. Stability of the Anti-Icing Coating
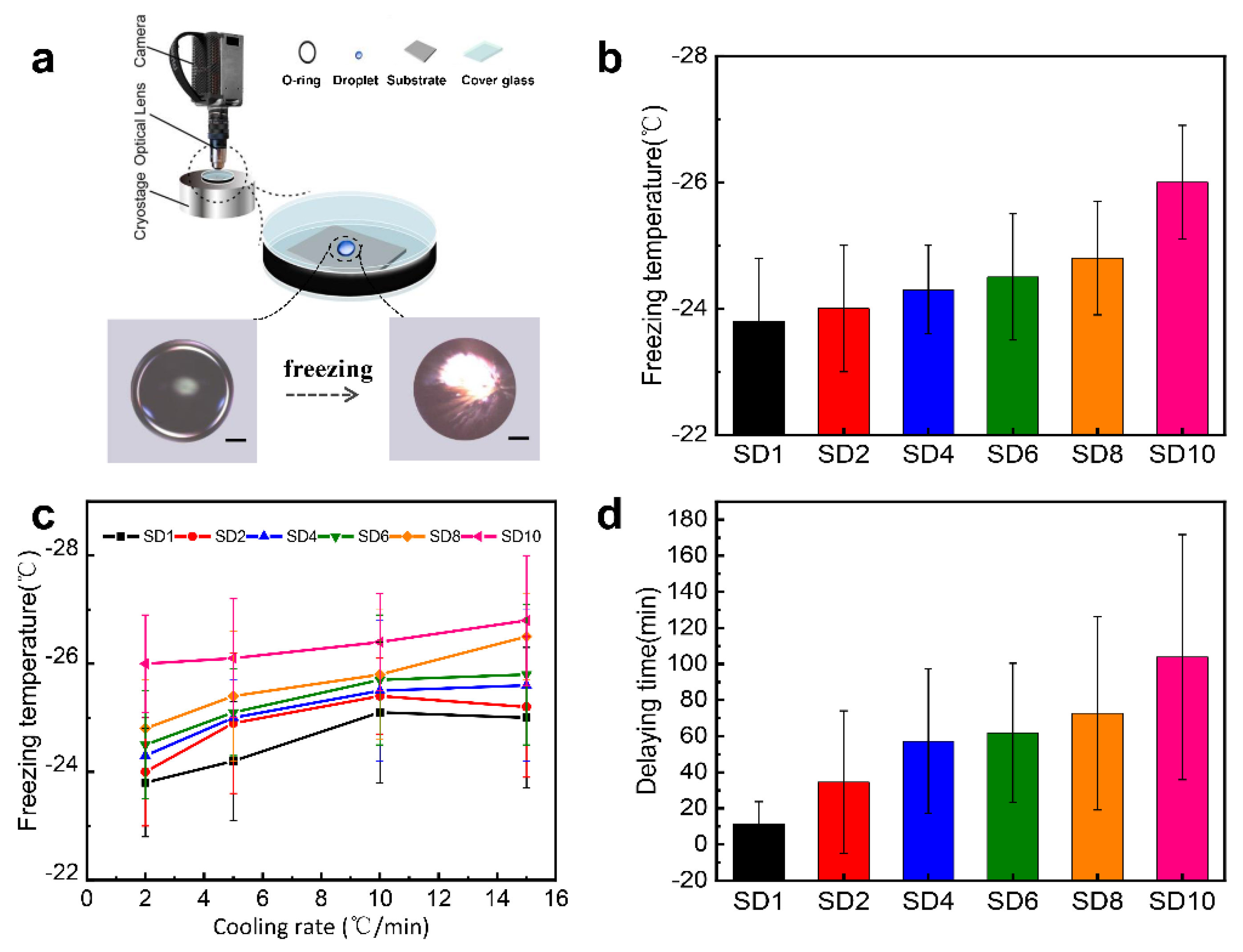
2.4. Anti-Icing Performances
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of SA-g-DA Conjugate
4.3. Fabrication of SA Hydrogel Surface
4.4. Characterization
4.5. Fabrication of Durable Hydrogel Coatings
4.6. Measurement of Ice Nucleation Temperature and Delay Time
4.7. Stability of the Hydrogel Surface
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ryzhkin, I.A.; Petrenko, V.F. Physical Mechanisms Responsible for Ice Adhesion. J. Phys. Chem. B 1997, 101, 6267–6270. [Google Scholar] [CrossRef]
- Ryerson, C.C. Ice protection of offshore platforms. Cold Reg. Sci. Technol. 2011, 65, 97–110. [Google Scholar] [CrossRef]
- Yao, X.; Song, Y.; Jiang, L. Applications of Bio-Inspired Special Wettable Surfaces. Adv. Mater. 2010, 23, 719–734. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Tiwari, M.K.; Doan, N.V.; Poulikakos, D. Mechanism of supercooled droplet freezing on surfaces. Nat. Commun. 2012, 3, 615. [Google Scholar] [CrossRef]
- Xiao, J.; Chaudhuri, S. Design of Anti-Icing Coatings Using Supercooled Droplets As Nano-to-Microscale Probes. Langmuir 2012, 28, 4434–4446. [Google Scholar] [CrossRef]
- Bragg, M.; Broeren, A.; Blumenthal, L. Iced-airfoil aerodynamics. Prog. Aerosp. Sci. 2005, 41, 323–362. [Google Scholar] [CrossRef]
- Kulinich, S.A.; Farzaneh, M. How Wetting Hysteresis Influences Ice Adhesion Strength on Superhydrophobic Surfaces. Langmuir 2009, 25, 8854–8856. [Google Scholar] [CrossRef]
- Tourkine, P.; Le Merrer, M.; QueéreéD. Delayed Freezing on Water Repellent Materials. Langmuir 2009, 25, 7214–7216. [Google Scholar] [CrossRef]
- Wang, F.; Li, C.; Lv, Y.; Lv, F.; Du, Y. Ice accretion on superhydrophobic aluminum surfaces under low-temperature conditions. Cold Reg. Sci. Technol. 2010, 62, 29–33. [Google Scholar] [CrossRef]
- Guo, P.; Zheng, Y.; Wen, M.; Song, C.; Lin, Y.; Jiang, L. Icephobic/Anti-Icing Properties of Micro/Nanostructured Surfaces. Adv. Mater. 2012, 24, 2642–2648. [Google Scholar] [CrossRef]
