Chiral Separation and Determination of Etoxazole Enantiomers in Vegetables by Normal-Phase and Reverse-Phase High Performance Liquid Chromatography
Abstract
1. Introduction
2. Results and Discussion
2.1. Chiral Separation of Etoxazole Enantiomers
2.2. Effects of Temperature on Etoxazole Separation
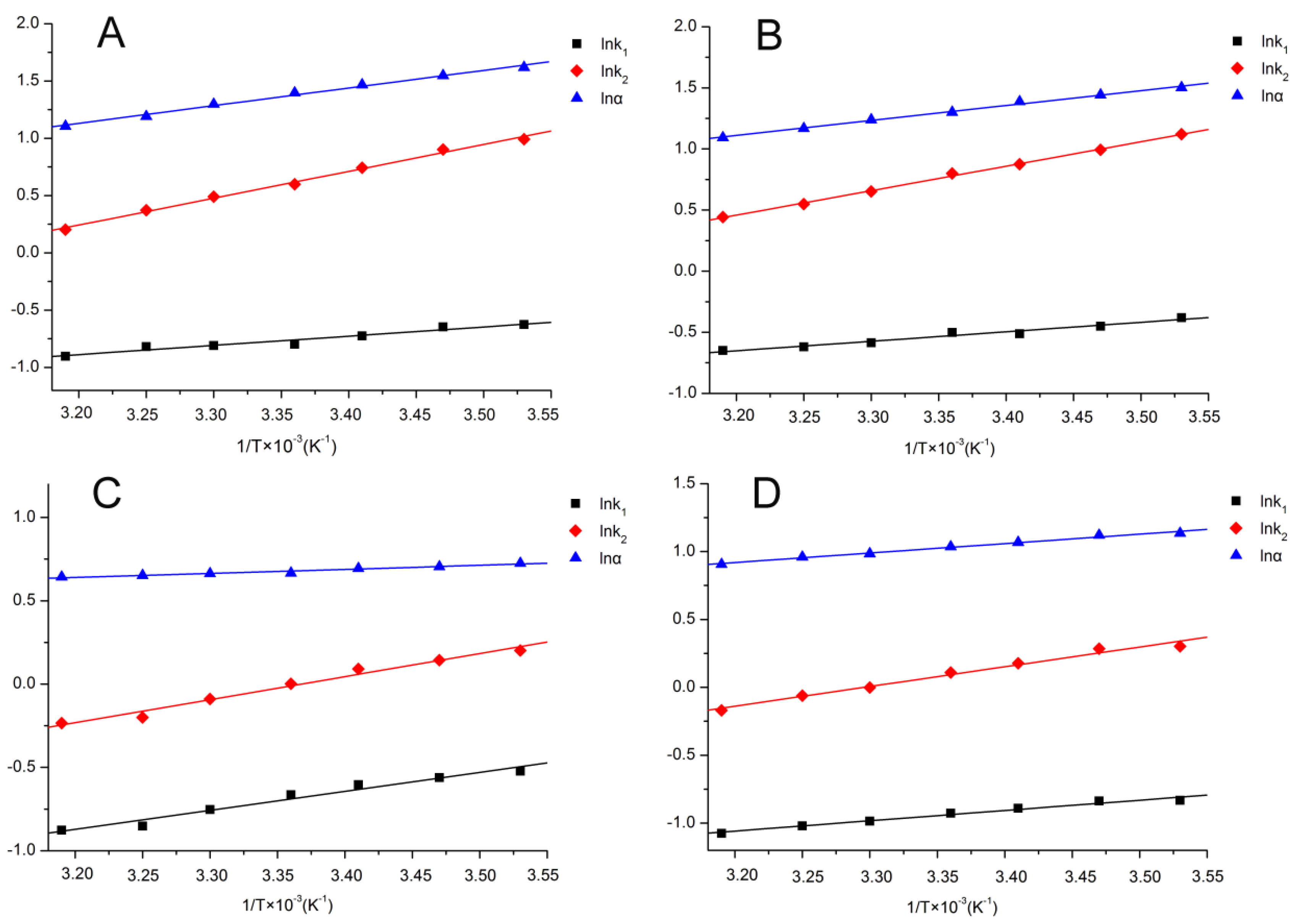
2.3. Thermodynamic Parameters
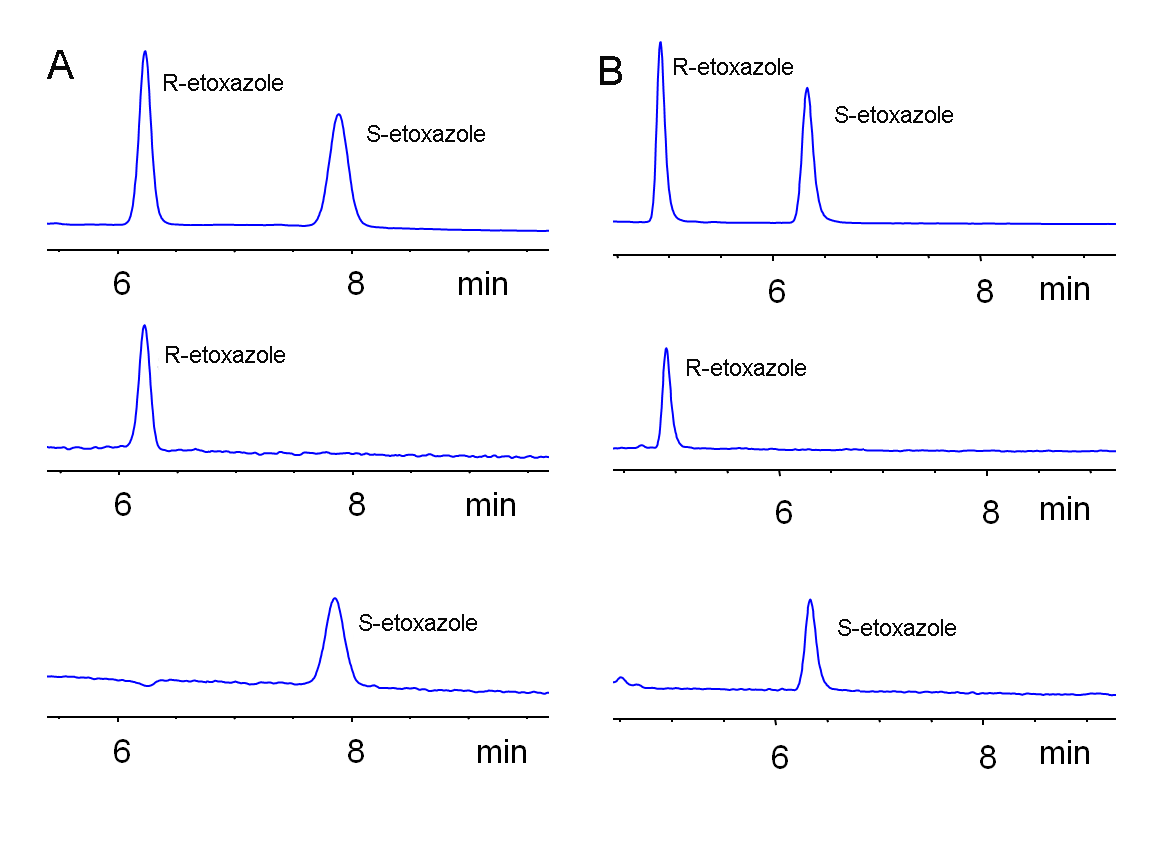
2.4. Elution Order of Etoxazole Enantiomers on Different Chiral Columns
2.5. Etoxazole Enantiomers Analysis in Vegetables
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Apparatus and Chromatographic Conditions
3.3. Method Validation
3.4. Sample Preparation
3.5. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| HPLC | high-performance liquid chromatography |
| CSP | chiral stationary phase |
| GC | gas chromatography |
| LOD | limit of detection |
| RSD | relative standard deviation. |
References
- Jeschke, P. Current status of chirality in agrochemicals. Pest Manag. Sci. 2018, 74, 2389–2404. [Google Scholar] [CrossRef] [PubMed]
- Williams, A. Opportunities for chiral agrochemicals. Pest Manag. Sci. 1996, 46, 3–9. [Google Scholar] [CrossRef]
- Chang, W.; Nie, J.; Yan, Z.; Wang, Y.; Farooq, S. Systemic Stereoselectivity Study of Etoxazole: Stereoselective Bioactivity, Acute Toxicity, and Environmental Behavior in Fruits and Soils. J. Agric. Food Chem. 2019, 67, 6708–6715. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hua, X.; Shi, H.-Y.; Liu, J.-S.; Tian, M.-M.; Wang, M. Enantioselective bioactivity, acute toxicity and dissipation in vegetables of the chiral triazole fungicide flutriafol. J. Hazard. Mater. 2015, 284, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, A.; Zhang, J.; Xie, X.; Liu, W. Enantiomeric Resolution and Growth-Retardant Activity in Rice Seedlings of Uniconazole. J. Agric. Food Chem. 2011, 60, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Liu, W.; Li, L.; Gan, J. Single and Joint Acute Toxicity of Isocarbophos Enantiomers toDaphnia magna. J. Agric. Food Chem. 2008, 56, 4273–4277. