Asymmetric Amination of meso-Epoxide with Vegetable Powder as a Low-Toxicity Catalyst
Abstract
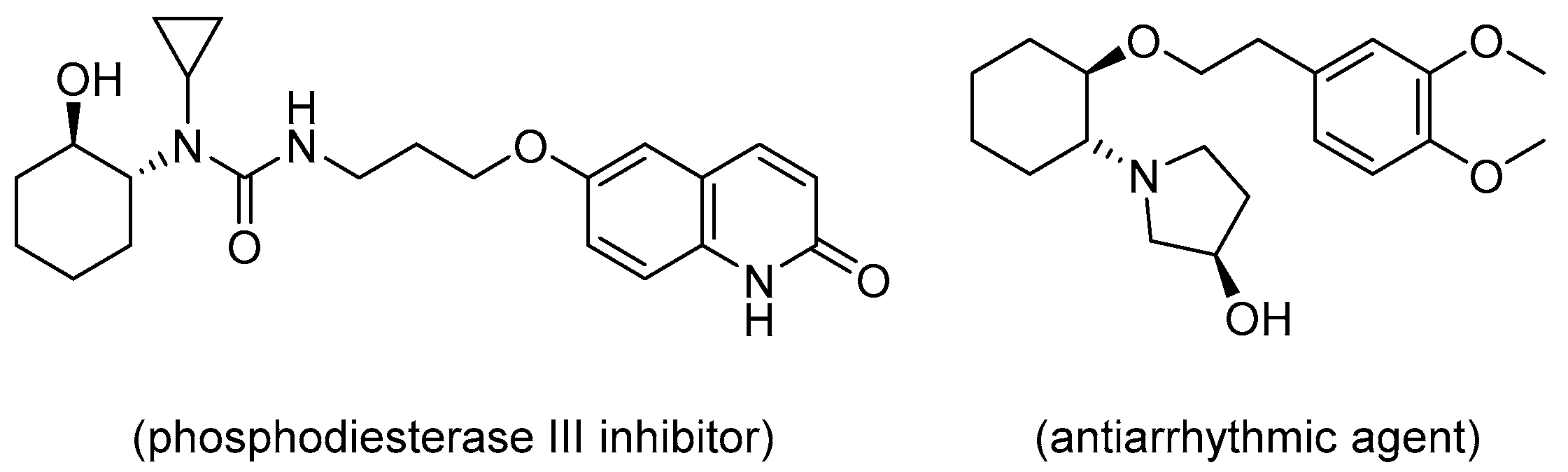
1. Introduction
2. Results and Discussion
2.1. Substrate Scope for Epoxide
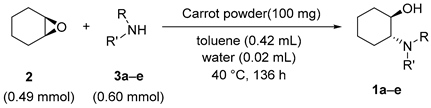
2.2. Substrate Scope for Amine
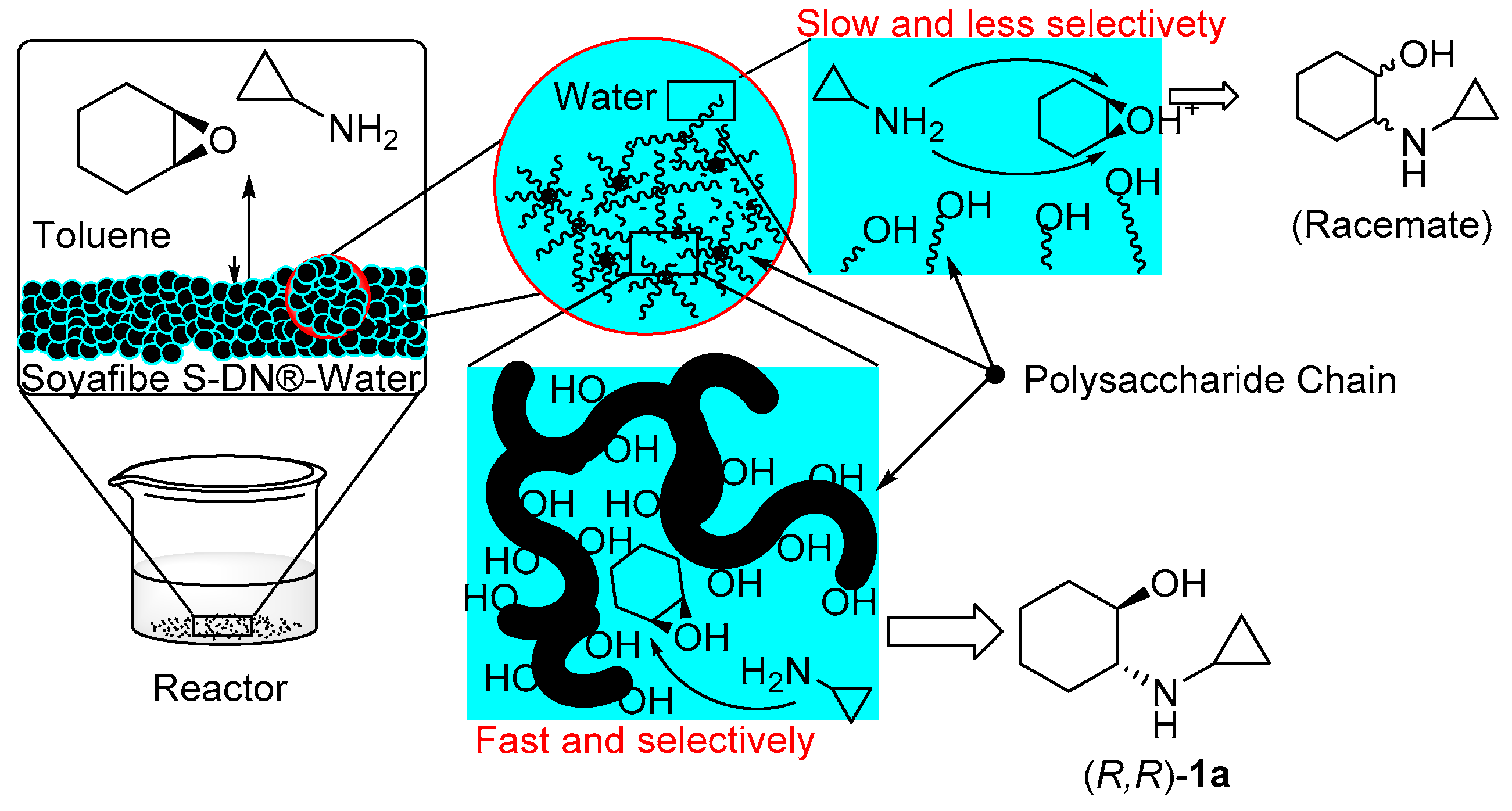
2.3. Proposed Reaction Mechanism
2.4. Screening of Polysaccharides as Catalysts
2.5. Screening of Vegetables
2.6. Evaluation of Catalytic Performance of Carrot Powder
3. Conclusions
4. Materials and Methods
4.1. General Procedure
4.2. Materials
4.3. Procedure and Analytical Data: General Procedure for Pretreatment of Vegetable
4.4. General Procedure for Catalytic Amination of Epoxide with Amine at the 0.5 mL Scale
- Conversion (%) = 100 × (Ap/Rp)/((Ap/Rp) + (As/RS))
- Ap = peak area for aminoalcohol from filtrate
- AS = peak area for epoxide from filtrate
- Rp = response of aminoalcohol
- RS = response of epoxide
4.5. General Procedure for Trimethylsilylation of the Product
4.6. General Procedure for Benzoation of the Product
4.7. General Procedure for Synthesis of Racemic Aminoalcohol
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Paulia, K.; Henryk, M.; Elzbita, P. Synthesis, Anticonvulsant Activity and Metabolism of 4-chlor-3-methylphenoxyethylamine Derivatives of Trans -2-aminocyclohexan-1-ol. Chirality 2015, 27, 163–169. [Google Scholar]
- Klingler, F.D. Asymmetric Hydrogenation of Prochiral Amino Ketones to Amino Alcohols for Pharmaceutical Use. Acc. Chem. Res. 2007, 40, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- De Costa, B.R.; Bowen, W.D.; Hellewell, S.B.; George, C.; Rothman, R.B.; Reid, A.A.; Walker, J.M.; Jacobson, A.E.; Rice, K.C. Alterations in the stereochemistry of the κ-selective opioid agonist U50,488 result in high-affinity σ ligands. J. Med. Chem. 1989, 32, 1996–2002. [Google Scholar] [CrossRef] [PubMed]
