Characterization of Fatty Acid Composition Underlying Hypothalamic Inflammation in Aged Mice
Abstract
1. Introduction
2. Results
2.1. Aged Mice Are Characterized by Enhanced Hypothalamic Inflammation
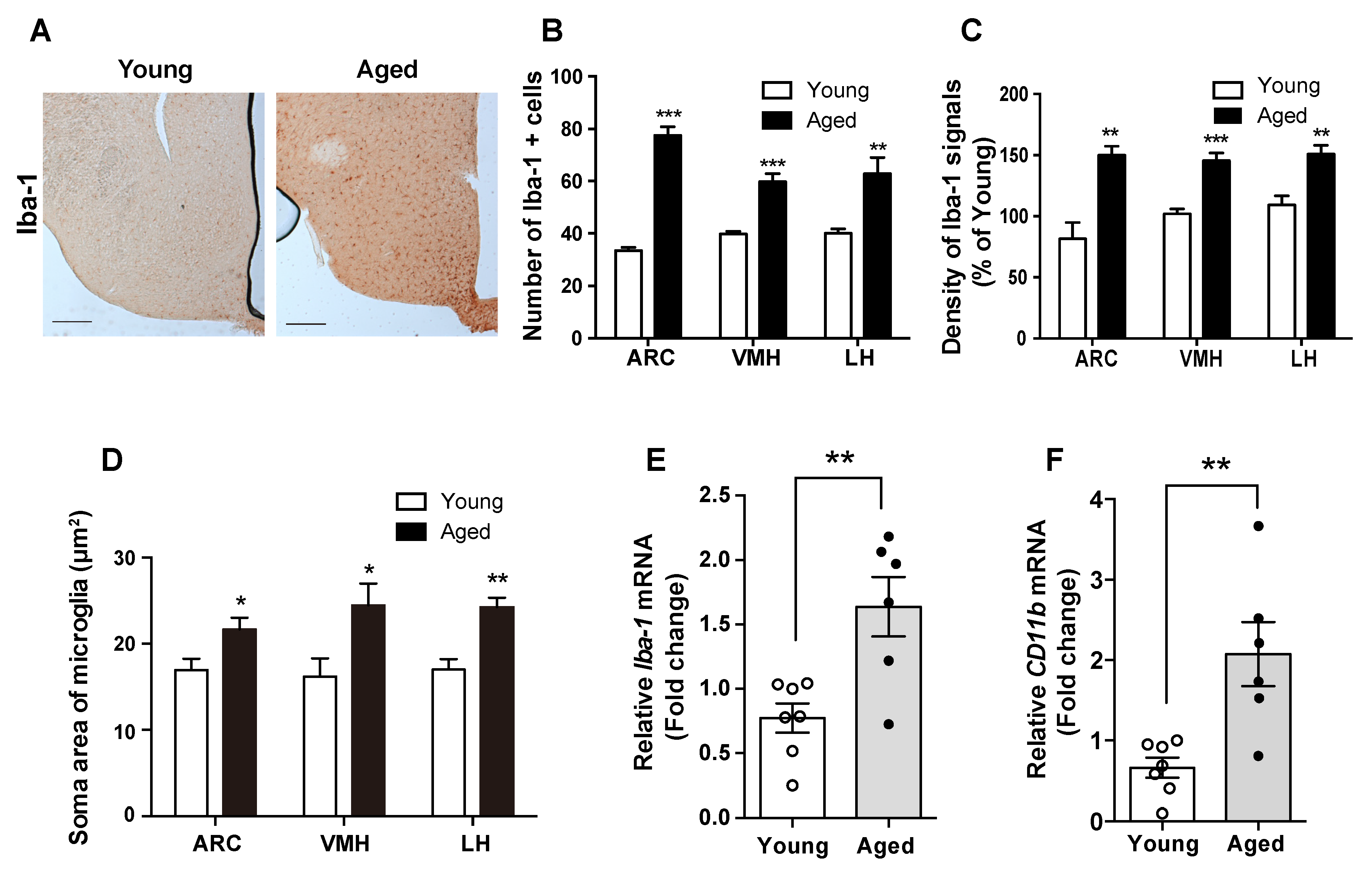
2.2. Hypothalamic Microgliosis Occurs in Aged Mice
2.3. Aged Mice Display Elevation in Hypothalamic sFA Levels
2.4. Enhanced Fatty Acid Utilization Is Observed in the Hypothalami of Aged Mice
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Analysis and Extraction of Long Chain Fatty Acids in Hypothalamus and Serum
4.3. Quantitative Real-Time PCR
4.4. Immunohistochemistry (IHC)
4.5. IHC Image Capture and Analyses
4.6. Statistical Analyses
Author Contributions
Funding
Conflicts of Interest
References
- Tchkonia, T.; Morbeck, D.E.; Von Zglinicki, T.; Van Deursen, J.; Lustgarten, J.; Scrable, H.; Khosla, S.; Jensen, M.D.; Kirkland, J.L. Fat tissue, aging, and cellular senescence. Aging Cell 2010, 9, 667–684. [Google Scholar] [CrossRef]
- Kim, D.H.; Bang, E.; Arulkumar, R.; Ha, S.; Chung, K.W.; Park, M.H.; Choi, Y.J.; Yu, B.P.; Chung, H.Y. Senoinflammation: A major mediator underlying age-related metabolic dysregulation. Exp. Gerontol. 2020, 134, 110891. [Google Scholar] [CrossRef] [PubMed]
- Floyd, R.A.; Hensley, K. Oxidative stress in brain aging: Implications for therapeutics of neurodegenerative diseases. Neurobiol. Aging 2002, 23, 795–807. [Google Scholar] [CrossRef]
- Hussain, S.G.; Ramaiah, K.V.A. Reduced eIF2α phosphorylation and increased proapoptotic proteins in aging. Biochem. Biophys. Res. Commun. 2007, 355, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Yu, J.; Zhu, A.; Nakanishi, H. Nutrients, microglia aging, and brain aging. Oxid. Med. Cell. Longev. 2016, 2016, 7498528. [Google Scholar] [CrossRef]
- Ransohoff, R.M. How neuroinflammation contributes to neurodegeneration. Science 2016, 353, 777–783. [Google Scholar] [CrossRef]
- Herrera, M.I.; Kölliker-Frers, R.; Barreto, G.; Blanco, E.; Capani, F. Glial Modulation by N-acylethanolamides in Brain Injury and Neurodegeneration. Front. Aging Neurosci. 2016, 8, 81. [Google Scholar] [CrossRef]
- Frasca, D.; Blomberg, B.B.; Paganelli, R. Aging, obesity, and inflammatory age-related diseases. Front. Immunol 2017, 8, 1745. [Google Scholar] [CrossRef]
