Abstract
A series of novel phenyl methoxyacrylate derivatives containing a 2-alkenylthiopyrimidine substructure were designed, synthesized, and evaluated in terms of acaricidal activity. The structures of the title compounds were identified by 1H NMR, 13C NMR and high-resolution mass spectra (HRMS). Compound (E)-methyl 2-(2-((2-(3,3-dichloroallylthio)-6-(trifluoromethyl)pyrimidin-4-yloxy)methyl)phenyl)-3-methoxyacr-ylate (4j) exhibited significant acaricidal activity against Tetranychus cinnabarinus (T. cinnabarinus) in greenhouse tests possessing nearly twice the larvicidal and ovicidal activity compared to fluacrypyrim. Furthermore, the results of the field trials demonstrated that compound 4j could effectively control Panonychuscitri with long-lasting persistence and rapid action. The toxicology data in terms of LD50 value confirmed that compound 4j has a relatively low acute toxicity to mammals, birds, and honeybees.
1. Introduction
Around 300,000 to 500,000 species of mites are distributed all over the world. Phytophagous mites are recognized as one of the most difficult pest communities to control, which can harm more than 150 crops, especially cotton, citrus, apples, vegetables, tea and flowers, causing great losses to agriculture and forestry every year [1,2,3,4,5,6,7,8]. On the other hand, due to the improper and frequent use of pesticides and a series of characteristics of mites themselves, such as the short generation cycle, rapid reproduction, high inbreeding rate, and high mutation rate, mites have already developed more and more serious resistance to the existing acaricides [9,10,11]. It is reported that 53 species of resistant ticks exist in the world [6]. Therefore, there is an urgent need for continually discovering and developing new acaricides with improved activity to safeguard crops against mites.
Compounds containing the methoxyacrylate moiety are widely applied as agrochemical acaricides [12]. Fluacrypyrim, the first strobilurin acaricide, was discovered by (Badische Anilin-und-Soda-Fabrik) BASF and developed by Nippon Soda [13]. It can effectively control all the growth stages of spider mites with a mode of action inhibiting mitochondrial electron transport in complex III within the respiratory chain [14]. Besides, Pyriminostrobin invented by the Liu group at Shenyang Sinochem Agrochemicals R&D Co., Ltd. and HNPC-A3066 discovered by Liu et al. at Hunan Research Institute of Chemical Industry, are another two typical strobilurin acaricides [15,16].
In recent years, molecules containing alkenyl and haloalkenyl moieties have played an important role in the R&D of new agrochemical chemistry. It is noteworthy that alkenyl/haloalkenyl is a multi-functional group that has been incorporated into essential structural motifs to improve the physical, chemical, and biological properties of many biologically active molecules, such as agricultural nematocides fluensulfone, 1,3-dicholorpropene; insecticides pyridalyl, flufiprole; fungicides fenpyrazamine, silthiofam and imazalil, and bactericides/fungicides/plant activators probenazole (Figure 1) [17]. Furthermore, the synthesis of new pyrimidine derivatives bearing the alkenyl/haloalkenyl group has attracted much attention from many organic chemists. Substituted 2- (alkenyl/haloalkenyl)thio)-pyrimidine showed good herbicidal, fungicidal and insecticidal activity [18,19,20,21,22,23].
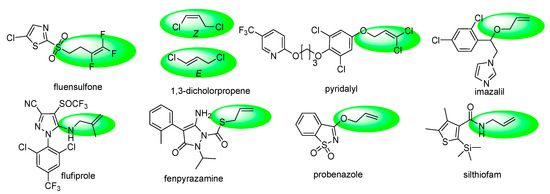
Figure 1.
The existing pesticides containing alkenyl/haloalkenyl moieties.
Organosulfur compounds are of fundamental and immense importance in organic chemistry. Sulfur-containing frameworks containing sulfur-containing heterocycles, sulfonamides, sulfonylureas, thioethers, sulfones, thioesters and thiocarbonyl compounds, have attracted considerable interest due to their unique physical and chemical properties [24,25,26,27], as well as their physiological activities [28,29,30]. Remarkably, in recent decades, thioether has emerged as the key functional groups widely used in natural products [31,32,33], medicine [24,25,34], pesticides [35,36], bioinformatics [37] and materials [28,38,39] (Figure 2). As a result, thioethers-containing molecules are attracting more and more attention based on their variety of biological activities [29].
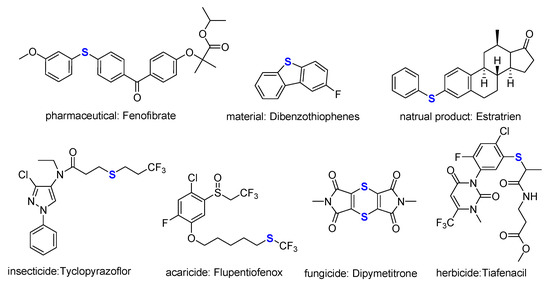
Figure 2.
Structures of the reported sulfur-containing compounds.
