Recovery of Anthocyanins from Passion Fruit Epicarp for Food Colorants: Extraction Process Optimization and Evaluation of Bioactive Properties
Abstract
1. Introduction
2. Results and Discussion
2.1. Colour Parameters of Fresh and Dried Epicarp
2.2. Anthocyanin Profile of the Epicarp Extract
2.3. Optimization of the Extraction Process for Obtaining Anthocyanin-Rich Extracts
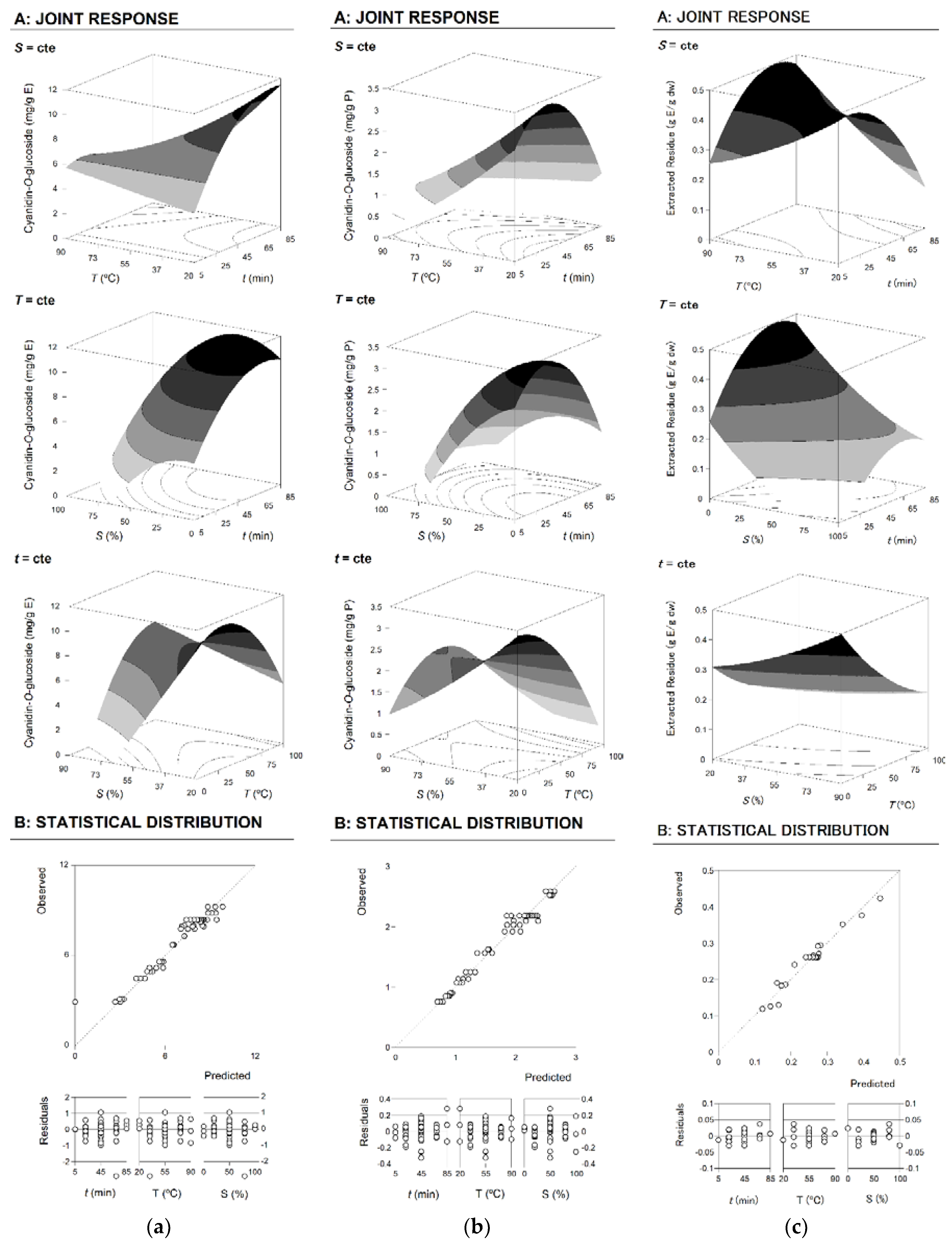
2.3.1. Response Criteria for RSM Analysis
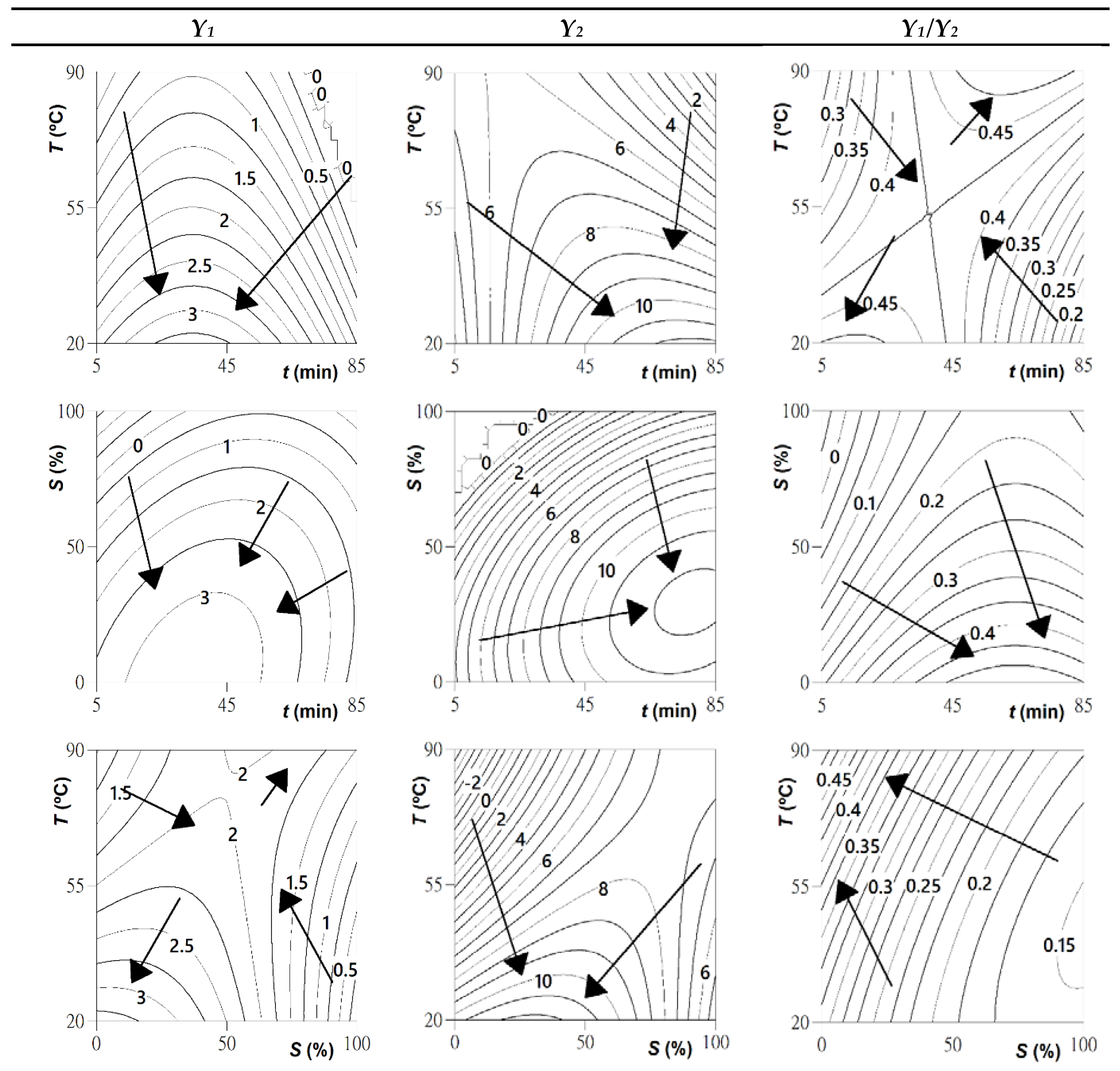
2.3.2. Model and Response Surface Analysis
2.3.3. Optimal Extraction Conditions and Verification of the Predictive Models
2.4. Effect of the Solid-to-Liquid Ratio
2.5. Bioactive Properties of Anthocyanin Extracts Obtained by the Conventional and Optimized Methods
2.5.1. Antioxidant Activity
2.5.2. Antimicrobial Activity
2.5.3. Cytotoxic Activity
3. Materials and Methods
3.1. Sample Preparation
3.2. Determination of Colour in Fresh and Dried Epicarp
3.3. Extraction and Analysis af Anthocyanins from Dried Epicarp
3.4. Experimental Design
3.5. Extraction Method
3.6. Extraction Process Optimization by Response Surface Methodology
3.7. Analysis of the Solid-to-Liquid Ratio
3.8. Production of Anthocyanin Extract under Optimal Extraction Conditions and Verification of the Predictive Models
3.9. Evaluation of bioactive Properties of the Anthocyanin Extracts Obtained by the Conventional and Optimized Methods
3.9.1. Antioxidant Activity
3.9.2. Antimicrobial Activity
3.9.3. Cytotoxic Activity
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Meldrum, R.J.; Little, C.L.; Sagoo, S.; Mithani, V.; McLauchlin, J.; de Pinna, E. Assessment of the microbiological safety of salad vegetables and sauces from kebab take-away restaurants in the United Kingdom. Food Microbiol. 2009, 26, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Sahu, J.; Pap, N.; Pongrácz, E.; Myllykoski, L.; Keiski, R. Waste Minimization and Utilization in the Food Industry: Valorization of Food Industry Wastes and Byproducts. In Introduction to Advanced Food Process Engineering; Sahu, J.K., Ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 595–630. [Google Scholar]