- Jais, A.; Brüning, J.C. Hypothalamic inflammation in obesity and metabolic disease. J. Clin. Investig. 2017, 127, 24–32. [Google Scholar] [CrossRef]
- Cai, D.; Khor, S. “Hypothalamic microinflammation” paradigm in aging and metabolic diseases. Cell Metab. 2019, 30, 19–35. [Google Scholar] [CrossRef]
- Thaler, J.P.; Yi, C.-X.; Schur, E.A.; Guyenet, S.J.; Hwang, B.H.; Dietrich, M.O.; Zhao, X.; Sarruf, D.A.; Izgur, V.; Maravilla, K.R.; et al. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Investig. 2012, 122, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Valdearcos, M.; Robblee, M.M.; Benjamin, D.I.; Nomura, D.K.; Xu, A.W.; Koliwad, S.K. Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell Rep. 2014, 9, 2124–2138. [Google Scholar] [CrossRef]
- Milanski, M.; Degasperi, G.; Coope, A.; Morari, J.; Denis, R.; Cintra, D.E.; Tsukumo, D.M.L.; Anhe, G.; Amaral, M.E.; Takahashi, H.K.; et al. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: Implications for the pathogenesis of obesity. J. Neurosci. 2009, 29, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Tu, T.H.; Kim, H.; Yang, S.; Kim, J.K.; Kim, J.G. Linoleic acid rescues microglia inflammation triggered by saturated fatty acid. Biochem. Biophys. Res. Commun. 2019, 513, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Valdearcos, M.; Douglass, J.D.; Robblee, M.M.; Dorfman, M.D.; Stifler, D.R.; Bennett, M.L.; Gerritse, I.; Fasnacht, R.; Barres, B.A.; Thaler, J.P.; et al. Microglial inflammatory signaling orchestrates the hypothalamic immune response to dietary excess and mediates obesity susceptibility. Cell Metab. 2018, 27, 1356. [Google Scholar] [CrossRef] [PubMed]
- Thaler, J.P.; Guyenet, S.J.; Dorfman, M.D.; Wisse, B.E.; Schwartz, M.W. Hypothalamic inflammation: Marker or mechanism of obesity pathogenesis? Diabetes 2013, 62, 2629–2634. [Google Scholar] [CrossRef]
- Johnson, A.A.; Stolzing, A. The role of lipid metabolism in aging, lifespan regulation, and age-related disease. Aging Cell 2019, 18, e13048. [Google Scholar] [CrossRef]
- Cesar, H.C.; Pisani, L.P. Fatty-acid-mediated hypothalamic inflammation and epigenetic programming. J. Nutr. Biochem. 2017, 42, 1–6. [Google Scholar] [CrossRef]
