Synthesis of Fluorinated 3,6-Dihydropyridines and 2-(Fluoromethyl)pyridines by Electrophilic Fluorination of 1,2-Dihydropyridines with Selectfluor®
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Methods
3.2. General Procedure for the Synthesis of 3-Fluoro-3,6-dihydropyridines 2a–k
3.2.1. Methyl 3-fluoro-2-methyl-5-nitro-6-phenyl-3,6-dihydropyridine-3-carboxylate (2a)
3.2.2. Methyl 6-([1,1′-biphenyl]-4-yl)-3-fluoro-2-methyl-5-nitro-3,6-dihydropyridine-3-carboxylate (2b)
3.2.3. Methyl 3-fluoro-6-(2-methoxyphenyl)-2-methyl-5-nitro-3,6-dihydropyridine-3-carboxylate (2c)
3.2.4. Methyl 3-fluoro-2-methyl-5-nitro-6-(3,4,5-trimethoxyphenyl)-3,6-dihydropyridine- 3-carboxylate (2d)
3.2.5. Methyl 2-ethyl-3-fluoro-6-(2-methoxyphenyl)-5-nitro-3,6-dihydropyridine-3-carboxylate (2e)
3.2.6. Methyl 3-fluoro-2-methyl-5-nitro-6-(4-nitrophenyl)-3,6-dihydropyridine-3-carboxylate (2f)
3.2.7. Methyl 3-fluoro-6-(3-fluorophenyl)-2-methyl-5-nitro-3,6-dihydropyridine-3-carboxylate (2g)
3.2.8. Methyl 6-(1,3-diphenyl-1H-pyrazol-4-yl)-3-fluoro-2-methyl-5-nitro-3,6-dihydropyridine- 3-carboxylate (2h)
3.2.9. Diethyl 5-fluoro-4,6-dimethyl-2-phenyl-2,5-dihydropyridine-3,5-dicarboxylate (2k)
3.3. General Procedure for the Synthesis of Pyridines 3a–k
3.3.1. Methyl 2-methyl-5-nitro-6-phenylnicotinate (3a)
3.3.2. Methyl 6-([1,1′-biphenyl]-4-yl)-2-methyl-5-nitronicotinate (3b)
3.3.3. Methyl 6-(2-methoxyphenyl)-2-methyl-5-nitronicotinate (3c)
3.3.4. Methyl 2-methyl-5-nitro-6-(3,4,5-trimethoxyphenyl)nicotinate (3d)
3.3.5. Methyl 2-ethyl-6-(2-methoxyphenyl)-5-nitronicotinate (3e)
3.3.6. Methyl 2-methyl-5-nitro-6-(4-nitrophenyl)nicotinate (3f)
3.3.7. Methyl 6-(3-fluorophenyl)-2-methyl-5-nitronicotinate (3g)
3.3.8. Methyl 6-(1,3-diphenyl-1H-pyrazol-4-yl)-2-methyl-5-nitronicotinate (3h)
3.3.9. Diethyl 2,4-dimethyl-6-phenylpyridine-3,5-dicarboxylate (3k)
3.4. General Procedure for the Synthesis of Methyl 2-(fluoromethyl)-5-nitro-6-arylnicotinates 5a–d
3.4.1. Methyl 2-(fluoromethyl)-5-nitro-6-phenylnicotinate (5a)
3.4.2. Methyl 2-(fluoromethyl)-6-(2-methoxyphenyl)-5-nitronicotinate (5b)
3.4.3. Methyl 2-(fluoromethyl)-5-nitro-6-(4-nitrophenyl)nicotinate (5c)
3.4.4. Methyl 2-(fluoromethyl)-6-(3-fluorophenyl)-5-nitronicotinate (5d)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Petrov, V.A. Fluorinated Heterocyclic Compounds: Synthesis, Chemistry, and Applications; Wiley: Hoboken, NJ, USA, 2009; pp. 1–432. ISBN 9780470452110. [Google Scholar]
