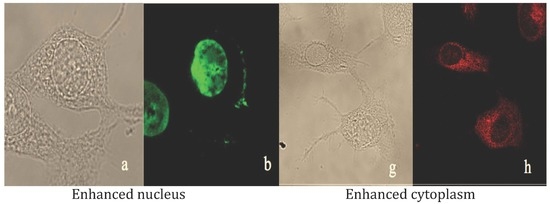
Non-Cytotoxic Dibenzyl and Difluoroborate Curcuminoid Fluorophores Allow Visualization of Nucleus or Cytoplasm in Bioimaging
Abstract
1. Introduction
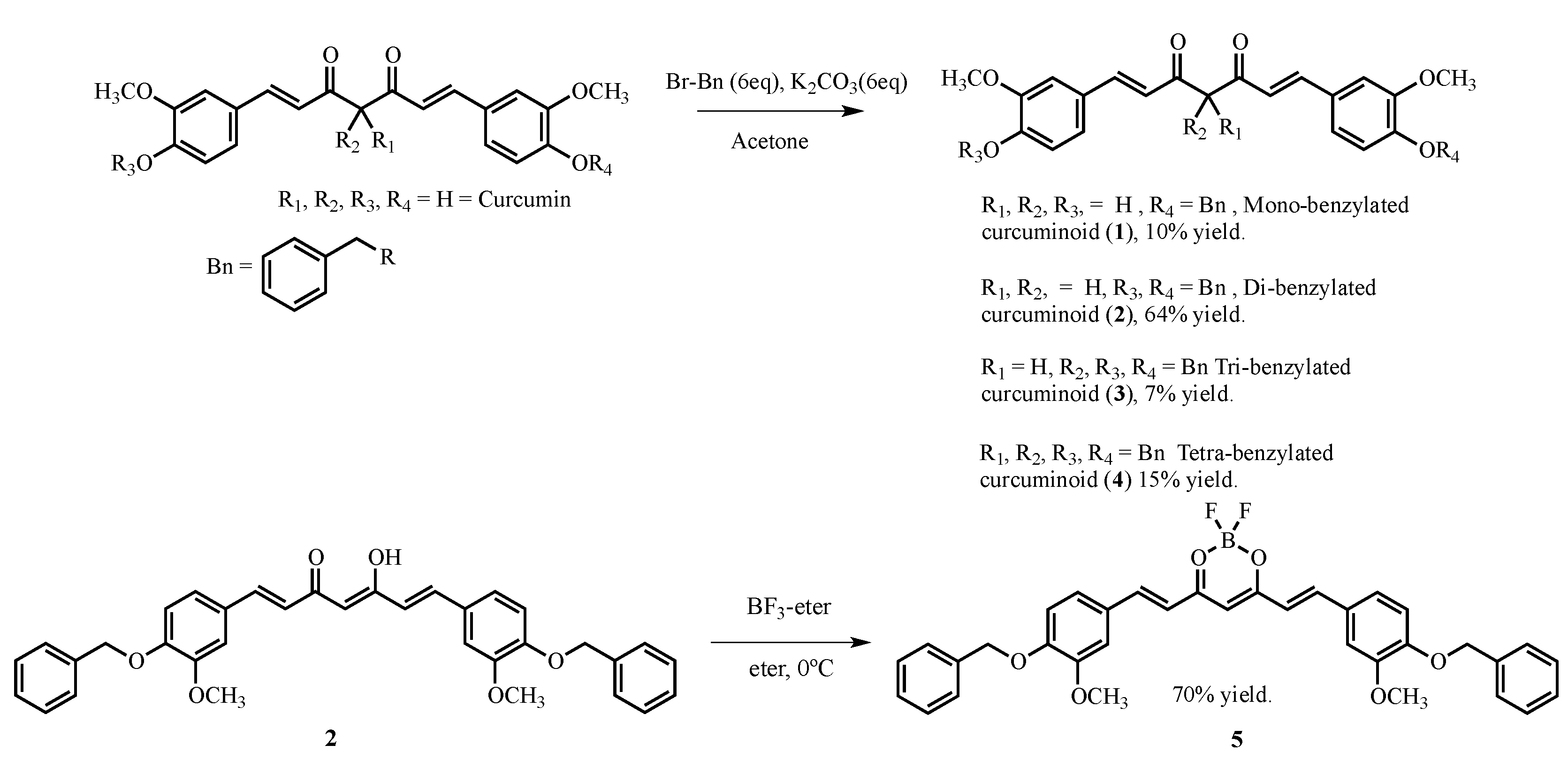
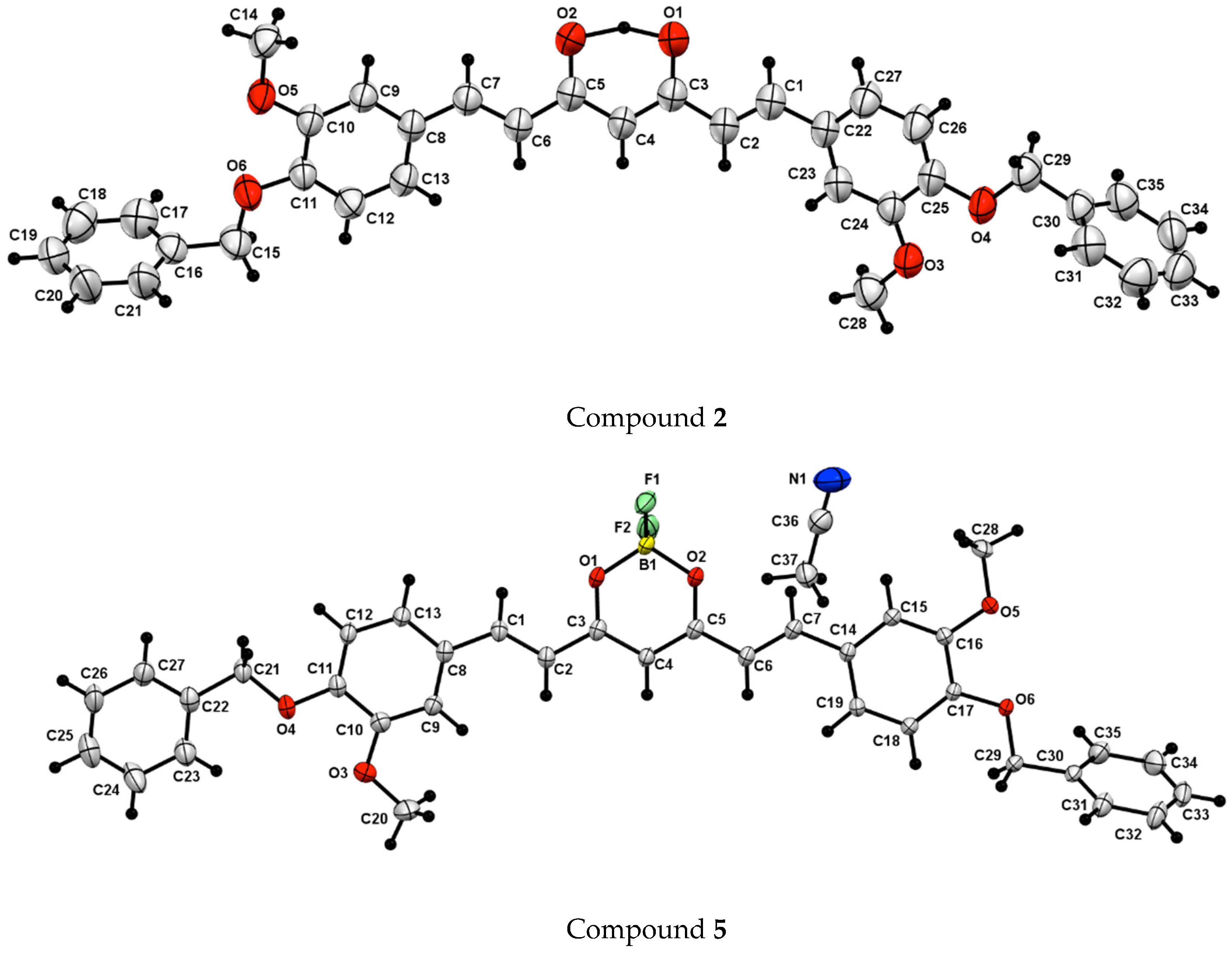
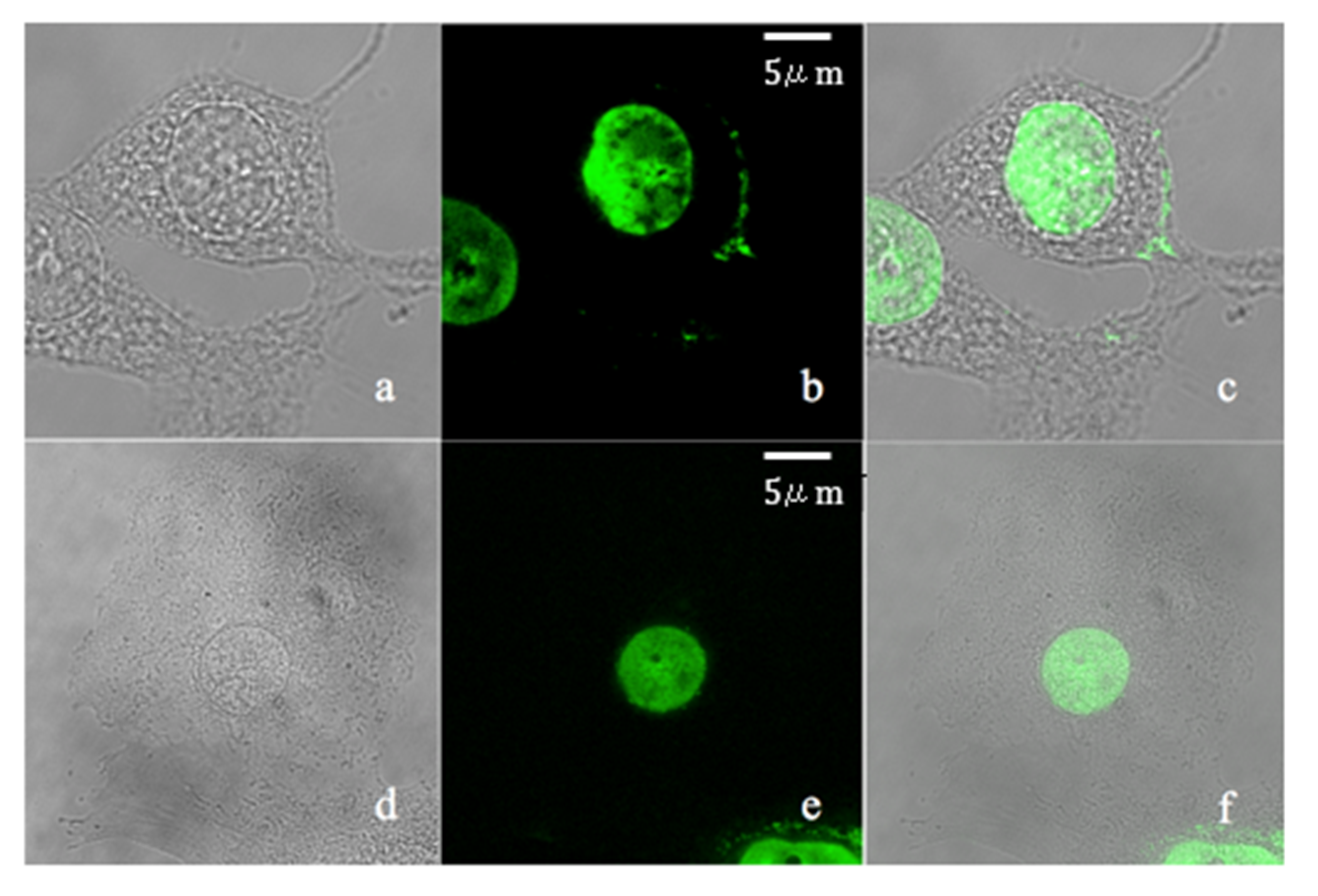
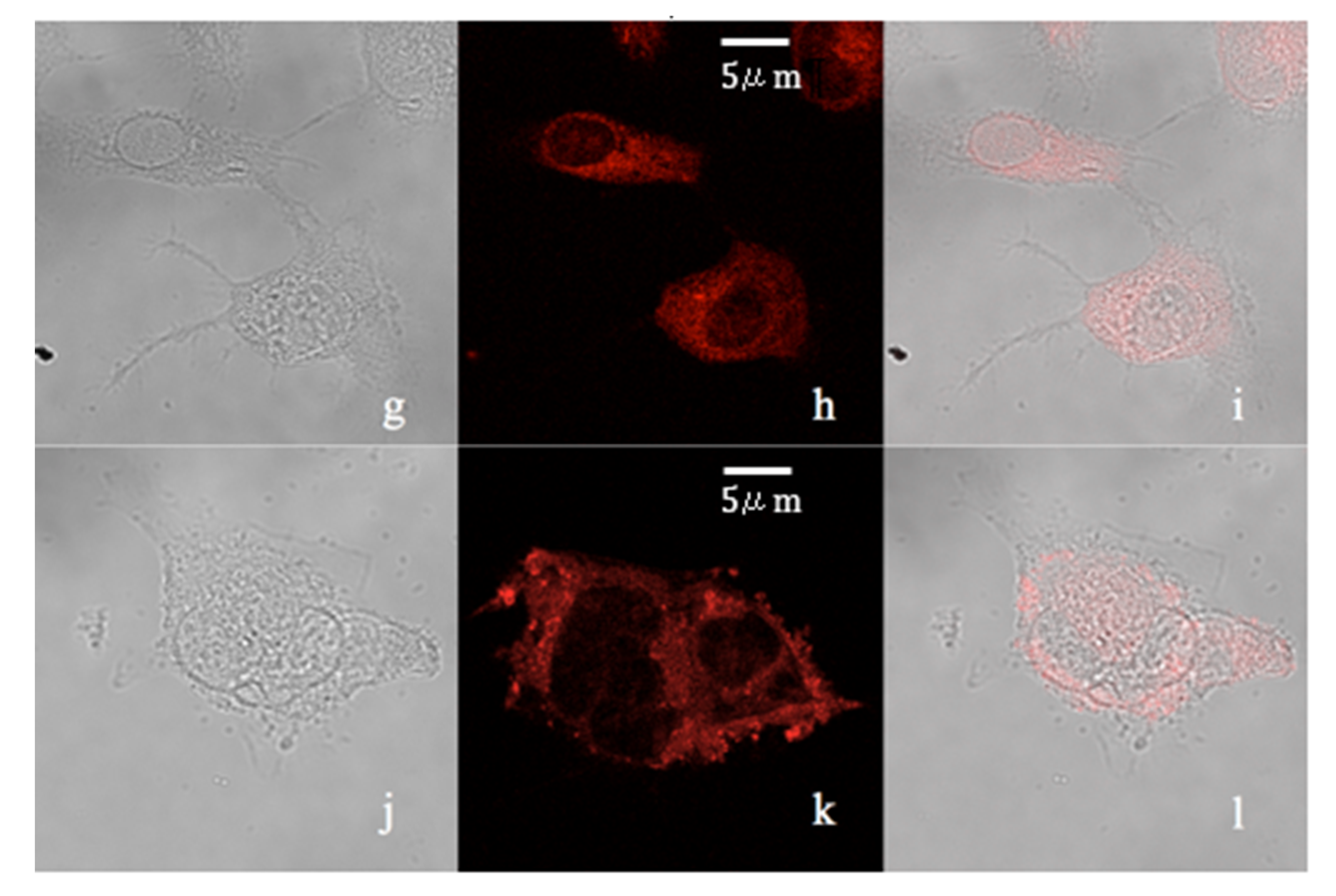
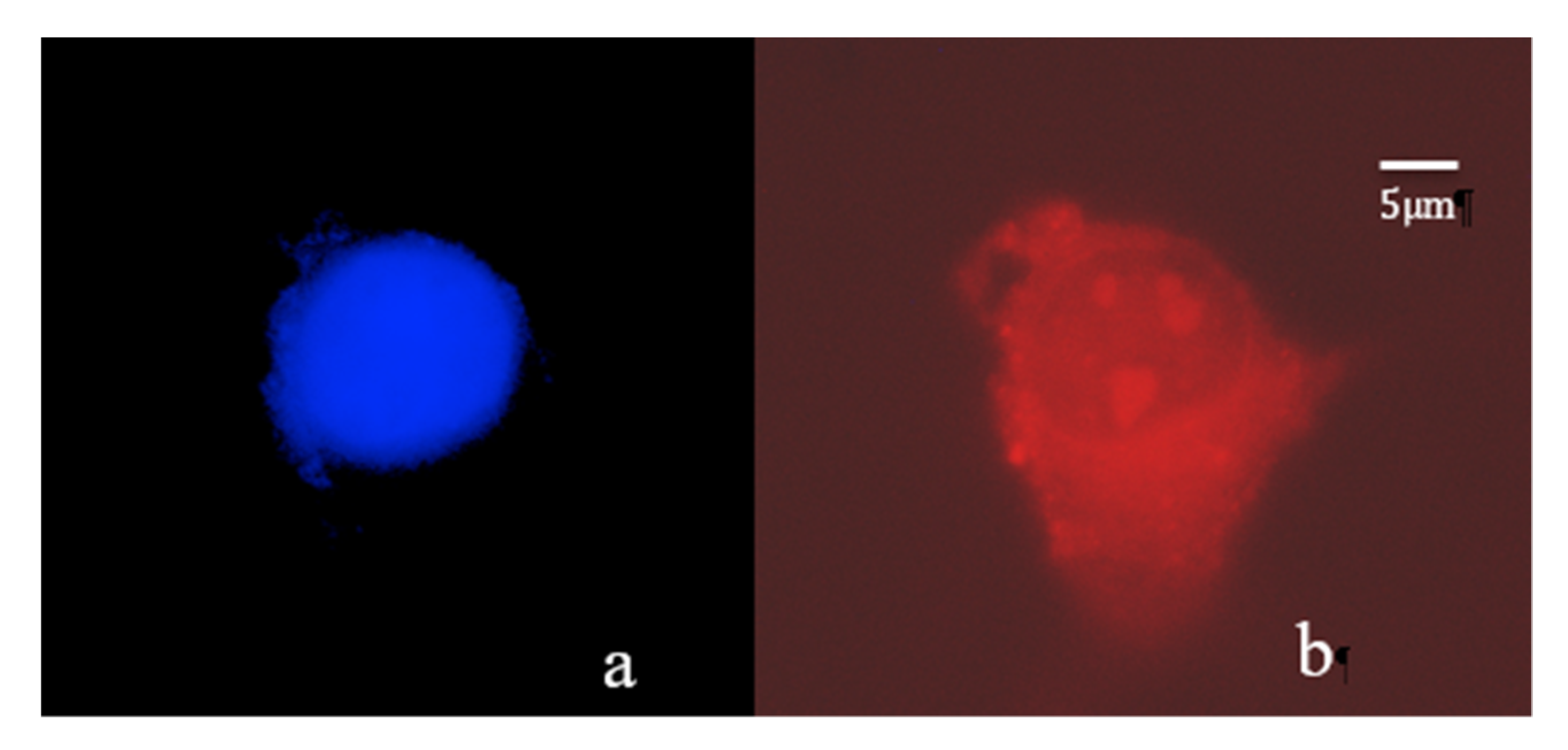
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Goel, A.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin as “Curecumin”: From kitchen to clinic. Biochem. Pharmacol. 2008, 75, 787–809. [Google Scholar] [CrossRef] [PubMed]
- Sanphui, P.; Bolla, G. Curcumin, a Biological Wonder Molecule: A Crystal Engineering Point of View. Cryst. Growth Des. 2018, 18, 5690–5711. [Google Scholar] [CrossRef]
- Wanninger, S.; Lorenz, V.; Subhan, A.; Edelmann, F.T. Metal complexes of curcumin—Synthetic strategies, structures and medicinal applications. Chem. Soc. Rev. 2015, 44, 4986–5002. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.-Y.; Sun, M.-Z.; Xiang, Y.-L.; Wu, Y.-M.; Tong, D.-Q. Curcumin as a colorimetric and fluorescent chemosensor for selective recognition of fluoride ion. J. Luminiscence 2010, 130, 304–308. [Google Scholar]
- Park, K.S.; Seo, Y.; Kim, M.K.; Kim, K.; Kim, Y.K.; Choo, H.; Chong, Y. A curcumin-based molecular probe for near-infrared fluorescence imaging of tau fibrils in Alzheimer’s disease. Org. Biomol. Chem. 2015, 13, 11194–11199. [Google Scholar] [CrossRef]
- Kunwar, A.; Barik, A.; Mishra, B.; Rathinasamy, K.; Pandey, R.; Priyadarsini, K.I. Quantitative cellular uptake, localization and cytotoxicity of curcumin in normal and tumor cells. Biochim. Biophys. Acta Gen. Subj. 2008, 1780, 673–679. [Google Scholar] [CrossRef]
- Ravindran, J.; Prasad, S.; Aggarwal, B.B. Curcumin and cancer cells: How many ways can curry kill tumor cells selectively? AAPS J. 2009, 11, 495–510. [Google Scholar] [CrossRef]