- Stephenson, J.; Nutma, E.; van der Valk, P.; Amor, S. Inflammation in CNS neurodegenerative diseases. Immunology 2018, 154, 204–219. [Google Scholar] [CrossRef]
- Spittau, B. Aging microglia-phenotypes, functions and implications for age-related neurodegenerative diseases. Front. Aging Neurosci. 2017, 9, 194. [Google Scholar] [CrossRef]
- Valdearcos, M.; Xu, A.W.; Koliwad, S.K. Hypothalamic inflammation in the control of metabolic function. Annu. Rev. Physiol. 2015, 77, 131–160. [Google Scholar] [CrossRef] [PubMed]
- Arner, P.; Rydén, M. Fatty acids, obesity and insulin resistance. Obes. Facts 2015, 8, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, J.; Zhang, M.J.; Tang, Y.; Rane, M.; Bhatnagar, A.; Spite, M. Increased saturated fatty acids in obesity alter resolution of inflammation in part by stimulating prostaglandin production. J. Immunol. 2013, 191, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Erbay, E. Nutrient sensing and inflammation in metabolic diseases. Nat. Rev. Immunol. 2008, 8, 923–934. [Google Scholar] [CrossRef] [PubMed]
- IS Sobczak, A.; A Blindauer, C.; J Stewart, A. Changes in plasma free fatty acids associated with type-2 diabetes. Nutrients 2019, 11, 2022. [Google Scholar] [CrossRef]
- Ulmann, L.; Mimouni, V.; Roux, S.; Porsolt, R.; Poisson, J.P. Brain and hippocampus fatty acid composition in phospholipid classes of aged-relative cognitive deficit rats. Prostaglandins Leukot. Essent. Fat. Acids 2001, 64, 189–195. [Google Scholar] [CrossRef]
- Rodriguez-Navas, C.; Morselli, E.; Clegg, D.J. Sexually dimorphic brain fatty acid composition in low and high fat diet-fed mice. Mol. Metab. 2016, 5, 680–689. [Google Scholar] [CrossRef]
- Mobbs, C.V.; Moreno, C.L.; Poplawski, M. Metabolic mystery: Aging, obesity, diabetes, and the ventromedial hypothalamus. Trends Endocrinol. Metab. 2013, 24, 488–494. [Google Scholar] [CrossRef][Green Version]
- Cintra, D.E.; Ropelle, E.R.; Moraes, J.C.; Pauli, J.R.; Morari, J.; De Souza, C.T.; Grimaldi, R.; Stahl, M.; Carvalheira, J.B.; Saad, M.J.; et al. Unsaturated fatty acids revert diet-induced hypothalamic inflammation in obesity. PLoS ONE 2012, 7, e30571. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |


| Fatty Acid (µg/g) | Hypothalamus | Serum | ||