- Gakh, A.A.; Kirk, K.L. Fluorinated Heterocycles. In ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2009; Volume 1003, pp. 3–20. ISBN 9780841269538. [Google Scholar]
- Nenajdenko, V. Fluorine in heterocyclic chemistry: Volume 2: 6-Membered heterocycles. In Fluorine in Heterocyclic Chemistry: Volume 2: 6-Membered Heterocycles; Springer International Publishing: Berlin, Germany, 2014; pp. 1–760. ISBN 9783319044354. [Google Scholar]
- Wang, J.; Scott, A.I. Fluoro-decarboxylation of pyrrolecarboxylic acids by F–TEDA–BF4—A convenient general synthesis of fluoropyrroles. J. Chem. Soc. Chem. Commun. 1995, 23, 2399–2400. [Google Scholar] [CrossRef]
- Hiramatsu, K.; Tamamura, H. A Mild Method for the Direct Fluorination of Pyrroles by Using a Lipophilic Anionic Phase-Transfer Catalyst. European J. Org. Chem. 2016, 2016, 3491–3494. [Google Scholar] [CrossRef]
- Forrest, A.K.; O’Hanlon, P.J. The preparation and lithiation of bromofluorofurans via a novel fluorodecarboxylation. Tetrahedron Lett. 1995, 36, 2117–2118. [Google Scholar] [CrossRef]
- Kobarfard, F.; Kauffman, J.M.; Boyko, W.J. Attempted syntheses of aminofluorothiophenes. J. Heterocycl. Chem. 1999, 36, 1247–1251. [Google Scholar] [CrossRef]
- Wang, X.; Seth, P.P.; Ranken, R.; Swayze, E.E.; Migawa, M.T. Synthesis and Biological Activity of 5-Fluorotubercidin. Nucleosides Nucleotides Nucleic Acids 2004, 23, 161–170. [Google Scholar] [CrossRef]
- Thiyagarajan, A.; Salim, M.T.A.; Balaraju, T.; Bal, C.; Baba, M.; Sharon, A. Structure based medicinal chemistry approach to develop 4-methyl-7-deazaadenine carbocyclic nucleosides as anti-HCV agent. Bioorganic Med. Chem. Lett. 2012, 22, 7742–7747. [Google Scholar] [CrossRef]
- O’Neill, P.M.; Storr, R.C.; Park, B.K. Synthesis of the 8-aminoquinoline antimalarial 5-fluoroprimaquine. Tetrahedron 1998, 54, 4615–4622. [Google Scholar] [CrossRef]
- Tang, P.; Ritter, T. Silver-mediated fluorination of aryl silanes. Tetrahedron 2011, 67, 4449–4454. [Google Scholar] [CrossRef]
- Gu, Q.; Vessally, E. N-Fluorobenzenesulfonimide: A useful and versatile reagent for the direct fluorination and amination of (hetero)aromatic C–H bonds. RSC Adv. 2020, 10, 16756–16768. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Tarui, T.; Shibata, N. A novel and efficient synthesis of 3-fluorooxindoles from indoles mediated by Selectfluor. Org. Lett. 2000, 2, 639–642. [Google Scholar] [CrossRef]