- Koga, Y.; Kihara, Y.; Okada, M.; Nishi, T.; Inoue, Y.; Kimura, Y.; Hidaka, H.; Fukuda, N. Carbostyril Derivatives as Antithrombotic Agents. PCT Int. Appl. WO9712869, 4 October 1997. [Google Scholar]
- Koga, Y.; Kihara, Y.; Okada, M.; Inoue, Y.; Tochizawa, S.; Toga, K.; Tachibana, K.; Kimura, Y.; Nishi, T.; Hidaka, H. 2(1H)-Quinolinone derivatives as novel anti-arteriostenotic agents showing anti-thrombotic and anti-hyperplastic activities. Bio. Med. Chem. Lett. 1998, 8, 1471–1476. [Google Scholar] [CrossRef]
- Beatch, G.N.; Ezrin, A. Uses of Ion Channel Modulating Compounds. PCT Int. Appl. WO2004098525, 3 May 2004. [Google Scholar]
- Bao, H.; Zhou, J.; Wang, Z.; Guo, Y.; You, T.; Ding, K. Insight into the Mechanism of the Asymmetric Ring-Opening Aminolysis of 4,4-Dimethyl-3,5,8-trioxabicyclo[5.1.0]octane Catalyzed by Titanium/BINOLate/Water System: Evidence for the Ti(BINOLate)2-Bearing Active Catalyst Entities and the Role of Water. J. Am. Chem. Soc. 2008, 130, 10116–10127. [Google Scholar] [CrossRef]
- Kumar, M.; Kureshy, R.I.; Ghosh, D.; Khan, N.H.; Abdi, S.H.R.; Bajaj, H.C. Synthesis of Chiral Ligands with Multiple Stereogenic Centers and Their Application in Titanium(IV)-Catalyzed Enantioselective Desymmetrization of meso-Epoxides. Chem. Cat. Chem. 2013, 5, 2336–2342. [Google Scholar] [CrossRef]
- More, G.V.; Bhanage, B.M. Asymmetric Ring Opening of meso -Epoxides with Aromatic Amines Using (R)-(+)-BINOL-Sc(OTf)3-NMM Complex as an Efficient Catalyst. Eur. J. Org. Chem. 2013, 2013, 6900–6906. [Google Scholar] [CrossRef]
- Sharma, A.; Agarwal, J.; Peddinti, R.K. Direct access to the optically active VAChT inhibitor vesamicol and its analogues via the asymmetric aminolysis of meso-epoxides with secondary aliphatic amines. Org. Biomol. Chem. 2017, 15, 1913–1920. [Google Scholar] [CrossRef]
- Bonollo, S.; Fringuelli, F.; Pizzo, F.; Vaccaro, L. Zn(II)-Catalyzed Desymmetrization of meso-Epoxides by Aromatic Amines in Water. Synlett 2008, 10, 1574–1578. [Google Scholar]