- Boinovich, L.; Emelyanenko, A.M.; Ivanov, V.; Pashinin, A.S. Durable Icephobic Coating for Stainless Steel. ACS Appl. Mater. Interfaces 2013, 5, 2549–2554. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, Y.; Lu, C.; Liu, Y.; Feng, S.; Liu, Y. Robust Slippery Liquid-Infused Porous Network Surfaces for Enhanced Anti-icing/Deicing Performance. ACS Appl. Mater. Interfaces 2020, 12, 25471–25477. [Google Scholar] [CrossRef] [PubMed]
- Piscitelli, F.; Chiariello, A.; Dabkowski, D.; Corraro, G.; Marra, F.; Di Palma, L. Superhydrophobic Coatings as Anti-Icing Systems for Small Aircraft. Aerospace 2020, 7, 2. [Google Scholar] [CrossRef]
- He, Z.; Wu, C.; Hua, M.; Wu, S.; Wu, D.; Zhu, X.; Wang, J.; He, X. Bioinspired Multifunctional Anti-icing Hydrogel. Phys. B Condens. Matter 2020, 2, 723–734. [Google Scholar] [CrossRef]
- Guo, Q.; He, Z.; Jin, Y.; Zhang, S.; Wu, S.; Bai, G.; Xue, H.; Liu, Z.; Jin, S.; Zhao, L.; et al. Tuning Ice Nucleation and Propagation with Counterions on Multilayer Hydrogels. Langmuir 2018, 34, 11986–11991. [Google Scholar] [CrossRef]
- Yang, H.; Ma, C.; Li, K.; Liu, K.; Loznik, M.; Teeuwen, R.; Van Hest, J.C.M.; Zhou, X.; Herrmann, A.; Wang, J. Tuning Ice Nucleation with Supercharged Polypeptides. Adv. Mater. 2016, 28, 5008–5012. [Google Scholar] [CrossRef]
- Ma, L.; Wang, J.; Zhao, F.; Wu, D.; Huang, Y.; Zhang, D.; Zhang, Z.; Fu, W.; Li, X.; Fan, Y. Plasmon-mediated photothermal and superhydrophobic TiN-PTFE film for anti-icing/deicing applications. Compos. Sci. Technol. 2019, 181, 107696. [Google Scholar] [CrossRef]
- Chen, D.; Gelenter, M.D.; Hong, M.; Cohen, R.E.; McKinley, G.H. Icephobic Surfaces Induced by Interfacial Nonfrozen Water. ACS Appl. Mater. Interfaces 2017, 9, 4202–4214. [Google Scholar] [CrossRef]
- Kim, P.; Wong, T.-S.; Alvareng, J.; Kreder, M.J.; Adorno-Martinez, W.E.; Aizenberg, J. Liquid-Infused Nanostructured Surfaces with Extreme Anti-Ice and Anti-Frost Performance. ACS Nano 2012, 6, 6569–6577. [Google Scholar] [CrossRef]
- Chen, J.; Dou, R.; Cui, D.; Zhang, Q.; Zhang, Y.; Xu, F.; Zhou, X.; Wang, J.; Song, Y.; Jiang, L. Robust Prototypical Anti-icing Coatings with a Self-lubricating Liquid Water Layer between Ice and Substrate. ACS Appl. Mater. Interfaces 2013, 5, 4026–4030. [Google Scholar] [CrossRef]