- Bisht, G.S.; Gnanaprakasam, B. Transition-metal-free addition reaction for the synthesis of 3-(aminobenzylidene/aminoalkylidene)indolin-2-ones and its synthetic applications. J. Org. Chem. 2019, 84, 13516–13527. [Google Scholar] [CrossRef] [PubMed]
- Shibata, N.; Tarui, T.; Doi, Y.; Kirk, K.L. Synthesis of fluorogypsetin and fluorobrevianamide E by a novel fluorination - Cyclization of cyclo-L-Trp-L-AAs. Angew. Chemie Int. Ed. 2001, 40, 4461–4463. [Google Scholar] [CrossRef]
- Yuan, X.; Yao, J.F.; Tang, Z.Y. Decarboxylative Fluorination of Electron-Rich Heteroaromatic Carboxylic Acids with Selectfluor. Org. Lett. 2017, 19, 1410–1413. [Google Scholar] [CrossRef] [PubMed]
- Hodson, H.F.; Madge, D.J.; Slawin, A.N.Z.; Widdowson, D.A.; Williams, D.J. Electrophilic fluorination in the synthesis of new fluoroindoles. Tetrahedron 1994, 50, 1899–1906. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, H.; He, H.; Wang, W.; Wang, Y.; Ke, Z.; Yeung, Y.-Y. Enantioseletive Fluorination of 3-Functionalized Oxindoles Using Electron-rich Amino Urea Catalyst. Adv. Synth. Catal. 2018, 360, 4710–4714. [Google Scholar] [CrossRef]
- Baudoux, J.; Salit, A.; Cahard, D.; Plaquevent, J.C. Ionic liquids as solvents of choice for electrophilic fluorination: Fluorination of indoles by F-TEDA-BF4. Tetrahedron Lett. 2002, 43, 6573–6574. [Google Scholar] [CrossRef]
- Yang, Q.; Dai, G.L.; Yang, Y.M.; Luo, Z.; Tang, Z.Y. Solvent Effects: Syntheses of 3,3-Difluorooxindoles and 3-Fluorooxindoles from Hydrazonoindolin-2-one by Selectfluor. J. Org. Chem. 2018, 83, 6762–6768. [Google Scholar] [CrossRef]
- Li, J.; Cai, Y.; Chen, W.; Liu, X.; Lin, L.; Feng, X. Highly enantioselective fluorination of unprotected 3-substituted oxindoles: One-step synthesis of BMS 204352 (MaxiPost). J. Org. Chem. 2012, 77, 9148–9155. [Google Scholar] [CrossRef] [PubMed]
- Shibata, N.; Suzuki, E.; Asahi, T.; Shiro, M. Enantioselective fluorination mediated by cinchona alkaloid derivatives/Selectfluor combinations: Reaction scope and structural information for N-fluorocinchona alkaloids. J. Am. Chem. Soc. 2001, 123, 7001–7009. [Google Scholar] [CrossRef]
