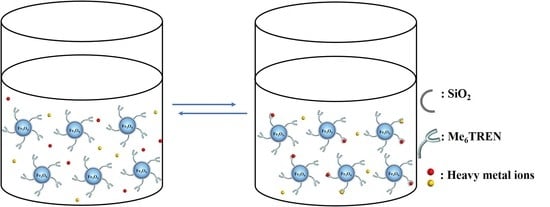
Reusable Magnetic Nanoparticle Immobilized Nitrogen-Containing Ligand for Classified and Easy Recovery of Heavy Metal Ions
Abstract
1. Introduction
2. Results and Discussion
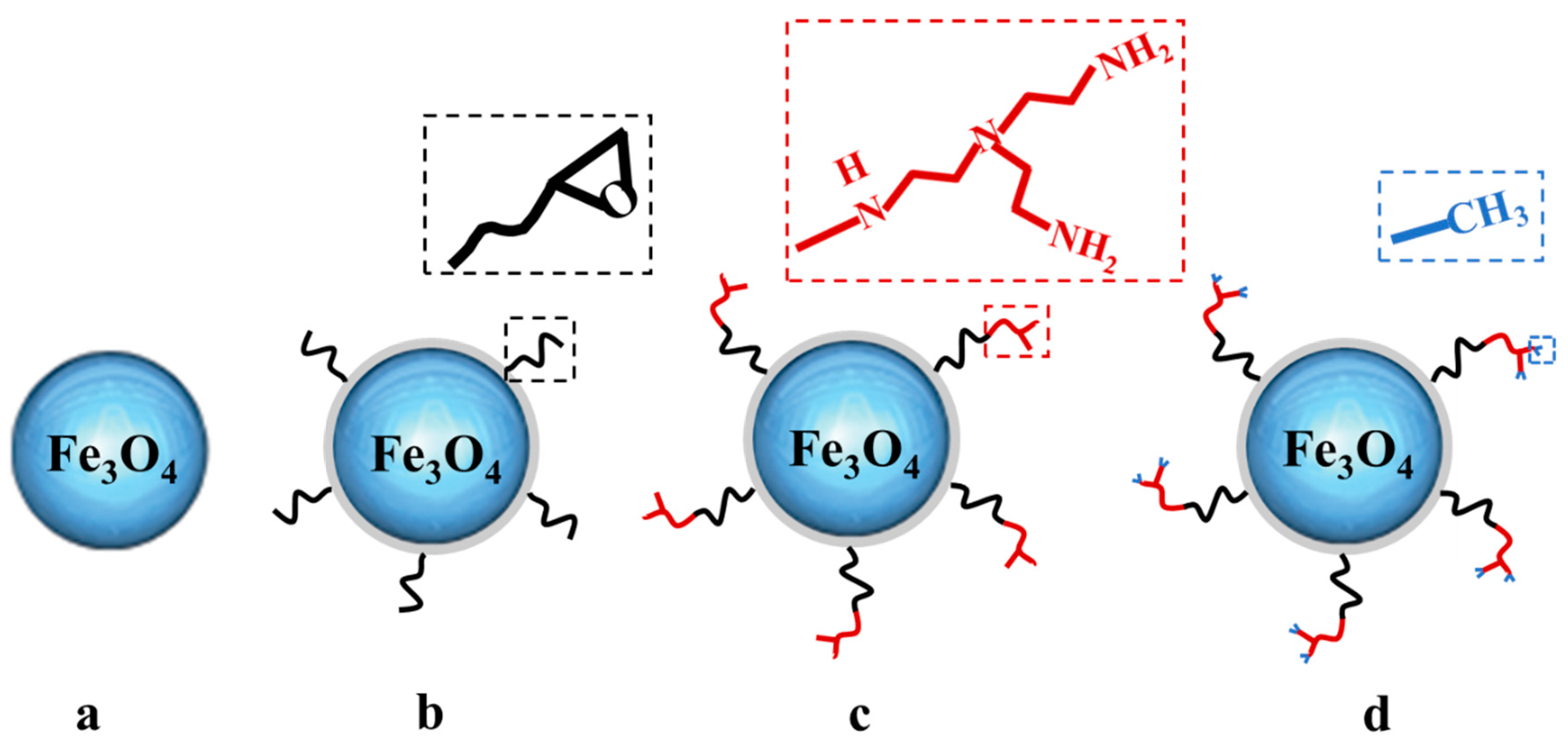
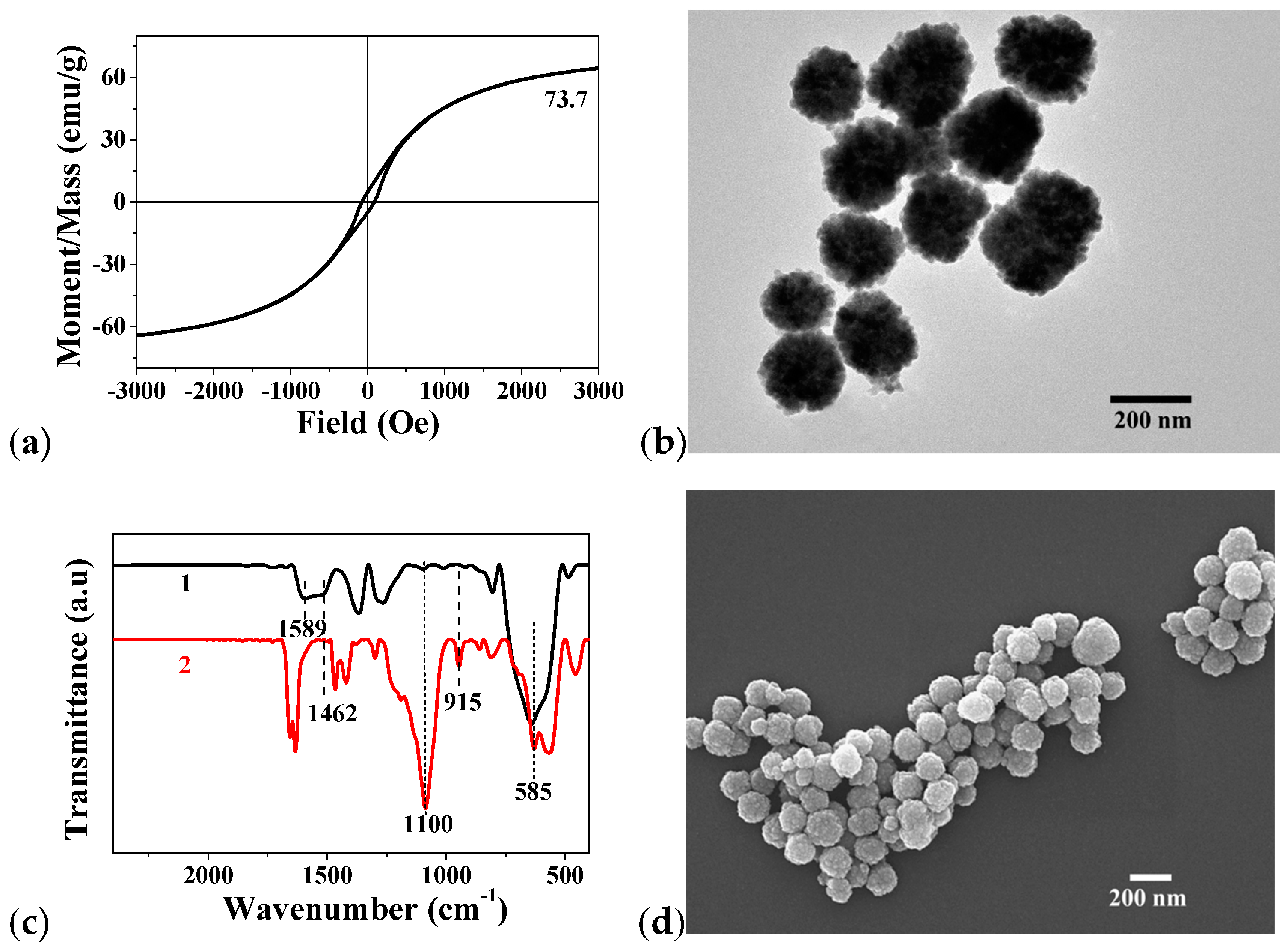
2.1. The Fe3O4@epoxide NPs
2.2. The Fe3O4@Me6TREN NP
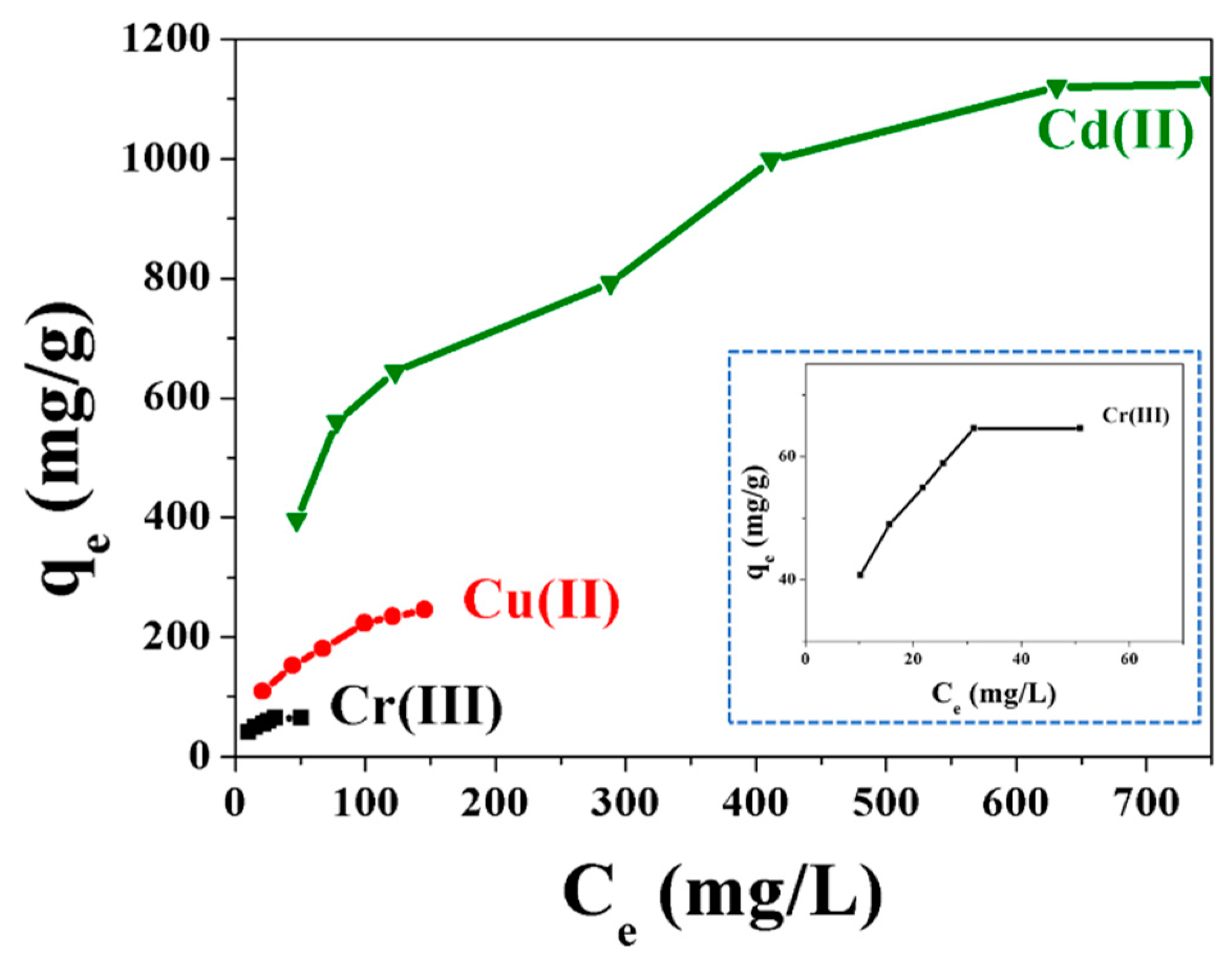
2.3. Adsorption of Heavy Metal Ions in Water with Fe3O4@Me6TREN NPs
2.4. Desorption of Heavy Metal Ions from Fe3O4@Me6TREN NPs
3. Materials and Methods
3.1. Materials
3.2. Synthesis of PEG-Capped Fe3O4 NPs
3.3. Synthesis of Epoxide-Capped Fe3O4 NPs (Fe3O4@epoxide)