Traditional synthetic procedures of fluacrypyrim involve a long synthesis step, low yield and poor cost performance. With an aim to find novel strobilurin acaricides with improved acaricidal activity and cost performance, and follow the isosteric design, we try to introduce the thioether group into strobilurin fluacrypyrim and construct a pyrimidine substructure bearing alkenyl/haloalkenyl at the same time. A series of novel strobilurin compounds were designed and synthesized (Figure 3). The synthetic routes for the target compounds 4a–4u and for the intermediate 2a–2o, are displayed in Figure 4, and have the advantages of convenient synthesis, simple post-processing, and high yield. The detailed synthesis, structure and activity relationship (SAR), and acute toxicology data are presented, which would provide the basis for further research.
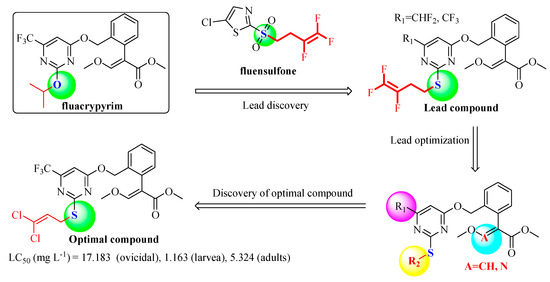
Figure 3.
Design strategy of the target compounds.
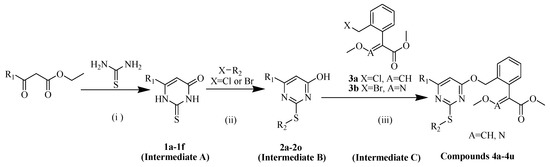
Figure 4.
Synthetic procedure for the title compounds. Reagents and conditions: (i) EtONa, EtOH, reflux, 4–6 h. (ii) K2CO3, N,N-Dimethylformamide (DMF), 40–50 °C, 3–5 h. (iii) K2CO3, DMF, 60–80 °C, 3–5 h.
2. Results and Discussion
2.1. Chemistry
The target compounds were characterized by 1H NMR, 13C NMR, melting point, high-resolution mass spectra (HRMS) analyses and some compounds were characterized by 19F NMR analyses. All the spectral and analytical data were consistent with the assigned structures (Supplementary Materials).
It is well known that the homologous elements in the same group with a higher atomic number have a lower electronegativity, so they have the stronger electron-donating ability and thus greater nucleophilicity [40,41]. Therefore, under the same conditions, the reaction between the thiol at the 2-position of the pyrimidine and halogenated alkenes could be well controlled by setting the reaction temperature between 40 and 50 °C, while the reaction between the halogenated alkenes and the hydroxyl at the 4-position of the pyrimidine could be reduced. The intermediate B is the key to synthesize the target compounds, and their synthesis procedures are shown in Figure 4. The physical properties of intermediates (1a–1f and 2a–2o) and target compounds (4a–4u) are presented in Table 1 and Table 2.

Table 1.
The physical properties of the intermediates 1a–1f and 2a–2o.

Table 2.
The physical properties of the compounds 4a–4u.
2.2. Acaricidal Activity
The acaricidal activity results of all the title compounds to control adult Tetranychus cinnabarinus (T. cinnabarinus) by using the spraying method in the greenhouse are listed in Table 3.

Table 3.
Acaricidal activities of the compounds 4a–4u against the adults of T. cinnabarinus.
In order to explore the bioactivity of our design thinking, considering the importance of alkenyl and fluorine [42,43], we firstly import the moiety 4,4-difluorobut-3-enyl of nematocide fluensulfone to acaricide fluacrypyrim while keeping the R1 group of an electron-withdrawing and hydrophobic CF3 and CHF2, and β-keto esters were used as the starting material to obtain the compounds 4a and 4b (Table 3). The bioassay results indicated that the compound 4a (R1 = CHF2) displayed around 90% control against adult T. cinnabarinus at 500 mg L−1, which was almost equal to the commercial fluacrypyrim showing 100% control at the same concentration. However, compound 4b (R1 = CF3) did not exhibit any activity even at 500 mg L−1. Based on the above result, the compound 4a was regarded as a primary lead compound for the further optimization focusing on the R2 group targeting T. cinnabarinus, while fixed R1 as CHF2 plus CF3 considered fluacrypyrim-bearing CF3 itself, although 4b had no activity. The modification on R2 was paid attention to for the allyl and the series of chloroallyl instead of 4,4-difluorobut-3-enyl, such as the 2-chloroallyl, 3-chloroallyl, 3,3-dichloroallyl, Yes 3-methylbut-2-enyl. Thus, the compounds 4c–4k were synthesized to evaluate the effect of the different R2 substituent on acaricidal activity (Table 3). Surprisingly, these compounds exhibited obviously better acaricidal activity than the lead compound 4a; among them, the acaricidal activity of the compounds 4c, 4f, 4i and 4j