- Djilas, S.; Čanadanović-Brunet, J.; Ćetković, G. By-products of fruits processing as a source of phytochemicals. Chem. Ind. Chem. Eng. Q. 2009, 15, 191–202. [Google Scholar] [CrossRef]
- Chockchaisawasdee, S.; Stathopoulos, C.E. Utilisation of bioactive compounds from agricultural and food production waste. In Extraction, Isolation and Utilization of Bioactive Compounds from Fruit Juice Industry Waste; Van Vuong, Q., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 272–313. ISBN 9781498741316. [Google Scholar]
- Pinela, J.; Prieto, M.A.; Barreiro, M.F.; Carvalho, A.M.; Oliveira, M.B.P.P.; Curran, T.P.; Ferreira, I.C.F.R. Valorisation of tomato wastes for development of nutrient-rich antioxidant ingredients: A sustainable approach towards the needs of the today’s society. Innov. Food Sci. Emerg. Technol. 2017, 41, 160–171. [Google Scholar] [CrossRef]
- Corrêa, R.C.G.; Peralta, R.M.; Haminiuk, C.W.I.; Maciel, G.M.; Bracht, A.; Ferreira, I.C.F.R. The past decade findings related with nutritional composition, bioactive molecules and biotechnological applications of Passiflora spp. (passion fruit). Trends Food Sci. Technol. 2016, 58, 79–95. [Google Scholar] [CrossRef]
- Lazarevic, D.; Buclet, N.; Brandt, N. The influence of the waste hierarchy in shaping European waste management: The case of plastic waste. Reg. Dev. Dialogue 2010, 31, 124–148. [Google Scholar]
- Liu, M.; Su, Y.J.; Lin, Y.L.; Wang, Z.W.; Gao, H.M.; Li, F.; Wei, X.Y.; Jiang, H.L. Optimization of green extraction of anthocyanins from purple passion fruit peels by response surface methodology. J. Food Process. Preserv. 2018, 42, e13756. [Google Scholar] [CrossRef]
- Pires, T.C.S.P.; Dias, M.I.; Barros, L.; Barreira, J.C.M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Incorporation of natural colorants obtained from edible flowers in yogurts. LWT 2018, 97, 668–675. [Google Scholar] [CrossRef]
- Teng, H.; Fang, T.; Lin, Q.; Song, H.; Liu, B.; Chen, L. Red raspberry and its anthocyanins: Bioactivity beyond antioxidant capacity. Trends Food Sci. Technol. 2017, 66, 153–165. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Jabeur, I.; Pereira, E.; Barros, L.; Calhelha, R.C.; Soković, M.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. Hibiscus sabdariffa L. as a source of nutrients, bioactive compounds and colouring agents. Food Res. Int. 2017, 100, 717–723. [Google Scholar] [CrossRef]