3.4. Synthesis of Tris(2-aminoethyl) Amine-Grafted Fe3O4 NPs (Fe3O4@TAEA)
3.5. Synthesis of Me6TREN-Grafted Fe3O4 NP Ligand (Fe3O4@Me6TREN)
3.6. Adsorption of Heavy Metal Ions in Water with Fe3O4@Me6TREN NPs
3.7. Desorption of Heavy Metal Ions from Fe3O4@Me6TREN NPs
3.8. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sharma, Y.C.; Srivastava, V.; Singh, V.K.; Kaul, S.N.; Weng, C.H. Nano-adsorbents for the removal of metallic pollutants from water and wastewater. Environ. Technol. 2009, 30, 583. [Google Scholar] [CrossRef] [PubMed]
- Awual, M.R. Assessing of lead(III) capturing from contaminated wastewater using ligand doped conjugate adsorbent. Chem. Eng. J. 2016, 289, 65–73. [Google Scholar] [CrossRef]
- Fu, F.L.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Carolin, C.F.; Kumar, P.S.; Saravanan, A.; Joshiba, G.J.; Naushad, M. Efficient techniques for the removal of toxic heavy metals from aquatic environment: A review. J. Environ. Chem. Eng. 2017, 5, 2782–2799. [Google Scholar] [CrossRef]
- Cao, J.S.; Wang, C.; Fang, F.; Lin, J.X. Removal of heavy metal Cu(II) in simulated aquaculture wastewater by modified palygorskite. Environ. Pollut. 2016, 219, 924–931. [Google Scholar] [CrossRef]
- Qu, J.H.; Tian, X.; Jiang, Z.; Cao, B.; Akindolie, M.S.; Hu, Q.; Feng, C.C.; Feng, Y.; Meng, X.L.; Zhang, Y. Multi-component adsorption of Pb(II), Cd(II) and Ni(II) onto microwave-functionalized cellulose: Kinetics, isotherms, thermodynamics, mechanisms and application for electroplating wastewater purification. J. Hazard. Mater. 2020, 387, 121718. [Google Scholar] [CrossRef]
- Isaac, R.A.; Gil, L.; Cooperman, A.N.; Hulme, K.; Eddy, B.; Ruiz, M.; Jacobson, K.; Larson, C.; Pancorbo, O.C. Corrosion in drinking water distribution systems: A major contributor of copper and lead to wastewaters and effluents. Environ. Sci. Technol. 1997, 31, 3198–3203. [Google Scholar] [CrossRef]
- Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011; pp. 398–403.