against adult T. cinnabarinus was comparable to fluacrypyrim at a lower concentration of 20 mg L−1 reaching the control of 80 ± 4%. Especially, compound 4i with a control effect of 70 ± 7%, and compound 4j with a control effect of 80 ± 4%, demonstrated a significantly higher acaricidal activity against adult T. cinnabarinus than fluacrypyrim with a control effect of 40 ± 7% at the same concentration of 4 mg L−1. In general, when R1 was fixed as CHF2, the acaricidal activity of the target compounds against T. cinnabarinus increased according to the order of R2: allyl ≈ 3,3-dichloroallyl > 2-chloroallyl > 3-chloroallyl. For example, at a concentration of 20 mg L−1, the acaricidal activity of the compounds 4c (R2 = allyl), 4d (R2 = 2-chloroallyl), 4e (R2 = 3-chloroallyl), and 4f (R2 = 3,3-dichloroallyl) was 80 ± 2, 50 ± 3, 20 ± 7, and 80 ± 5%, respectively. Similarly, when R1 was fixed as CF3, the acaricidal activity of the target compounds against T. cinnabarinus increased according to the order of R2: 3,3-dichloroallyl > 3-chloroallyl > 3-methylbut-2-enyl > 2-chloroallyl > allyl (Figure 5). For example, at a concentration of 20 mg L−1, the acaricidal activity of the compounds 4g (R2 = allyl), 4h (R2 = 2-chloroallyl), 4i (R2 = 3-chloroallyl), 4j (R2 = 3,3-dichloroallyl), and 4k (R2 = 3-methylbut-2-enyl) was 0, 50 ± 6, 80 ± 10, 80 ± 2, and 80 ± 3%, respectively. Additionally, the acaricidal activities of 4i and 4j were stronger than those of 4e and 4f, respectively, it seems that CF3 (R1) was required for the development of optimal acaricidal activity, rather than CHF2 (R1). Based on the analyses mentioned above, valuable information about the R2 group was revealed: that 3,3-dichloroallyl, 3-chloroallyl and allyl could be considered as optimal moieties for further optimization.
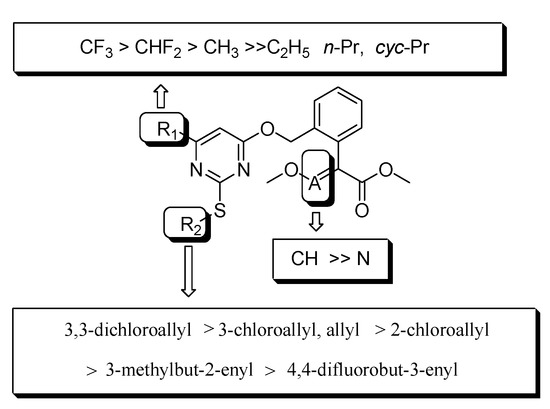
Figure 5.
Structure activity relationships for the compounds 4a–4u against adult T. cinnabarinus.
Encouraged by the above findings, R1 was still kept as CHF2, CF3 and R2 selected the three optimal moieties allyl, 3-chloroallyl and 3,3-dichloroallyl, respectively. We then turned our attention to the replacement of methoxyacrylate with methoxyiminoacetate. Six compounds (4l–4q) were prepared. To our disappointment, their acaricidal activity of the compounds 4l–4q did not show better activity than their corresponding methoxyacrylate compounds, except the pairs of 4g and 4o (4o with a little higher activity than 4g). Therefore, the methoxyacrylate group was maintained continuously in the following optimization.
Finally, based on the valuable SARs information achieved above, the R2 of 3,3-dichloroallyl and methoxyacrylate, we went back to modify the R1 of the pyrimidine ring with opposite electron effect groups like electron-donating methyl, ethyl, n-Pr and cyc-Pr. Unfortunately, all of the four compounds 4r–4u did not display any activity, even at 500 mg L−1.
Therefore, the compounds 4i (R1 = CF3, R2 = 3-chloroallyl, A = CH) and 4j (R1 = CF3, R2 = 3,3-dichloroallyl, A = CH) with methoxyacrylate were regarded as the optimal structures with acaricidal activity at 4 mg L−1, respectively, which is significantly higher than that of commercialized control fluacrypyrim.
In order to further evaluate the potential of compounds 4i and 4j, their acaricidal activity comparison was conducted with fluacrypyrim as a control against all stages of T. cinnabarinus including adults, larvae, and eggs. As the data shown in Table 4, the acaricidal activity of compound 4j against adult T. cinnabarinus (LC50: 5.324 mg L−1) was roughly equivalent to that of fluacrypyrim, (LC50: 4.178 mg L−1) and greatly higher than that of the compound 4i (LC50: 138.626 mg L−1). More importantly, compound 4j presented excellent ovicidal (LC50: 17.183 mg L−1) and larvicidal (LC50: 1.163 mg L−1) activity, nearly twice as much as fluacrypyrim (LC50: ovicidal, 43.332 mg L−1; larvicidal, 2.009 mg L−1). To date, compound 4j has shown the best acaricidal activity, so we then examined the field-trial potential of a new acaricide candidate, compound 4j ((E)-methyl 2-(2-((2-(3,3-dichloroallylthio)-6-(trifluoromethyl)pyrimidin-4-yloxy)methyl)phenyl)-3-methoxyacry-late).

Table 4.
LC50 of the acaricidal activity against T. cinnabarinus.