- Abramovitch, R.A. The Chemistry of Heterocyclic Compounds, 14, Parts 1-4, Supplement: Pyridine and Its Derivatives. In The Chemistry of Heterocyclic Compounds; John Wiley & Sons: Hoboken, NJ, USA, 2007; ISBN 9780470188132. [Google Scholar]
- Van Der Puy, M. Direct fluorination of substituted pyridines. Tetrahedron Lett. 1987, 28, 255–258. [Google Scholar] [CrossRef]
- Anders, E.; Opitz, A.; Bauer, W. Remote Controlled Nucleophilicity, 21: Lithiated Cα-Substituted 4-Methylpyridines. Synthesis (Stuttg) 1991, 1991, 1221–1227. [Google Scholar] [CrossRef]
- Ying, W.; DesMarteau, D.D.; Gotoh, Y. N-fluoro-bis[(trifluoromethyl)sulfonyl]imide: Electrophilic fluorination of imines and some methyl-substituted pyridines. Tetrahedron 1996, 52, 15–22. [Google Scholar] [CrossRef]
- Baudoux, J.; Cahard, D. Electrophilic Fluorination with N-F Reagents. In Organic Reactions; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; pp. 1–326. [Google Scholar]
- Moberg, C.; Adolfsson, H.; Wärnmark, K.; Norrby, P.-O.; Marstokk, K.-M.; Møllendal, H. Conformational Preference of 2-(Halomethyl)- and 2-(Oxymethyl)Pyridines: Microwave Spectrum, Ab Initio, and MM3 Studies of 2-(Fluoromethyl)Pyridine. Chem. A Eur. J. 1996, 2, 516–522. [Google Scholar] [CrossRef]
- Andrews, S.P.; Brown, G.A.; Congreve, M.S.; Mason, J.S.; Richardson, C.M. 1,2,4-Triazine-4-amine derivatives. PCT Int. Appl. WO2011095625 A, 2011. [Google Scholar]
- Pikun, N.V.; Kolesnyk, N.V.P.; Rusanov, E.B.; Plotniece, A.; Rucins, M.; Sobolev, A.; Shermolovich, Y.G. Synthesis of fluorinated 2,6-heptanediones and 2-oxa-6-azabicyclo[2.2.2]octanes from 1,4-dihydropyridines. Tetrahedron 2018, 74, 2884–2890. [Google Scholar] [CrossRef]
- Pikun, N.V.; Kolesnyk, N.V.P.; Rusanov, E.B.; Plotniece, A.; Sobolev, A.; Domracheva, I.; Shermolovich, Y.G. Contrasting reactivity of fluorinated 2,6-heptanediones towards amines and ammonia, leading to cyclohexanediones or 2-oxa-6-azabicyclo[2.2.2]octanes and evaluation of their cytotoxicity. New J. Chem. 2019, 43, 10537–10544. [Google Scholar] [CrossRef]
- Ananthakrishnan, R.; Gazi, S. [Ru(bpy)3]2+ aided photocatalytic synthesis of 2-arylpyridines via Hantzsch reaction under visible irradiation and oxygen atmosphere. Catal. Sci. Technol. 2012, 2, 1463–1471. [Google Scholar] [CrossRef]
- Eisner, U.; Sadeghi, M.M. Isomerisation of dihydropyridines. Tetrahedron Lett. 1978, 19, 299–302. [Google Scholar] [CrossRef]