- Chen, Q.Y.; Yao, Y.; Li, X.Y.; Lu, J.; Zhou, J.; Huang, Z.L. Comparison of heavy metal removals from aqueous solutions by chemical precipitation and characteristics of precipitates. J. Water Process. Eng. 2018, 26, 289–300. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Luo, Z.; Hills, C.; Xue, G.; Tyrer, M. Precipitation of heavy metals from wastewater using simulated flue gas: Sequent additions of fly ash, lime and carbon dioxide. Water Res. 2009, 43, 2605–2614. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Pintauer, T.; Gaynor, S. Removal of copper-based catalyst in atom transfer radical polymerization using ion exchange resins. Macromolecules 2000, 33, 1476–1478. [Google Scholar] [CrossRef]
- Beker, U.G.; Guner, F.S.; Dizman, M.; Erciyes, A.T. Heavy metal removal by ion exchanger based on hydroxyethyl cellulose. J. Appl. Polym. Sci. 1999, 74, 3501–3506. [Google Scholar] [CrossRef]
- Sheng, D.; Liu, X.H.; Liao, J.B.; Lin, H.; Liu, F. PEI modified multiwalled carbon nanotube as a novel additive in PAN nanofiber membrane for enhanced removal of heavy metal ions. Chem. Eng. J. 2019, 375, 122086. [Google Scholar]
- Zhao, F.Y.; Ji, Y.L.; Weng, X.D.; Mi, Y.F.; Ye, C.C.; An, Q.F.; Gao, C.J. High-flux positively charged nanocomposite nanofiltration membranes filled with poly(dopamine) modified multiwall carbon nanotubes. ACS Appl. Mater. Inter. 2016, 8, 6693–6700. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.L.; Hu, W.; Guo, M.M.; Wang, Y.L.; Jia, J.P.; Hu, Z.B. Preparation of cotton-based fibrous adsorbents for the removal of heavy metal ions. Carbohyd. Polym. 2019, 225, 1152182. [Google Scholar] [CrossRef] [PubMed]
- Lia, Y.Q.; Guo, C.F.; Shi, R.H.; Zhang, H.; Gong, L.Z.; Dai, L.B. Chitosan/ nanofibrillated cellulose aerogel with highly oriented microchannel structure for rapid removal of Pb(II) ions from aqueous solution. Carbohyd. Polym. 2019, 223, 115048. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zheng, F.; Wang, J.; Zhou, J.M.; Huang, X.H.; Chen, L.; Hu, P.F.; Gao, J.M.; Zhen, Q.; Bashir, S. Facile preparation of zeolite-activated carbon composite from coal gangue with enhanced adsorption performance. Chem. Eng. J. 2020, 390, 124513. [Google Scholar] [CrossRef]
- Li, C.; Ma, H.Y.; Venkateswaran, S.; Hsiao, B.S. Highly efficient and sustainable carboxylated cellulose filters for removal of cationic dyes/heavy metals ions. Chem. Eng. J. 2020, 389, 123458. [Google Scholar] [CrossRef]
- Salam, M.A. Removal of heavy metal ions from aqueous solutions with multi-walled carbon nanotubes: Kinetic and thermodynamic studies. Int. J. Environ. Sci. Technol. 2013, 10, 677–688. [Google Scholar] [CrossRef]