- Chen, J.; Luo, Z.; Fan, Q.; Lv, J.; Wang, J. Anti-Ice Coating Inspired by Ice Skating. Small 2014, 10, 4693–4699. [Google Scholar] [CrossRef] [PubMed]
- Dou, R.; Chen, J.; Zhang, Y.; Wang, X.; Cui, D.; Song, Y.; Jiang, L.; Wang, J. Anti-icing Coating with an Aqueous Lubricating Layer. ACS Appl. Mater. Interfaces 2014, 6, 6998–7003. [Google Scholar] [CrossRef] [PubMed]
- Okoroafor, E.U.; Newborough, M.; Highgate, D. Effects of thermal cycling on the crystallization characteristics of water within crosslinked hydro-active polymeric structures. J. Phys. D Appl. Phys. 1998, 31, 3130–3138. [Google Scholar] [CrossRef]
- Ozbay, S.; Yuceel, C.; Erbil, H.Y. Improved Icephobic Properties on Surfaces with a Hydrophilic Lubricating Liquid. ACS Appl. Mater. Interfaces 2015, 7, 22067–22077. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yao, X.; Chen, J.; He, Z.; Liu, J.; Li, Q.; Wang, J.; Jiang, L. Organogel as durable anti-icing coatings. Sci. China Mater. 2015, 58, 559–565. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, X.; Wu, S.; Li, Q.; Lv, J.; Wang, J.; Jiang, L. Bioinspired Solid Organogel Materials with a Regenerable Sacrificial Alkane Surface Layer. Adv. Mater. 2017, 29, 1700865. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhou, Y.; Peng, J.; Xu, C.; Xu, Q.; Xing, M.; Chang, J. A novel dual-adhesive and bioactive hydrogel activated by bioglass for wound healing. NPG Asia Mater. 2019, 11, 1–11. [Google Scholar] [CrossRef]
- Guo, Z.; Mi, S.; Sun, W. A Facile Strategy for Preparing Tough, Self-Healing Double-Network Hyaluronic Acid Hydrogels Inspired by Mussel Cuticles. Macromol. Mater. Eng. 2019, 304, 1800715. [Google Scholar] [CrossRef]
- Lee, C.; Shin, J.; Lee, J.S.; Byun, E.; Ryu, J.H.; Um, S.H.; Kim, D.-I.; Lee, H.; Cho, S.-W. Bioinspired, Calcium-Free Alginate Hydrogels with Tunable Physical and Mechanical Properties and Improved Biocompatibility. Biomacromolecules 2013, 14, 2004–2013. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef]