- Reine, I.; Muceniece, D.; Lusis, V.; Kemme, A. 2-Azabicyclo[2.2.2]oct-7-enes. 1. Synthesis from polysubstituted 1,2-dihydropyridines. Heterocycl. Commun. 2002, 8, 173–178. [Google Scholar] [CrossRef][Green Version]
- Ondrus, T.A.; Knaus, E.E.; Ciam, C.S. Some reactions of 1,2-dihydropyridines with cyanogen azide. Synthesis of üiazabieyclo[4.1.0]hept-4-enes. J. Heterocycl. Chem. 1979, 16, 409–410. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Y.; Zhu, S.; Zhu, G.; Jia, Y.; Huang, Q. Synthesis of N-(1,2,3,6-tetrahydropyridylidene)-fluoroalkanesulfonylamides from reactions of per(poly)fluoroalkanesulfonyl azides with 1,2-dihydropyridines. J. Fluor. Chem. 2000, 106, 133–138. [Google Scholar] [CrossRef]
- Francis, R.F.; Crews, C.D.; Scott, B.S. Identification of 2,5-Dihydropyridine Intermediates in the Reactions of 2-Alkyl(Phenyl)-1-lithio-1,2-dihydropyridines with Alkyl Halides. J. Org. Chem. 1978, 43, 3227–3230. [Google Scholar] [CrossRef]
- Giam, C.S.; Knaus, E.E.; Pasutto, F.M. Carbon vs. Nitrogen Acylation in Reactions of Organolithium-Pyridine Adducts with Acid Chlorides and Esters. J. Org. Chem. 1974, 39, 3565–3568. [Google Scholar] [CrossRef]
- Giam, C.S.; Knaus, E.E.; Lockhart, R.A.; Keener, I.G. Some Reactions of 1-Lithio-2-phenyl-1,2-dihydropyridine. IV. Synthesis of β-Substituted Pyridines. Can. J. Chem. 1975, 53, 2305–2310. [Google Scholar] [CrossRef]
- Ondrus, T.A.; Pasutto, F.M.; Knaus, E.E.; Giam, C.S. Some reactions of 2- n -butyl(phenyl)-1,2-dihydropyridines with isocyanates, pyridyl esters, and diethyl chlorophosphate. Can. J. Chem. 1978, 56, 1913–1918. [Google Scholar] [CrossRef]
- Vigante, B.; Plotniece, A.; Rucins, M.; Petrova, M.; Muhamadejev, R.; Pajuste, K.; Belyakov, S.; Shermolovich, Y.G.; Sobolev, A. An efficient synthesis of multisubstituted 4-nitrobuta-1,3-dien-1-amines and application in cyclisation reactions. Tetrahedron 2018, 74, 2596–2607. [Google Scholar] [CrossRef]
- Hennig, M.; Munzarová, M.L.; Bermel, W.; Scott, L.G.; Sklenář, V.; Williamson, J.R. Measurement of long-range 1H-19F scalar coupling constants and their glycosidic torsion dependence in 5-fluoropyrimidine- substituted RNA. J. Am. Chem. Soc. 2006, 128, 5851–5858. [Google Scholar] [CrossRef]