- Bao, H.; Wu, J.; Li, H.; Wang, Z.; You, T.; Ding, K. Enantioselective Ring Opening Reaction of meso -Epoxides with Aromatic and Aliphatic Amines Catalyzed by Magnesium Complexes of BINOL Derivatives. Eur. J. Org. Chem. 2010, 2010, 6722–6726. [Google Scholar] [CrossRef]
- Arai, K.; Lucarini, S.; Salter, M.M.; Ohta, K.; Yamashita, Y.; Kobayashi, S. The Development of Scalemic Multidentate Niobium Complexes as Catalysts for the Highly Stereoselective Ring Opening of meso-Epoxides and meso-Aziridines. J. Am. Chem. Soc. 2007, 129, 8103–8111. [Google Scholar] [CrossRef] [PubMed]
- Cue, B.W.; Zhang, J. Green process chemistry in the pharmaceutical industry. Green Chem. Lett. Rev. 2009, 2, 193–211. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Asano, T.; Tsuzaki, K.; Wada, K. Catalytic Asymmetric Amination of meso-Epoxide Using Soy Polysaccharide (Soyafibe S-DN). Bull. Chem. Soc. Jpn. 2018, 91, 678–683. [Google Scholar] [CrossRef]
- Takahashi, T.; Nakamura, A.; Kato, M.; Maeda, H.; Mandella, R.C.; Broadmeadow, A.; Ruckman, S.A. Soluble soybean fiber: A 3-month dietary toxicity study in rats. Food Chem. Toxicol. 2003, 41, 1111–1121. [Google Scholar] [CrossRef]
- Nakamura, A. Development of Soybean Soluble Polysaccharide Derived from “Okara”, and Application as a Functional Food Ingredient. Nippon Shokuhin Kagaku Kougakukaishi 2011, 58, 559–566. [Google Scholar] [CrossRef]
- Nakamura, A.; Furuta, H.; Maeda, H.; Takao, T.; Nagamatsu, Y. Structural Studies by Stepwise Enzymatic Degradation of the Main Backbone of Soybean Soluble Polysaccharides Consisting of Galacturonan and Rhamnogalacturonan. Biosci. Biotechnol. Biochem. 2002, 66, 1301–1313. [Google Scholar] [CrossRef]
- Azizi, N.; Saidi, M.R. Highly Chemoselective Addition of Amines to Epoxides in Water. Org. Lett. 2005, 7, 3649–3651. [Google Scholar] [CrossRef]