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, L.; Li, D.; Teng, M.; Zhang, R.; Zhou, Z.; Zhu, W. Enantioselective bioaccumulation of hexaconazole and its toxic effects in adult zebrafish (Danio rerio). Chemosphere 2015, 138, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.R.; Afonso, C.M.; Castro, P.M.; Tiritan, M.E. Enantioselective biodegradation of pharmaceuticals, alprenolol and propranolol, by an activated sludge inoculum. Ecotoxicol. Environ. Saf. 2013, 87, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dong, F.; Liu, X.; Xu, J.; Li, J.; Kong, Z.; Chen, X.; Liang, X.; Zheng, Y. Simultaneous enantioselective determination of triazole fungicides in soil and water by chiral liquid chromatography/tandem mass spectrometry. J. Chromatogr. A 2012, 1224, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Qian, M.; Zhang, H.; Nie, J.; Ye, J.; Li, Z. Etoxazole is Metabolized Enantioselectively in Liver Microsomes of Rat and Human in Vitro. Environ. Sci. Technol. 2016, 50, 9682–9688. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, J.; Ishida, T.; Kikuchi, Y.; Ito, Y.; Morikawa, C.; Tsukidate, Y.; Tanji, I.; Ota, Y.; Toda, K. Synthesis and Activity of Novel Acaricidal/Insecticidal 2, 4-Diphenyl-1, 3-oxazolines. J. Pestic. Sci. 2002, 27, 1–8. [Google Scholar] [CrossRef]
- A Dekeyser, M. Acaricide mode of action. Pest Manag. Sci. 2005, 61, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Li, Z.G.; Zhuang, S.; Li, X.; Xu, M.; Lin, M.; Wang, Q.; Zhang, H. Enantioselective determination of acaricide etoxazole in orange pulp, peel, and whole orange by chiral liquid chromatography with tandem mass spectrometry. J. Sep. Sci. 2015, 38, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Malhat, F.M.; Badawy, H.; Barakat, D.; Saber, A. Determination of etoxazole residues in fruits and vegetables by SPE clean-up and HPLC-DAD. J. Environ. Sci. Heal. Part B 2013, 48, 331–335. [Google Scholar] [CrossRef]
- Chen, H.; Li, W.; Guo, L.; Weng, H.; Wei, Y.; Guo, Q. Residue, dissipation, and safety evaluation of etoxazole and pyridaben in Goji berry under open-field conditions in the China’s Qinghai–Tibet Plateau. Environ. Monit. Assess. 2019, 191, 517. [Google Scholar] [CrossRef]
- Hwang, J.-I.; Kim, J.-E. Residual Patterns of Acaricides, Etoxazole and Flufenoxuron in Apples. Korean J. Pestic. Sci. 2014, 18, 61–68. [Google Scholar] [CrossRef]
- Wang, P.; Jiang, S.; Liu, D.; Zhang, H.; Zhou, Z. Enantiomeric Resolution of Chiral Pesticides by High-Performance Liquid Chromatography. J. Agric. Food Chem. 2006, 54, 1577–1583. [Google Scholar] [CrossRef]