- Bandyopadhyay, D. Farmer to pharmacist: Curcumin as an anti-invasive and antimetastatic agent for the treatment of cancer. Front. Chem. 2014, 2, 1–11. [Google Scholar] [CrossRef]
- Xu, G.; Wei, D.; Wang, J.; Jiang, B.; Wang, M.; Xue, X.; Zhou, S.; Wu, B.; Jiang, M. Crystal structure, optical properties and biological imaging of two curcumin derivatives. Dye. Pigment. 2014, 101, 312–317. [Google Scholar] [CrossRef]
- Xu, G.; Wang, J.; Si, G.; Mahong, W.; Hualin, C.; Bin, C.; Zhou, S. Preparation, photoluminescence properties and application for in vivo tumor imaging of curcumin derivative-functionalized graphene oxide composite. Dye. Pigment. 2017, 141, 470–478. [Google Scholar] [CrossRef]
- Si, G.; Zhou, S.; Xu, G.; Wang, J.; Wu, B.; Zhou, S. A curcumin-based NIR fluorescence probe for detection of amyloid-beta (Aβ) plaques in Alzheimer’s disease. Dye. Pigment. 2019, 163, 509–515. [Google Scholar] [CrossRef]
- Naksuriya, O.; Okonogi, S.; Schiffelers, R.M.; Hennink, W.E. Curcumin nanoformulations: A review of pharmaceutical properties and preclinical studies and clinical data related to cancer treatment. Biomaterials 2014, 35, 3365–3383. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Banerjee, S.; Sil, P.C. The beneficial role of curcumin on inflammation, diabetes and neurodegenerative disease: A recent update. Food Chem. Toxicol. 2015, 83, 111–124. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, C.; Shi, H.; Yang, M.; Liu, Y.; Ji, P.; Chen, H.; Tan, R.X.; Li, E. Curcumin is a biologically active copper chelator with antitumor activity. Phytomedicine 2016, 23, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sumanont, Y.; Murakami, Y.; Tohda, M.; Vajragupta, O.; Watanabe, H.; Matsumoto, K. Effects of manganese complexes of curcumin and diacetylcurcumin on kainic acid-induced neurotoxic responses in the rat hippocampus. Biol. Pharm. Bull. 2007, 30, 1732–1739. [Google Scholar] [CrossRef]