- Hilton, J.; Sutcliffe, L.H. The “through-space” mechanism in spin spin coupling. Prog. Nucl. Magn. Reson. Spectrosc. 1975, 10, 27–39. [Google Scholar] [CrossRef]
- Myhre, P.C.; Edmonds, J.W.; Kruger, J.D. Long-Range Spin-Spin Coupling in Alkylfluorobenzenes. The Stereochemical Requirements for Coupling of Fluorine and Hydrogen Separated by Five Bonds. J. Am. Chem. Soc. 1966, 88, 2459–2466. [Google Scholar] [CrossRef]
- Abraham, R.J.; Mobli, M. Modelling H-NMR Spectra of Organic Compounds: Theory, Applications and NMR Prediction Software. In Modelling H-NMR Spectra of Organic Compounds: Theory, Applications and NMR Prediction Software; John Wiley & Sons: Hoboken, NJ, USA, 2008; pp. 1–380. ISBN 9780470723012. [Google Scholar]
- Grossel, M.C.; Cheetham, A.K.; Newsam, J.M. The effect of bulky substituents on the conformation of cyclohexa-1,4-diene. Tetrahedron Lett. 1978, 19, 5229–5232. [Google Scholar] [CrossRef]
- Atkinson, D.J.; Perkins, M.J. A comment on the magnitude of homoallylic coupling and the conformation of 1,4-cyclohexadiene. Tetrahedron Lett. 1969, 10, 2335–2338. [Google Scholar] [CrossRef]
- Grossel, M.C.; Cheetham, A.K.; Hope, D.A.O.; Lam, K.P.; Perkins, M.J. The preferred conformation of cis-1,4-dihydro-4-tritylbiphenyl: A flexible cyclohexa-1,4-diene. Tetrahedron Lett. 1979, 20, 1351–1354. [Google Scholar] [CrossRef]
- Cheetham, A.K.; Newsam, J.M.; Grossel, M.C. Conformational Studies of Dihydrotetraphenylmethanes. 1. X-ray Crystallographic and Solution 1H-NMR Studies of trans-1,4-Dihydro-4-tritylbiphenyl and Its 4’-Bromo Derivative: Boat-Boat Inversion in a Congested Cyclohexa-1,4-diene. J. Am. Chem. Soc. 1981, 103, 5363–5372. [Google Scholar] [CrossRef]
- Garbisch, E.W.; Griffith, M.G. The Conformation of 1,4-Cyclohexadiene from Stereoisomeric Allylic-Allylic Proton Couplings. J. Am. Chem. Soc. 1968, 90, 3590–3592. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 3a,c,e and 5a,d are available from the authors. |


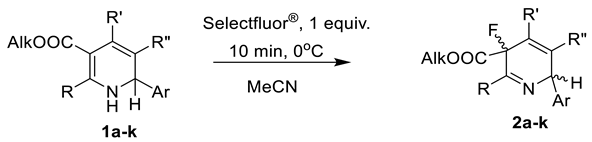
| Comp. | R | Alk | R′ | R″ | Ar | The Ratio of Diastereomers, % 1 | Yield, %2 |
|---|---|---|---|---|---|---|---|
| 2a | Me | Me | H | NO2 | Ph | 45:55 | 94 |
| 2b | Me | Me | H | NO2 | Ph-Ph | 40:60 | 96 |
| 2c | Me | Me | H | NO2 | o-OMe-Ph | 45:55 | 90 |
| 2d | Me | Me | H | NO2 | 3,4,5-OMe-Ph | 20:80 | 90 |
| 2e | Et | Me | H | NO2 | o-OMe-Ph | 50:50 | 97 |
| 2f | Me | Me | H | NO2 | p-NO2-Ph | 50:50 | 94 |