- Kidøy, L.; Mette, A.; Rd, N.; Andersen, Ø.M.; Pedersen, A.T.; Aksnes, D.W.; Kiremire, B.T. Anthocyanins in fruits of Passiflora edulis and P. suberosa. J. Food Compos. Anal. 1997, 10, 49–54. [Google Scholar] [CrossRef]
- Jiménez, A.M.; Sierra, C.A.; Rodríguez-Pulido, F.J.; González-Miret, M.L.; Heredia, F.J.; Osorio, C. Physicochemical characterisation of gulupa (Passiflora edulis Sims. fo edulis) fruit from Colombia during the ripening. Food Res. Int. 2011, 44, 1912–1918. [Google Scholar] [CrossRef]
- Dos Reis, L.C.R.; Facco, E.M.P.; Salvador, M.; Flôres, S.H.; de Oliveira Rios, A. Antioxidant potential and physicochemical characterization of yellow, purple and orange passion fruit. J. Food Sci. Technol. 2018, 55, 2679–2691. [Google Scholar] [CrossRef] [PubMed]
- Pinela, J.; Prieto, M.A.; Pereira, E.; Jabeur, I.; Barreiro, M.F.; Barros, L.; Ferreira, I.C.F.R. Optimization of heat- and ultrasound-assisted extraction of anthocyanins from Hibiscus sabdariffa calyces for natural food colorants. Food Chem. 2019, 275, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento, E.M.G.C.; Mulet, A.; Ascheri, J.L.R.; De Carvalho, C.W.P.; Cárcel, J.A. Effects of high-intensity ultrasound on drying kinetics and antioxidant properties of passion fruit peel. J. Food Eng. 2015, 170, 108–118. [Google Scholar] [CrossRef]
- Rotta, E.M.; Rodrigues, C.A.; Jardim, I.C.S.F.; Maldaner, L.; Visentainer, J.V. Determination of phenolic compounds and antioxidant activity in passion fruit pulp (Passiflora spp.) using a modified QuEChERS method and UHPLC-MS/MS. LWT 2019, 100, 397–403. [Google Scholar] [CrossRef]
- Janzantti, N.S.; Macoris, M.S.; Garruti, D.S.; Monteiro, M. Influence of the cultivation system in the aroma of the volatile compounds and total antioxidant activity of passion fruit. LWT Food Sci. Technol. 2012, 46, 511–518. [Google Scholar] [CrossRef]