- Asti, M.; Ferrari, E.; Croci, S.; Atti, G.; Rubagotti, S.; Iori, M.; Capponi, P.C.; Zerbini, A.; Saladini, M.; Versari, A. Synthesis and characterization of 68Ga-labeled curcumin and curcuminoid complexes as potential radiotracers for imaging of cancer and Alzheimer´s disease. Inorg. Chem. 2014, 53, 4922–4933. [Google Scholar] [CrossRef]
- Meza-Morales, W.; Mirian Estévez-Carmona, M.; Alvarez-Ricardo, Y.; Obregón-Mendoza, M.A.; Cassani, J.; Ramírez-Apan, M.T.; Escobedo-Martínez, C.; Soriano-García, M.; Reynolds, W.F.; Enríquez, R.G. Full structural characterization of homoleptic complexes of diacetylcurcumin with Mg, Zn, Cu, and Mn: Cisplatin-level Cytotoxicity in Vitro with Minimal Acute Toxicity in Vivo. Molecules 2019, 24, 1598. [Google Scholar] [CrossRef]
- He, Y.; Huang, Y.; Cheng, Y. Structure evolution of curcumin nanoprecipitation from a micromixer. Cryst. Growth Des. 2010, 10, 1021–1024. [Google Scholar] [CrossRef]
- Sanphui, P.; Goud, N.R.; Khandavilli, U.B.R.; Nangia, A. Fast dissolving curcumin cocrystals. Cryst. Growth Des. 2011, 11, 4135–4145. [Google Scholar] [CrossRef]
- Anand, P.; Thomas, S.G.; Kunnumakkara, A.B.; Sundaram, C.; Harikumar, K.B.; Sung, B.; Tharakan, S.T.; Misra, K.; Priyadarsini, I.K.; Rajasekharan, K.N.; et al. Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature. Biochem. Pharmacol. 2008, 76, 1590–1611. [Google Scholar] [CrossRef]
- Rumi, M.; Ehrlich, J.E.; Heikal, A.A.; Perry, J.W.; Barlow, S.; Hu, Z.; McCord-Maughon, D.; Parker, T.C.; Röckel, H.; Thayumanavan, S.; et al. Structure—Property relationships for two-photon absorbing chromophores: Bis-donor diphenylpolyene and bis(styryl)benzene derivatives. J. Am. Chem. Soc. 2000, 122, 9500–9510. [Google Scholar] [CrossRef]