| 2g | Me | Me | H | NO2 | m-F-Ph | 50:50 | 88 |
| 2h | Me | Me | H | NO2 | 1,3-Ph-1H-pyrazol-4-yl | 10:90 | 89 |
| 2k | Me | Et | Me | COOEt | Ph | 15:85 | 82 |
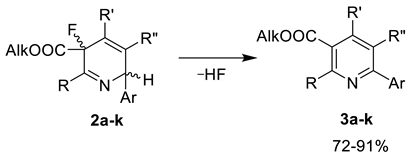
| Comp. | R | Alk | R′ | R″ | Ar | Yield, %1 |
|---|---|---|---|---|---|---|
| 3a | Me | Me | H | NO2 | Ph | 91 |
| 3b | Me | Me | H | NO2 | Ph-Ph | 85 |
| 3c | Me | Me | H | NO2 | o-OMe-Ph | 72 |
| 3d | Me | Me | H | NO2 | 3,4,5-OMe-Ph | 84 |
| 3e | Et | Me | H | NO2 | o-OMe-Ph | 82 |
| 3f | Me | Me | H | NO2 | p-NO2-Ph | 93 |
| 3g | Me | Me | H | NO2 | m-F-Ph | 87 |
| 3h | Me | Me | H | NO2 | 1,3-Ph-1H-pyrazol-4-yl | 79 |
| 3k | Me | Et | Me | COOEt | Ph | 85 |
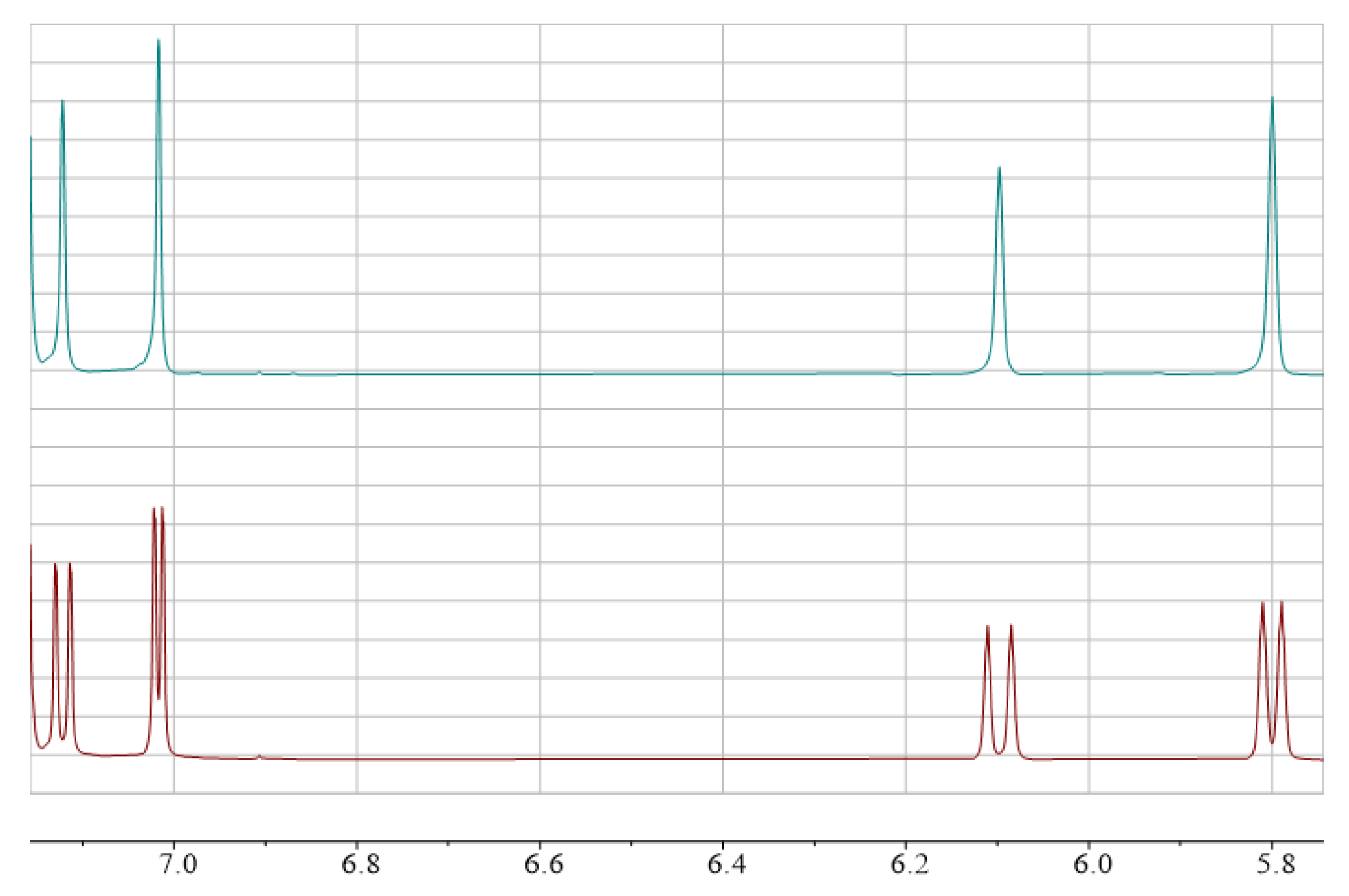
| Comp. | The Ratio of Diastereo-mers, %1 | δ C6H Proton for Both Diastereomers in 1H-NMR Spectra, ppm | δ F for both Diastereomers in 19F-NMR Spectra, ppm | Homoallylic Coupling Constant 5JH-F(F-H) for Both Diastereomers, Hz2 | 3JH-F(F-H) for Both Diastereomers, Hz2 | Allylic Coupling Constant 4JH-H for both Diastereomers, Hz3 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Minor | Major | Minor | Major | Minor | Major | Minor | Major | Minor | Major | ||
| 2a | 45:55 | 6.09 | 5.79 | −140.6 | −142.4 | 15.4 | 12.1 | 9.4 | 5.6 | 1.3 | 1.2 |
| 2b | 40:60 | 6.22 | 5.94 | −140.3 | −142.4 | 15.4 | 12.1 | 9.3 | 5.6 | 1.2 | 1.2 |
| 2c | 45:55 | 6.70 | 6.22 | −139.9 | −144.3 | 16.6 | 12.6 | 9.0 | 6.2 | 1.4 | 1.6 |
| 2d | 20:80 | 5.79 | 6.02 | −141.5 | −140.4 | 12.2 | 15.8 | 5.4 | 9.2 | 1.1 | 1.2 |
| 2e | 50:50 | 6.72 | 6.21 | −140.5 | −145.1 | 17.0 | 12.6 | 9.1 | 6.5 | 1.2 | 1.6 |
| 2f | 50:50 | 6.26 | 5.94 | −141.6 | −143.0 | 14.4 | 11.4 | 9.5 | 5.7 | 1.1 | 1.3 |
| 2g | 50:50 | 6.16 | 5.85 | −140.7 | −142.8 | 14.9 | 11.8 | 9.4 | 5.6 | 1.1 | 1.2 |
| 2h | 10:90 | 6.05 | 6.30 | −144.2 | −142.6 | 11.9 | 14.0 | 5.8 | 9.5 | 1.5 | 1.1 |
| 2k | 15:85 | 5.38 | 5.70 | −150.4 | −145.9 | 12.0 | 15.5 | - | - | - | - |
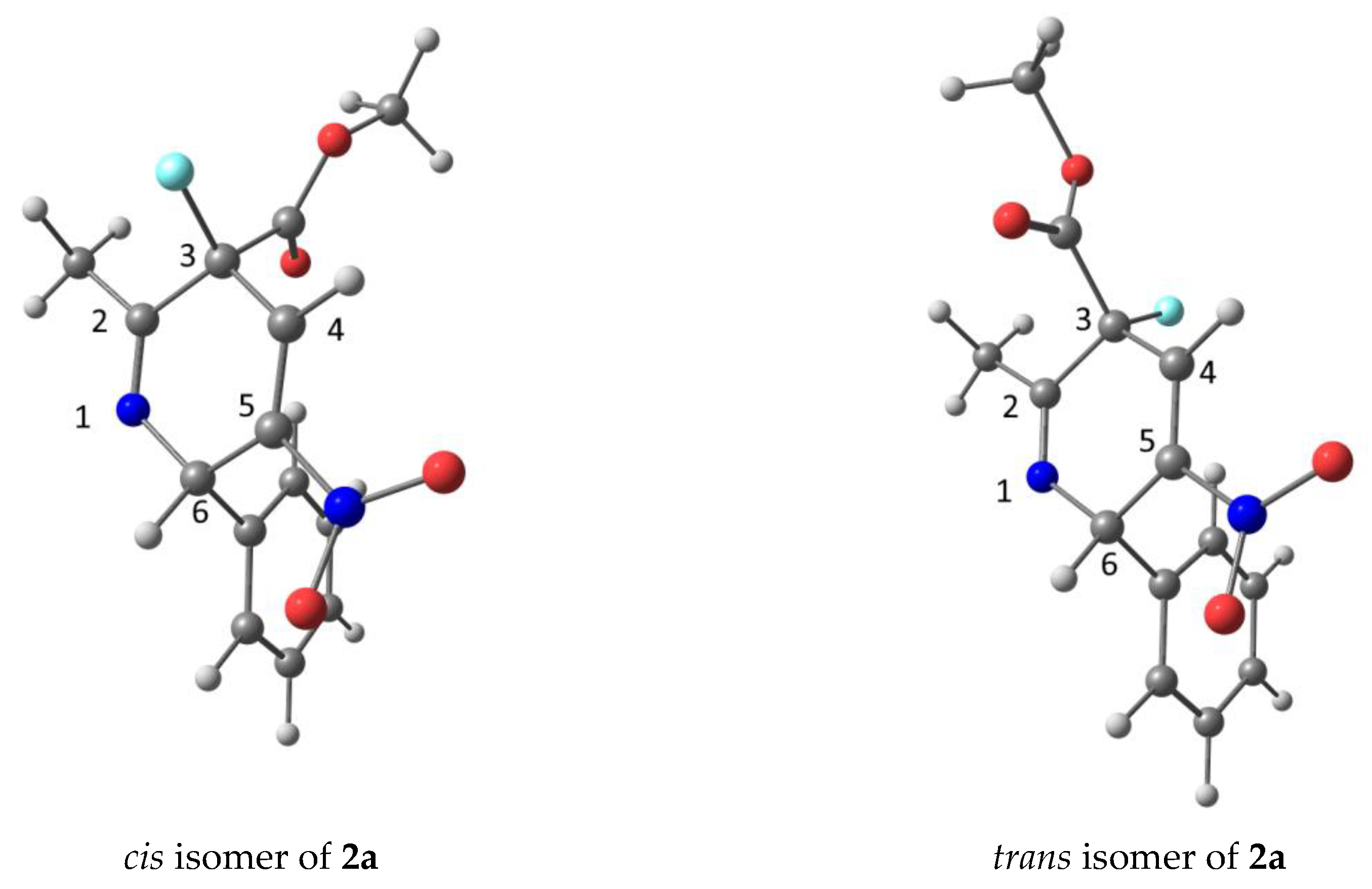
| Comp. | d(H…F), Å | d(C6 to N1-C2-C4-C5 plane), Å | d(C3 to N1-C2-C4-C5 plane), Å | The angle between C6-N1-C2-C3 and C6-C5-C4-C3 Planes, ° | ||||
|---|---|---|---|---|---|---|---|---|
| cis | trans | cis | trans | cis | trans | cis | trans | |
| 2a | 4.60 | 4.73 | 0.11 | 0.11 | 0.12 | 0.13 | 169.38 | 168.88 |
| 2b | 4.60 | 4.85 | 0.11 | 0.02 | 0.13 | 0.05 | 169.18 | 178.46 |