2.3. Field Trials
In this study, the compound 4j was selected as the most potent compound with the highest acaricidal activity against T. cinnabarinus. Field trials were carried out to confirm its field performance in November, 2018 in Nanning, Guangxi Province, China. The field trials (Table 5) indicated that the acaricidal activity of compound 4j at 100 mg L−1 against citrus red mites was roughly equivalent to that of cyenopyrafen and slightly higher than that of cyetpyrafen, a newly commercialized acaricide from Sinochem Group. At a concentration of 100 mg L−1, the effect of compound 4j on controlling the citrus red mite was 96.61, 97.36, 96.25, and 81.02% after 3, 7, 10, and 20 days of treatment, respectively, while cyenopyrafen revealed a controlling effect of 92.26, 97.85, 96.43, and 80.87% against Panonychuscitri after 3, 7, 10, and 20 days of treatment, respectively. At the same concentration, the effect of cyetpyrafenon controlling citrus red mite was also 97.70, 100, 99.38, and 89.79% after 3, 7, 10 and 20 days of treatment, respectively. The comparison of the above-treatment results indicated that the compound 4j was a highly active and rapid-acting acaricidal compound, which can effectively control Panonychuscitri at an application rate of 100 mg L−1.

Table 5.
Acaricidal activity of the compound 4j against P. Citri in field tests (Nanning, China).
2.4. The Toxicity of Compound 4j
The toxicity of compound 4j on primary mammals, birds, and honeybees was evaluated by The Safety Evaluation Center of Shenyang Research Institute of Chemical Industry (National Shenyang New Drug Safety Evaluation and Research Center, Shenyang, China) and Zhejiang Academy of Agricultural Science in 2019 as presented in Table 6. It was found that (1) the acute oral median lethal dose (LD50) of compound 4j for rats is higher than 500 mg kg−1; (2) it has a low toxicity for birds (>2090 mg a.i./kg) and honeybees (>116 µg a.i./honeybee); and (3) it moderately irritates rabbits’ eyes.

Table 6.
Toxicity results of the compound 4j.
3. Materials and Methods
3.1. Reagents and Instruments
The reagents were all high purity analytical or chemical grades, purchased from commercial sources and were used as received. All anhydrous solvents were dried and distilled by standard techniques before use. The melting points were determined by using an RY-1 melting point apparatus (TaiKe, Beijing, China). The 1H NMR, 13C NMR, and 19F NMR spectra were recorded utilizing an AV 400/500 spectrometer (Bruker, Karlsruhe, Germany) in CDCl3 or DMSO-d6 solution using tetramethylsilane as an internal standard, and the chemical shifts (δ) were given in parts per million (ppm). High-resolution mass spectra (HRMS) data were obtained by employing Fourier transform ion cyclotron resonance mass spectrometry (FTICR-MS) (ionspec, 7.0 T). The reaction progress was monitored by thin-layer chromatography (TLC) on silica gel GF254 (Tianzhe, Qingdao, China), and the spots were visualized with ultraviolet (UV) light (Gonyi, Zhengzhou, China).
3.2. Synthetic Chemistry
All the title compounds were synthesized as described in Figure 4 (Page 6). General procedures for the preparation of the title compounds (4a–4u) and intermediates (1a–1f and 2a–2o) according to the related literature [44,45,46] and spectra data of the title compounds (4a–4u) are provided in the Supplementary Materials. Intermediate 3a was obtained from 1H-isochromene-3(4H)-one as the starting material via a multi-step synthesis referring to the literatures [47,48]. Intermediate 3b was synthesized using a 2-methylbenzoic acid as the starting material [49,50].
3.2.1. Synthesis of 6-Substituent-2-thioxo-2,3-dihydropyrimidin-4(1H)-one (1a–1f)
All of the thiouracil derivatives (R1 = CH3, C2H5, n-Pr, cyc-Pr, CHF2, CF3) were prepared as followed. To a 1000 mL three-neck flask equipped with a magnetic stirrer, a thermometer, and a dropping funnel, 500 mL of methanol and 59.4 g (1.1 mol) of solid sodium methoxide were added, and the mixture was then stirred at room temperature. After 0.5 h, 0.5 mol (93.0 g) of ethyl 4,4,4-trifluoro-3-oxobutanoate and 0.6 mol of thiourea were added to the flask, and the mixture was heated to reflux for about 4 h. Finally, a 2 M HCl aqueous solution was added to the flask until the reaction mixture was neutralized. The resultant solid was separated by filtration, then washed with water, and finally dried in a desiccator to produce 88.3 g of intermediate 1b as a white solid at a yield of 90%; melting point: 247.5–248.2 °C; 1H NMR (500 MHz, DMSO-d6) δ 13.37 (br, 1H), 12.78 (s, 1H), 6.34 (s, 1H).
3.2.2. Synthesis of 2-(3,4,4-Trifluorobut-3-enylthio)-6-(trifluoromethyl)pyrimidin-4-ol (2f)
Potassium carbonate (15.5 g, 98.0%, 0.11 mol) was added into a solution of 14.5 g (0.10 mol) of 6-trifluoromethyl-2-thiouracil (1b, R1 = CF3) in 100 mL of DMF, and the reaction mixture was stirred at 40 °C for 0.5 h, followed by the addition of 19.1 g (0.10 mol) of 4-bromo-1,1,2-trifluorobut-1-ene; then, the reaction mixture was heated to 50 °C for about 3 h and monitored by TLC until the reaction was complete; afterward, the mixture was poured into water, and a 2 M HCl aqueous solution was added to the flask until the reaction mixture was neutralized. The resultant solid was separated by filtration, then washed with water, and finally dried in a desiccator to obtain 28.0 g of the intermediate 2f as a white solid at a yield of 92%; melting point: 82.6–83.7 °C; 1H NMR (500 MHz, DMSO-d6) δ13.33 (br, 1H, OH), 6.55 (s,1H, pyrimidy1-H), 3.26 (t, J = 9.0 Hz, 2H, CH2), 2.69–2.75 (m, 2H, CH2).