- López, C.J.; Caleja, C.; Prieto, M.A.; Sokovic, M.; Calhelha, R.C.; Barros, L.; Ferreira, I.C.F.R. Stability of a cyanidin-3-O-glucoside extract obtained from Arbutus unedo L. and incorporation into wafers for colouring purposes. Food Chem. 2019, 275, 426–438. [Google Scholar] [CrossRef]
- Bessada, S.M.F.; Barreira, J.C.M.; Barros, L.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P. Phenolic profile and antioxidant activity of Coleostephus myconis (L.) Rchb.f.: An underexploited and highly disseminated species. Ind. Crops Prod. 2016, 89, 45–51. [Google Scholar] [CrossRef]
- Pinela, J.; Prieto, M.A.; Carvalho, A.M.; Barreiro, M.F.; Oliveira, M.B.P.P.; Barros, L.; Ferreira, I.C.F.R. Microwave-assisted extraction of phenolic acids and flavonoids and production of antioxidant ingredients from tomato: A nutraceutical-oriented optimization study. Sep. Purif. Technol. 2016, 164, 114–124. [Google Scholar] [CrossRef]
- Prieto, M.A.; Vázquez, J.A. In vitro determination of the lipophilic and hydrophilic antioxidant capacity of unroasted coffee bean extracts and their synergistic and antagonistic effects. Food Res. Int. 2014, 62, 1183–1196. [Google Scholar] [CrossRef]
- Lockowandt, L.; Pinela, J.; Roriz, C.L.; Pereira, C.; Abreu, R.M.V.; Calhelha, R.C.; Alves, M.J.; Barros, L.; Bredol, M.; Ferreira, I.C.F.R. Chemical features and bioactivities of cornflower (Centaurea cyanus L.) capitula: The blue flowers and the unexplored non-edible part. Ind. Crops Prod. 2019, 128, 496–503. [Google Scholar] [CrossRef]
- Soković, M.; Glamočlija, J.; Marin, P.D.; Brkić, D.; Griensven, L.J.L.D. van Antibacterial effects of the essential oils of commonly consumed medicinal herbs using an in vitro model. Molecules 2010, 15, 7532–7546. [Google Scholar] [CrossRef] [PubMed]
- Soković, M.; van Griensven, L.J.L.D. Antimicrobial activity of essential oils and their components against the three major pathogens of the cultivated button mushroom, Agaricus bisporus. Eur. J. Plant Pathol. 2006, 116, 211–224. [Google Scholar] [CrossRef]
- Guimarães, R.; Barros, L.; Dueñas, M.; Calhelha, R.C.; Carvalho, A.M.; Santos-Buelga, C.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Infusion and decoction of wild German chamomile: Bioactivity and characterization of organic acids and phenolic compounds. Food Chem. 2013, 136, 947–954. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