|---|---|---|---|---|
| 4 Months (Young) | 24 Months (Old) | 4 Months (Young) | 24 Months (Old) | |
| C14:0 (myristic acid) | 42.38 ± 3.11 1 | 55.51 ± 3.53 * | 4.02 ± 0.31 | 4.60 ± 0.55 |
| C16:0 (palmitic acid, mg/g) | 6.63 ± 0.16 | 7.21 ± 0.11 * | 0.47 ± 0.05 | 0.44 ± 0.03 |
| C16:1n7 (palmitoleic acid) | 213.03 ± 6.78 | 204.30 ± 4.35 | 45.43 ± 8.62 | 45.06 ± 5.77 |
| C18:0 (stearic acid, mg/g) | 5.61 ± 0.09 | 5.72 ± 0.04 | 0.19 ± 0.01 | 0.19 ± 0.01 |
| C18:1n9 (oleic acid, mg/g) | 6.15 ± 0.07 | 5.51 ± 0.03 *** | 0.22 ± 0.04 | 0.24 ± 0.02 |
| C18:2n6 (linoleic acid) | 200.00± 14.67 | 238.33 ± 5.55 * | 584.20 ± 71.75 | 515.85 ± 47.49 |
| C18:3n3 (α-Linolenic acid) | ND 2 | ND | 80.21 ± 5.10 | 74.94 ± 5.67 |
| C18:3n6 (γ-Linolenic acid) | 143.13 ± 13.58 | 202.73 ± 11.35 * | 9.76 ± 1.00 | 9.69 ± 0.85 |
| C20:0 (arachidic acid) | 78.10 ± 5.44 | 122.05 ± 6.68 ** | ND | ND |
| C20:3n6 (dihomo-γ-linolenic acid) | 105.32 ± 5.74 | 114.18 ± 3.25 | 22.97 ± 3.20 | 22.36 ± 2.60 |
| C20:4n6 (arachidonic acid, mg/g) | 3.68 ± 0.07 | 3.78 ± 0.33 | 0.15 ± 0.01 | 0.16 ± 0.01 |
| C20:5n3 (eicosapentaenoic acid) | 182.74 ± 11.32 | 218.70 ± 11.53 | 32.89 ± 0.30 | 26.99 ± 1.31 |
| C24:0 (lignoceric acid, mg/g) | 4.25 ± 0.10 | 4.43 ± 0.06 | 0.08 ± 0.009 | 0.07 ± 0.006 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.J.; Tu, T.H.; Yang, S.; Kim, J.K.; Kim, J.G. Characterization of Fatty Acid Composition Underlying Hypothalamic Inflammation in Aged Mice. Molecules 2020, 25, 3170. https://doi.org/10.3390/molecules25143170
Kim YJ, Tu TH, Yang S, Kim JK, Kim JG. Characterization of Fatty Acid Composition Underlying Hypothalamic Inflammation in Aged Mice. Molecules. 2020; 25(14):3170. https://doi.org/10.3390/molecules25143170
Chicago/Turabian StyleKim, Ye Jin, Thai Hien Tu, Sunggu Yang, Jae Kwang Kim, and Jae Geun Kim. 2020. "Characterization of Fatty Acid Composition Underlying Hypothalamic Inflammation in Aged Mice" Molecules 25, no. 14: 3170. https://doi.org/10.3390/molecules25143170
APA StyleKim, Y. J., Tu, T. H., Yang, S., Kim, J. K., & Kim, J. G. (2020). Characterization of Fatty Acid Composition Underlying Hypothalamic Inflammation in Aged Mice. Molecules, 25(14), 3170. https://doi.org/10.3390/molecules25143170