3.2.3. Synthesis of 2-(2-Chloroallylthio)-6-(trifluoromethyl)pyrimidin-4-ol (2h)
Potassium carbonate (15.5 g, 98.0%, 0.11 mol) was added into a solution of 14.5 g (0.10 mol) of 6-trifluoromethyl-2-thiouracil (1b) in 100 mL of DMF, and the reaction mixture was stirred at 40 °C for 0.5 h, followed by the addition of 11.0 g (0.10 mol) of 2,3-dichloroprop-1-ene; afterward, the reaction mixture was heated to 50 °C for about 3 h and monitored by thin-layer chromatography until the reaction was complete; the mixture was then poured into water, and a 2 M HCl aqueous solution was added to the flask until the reaction mixture was neutralized. The resultant solid was separated by filtration, then washed with water (60 mL), and finally dried in a desiccator to form 25.0 g of intermediate 2h as a white solid at a yield of 93%; melting point: 116.2–116.8 °C; 1H NMR (500 MHz, DMSO-d6) δ 13.36 (br, 1H, OH), 6.58 (s, 1H, pyrimidy1-H), 5.54 (s, 1H, CH2), 5.32 (s, 1H, CH2), 4.10 (s, 2H, CH2).
3.2.4. Synthesis of (E)-Methyl 3-methoxy-2-(2-((2-(3,4,4-trifluorobut-3-enylthio)-6-(trifluoromethyl)pyrimidin-4-yloxy)methyl)phenyl)acrylate Compound 4b
Potassium carbonate (1.55 g, 98.0%, 0.011 mol) and 2-(3,4,4-trifluorobut-3-enylthio)-6-(trifluoromethyl)pyrimidin-4-ol (2f) (2.5 g, 0.01 mol) were added to 50 mL of DMF and reacted for 1 h at 60 °C; then, 2.55 g (0.011 mol) of (E)-methyl 2-(2-(chloromethyl)phenyl)-3-methoxyacrylate 3a was introduced into the batches. The reaction temperature was raised to 80 °C and maintained for 2 h; the reaction was monitored by TLC. Afterward, the reaction mixture was poured into water and extracted with ethyl acetate (50 mL). The organic phase was washed successively with water (20 mL) and saturated brine (20 mL), then dried, filtered, and evaporated under reduced pressure. The residue was purified by silica gel chromatography using ethyl acetate/petroleum ether (1:10, v/v) as the eluent in a temperature range of 60–90 °C to obtain 4.4 g of the compound 4b as a yellow oil at a yield of 87%. 1H NMR (500 MHz, DMSO-d6) δ 7.61 (s, 1H, CH), 7.48–7.50 (m, 1H, Ar-H), 7.22–7.36 (m, 2H, Ar-H), 7.13–7.15 (m, 1H, Ar-H), 7.11 (s, 1H, pyrimidy1-H), 5.35 (s, 2H, CH2), 3.78 (s, 3H, CH3), 3.57 (s, 3H, CH3), 3.33 (t, J = 7.0 Hz, 2H, CH2), 2.73–2.81 (m, 2H, CH2); 13C NMR (125 MHz, DMSO-d6) δ 171.76, 169.42, 166.85, 160.87, 160.81, 154.89 (q, J = 36.3 Hz), 153.24 (m), 134.29, 132.65, 131.30, 128.64, 128.14, 127.62, 127.81 (m), 120.24 (q, J = 272.5 Hz), 108.66, 101.43(q, J = 2.5 Hz), 67.12, 61.79, 51.16, 25.49, 25.35 (dd, J1 = 21.3 Hz, J2 = 2.5 Hz); HRMS: m/z 531.0787 (M + Na)+(calcd. [M + Na]+ 531.0784.
3.3. Acaricidal Activities Assay
The detailed procedure of the acaricidal activity against adult T. cinnabarinus was measured according to the related literature [51].
Each of the test compounds was first dissolved in a mixture of acetone and water (at a volume ratio of 9 to 1) containing 0.1% TWEEN® 80 to furnish the stock solutions. A series of test solutions were then prepared by diluting the stock solutions with water containing 0.1% TWEEN® 80. Afterward, the kidney bean plants with one true leaf were infested with T. cinnabarinus (carmine spider mite) prior to spraying. An airbrush was used to spray the plants with the test solutions, and each bioassay was replicated three times at a temperature of 25 ± 1 °C to meet the statistical requirements. After the plants were dried, they were transferred to a maintenance room for observation. The mortality rate of the spider mite was investigated after 72 h. The mites that did not either fly away or respond to the touch of a fine brush were considered to be dead. The rate of mortality was evaluated according to a percentage scale of 0–100, in which 0 indicates no activity of the test solutions against the spider mites, and 100 indicates totally killing them. When the percentage of the mortality of the blank control was less than 5%, the results of the treatment were directly used. However, if the percentage of the mortality of the blank control ranged from 5 to 20%, the results were corrected by the means of the following equation:
where, V is the value of the corrected percentage of mortality, and X represents the viability of the blank control; Y stands for the viability of the treatment. As a positive control, commercial fluacrypyrim was also evaluated using exactly the same procedure.