| Samples | L* (Lightness) | a* (Redness) | b* (Yellowness) | RGB Colour |
|---|---|---|---|---|
| Fresh epicarp | 34.2 ± 0.9 | 6.9 ± 0.3 | 5.1 ± 0.2 | |
| Dried epicarp | 34.3 ± 1.5 | 16.0 ± 0.6 | 2.8 ± 0.1 | |
| p>-value | 0.761 | <0.01 | <0.01 |
| Coded Values | Natural Values | ||
|---|---|---|---|
| t (min) | T (°C) | S (%) | |
| −1.68 | 5 | 20 | 0 |
| −1 | 21.2 | 34.2 | 20.3 |
| 0 | 45 | 55 | 50 |
| +1 | 21.2 | 75.8 | 79.7 |
| +1.68 | 85 | 90 | 100 |
| Run | Coded Values | Natural Values | Experimental Responses | ||||||
|---|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X1: t | X2: T | X3: S | Y1 | Y2 | Y1/Y2 | |
| min | °C | % | mg Anthocyanin/g Dried Epicarp | mg Anthocyanin/g Extract | g Extract/g Dried Epicarp | ||||
| 1 | −1 | −1 | −1 | 21.2 | 34.2 | 20.3 | 2.58 | 6.54 | 0.395 |
| 2 | −1 | −1 | +1 | 21.2 | 34.2 | 79.7 | 1.96 | 9.34 | 0.210 |
| 3 | −1 | +1 | −1 | 21.2 | 75.8 | 20.3 | 1.48 | 5.41 | 0.274 |
| 4 | −1 | +1 | +1 | 21.2 | 75.8 | 79.7 | 1.06 | 3.11 | 0.342 |
| 5 | +1 | −1 | −1 | 68.8 | 34.2 | 20.3 | 0.74 | 2.84 | 0.262 |
| 6 | +1 | −1 | +1 | 68.8 | 34.2 | 79.7 | 1.25 | 7.51 | 0.166 |
| 7 | +1 | +1 | −1 | 68.8 | 75.8 | 20.3 | 1.13 | 7.88 | 0.143 |
| 8 | +1 | +1 | +1 | 68.8 | 75.8 | 79.7 | 1.55 | 8.39 | 0.185 |
| 9 | −1.68 | 0 | 0 | 5 | 55 | 50 | 0.87 | 4.98 | 0.174 |
| 10 | +1.68 | 0 | 0 | 85 | 55 | 50 | 0.88 | 7.28 | 0.121 |
| 11 | 0 | −1.68 | 0 | 45 | 20 | 50 | 2.57 | 9.12 | 0.282 |
| 12 | 0 | +1.68 | 0 | 45 | 90 | 50 | 2.17 | 7.84 | 0.277 |
| 13 | 0 | 0 | −1.68 | 45 | 55 | 0 | 1.95 | 4.36 | 0.446 |
| 14 | 0 | 0 | +1.68 | 45 | 55 | 100 | 0.93 | 5.74 | 0.162 |
| 15 | 0 | 0 | 0 | 45 | 55 | 50 | 2.14 | 8.56 | 0.251 |
| 16 | 0 | 0 | 0 | 45 | 55 | 50 | 2.27 | 8.38 | 0.271 |
| 17 | 0 | 0 | 0 | 45 | 55 | 50 | 2.06 | 7.93 | 0.260 |
| 18 | 0 | 0 | 0 | 45 | 55 | 50 | 2.27 | 8.27 | 0.274 |
| 19 | 0 | 0 | 0 | 45 | 55 | 50 | 2.27 | 8.46 | 0.268 |
| 20 | 0 | 0 | 0 | 45 | 55 | 50 | 2.06 | 8.55 | 0.241 |
| Y1 | Y2 | Y1/Y2 | |||||
|---|---|---|---|---|---|---|---|
| (A) Parametric information | |||||||