- Albota, M.; Beljonne, D.; Brédas, J.L.; Ehrlich, J.E.; Fu, J.Y.; Heikal, A.A.; Hess, S.E.; Kogej, T.; Levin, M.D.; Marder, S.R.; et al. Design of organic molecules with large two-photon absorption cross sections. Science 1998, 281, 1653–1656. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Tian, R.; Antaris, A.L.; Chen, X.; Dai, H. Near-Infrared-II Molecular Dyes for Cancer Imaging and Surgery. Adv. Mater. 2019, 31, 1–25. [Google Scholar] [CrossRef]
- Obregón-Mendoza, M.A.; Estévez-Carmona, M.M.; Alvarez-Ricardo, Y.; Meza-Morales, W.; Escobedo-Martínez, C.; Soriano-García, M.; Enríquez, R.G. Crystal Structure, Synthesis and Biological Activity of Ether and Ester Trans-Ferulic Acid Derivatives. Int. J. Org. Chem. 2018, 8, 359–377. [Google Scholar] [CrossRef]
- Chongzhao, R.; Xiaoyin, X.; Raymond, S.B.; Ferrara, B.J.; Neal, K.; Bacskai, B.J.; Medarova, Z.; Moore, A. Design, synthesis, and testing of difluoroboron-derivatized curcumins as near-infrared probes for in vivo detection of amyloid-β deposits. J. Am. Chem. Soc. 2009, 131, 15257–15261. [Google Scholar]
- Yuyang, Z.; Ren-Cheng, T. Modification of curcumin with a reactive UV absorber and its dyeing and functional properties for silk. Dye. Pigment. 2016, 134, 203–211. [Google Scholar]
- Lozada, M.C.; Lobato, C.E.; Enríquez, R.G.; Ortíz, B.; Gnecco, D.; Reynolds, W.F.; Soriano-García, M. Crystal Structure of {Acetic acid 4-[7-(4-acetoxy-3-methoxyphenyl)-3,5-dioxoheptyl]-2-methoxy ester-03.05}-boron difluoride: A Boron Complex of Acetylated Tetrahydrocurcumin Derivative. Anal. Sci. 2004, 20, 167–168. [Google Scholar] [CrossRef][Green Version]
- Laali, K.K.; Greves, W.J.; Correa-Smits, S.J.; Zwarycz, A.T.; Bunge, S.D.; Borosky, G.L.; Manna, A.; Paulus, A.; Chanan-Khan, A. Novel fluorinated curcuminoids and their pyrazole and isoxazole derivatives: Synthesis, structural studies, Computational/Docking and in-vitro bioassay. J. Fluor. Chem. 2018, 206, 82–98. [Google Scholar] [CrossRef]