- Militaru, B.A.; Pode, R.; Lupa, L.; Schmidt, W.; Tekle-Röttering, A.; Kazamer, N. Using sewage sludge ash as an efficient adsorbent for Pb(II) and Cu(II) in single and binary systems. Molecules 2020, 25, 2559. [Google Scholar] [CrossRef]
- Liang, X.X.; Wang, N.; Qu, Y.L.; Yang, L.Y.; Wang, Y.G.; Ouyang, X.K. Facile preparation of metal-organic framework (MIL-125)/chitosan beads for adsorption of Pb(II) from aqueous solutions. Molecules 2018, 23, 1524. [Google Scholar] [CrossRef]
- Zhuang, P.F.; Zhang, P.; Li, K.; Kumari, B.; Li, D.; Mei, X.F. Silver nanoclusters encapsulated into metal–organic frameworks for rapid removal of heavy metal ions from water. Molecules 2019, 24, 2442. [Google Scholar] [CrossRef] [PubMed]
- Kulal, P.; Badalamoole, V. Magnetite nanoparticle embedded Pectin-graft-poly(N-hydroxyethylacrylamide) hydrogel: Evaluation as adsorbent for dyes and heavy metal ions from waste water. Int. J. Biol. Macromol. 2020, 156, 1408–1417. [Google Scholar] [CrossRef] [PubMed]
- Ngambia, A.; Ifthikar, J.; Shahib, I.I.; Jawad, A.; Shahzad, A.; Zhao, M.M.; Wang, J.; Chen, Z.L.; Chen, Z.Q. Adsorptive purification of heavy metal contaminated wastewater with sewage sludge derived carbon-supported Mg(II) composite. Sci. Total Environ. 2019, 691, 306–321. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.C.; Fang, S.W.; Shi, P.; Duan, M. Hydrazine-functionalized guar-gum material capable of capturing heavy metal ions. Carbohyd. Polym. 2019, 223, 115137. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.W.; Hu, Q.Y.; Fang, Z.; Zhang, X.J.; Zhang, B.B. Magnetic chitosan nanocomposites: A useful recyclable tool for heavy metal ion removal. Langmuir 2009, 25, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Kong, H.; Jang, J. Adsorption of heavy metal ions from aqueous solution by polyrhodanine-encapsulated magnetic nanoparticles. J. Colloid. Interf. Sci. 2011, 395, 505–511. [Google Scholar] [CrossRef]
- Ling, L.; Huang, X.Y.; Zhang, W.X. Enrichment of precious metals from wastewater with core-shell nanoparticles of iron. Adv. Mater. 2018, 30, e1705703. [Google Scholar] [CrossRef]
- Huang, J.L.; Fan, L.Q.; Gu, Y.; Geng, C.L.; Luo, H.; Huang, Y.F.; Lin, J.M.; Wu, J.H. One-step solvothermal synthesis of high-capacity Fe3O4/reduced graphene oxide composite for use in Li-ion capacitor. J. Alloy. Compd. 2019, 788, 1119e1126. [Google Scholar] [CrossRef]
- Weng, S.F.; Xu, Y.; Zhuang, Z. Fourier Transform Infrared Spectroscopy, 3rd ed.; Chemical Industry Press: Beijing, China, 2016. [Google Scholar]