| Intercept | b0 | 2.18 | ±0.06 | 8.4 | ±0.2 | 0.26 | ±0.01 |
| Linear effect | b1 | ns | 0.7 | ±0.1 | −0.02 | ±0.01 | |
| b2 | −0.15 | ±0.04 | −0.3 | ±0.1 | ns | ||
| b3 | −0.30 | ±0.04 | 0.3 | ±0.1 | −0.07 | ±0.01 | |
| Quadratic effect | b11 | −0.47 | ±0.04 | −0.8 | ±0.1 | −0.04 | ±0.01 |
| b22 | 0.06 | ±0.04 | ns | ns | |||
| b33 | −0.27 | ±0.04 | −1.2 | ±0.1 | 0.02 | ±0.01 | |
| Interactive effect | b12 | ns | −1.2 | ±0.2 | 0.05 | ±0.01 | |
| b13 | 0.25 | ±0.05 | 0.6 | ±0.2 | ns | ||
| b23 | 0.34 | ±0.05 | 1.7 | ±0.2 | ns | ||
| (B) Statistical information | |||||||
| R2 | 0.9677 | 0.9546 | 0.9542 | ||||
| R2 adjusted | 0.962 | 0.951 | 0.951 | ||||
| (C) Optimal variable conditions for response maximization | |||||||
| Individual conditions | Time (min) | 35 | ±2 | 78 | ±8 | 64 | ±8 |
| Temperature (°C) | 20 | ±4 | 20 | ±4 | 90 | ±3 | |
| Solvent (%) | 0 | ±2 | 29 | ±4 | 0.0 | ±0.9 | |
| Response: | 3.4 | ±0.6 | 12 | ±2 | 0.29 | ±0.04 | |
| Global conditions | Time (min) | 38 ± 4 | |||||
| Temperature (°C) | 20 ± 2 | ||||||
| Solvent (%) | 0 ± 2 | ||||||
| Response: | 3.4 | ±0.5 | 9 | ±1 | 0.37 | ±0.05 | |
| Optimal Extract | Normal Extract | Positive Control | ||||
|---|---|---|---|---|---|---|
| Antioxidant activity | Trolox | |||||
| TBARS (EC50, µg/mL) | 115 ± 3 | 136 ± 4 | 20.4 ± 0.5 | |||
| OXHLIA (IC50, µg/mL) | 78 ± 3 | 144 ± 4 | 5.4 ± 0.3 | |||
| Cytotoxicity to tumor cells | Ellipticine | |||||
| NCI-H460 (GI50, µg/mL) | >400 | >400 | 1.03 ± 0.09 | |||
| MCF-7 (GI50, µg/mL) | >400 | >400 | 1.1 ± 0.2 | |||
| HepG2 (GI50, µg/mL) | 363 ± 15 | >400 | 1.1 ± 0.2 | |||
| HeLa (GI50, µg/mL) | >400 | >400 | 1.91 ± 0.06 | |||
| Hepatotoxicity | Ellipticine | |||||
| PLP2 (GI50, µg/mL) | >400 | >400 | 3.2 ± 0.7 | |||
| Antibacterial activity | Ampicillin | |||||
| MIC (mg/mL) | MBC (mg/mL) | MIC (mg/mL) | MBC (mg/mL) | MIC (mg/mL) | MBC (mg/mL) | |
| Staphylococcus aureus | 8.00 | 8.00 | 8.00 | >8.00 | 0.012 | 0.025 |
| Listeria monocytogenes | 4.00 | 8.00 | 8.00 | >8.00 | 0.40 | 0.50 |
| Escherichia coli | 4.00 | 8.00 | 8.00 | 8.00 | 0.40 | 0.50 |
| Enterobacter cloacae | 8.00 | 8.00 | 8.00 | >8.00 | 0.006 | 0.012 |