- Kamada, K.; Namikawa, T.; Senatore, S.; Matthews, C.; Lenne, P.F.; Maury, O.; Andraud, C.; Ponce-Vargas, M.; Le Guennic, B.; Jacquemin, D.; et al. Boron Difluoride Curcuminoid Fluorophores with Enhanced Two-Photon Excited Fluorescence Emission and Versatile Living-Cell Imaging Properties. Chem. A Eur. J. 2016, 22, 5219–5232. [Google Scholar] [CrossRef]
- Editors Dementia: A Situation for Concern. World Health Popul. 2019, 18, 3–5. [CrossRef]
- Roy, M.; Chakraborty, S.; Siddiqi, M.; Bhattacharya, R.K. Induction of apoptosis in tumor cells by natural phenolic compounds. Asian Pac. J. Cancer Prev. 2012, 3, 61–67. [Google Scholar]
- Xu, G.; Wang, J.; Si, G.; Mahong, W.; Wu, B.; Zhou, S. Two-photon absorption and cell imaging of two multi-branched dyes based on curcumin. Dye. Pigment. 2015, 123, 267–273. [Google Scholar] [CrossRef]
- Chen, J.H.; Ho, C.T. Antioxidant Activities of Caffeic Acid and Its Related Hydroxycinnamic Acid Compounds. J. Agric. Food Chem. 1997, 45, 2374–2378. [Google Scholar] [CrossRef]
- Janicke, B.; Hegardt, C.; Krogh, M.; Onning, G.; Åkesson, B.; Cirenajwis, H.M.; Oredsson, S.M. The antiproliferative effect of dietary fiber phenolic compounds ferulic acid and p-coumaric acid on the cell cycle of Caco-2 cells. Nutr. Cancer 2011, 63, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, C.; Santangelo, R. Ferulic acid: Pharmacological and toxicological aspects. Food Chem. Toxicol. 2014, 65, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, V.; Liebing, P.; Suta, M.; Felix, E.; Liane, H.; Busse, S.; Wang, S.; Wickleder, C.; Edelmann, F.T. Synthesis, structure, complexation, and luminescence properties of the first metal-organic curcumin compound Bis(4-triphenylsiloxy)curcumin. J. Luminiscence 2019, 211, 243–250. [Google Scholar] [CrossRef]
- Brouwer, A.M. Standards for photoluminescence quantum yield measurements in solution (IUPAC technical report). Pure Appl. Chem. 2011, 83, 2213–2228. [Google Scholar] [CrossRef]