| 2c | 4.59 | 4.85 | 0.10 | 0.01 | 0.13 | 0.08 | 169.72 | 175.66 |
| 2d | 4.61 | 4.72 | 0.12 | 0.12 | 0.13 | 0.15 | 168.90 | 167.86 |
| 2e | 4.57 | 4.77 | 0.09 | 0.07 | 0.12 | 0.07 | 170.45 | 173.73 |
| 2f | 4.59 | 4.85 | 0.10 | 0.03 | 0.13 | 0.10 | 169.74 | 174.07 |
| 2g | 4.60 | 4.85 | 0.11 | 0.01 | 0.13 | 0.08 | 169.40 | 175.94 |
| 2h | 4.63 | 4.77 | 0.13 | 0.08 | 0.14 | 0.08 | 167.81 | 172.97 |
| 2k | 4.98 | 4.80 | 0.30 | 0.02 | 0.42 | 0.05 | 148.20 | 176.79 |
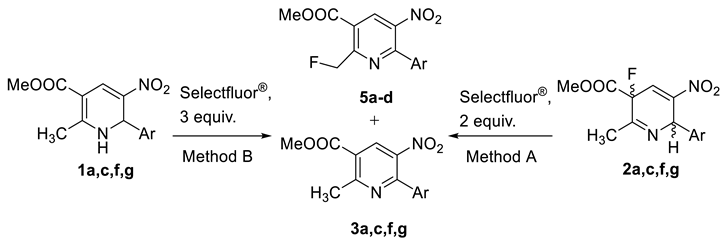
| Comp. | Ar | Ratio 3:5, %1 | Yields, %2 | ||
|---|---|---|---|---|---|
| Method A | Method B | Comp. 3 | Comp. 5 | ||
| 3a, 5a | Ph | 50:50 | 55:45 | 44 | 32 |
| 3c, 5b | o-OMe-Ph | 60:40 | 55:45 | 52 | 21 |
| 3f, 5c | p-NO2-Ph | 15:75 | 20:80 | 10 | 38 |
| 3g, 5d | m-F-Ph | 50:50 | 50:50 | 36 | 43 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pikun, N.V.; Sobolev, A.; Plotniece, A.; Rucins, M.; Vigante, B.; Petrova, M.; Muhamadejev, R.; Pajuste, K.; Shermolovich, Y.G. Synthesis of Fluorinated 3,6-Dihydropyridines and 2-(Fluoromethyl)pyridines by Electrophilic Fluorination of 1,2-Dihydropyridines with Selectfluor®. Molecules 2020, 25, 3143. https://doi.org/10.3390/molecules25143143
Pikun NV, Sobolev A, Plotniece A, Rucins M, Vigante B, Petrova M, Muhamadejev R, Pajuste K, Shermolovich YG. Synthesis of Fluorinated 3,6-Dihydropyridines and 2-(Fluoromethyl)pyridines by Electrophilic Fluorination of 1,2-Dihydropyridines with Selectfluor®. Molecules. 2020; 25(14):3143. https://doi.org/10.3390/molecules25143143
Chicago/Turabian StylePikun, Nadiia V., Arkadij Sobolev, Aiva Plotniece, Martins Rucins, Brigita Vigante, Marina Petrova, Ruslan Muhamadejev, Karlis Pajuste, and Yuriy G. Shermolovich. 2020. "Synthesis of Fluorinated 3,6-Dihydropyridines and 2-(Fluoromethyl)pyridines by Electrophilic Fluorination of 1,2-Dihydropyridines with Selectfluor®" Molecules 25, no. 14: 3143. https://doi.org/10.3390/molecules25143143
APA StylePikun, N. V., Sobolev, A., Plotniece, A., Rucins, M., Vigante, B., Petrova, M., Muhamadejev, R., Pajuste, K., & Shermolovich, Y. G. (2020). Synthesis of Fluorinated 3,6-Dihydropyridines and 2-(Fluoromethyl)pyridines by Electrophilic Fluorination of 1,2-Dihydropyridines with Selectfluor®. Molecules, 25(14), 3143. https://doi.org/10.3390/molecules25143143