- Kim, S.; Moon, J.; Choi, J.S.; Cho, W.K.; Kang, S.M. Mussel-Inspired Approach to Constructing Robust Multilayered Alginate Films for Antibacterial Applications. Adv. Funct. Mater. 2016, 26, 4099–4105. [Google Scholar] [CrossRef]
- Hong, S.H.; Shin, M.; Lee, J.; Ryu, J.H.; Lee, S.; Yang, J.W.; Kim, W.D.; Lee, H. STAPLE: Stable Alginate Gel Prepared by Linkage Exchange from Ionic to Covalent Bonds. Adv. Heal. Mater. 2015, 5, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Cheng, C.; Nie, C.; He, C.; Deng, J.; Wang, L.; Xia, Y.; Zhao, C. Anticoagulant sodium alginate sulfates and their mussel-inspired heparin-mimetic coatings. J. Mater. Chem. B 2016, 4, 3203–3215. [Google Scholar] [CrossRef] [PubMed]
- Borchard, W.; Kenning, A.; Kapp, A.; Mayer, C. Phase diagram of the system sodium alginate/water: A model for biofilms. Int. J. Boil. Macromol. 2005, 35, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, V.; Jayakrishnan, A. Synthesis and evaluation of anti-fungal activities of sodium alginate-amphotericin B conjugates. Int. J. Boil. Macromol. 2018, 108, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Rho, J.; Messersmith, P.B. Facile Conjugation of Biomolecules onto Surfaces via Mussel Adhesive Protein Inspired Coatings. Adv. Mater. 2009, 21, 431–434. [Google Scholar] [CrossRef]
- Liu, K.; Wang, C.-L.; Ma, J.; Shi, G.; Yao, X.; Fang, H.; Song, Y.; Wang, J. Janus effect of antifreeze proteins on ice nucleation. Proc. Natl. Acad. Sci. USA 2016, 113, 14739–14744. [Google Scholar] [CrossRef]
- Yang, H.; Diao, Y.; Huang, B.; Li, K.; Wang, J. Metal–catechol complexes mediate ice nucleation. Chem. Commun. 2019, 55, 6413–6416. [Google Scholar] [CrossRef]
- Chen, J.; Li, K.; Wu, S.; Liu, J.; Liu, K.; Fan, Q. Durable Anti-Icing Coatings Based on Self-Sustainable Lubricating Layer. ACS Omega 2017, 2, 2047–2054. [Google Scholar] [CrossRef]
- Ping, Z.; Nguyen, Q.; Chen, S.; Zhou, J.; Ding, Y. States of water in different hydrophilic polymers—DSC and FTIR studies. Polymer 2001, 42, 8461–8467. [Google Scholar] [CrossRef]