- Baker, R.A. Reassessment of Some Fruit and Vegetable Pectin Levels. J. Food Sci. 1997, 62, 225–229. [Google Scholar] [CrossRef]
- Voragen, A.G.J.; Goenen, G.J.; Verhoef, R.P.; Schols, H.A. Pectin, a versatile polysaccharide present in plant cell walls. Struct. Chem. 2009, 20, 263–275. [Google Scholar] [CrossRef]
- Mohnen, D. Pectin Structure and Biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, J.T.; Willis, D.E. Calculation of Flame Ionization Detector Relative Response Factors Using the Effective Carbon Number Concept. J. Chromatogr. Sci. 1985, 23, 333–340. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

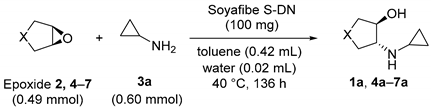
| Entry | Epoxide | X | Product | Conv. (%) | % ee |
|---|---|---|---|---|---|
| Control a | 2 | -C2H4- | 1a | 1 | - |
| 1 | 2 | -C2H4- | 1a | 98 | 65 |
| 2 b | 2 | -C2H4- | 1a | 95 | 64 |
| 3 | 4 | -CH2- | 4a | 98 | 67 |
| 4 | 5 | -O- | 5a | 83 | 21 c |
| 5 | 6 | -C3H6- | 6a | 17 | 35 c |
| 6 | 7 | H, H | 7a | 55 | 4 |

| Entry | Amine | R | R’ | Product | Conv. (%) | % ee |
|---|---|---|---|---|---|---|
| 1 | 3a | Cyclopropyl | H | 1a | 98 | 65 |
| 2 | 3b | Propyl | H | 1b | 87 | 39 |
| 3 | 3c | Isopropyl | H | 1c | 97 | 58 |
| 4 | 3d | Allyl | H | 1d | 96 | 32 |
| 5 | 3e | Propargyl | H | 1e | 98 | 53 |
| 6 | 3f | tert-Butyl | H | 1f | 4 | 32 |
| 7 | 3g | 3-Pentyl | H | 1g | 37 | 81 |
| 8 | 3h | Cyclopentyl | H | 1h | 99 | 48 |
| 9 | 3i | Benzyl | H | 1i | 90 | 30 a |
| 10 | 3j | 2-Phenylethyl | H | 1j | 100 | 59 a |
| 11 | 3k | Methyl | Methyl | 1k | 74 | 29 |
| 12 | 3m b | –(CH2)4- | 1m | 99 | 29 | |
| 13 | 3n | Phenyl | H | 1n | 57 | 18 a |
| 14 | 3o | H | H | 1o | 22 | 26 |

| Entry | Polysaccharide | Conv. (%) a | %ee a |
|---|---|---|---|
| 1 | Pectin (from citrus) b | 9 | 42 |
| 2 | Dextran | 1 | 0 |
| 3 | Chitosan | 2 | 0 |
| 4 | Carrageenan | 4 | 0 |
| 5 | Curdlan | 4 | −1 |
| 6 | (+)-Arabinogalactan (from lurch wood) | 0 | - |