- Zhu, H.; Fan, J.; Du, J.; Peng, X. Fluorescent Probes for Sensing and Imaging within Specific Cellular Organelles. Acc. Chem. Res. 2016, 49, 2115–2126. [Google Scholar] [CrossRef]
- Hong, G.; Antaris, A.L.; Dai, H. Near-infrared fluorophores for biomedical imaging. Nat. Biomed. Eng. 2017, 1. [Google Scholar] [CrossRef]
- Gao, P.; Pan, W.; Li, N.; Tang, B. Fluorescent probes for organelle-targeted bioactive species imaging. Chem. Sci. 2019, 10, 6035–6071. [Google Scholar] [CrossRef]
- Alamudi, S.H.; Chang, Y.T. Advances in the design of cell-permeable fluorescent probes for applications in live cell imaging. Chem. Commun. 2018, 54, 13641–13653. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Im, Y.H.; Ahn, J.; Yang, J.; Choi, J.Y.; Lee, K.H.; Kim, B.T.; Choe, Y.S. Synthesis and in vivo characterization of 18 F-labeled difluoroboron-curcumin derivative for β-amyloid plaque imaging. Sci. Rep. 2019, 9, 1–13. [Google Scholar]
- Escobedo-Martínez, C.; Guzmán-Gutiérrez, S.L.; Carrillo-López, M.I.; Deveze-Álvarez, M.A.; Trujillo-Valdivia, A.; Meza-Morales, W.; Enríquez, R.G. Diacetylcurcumin: Its Potential Antiarthritic Effect on a Freund’s Complete Adjuvant-Induced Murine Model. Molecules 2019, 24, 2643. [Google Scholar] [CrossRef] [PubMed]
- Mondal, R.; Mallik, A.K. Recent Applications of Potassium Carbonate in Organic Synthesis. Org. Prep. Proced. Int. 2014, 46, 391–434. [Google Scholar] [CrossRef]
- Bong, P.H. Spectral and photophysical behaviors of curcumin and curcuminoids. Bull. Korean Chem. Soc. 2000, 21, 81–86. [Google Scholar]
- Meza-Morales, W.; Machado-Rodriguez, J.C.; Alvarez-Ricardo, Y.; Obregón-Mendoza, M.A.; Nieto-Camacho, A.; Toscano, R.A.; Soriano-García, M.; Cassani, J.; Enríquez, R.G. A new family of homoleptic copper complexes of curcuminoids: Synthesis, characterization and biological properties. Molecules 2019, 24, 910. [Google Scholar] [CrossRef] [PubMed]