- De Palma, R.; Peeters, S.; Van Bael, M.J.; Van den Rul, H.; Bonroy, K.; Laureyn, W.; Mullens, J.; Borghs, G.; Maes, G. Silane ligand exchange to make hydrophobic superparamagnetic nanoparticles water-dispersible. Chem. Mater. 2007, 19, 1821–1831. [Google Scholar] [CrossRef]
- Boamah, P.O.; Huang, Y.; Hua, M.Q.; Onumah, J.; Sam-Amoah, L.K.; Qian, Y.; Zhang, Q. Sorption of copper onto low molecular weight chitosan derivative from aqueous solution. Ecotox. Environ. Safety 2016, 129, 154–163. [Google Scholar] [CrossRef]
- Ertugay, N.; Malkoc, E. Adsorption isotherm, kinetic, and thermodynamic studies for methylene blue from aqueous solution by needles of Pinus sylvestris L. Pol. J. Environ. Stud. 2014, 23, 1995–2006. [Google Scholar]
Sample Availability: Not available. |


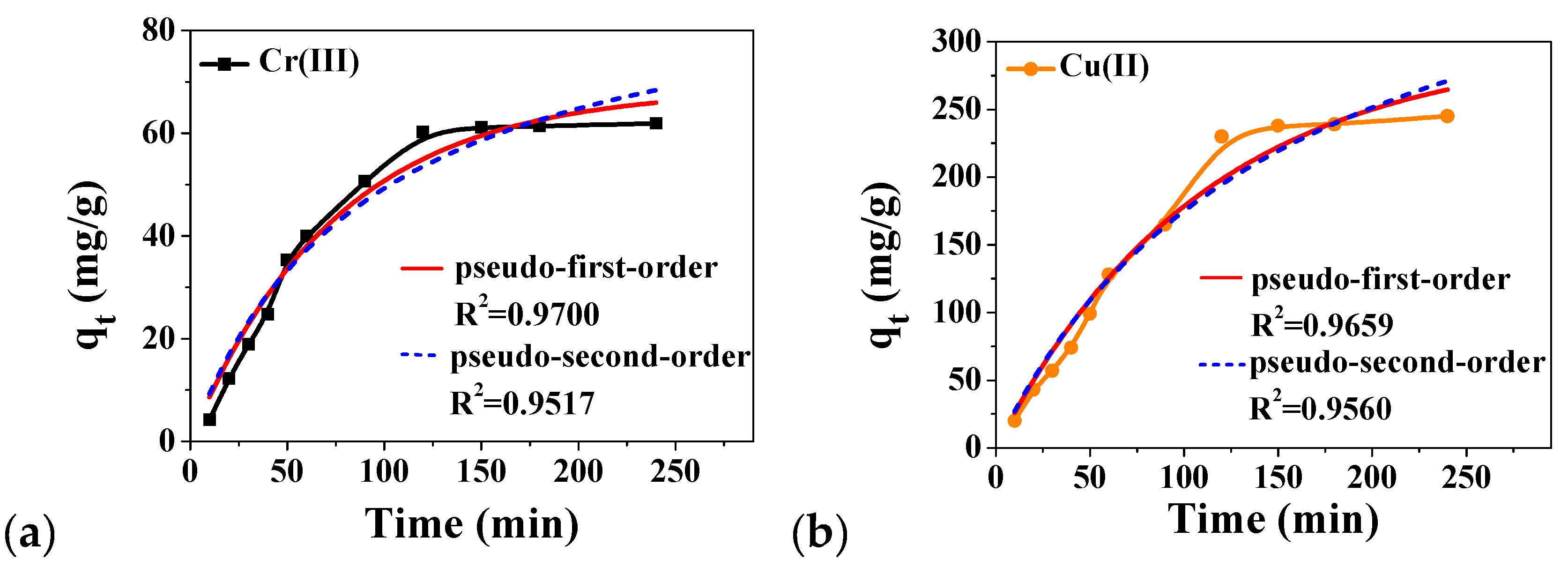


| Metal Ions | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|
| qe (mg·g−1) | k1 (min−1) | R2 | qe (mg·g−1) | k2 (mg·g−1·min−1) | R2 | |
| Cr(III) | 68.7 | 1.34 × 10−2 | 0.9700 | 94.5 | 2.20 × 10−5 | 0.9517 |
| Cu(II) | 298.0 | 0.91 × 10−2 | 0.9659 | 446.2 | 1.47 × 10−5 | 0.9556 |
| Pb(II) | 5.4 | 2.14 × 10−2 | 0.9724 | 6.6 | 3.60 × 10−3 | 0.9780 |
| Cd(II) | 1298.0 | 1.11 × 10−2 | 0.9691 | 1842.8 | 4.67 × 10−6 | 0.9543 |
| Metal Ions | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| qm(mg/g) | KL (L/g) | R2 | KF | 1/n | R2 | |
| Cr(III) | 76.6 | 0.122 | 0.989 | 15.85 | 0.404 | 0.987 |
| Cu(II) | 323.6 | 0.023 | 0.990 | 33.11 | 0.411 | 0.983 |
| Cd(II) | 1312.3 | 0.008 | 0.987 | 100.00 | 0.376 | 0.975 |
| Metal Ions | Adsorption | Desorption | ||
|---|---|---|---|---|
| C1 (ng/g) | m1 (mg) | C2 (ng/g) | m2 (mg) | |
| Cr(III) | 157.52 | 0.059 | 44.60 | 0.061 |
| Cu(II) | 208.51 | 0.254 | 127.56 | 0.242 |
| Pb(II) | 775.97 | 0.004 | 6.29 | 0.003 |
| Cd(II) | 236.23 | 1.003 | 435.71 | 0.956 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jing, J.; Shi, C. Reusable Magnetic Nanoparticle Immobilized Nitrogen-Containing Ligand for Classified and Easy Recovery of Heavy Metal Ions. Molecules 2020, 25, 3204. https://doi.org/10.3390/molecules25143204
Jing J, Shi C. Reusable Magnetic Nanoparticle Immobilized Nitrogen-Containing Ligand for Classified and Easy Recovery of Heavy Metal Ions. Molecules. 2020; 25(14):3204. https://doi.org/10.3390/molecules25143204
Chicago/Turabian StyleJing, Jingyun, and Congling Shi. 2020. "Reusable Magnetic Nanoparticle Immobilized Nitrogen-Containing Ligand for Classified and Easy Recovery of Heavy Metal Ions" Molecules 25, no. 14: 3204. https://doi.org/10.3390/molecules25143204
APA StyleJing, J., & Shi, C. (2020). Reusable Magnetic Nanoparticle Immobilized Nitrogen-Containing Ligand for Classified and Easy Recovery of Heavy Metal Ions. Molecules, 25(14), 3204. https://doi.org/10.3390/molecules25143204