| Salmonella typhimurium | 8.00 | 8.00 | 8.00 | >8.00 | 0.75 | 1.20 |
| Antifungal activity | Ketoconazol | |||||
| MIC (mg/mL) | MFC (mg/mL) | MIC (mg/mL) | MFC (mg/mL) | MIC (mg/mL) | MFC (mg/mL) | |
| Aspergillus fumigatus | 8.00 | >8.00 | >8.00 | >8.00 | 0.20 | 0.50 |
| Aspergillus versicolor | 8.00 | 8.00 | >8.00 | >8.00 | 0.20 | 0.50 |
| Aspergillus niger | 4.00 | 8.00 | >8.00 | >8.00 | 0.20 | 0.50 |
| Penicillium funiculosum | 8.00 | 8.00 | >8.00 | >8.00 | 0.20 | 0.50 |
| Penicillium ochrochloron | 1.00 | 1.00 | >8.00 | >8.00 | 0.20 | 0.50 |
| Trichoderma viride | 4.00 | 8.00 | >8.00 | >8.00 | 0.20 | 0.30 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghada, B.; Pereira, E.; Pinela, J.; Prieto, M.A.; Pereira, C.; Calhelha, R.C.; Stojković, D.; Sokóvić, M.; Zaghdoudi, K.; Barros, L.; et al. Recovery of Anthocyanins from Passion Fruit Epicarp for Food Colorants: Extraction Process Optimization and Evaluation of Bioactive Properties. Molecules 2020, 25, 3203. https://doi.org/10.3390/molecules25143203
Ghada B, Pereira E, Pinela J, Prieto MA, Pereira C, Calhelha RC, Stojković D, Sokóvić M, Zaghdoudi K, Barros L, et al. Recovery of Anthocyanins from Passion Fruit Epicarp for Food Colorants: Extraction Process Optimization and Evaluation of Bioactive Properties. Molecules. 2020; 25(14):3203. https://doi.org/10.3390/molecules25143203
Chicago/Turabian StyleGhada, Bejaoui, Eliana Pereira, José Pinela, Miguel A. Prieto, Carla Pereira, Ricardo C. Calhelha, Dejan Stojković, Marina Sokóvić, Khalil Zaghdoudi, Lillian Barros, and et al. 2020. "Recovery of Anthocyanins from Passion Fruit Epicarp for Food Colorants: Extraction Process Optimization and Evaluation of Bioactive Properties" Molecules 25, no. 14: 3203. https://doi.org/10.3390/molecules25143203
APA StyleGhada, B., Pereira, E., Pinela, J., Prieto, M. A., Pereira, C., Calhelha, R. C., Stojković, D., Sokóvić, M., Zaghdoudi, K., Barros, L., & Ferreira, I. C. F. R. (2020). Recovery of Anthocyanins from Passion Fruit Epicarp for Food Colorants: Extraction Process Optimization and Evaluation of Bioactive Properties. Molecules, 25(14), 3203. https://doi.org/10.3390/molecules25143203