- Bruker AXS Inc. APEX2 and SAINT-Plus; Bruker AXS Inc.: Madison, WI, USA, 2013. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Nardelli, M. PARST: A system of fortran routines for calculating molecular structure parameters from results of crystal structure analyses. J. Appl. Crystallogr. 1995, 28, 659. [Google Scholar] [CrossRef]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; Van De Streek, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Crystallogr. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Chen, R.F. Fluorescence Quantum Yield Measurements: Vitamin B6 Compounds. Science 1965, 150, 1593–1595. [Google Scholar] [CrossRef]
- Jamalzadeh, L.; Ghafoori, H.; Sariri, R.; Rabuti, H.; Nasirzade, J.; Hasani, H.; Aghamaali, M.R. Cytotoxic Effects of Some Common Organic Solvents on MCF-7, RAW-264.7 and Human Umbilical Vein Endothelial Cells. Avicenna J. Med. Biochem 2016, 1, 1–6. [Google Scholar] [CrossRef]
- Andrés, A.; Rosés, M.; Ràfols, C.; Bosch, E.; Espinosa, S.; Segarra, V.; Huerta, J.M. Setup and validation of shake-flask procedures for the determination of partition coefficients (log D) from low drug amounts. Eur. J. Pharm. Sci. 2015, 76, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Sangster, J. Octanol-Water Partition Coefficients of Simple Organic Compounds Octanol-Water Partition Coefficients of Simple Organic Compounds. J. Phys. Chem. Ref. Data 1989, 18, 1111. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1–5 are available from the authors. |
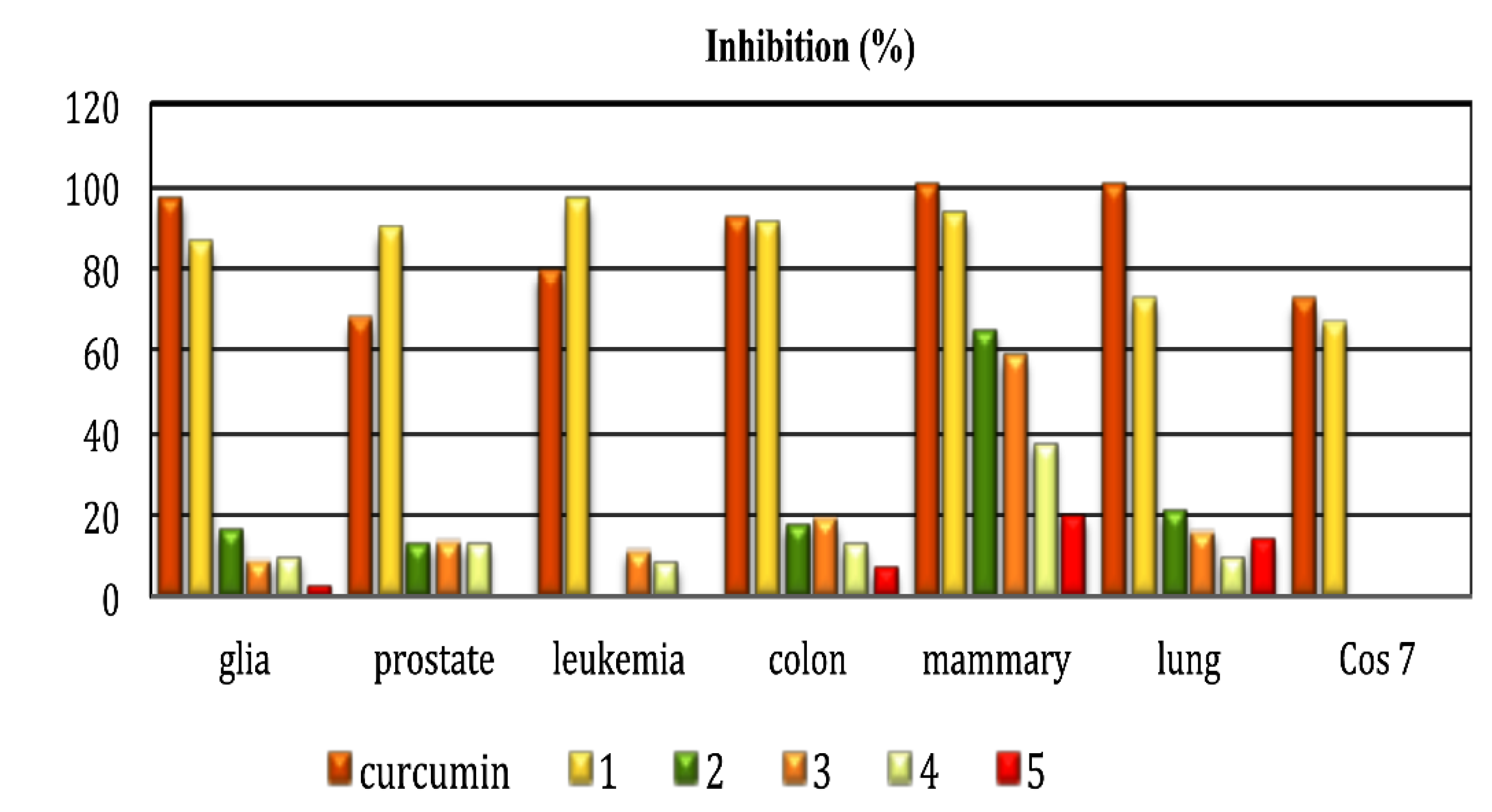

| Compound | Cytotoxic Activity Percentage of Inhibition at 25 μm, after 48 h of Incubation a | ||||||
|---|---|---|---|---|---|---|---|
| U-251 | PC-3 | K562 | HCT-15 | MCF-7 | SKLU-1 | COS-7 | |
| Curcumin | 96.55 | 66.9 | 78.57 | 91.7 | 100 | 100 | 71.6 |
| 1 | 85.4 | 88.8 | 96.1 | 90.7 | 93.0 | 71.9 | 66.4 |
| 2 | 16.0 | 12.9 | NC | 17.4 | 64.8 | 20.6 | NC |
| 3 | 8.5 | 12.7 | 10.9 | 18.3 | 58.3 | 15.5 | NC |
| 4 | 9.4 | 12.8 | 7.8 | 12.5 | 37.4 | 9.3 | NC |