V = (X − Y)/Y × 100
4. Conclusions
In summary, a series of phenyl methoxyacrylates containing 2-alkenylthiopyrimidine derivatives were designed, synthesized, and evaluated in terms of acaricidal activity. Compound 4j exhibited significant acaricidal activity against the adults, larvae, and eggs of T. cinnabarinus (Boisduval), significantly higher than fluacrypyrim. Furthermore, the field trials proved that the acaricidal efficacy of compound 4j was approximately equivalent to those of cyetpyrafen and cyenopyrafen. The field trials also demonstrated that compound 4j at a concentration of 100 mg L−1 presented a control effect of 96.25% and 81.02% against Panonychuscitri after 10 and 20 days of treatment, respectively, indicating that it has long-lasting persistence (20 days) in field trials. It also acts rapidly against Panonychuscitri by reaching a controlling effect of 96.61% after 3 days of treatment. The compound 4j has quite low acute toxicity to mammals, birds, and honeybees. Finally, the findings of the current work suggested that the compound 4j (3,3-dichloroallylthio, trifluoromethyl, pyrimidine) could be a novel acaricide candidate for the control of spider mites, which is worthy of being further studied.
Supplementary Materials
The following are available online, the data of title compounds 4a–4u and spectrogram of title compounds 4a–4u (Figures S1–S36) are presented as Supporting Information.
Author Contributions
X.D. and S.H. conceived and designed the experiments; S.H. and Y.C. realized the synthetic work; S.H., Z.C. and Y.C. analyzed the data and wrote the paper; manuscript review and edit, X.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research & Development Program of China of Funder, grant number 2016YFD0300708.
Acknowledgments
The acaricidal activity assay of this work was evaluated by the Bioassay Testing and Safety Assessment Center in Zhejiang Research Institute of Chemical Industry and the Bioassay Testing Center in Shenyang Sinochem Agrochemicals R&D Co. Ltd. The toxicity test was carried out by the National Safety and Evaluation Center of New Drug (Shenyang) and Zhejiang Academy of Agricultural Sciences.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, Q.S.; Zhao, S.; Zou, J.; Shi, L.; He, L. Monitoring of acaricide resistance in Tetranychus Cinnabarinus. Chin. J. Appl. Entomol. 2012, 4, 364–369. [Google Scholar]
- Ma, L.N.; Hu, J.H.; Lei, H.D. Research progress of botanical acaricides. Chin. Agric. Sci. Bull. 2008, 24, 377–380. [Google Scholar]
- Hua, N.Z. A review of new high efficiency and low toxicity acaricides. World Pestic. 2016, 3, 25–34. [Google Scholar]
- Van Leeuwen, T.; Vontas, J.; Tsagkarakou, A.; Dermauw, W.; Tirry, L. Acaricide resistance mechanisms in the two-spotted spider mite Tetranychusurticae and other important acari: A review. Insect Biochem. Mol. Biol. 2010, 40, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.L.; Montez, G.H.; Liu, L.; Grafton-Cardwella, E.E. Spirodiclofen and spirotetramat bioassays for monitoring resistance in citrus red mite panonychus citri (Acari: Tetranychidae). Pest Manage. Sci. 2012, 68, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Shen, J.Z.; You, W.L. Research on advances of acaricide mechanism. Pestic. Sci. Adm. 1999, 20, 16–20. [Google Scholar]
- Mark, A.D. Review acaricide mode of action. Pest Manag. Sci. 2005, 61, 103–110. [Google Scholar]
- Van Leeuwen, T.; Tirry, L.; Yamamoto, A.; Nauen, R.; Wannes, D. The economic importance of acaricides in the control of phytophagous mites and an update on recent acaricide mode of action research. Pestic. Biochem. Physiol. 2015, 121, 12–21. [Google Scholar] [CrossRef]
- Khalighi, M.; Dermauw, W.; Wybouw, N.; Bajda, S.; Osakabe, M.; Tirrya, L.; Van Leeuwena, T. Molecular analysis of cyenopyrafen resistance in the two-spotted spider mite Tetranychus. Pest Manag. Sci. 2016, 72, 103–112. [Google Scholar] [CrossRef]
- Kramer, T.; Nauen, R. Monitoring of spirodiclofen susceptibility in field populations of European red mites, Panonychusulmi (Koch) (Acari: Tetranychidae), and the cross-resistance pattern of a laboratory-selected strain. Pest Manag. Sci. 2011, 67, 1285–1293. [Google Scholar] [CrossRef]
- Feng, K.Y.; Yang, Y.W.; Wen, X.; Ou, S.Y.; Zhang, P.; Yu, Q.; Zhang, Y.C.; Shen, G.M.; Xu, Z.F.; Li, J.H.; et al. Stability of cyflumetofen resistance in Tetranychus Cinnabarinus and its correlation with glutathione-S-transferase gene expression. Pest Manag. Sci. 2019, 75, 2802–2809. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.T.; Liu, J.L.; Song, Y.Q.; Liu, S.W. Research progress on strobilurin acaricides. In Proceedings of the 14th Annual Meeting of the Pesticide Committee of the Chinese Chemical Society, Shenyang, China, 18–20 June 2010. [Google Scholar]