- Yang, W.; Qiu, J.; Chen, T.; Yang, S.; Hou, S. Direct Enantioseparation of Nitrogen-heterocyclic Pesticides on Amylose-tris-(5-chloro-2-methylphenylcarbamate) by Reversed-Phase High-Performance Liquid Chromatography. Chirality 2012, 24, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Dai, S.; Zheng, C.; Yang, S.; Chai, T.; Bie, M. Enantiomeric separation of triazole fungicides with 3-μm and 5-μml particle chiral columns by reverse-phase high-performance liquid chromatography. Chirality 2011, 23, 479–486. [Google Scholar] [CrossRef]
- Fox, S.; Strasdeit, H.; Haasmann, S.; Brückner, H. Gas chromatographic separation of stereoisomers of non-protein amino acids on modified γ-cyclodextrin stationary phase. J. Chromatogr. A 2015, 1411, 101–109. [Google Scholar] [CrossRef]
- Fernandez, V.P.; Vega, E.D.; Chankvetadze, B.; Crego, A.L.; Garcia, M.A.; Marina, M.L. Evaluation of new cellulose-based chiral stationary phases Sepapak-2 and Sepapak-4 for the enantiomeric separation of pesticides by nano liquid chromatography and capillary electrochromatography. J. Chromatogr. A 2012, 1234, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Dascalu, A.-E.; Ghinet, A.; Chankvetadze, B.; Lipka, E. Comparison of dimethylated and methylchlorinated amylose stationary phases, coated and covalently immobilized on silica, for the separation of some chiral compounds in supercritical fluid chromatography. J. Chromatogr. A 2020, 1621, 461053. [Google Scholar] [CrossRef]
- Dascalu, A.-E.; Speybrouck, D.; Billamboz, M.; Corens, D.; Ghinet, A.; Lipka, E. Analytical and preparative enantioseparations in supercritical fluid chromatography using different brands of immobilized cellulose tris (3,5-dichlorophenylcarbamate) columns: Some differences. J. Chromatogr. A 2020, 1622, 461125. [Google Scholar] [CrossRef] [PubMed]
- Dubey, R.; Bhushan, R. Enantioseparation by Thin-Layer Chromatography. In Chiral Separations: Methods and Protocols, 3rd ed.; Scriba, G.K.E., Ed.; Springer Science+Business Media, LLC: New York, NY, USA, 2019; Volume 1985, pp. 35–44. [Google Scholar]
- Marina, M.L.; Benito, I.; DiezMasa, J.C.; Gonzalez, M.J. Chiral separation of polychlorinated biphenyls by micellar electrokinetic chromatography with gamma-cyclodextrin as modifier in the separation buffer. Chromatographia 1996, 42, 269–272. [Google Scholar] [CrossRef]
- Choy, T.M.H.; Chan, W.H.; Lee, A.W.; Huie, C.W. Stacking and separation of enantiomers by acetonitrile-salt mixtures in micellar electrokinetic chromatography. Electrophor. 2003, 24, 3116–3123. [Google Scholar] [CrossRef] [PubMed]