| 5 | 2.4 | NC | NC | 6.7 | 19.6 | 13.8 | NC |
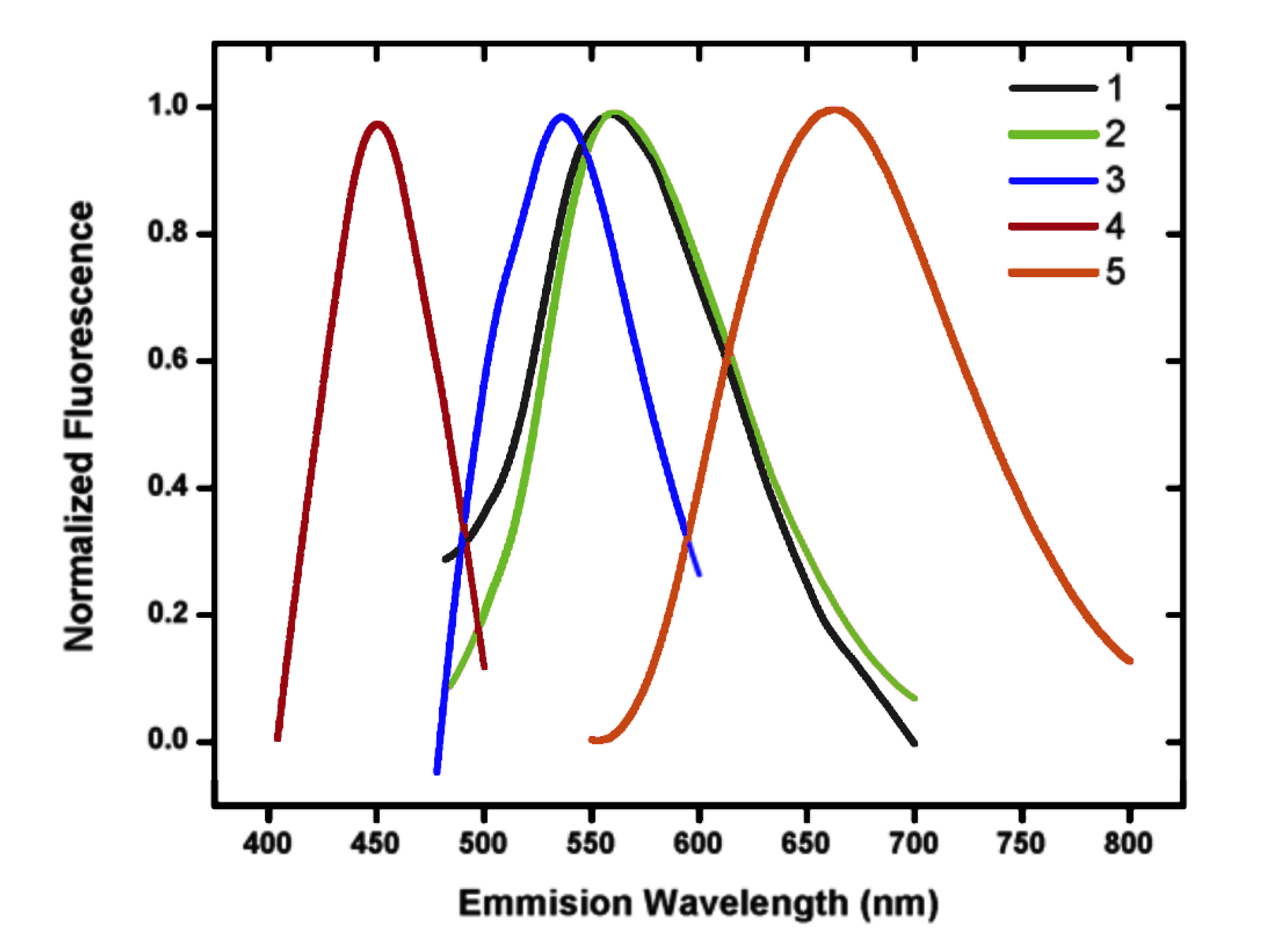
| Compound | /nm | ε (M−1cm−1) a | /nm | Φ b |
|---|---|---|---|---|
| Curcumin | 420 * | ----- | 520 * | 0.01 * |
| 1 | 428 | 76,900 | 556 | 0.0016 |
| 2 | 425 | 28,200 | 560 | 0.023 |
| 3 | 442 | 29,500 | 534 | 0.025 |
| 4 | 350 | 37,900 | 450 | 0.01 |
| 5 | 510 | 76,500 | 662 | 0.021 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obregón-Mendoza, M.A.; Arias-Olguín, I.I.; Estévez-Carmona, M.M.; Meza-Morales, W.; Alvarez-Ricardo, Y.; Toscano, R.A.; Arenas-Huertero, F.; Cassani, J.; Enríquez, R.G. Non-Cytotoxic Dibenzyl and Difluoroborate Curcuminoid Fluorophores Allow Visualization of Nucleus or Cytoplasm in Bioimaging. Molecules 2020, 25, 3205. https://doi.org/10.3390/molecules25143205
Obregón-Mendoza MA, Arias-Olguín II, Estévez-Carmona MM, Meza-Morales W, Alvarez-Ricardo Y, Toscano RA, Arenas-Huertero F, Cassani J, Enríquez RG. Non-Cytotoxic Dibenzyl and Difluoroborate Curcuminoid Fluorophores Allow Visualization of Nucleus or Cytoplasm in Bioimaging. Molecules. 2020; 25(14):3205. https://doi.org/10.3390/molecules25143205
Chicago/Turabian StyleObregón-Mendoza, Marco A., Imilla I. Arias-Olguín, M. Mirian Estévez-Carmona, William Meza-Morales, Yair Alvarez-Ricardo, Rubén A. Toscano, Francisco Arenas-Huertero, Julia Cassani, and Raúl G. Enríquez. 2020. "Non-Cytotoxic Dibenzyl and Difluoroborate Curcuminoid Fluorophores Allow Visualization of Nucleus or Cytoplasm in Bioimaging" Molecules 25, no. 14: 3205. https://doi.org/10.3390/molecules25143205
APA StyleObregón-Mendoza, M. A., Arias-Olguín, I. I., Estévez-Carmona, M. M., Meza-Morales, W., Alvarez-Ricardo, Y., Toscano, R. A., Arenas-Huertero, F., Cassani, J., & Enríquez, R. G. (2020). Non-Cytotoxic Dibenzyl and Difluoroborate Curcuminoid Fluorophores Allow Visualization of Nucleus or Cytoplasm in Bioimaging. Molecules, 25(14), 3205. https://doi.org/10.3390/molecules25143205