- Liu, C.L.; Guan, A.Y.; Liu, Z.L. The novel (E)-methyl-β-methoxyacrylate acaricides fluacrypyrim. Word Pestic. 2002, 24, 44–45. [Google Scholar]
- Beautement, K.; Clough, J.M.; de Fraine, P.J.; Godfrey, C.R.A. Fungicidal beta-methoxyacrylates: From natural products to novel synthetic agricultural fungicides. Pestic. Sci. 1991, 31, 499–519. [Google Scholar] [CrossRef]
- Chai, B.S.; Liu, C.L.; Li, H.C.; Zhang, H.; Liu, S.W.; Huang, G.; Chang, J.B. The discovery of SYP-10913 and SYP-11277: Novel strobilurin acaricides. Pest Manag. Sci. 2011, 67, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.P.; Wang, X.G.; Chen, C.; Pei, H.; Mao, C.H.; Wang, Y.J.; He, H.J.; Huang, L.; Liu, X.P.; Hu, Z.B.; et al. The discovery of HNPC-A3066: A novel strobilurin acaricide. Pest Manag. Sci. 2009, 65, 229–234. [Google Scholar] [CrossRef]
- Liu, C.L.; Yang, J.C. Handbook of Modern Pesticide; Chemical Industry Press: Beijing, China, 2018. [Google Scholar]
- D’Amico, J. Certain 2-(Haloalkenylthio)-4,6-dimethylpyrimidines. U.S. Parent US3557112A, 19 January 1971. [Google Scholar]
- Tetsuo, T.; Yasutomo, T.; Mitsuaki, T.; Seiji, T.; Hiroshi, H. Pyrimidinyloxyalkanamide Derivatives and Herbicide Compositions Containing Them. European Patent EP212969, 3 March 1987. [Google Scholar]
- Edwards, L.H. Fungicidal and Insecticidal 2-Thiohaloalkenyl-4-dialkoxyphosphino-thioyloxy-6-alkyl-1,3-pyrimidines. U.S. Patent US4402952A, 6 September 1983. [Google Scholar]
- Katsumi, M.; Ikumi, U.; Tsuyoshi, A.; Katsumi, F.; Yoshiyuki, K.; Norimichi, M. Preparation of Pyrimidinyloxyalkanoic Amide Derivatives as Fungicides for Agricultural and Horticultural Use. World Patent WO9818766, 7 May 1998. [Google Scholar]
- Fitzjohn, S.; Robinson, M.P.; Turnbull, M.D. Heterocyclic Compounds with Parasitical Activity. World Patent WO9406777A1, 31 March 1994. [Google Scholar]
- Turnbull, M.D. Nematicide Pyrimidine Derivatives. European Patent EP506269A1, 30 September 1992. [Google Scholar]
- Ilardi, E.A.; Vitaku, E.; Njardarson, J.T. Data-mining for sulfur and fluorine: An evaluation of pharmaceuticals to reveal opportunities for drug design and discovery. J. Med. Chem. 2014, 57, 2832–2842. [Google Scholar] [CrossRef]
- Feng, M.; Tang, B.; Liang, S.; Jiang, X. Sulfur containing scaffolds in drugs: Synthesis and application in medicinal chemistry. Curr. Top. Med. Chem. 2016, 16, 1200–1216. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.R.; Eastman, C.M.; Njardarson, J.T. Beyond C,H,O, and N analysis of the elemental composition of U.S. FDA approved drug architectures. J. Med. Chem. 2014, 57, 9764–9773. [Google Scholar] [CrossRef]
- Xiong, H.Y.; Pannecoucke, X.; Besset, T. Recent advances in the synthesis of SCF2H- and SCF2FG-containing molecules. Chem. Eur. J. 2016, 22, 16734–16749. [Google Scholar] [CrossRef]
- Mishra, A.; Ma, C.Q.; Baüerle, P. Functional oligothiophenes: Molecular design for multidimensional nanoarchitectures and their applications. Chem. Rev. 2009, 109, 1141–1276. [Google Scholar] [CrossRef]
- Scott, K.A.; Njardarson, J.T. Analysis of US FDA-approved drugs containing sulfur atoms. Top. Curr. Chem. 2018, 376, 5. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Fan, Q.; Jiang, X. Transition-metal-free diarylannulated sulfide and selenide construction via radical/anion-mediated sulfur−iodine and selenium−iodine exchange. Org. Lett. 2016, 18, 5756–5759. [Google Scholar] [CrossRef] [PubMed]
- Borthwick, A.D. 2,5-Diketopiperazines: Synthesis, reactions, medicinal chemistry, and bioactive natural products. Chem. Rev. 2012, 112, 3641–3716. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Hale, C.R.H.; Nilewski, C.; Ioannidou, H.A. Constructing molecular complexity and diversity: Total synthesis of natural products of biological and medicinal importance. Chem. Soc. Rev. 2012, 41, 5185–5238. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, X. Transfer of sulfur: From simple to diverse. Chem. Asian J. 2013, 8, 2546–2563. [Google Scholar] [CrossRef]
- Davison, E.K.; Sperry, J. Natural products with heteroatom-rich ring systems. J. Nat. Prod. 2017, 80, 3060–3079. [Google Scholar] [CrossRef]
- Available online: http://www.alanwood.net/pesticides/index.html (accessed on 21 May 2020).