| 7 | Gum Arabic | 1 | 0 |
| 8 | Xanthan gum | 0 | - |
| 9 | Pectinic acid b | 0 | - |
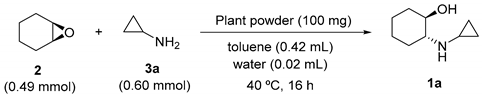
| Entry | Vegetable | Conv. (%) c | % ee c |
|---|---|---|---|
| 1 | Kiwifruit (peel) a | 9 | 71 |
| 2 | Carrot b | 12 | 62 |
| 3 | Pomelo (seed) a | 9 | 54 |
| 4 | Pumpkin b | 8 | 54 |
| 5 | Pistachio (seed) a | 7 | 47 |
| 6 | Potato (Mashed) b | 7 | 33 |
| 7 | Citron (peel) a | 4 | 32 |
| 8 | Lotus root b | 4 | 31 |
| 9 | Apple (seed) a | 3 | 26 |
| 10 | Tea (leaf) a | 29 | 28 |
| 11 | Kidney bean (seed) a | 5 | 27 |
| 12 | Green pea (seed) a | 4 | 14 |
| 13 | Turmeric b | 11 | −14 |
| Entry | Amine | R | R’ | Product | Conv. (%) | % ee |
|---|---|---|---|---|---|---|
| 1 | 3a | Cyclopropyl | H | 1a | 47 | 59 |
| 2 | 3b | Propyl | H | 1b | 21 | 13 |
| 3 | 3c | Isopropyl | H | 1c | 23 | 51 |
| 4 | 3d | Allyl | H | 1d | 19 | 8 |
| 5 | 3e | Propargyl | H | 1e | 27 | 2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takeuchi, Y.; Asano, T.; Tsuzaki, K.; Wada, K.; Kurata, H. Asymmetric Amination of meso-Epoxide with Vegetable Powder as a Low-Toxicity Catalyst. Molecules 2020, 25, 3197. https://doi.org/10.3390/molecules25143197
Takeuchi Y, Asano T, Tsuzaki K, Wada K, Kurata H. Asymmetric Amination of meso-Epoxide with Vegetable Powder as a Low-Toxicity Catalyst. Molecules. 2020; 25(14):3197. https://doi.org/10.3390/molecules25143197
Chicago/Turabian StyleTakeuchi, Yuki, Tatsuhiro Asano, Kazuya Tsuzaki, Koichi Wada, and Hiroyuki Kurata. 2020. "Asymmetric Amination of meso-Epoxide with Vegetable Powder as a Low-Toxicity Catalyst" Molecules 25, no. 14: 3197. https://doi.org/10.3390/molecules25143197
APA StyleTakeuchi, Y., Asano, T., Tsuzaki, K., Wada, K., & Kurata, H. (2020). Asymmetric Amination of meso-Epoxide with Vegetable Powder as a Low-Toxicity Catalyst. Molecules, 25(14), 3197. https://doi.org/10.3390/molecules25143197