- Li, W.; Xue, F.; Cheng, R. States of water in partially swollen poly(vinyl alcohol) hydrogels. Polymer 2005, 46, 12026–12031. [Google Scholar] [CrossRef]
- Li, C.; Xian, G.; Li, H. Water Absorption and Distribution in a Pultruded Unidirectional Carbon/Glass Hybrid Rod under Hydraulic Pressure and Elevated Temperatures. Polymer 2018, 10, 627. [Google Scholar] [CrossRef] [PubMed]
- Ozmen, M.M.; Okay, O. Superfast responsive ionic hydrogels with controllable pore size. Polymer 2005, 46, 8119–8127. [Google Scholar] [CrossRef]
- Chavan, S.; Park, D.; Singla, N.; Sokalski, P.; Boyina, K.; Miljkovic, N. Effect of Latent Heat Released by Freezing Droplets during Frost Wave Propagation. Langmuir 2018, 34, 6636–6644. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
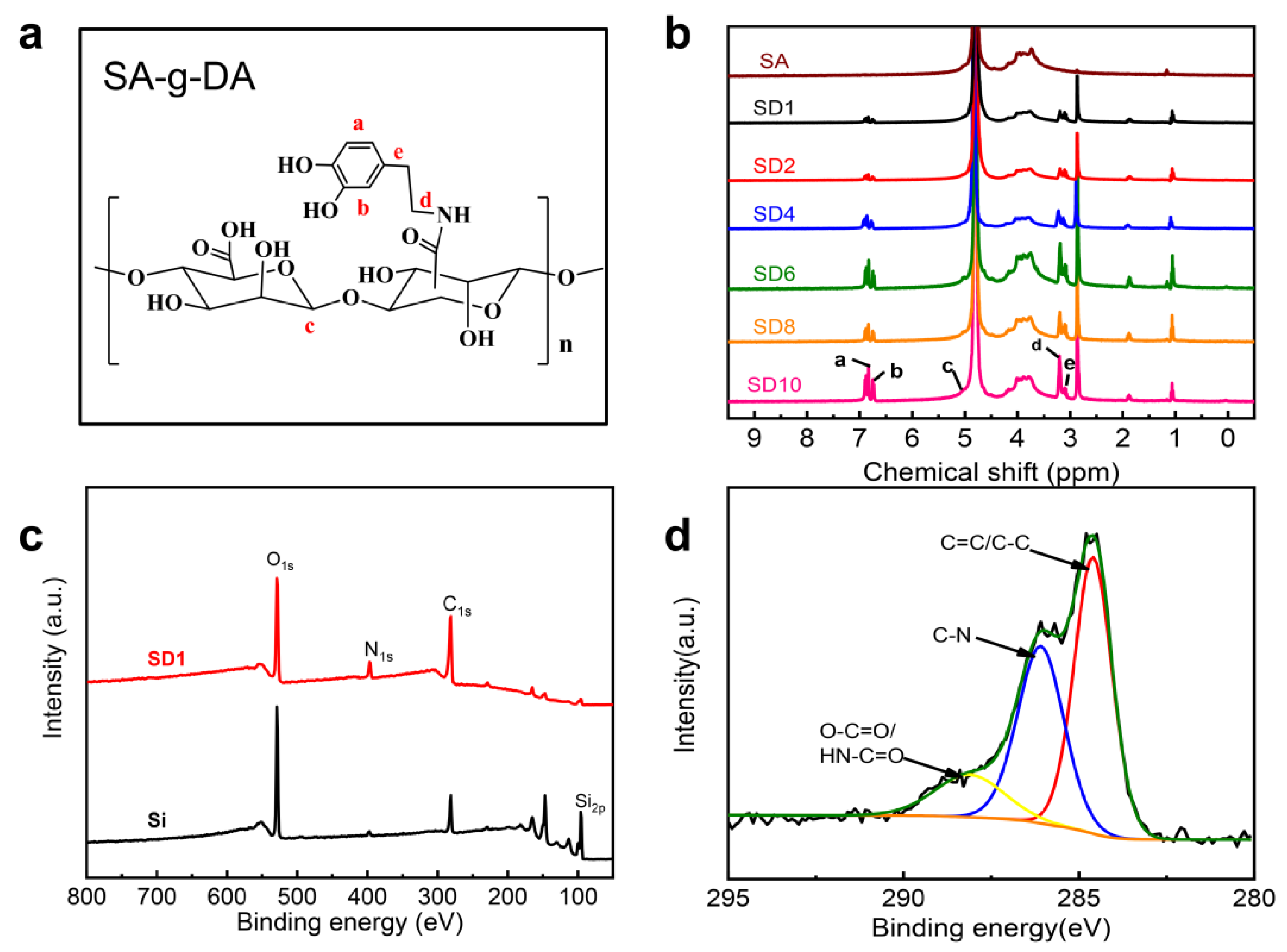


| Conjugate | SA (mmol) | DA (mmol) | SA-DA a | f b | MWU |
|---|---|---|---|---|---|
| SD1 | 4.5 | 0.45 | 10:1 | 50.4% | 32.4% |
| SD2 | 4.5 | 0.9 | 10:2 | 58.8% | 21.9% |
| SD4 | 4.5 | 1.8 | 10:4 | 59.1% | 16.3% |
| SD6 | 4.5 | 2.7 | 10:6 | 60.6% | 10.1% |
| SD8 | 4.5 | 3.6 | 10:8 | 61.3% | 6.1% |
| SD10 | 4.5 | 4.5 | 10:10 | 83.3% | 5.0% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, B.; Jiang, S.; Diao, Y.; Liu, X.; Liu, W.; Chen, J.; Yang, H. Hydrogels as Durable Anti-Icing Coatings Inhibit and Delay Ice Nucleation. Molecules 2020, 25, 3378. https://doi.org/10.3390/molecules25153378
Huang B, Jiang S, Diao Y, Liu X, Liu W, Chen J, Yang H. Hydrogels as Durable Anti-Icing Coatings Inhibit and Delay Ice Nucleation. Molecules. 2020; 25(15):3378. https://doi.org/10.3390/molecules25153378
Chicago/Turabian StyleHuang, Beili, Shanshan Jiang, Yunhe Diao, Xuying Liu, Wentao Liu, Jinzhou Chen, and Huige Yang. 2020. "Hydrogels as Durable Anti-Icing Coatings Inhibit and Delay Ice Nucleation" Molecules 25, no. 15: 3378. https://doi.org/10.3390/molecules25153378
APA StyleHuang, B., Jiang, S., Diao, Y., Liu, X., Liu, W., Chen, J., & Yang, H. (2020). Hydrogels as Durable Anti-Icing Coatings Inhibit and Delay Ice Nucleation. Molecules, 25(15), 3378. https://doi.org/10.3390/molecules25153378