- Liu, C.L.; Chai, B.S. New Agrochemicals Discovery and Synthesis; Chemical Industry Press: Beijing, China, 2013. [Google Scholar]
- Lin, Y.; Zhang, S.Z.; Block, E.; Katz, L.C. Encoding social signals in the mouse main olfactory bulb. Nature 2005, 434, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Nair, D.P.; Podgórski, M.; Shunsuke, C.; Gong, T.; Xi, W.X.; Fenoli, C.R.; Bowman, C.N. The thiol-Michael Addition Click Reaction: A powerful and widely used tool in materials chemistry. Chem. Mater. 2014, 26, 724–744. [Google Scholar] [CrossRef]
- Bürchstümmer, H.; Weissenstein, A.; Bilalas, D.; Würthner, F. Synthesis and characterization of optical and redox properties of bithiophene-functionalized diketopyrrolopyrrole chromophores. J. Org. Chem. 2011, 76, 2426–2432. [Google Scholar]
- Rakhimov, A.I.; Titova, E.S.; Fedunov, R.G.; Babkin, V.A. Special features of the nucleophilic substitution of halogen in alkyl and benzyl halides with anions generated from 4-hydroxy-2-mercapto-6-methylpyrimidine. Chem. Heterocycl. Compd. 2008, 44, 700–708. [Google Scholar] [CrossRef]
- Harold, W.B.; Irving, G.; Karl, D. The synthesis of 5-halogeno-2-thiouracil and 6-methyl-5-halogeno-2-thiouracil derivatives. J. Am. Chem. Soc. 1948, 70, 1753–1756. [Google Scholar]
- Uneyama, K. Organofluorine Chemistry; Blackwell: Oxford, UK, 2006. [Google Scholar]
- Kirsch, P. Modern Fluoroorganic Chemistry: Synthesis, Reactivity, Applications; Wiley-VCH: Weinheim, Germany, 2013. [Google Scholar]
- Erkin, A.V.; Krutikov, V.I.; Chubraev, M.A. Synthesis of 2-(pyrazol-1-yl)pyrimidine derivatives by cyclocondensation of ethyl acetoacetate (6-methyl-4-oxo-3,4-dihydropyrimidin-2-yl)hydrazone with aromatic aldehydes. Russ. J. Gen. Chem. 2004, 74, 423–427. [Google Scholar] [CrossRef]
- Tang, M.P.; Jia, J.X.; Xue, J.J.; Liu, J.P. Action of thiouracil derivatives as novel thermal stabilizers for poly (vinyl chloride) and the synergistic effect with calcium stearate. Russ. J. Appl. Chem. 2017, 90, 129–137. [Google Scholar] [CrossRef]
- Slivka, N.Y.; Gevaza, Y.I.; Staninets, V.I. Halocyclization of substituted 2-(alkenylthio)pyrimidin-6-ones. Chem. Heterocycl. Compd. 2004, 40, 660–666. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Hao, S.L. Efficient Green Synthesis Method of Agricultural Fungicide Picoxystrobin. Chinese Patent CN104230794, 24 December 2014. [Google Scholar]
- Yasuyuki, M.; Takahiro, S.; Yutaka, I.; Hiroyuki, Y.; Makoto, F.; Mitsuru, T.; Yoshiyuki, I.; Satoru, Y.; Noriaki, K. Process for Producing Acrylic Acid Derivative. U.S. Parent US20040152894, 5 August 2004. [Google Scholar]
- Bernd, W.; Siegbert, B.; Franz, S.; Thomas, K.; Franz, R.; Eberhard, A.; Gisela, L. Preparation of (pyridinyloxy)phenylglyoxylate O-methyl Oximes as Agrochemical Fungicides. U.S. Parent US5554578, 10 September 1996. [Google Scholar]
- Sanghyuck, L.; Oh Seok, K.; Chang-Soo, L.; Won, M.; Ban, H.S.; Ra, C.S. Synthesis and biological evaluation of kresoxim-methyl analogues as novel inhibitors of hypoxia-inducible factor (HIF)-1 accumulation in cancer cells. Bioorg. Med. Chem. Lett. 2017, 27, 3026–3029. [Google Scholar]
- Xie, Y.; Xu, Y.; Liu, C.L.; Guan, A.Y.; Ban, L.F.; Ding, F.; Peng, W. Intermediate derivatization method in the discovery of new acaricide candidate: Synthesis of N-substituted piperazines derivatives and their activity against phytophagous mites. Pest Manag. Sci. 2017, 73, 945–952. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).