- Ward, T.J.; Ward, K.D. Chiral Separations: Fundamental Review 2010. Anal. Chem. 2010, 82, 4712–4722. [Google Scholar] [CrossRef]
- Liu, N.; Dong, F.; Xu, J.; Liu, X.; Chen, Z.; Pan, X.; Chen, X.; Zheng, Y. Enantioselective separation and pharmacokinetic dissipation of cyflumetofen in field soil by ultra-performance convergence chromatography with tandem mass spectrometry. J. Sep. Sci. 2016, 39, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Wu, J.; Liu, W. Enantioselective separation and analysis of chiral pesticides by high-performance liquid chromatography. TrAC Trends Anal. Chem. 2009, 28, 1148–1163. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, R.; Wang, X.; Wang, Y.; Wang, D.; Zhou, Z.; Zhu, W. Enantiomeric Separation of Chiral Pesticides by Permethylated β-Cyclodextrin Stationary Phase in Reversed PhaseLiquid Chromatography. Chirality 2016, 28, 409–414. [Google Scholar] [CrossRef]
- Sun, D.; Pang, J.; Fang, Q.; Zhou, Z.; Jiao, B.N. Stereoselective toxicity of etoxazole to MCF-7 cells and its dissipation behavior in citrus and soil. Environ. Sci. Pollut. Res. 2016, 23, 24731–24738. [Google Scholar] [CrossRef]
- Sun, D.; Wang, Y.; Zhang, Q.; Pang, J. Investigation of etoxazole metabolites in citrus, soil and earthworms by ultra-performance liquid chromatography with time-of-flight mass spectrometry. Chemosphere 2019, 226, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yu, Q.; He, Y.; Zhu, W.; Zhou, Z.; He, L. Chiral pyrethroid insecticide fenpropathrin and its metabolite: Enantiomeric separation and pharmacokinetic degradation in soils by reverse-phase high-performance liquid chromatography. Anal. Methods 2017, 9, 4439–4446. [Google Scholar] [CrossRef]
- Wang, P.; Liu, D.; Jiang, S.; Xu, Y.; Gu, X.; Zhou, Z. The chiral resolution of pesticides on amylose-tris(3,5-dimethylphenylcarbamate) CSP by HPLC and the enantiomeric identification by circular dichroism. Chirality 2007, 20, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yu, Q.; He, X.; Qian, K.; Xiao, W.; Xu, Z.; Li, T.; He, L. Enantiomeric separation of type I and type II pyrethroid insecticides with different chiral stationary phases by reversed-phase high-performance liquid chromatography. Chirality 2017, 30, 420–431. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |
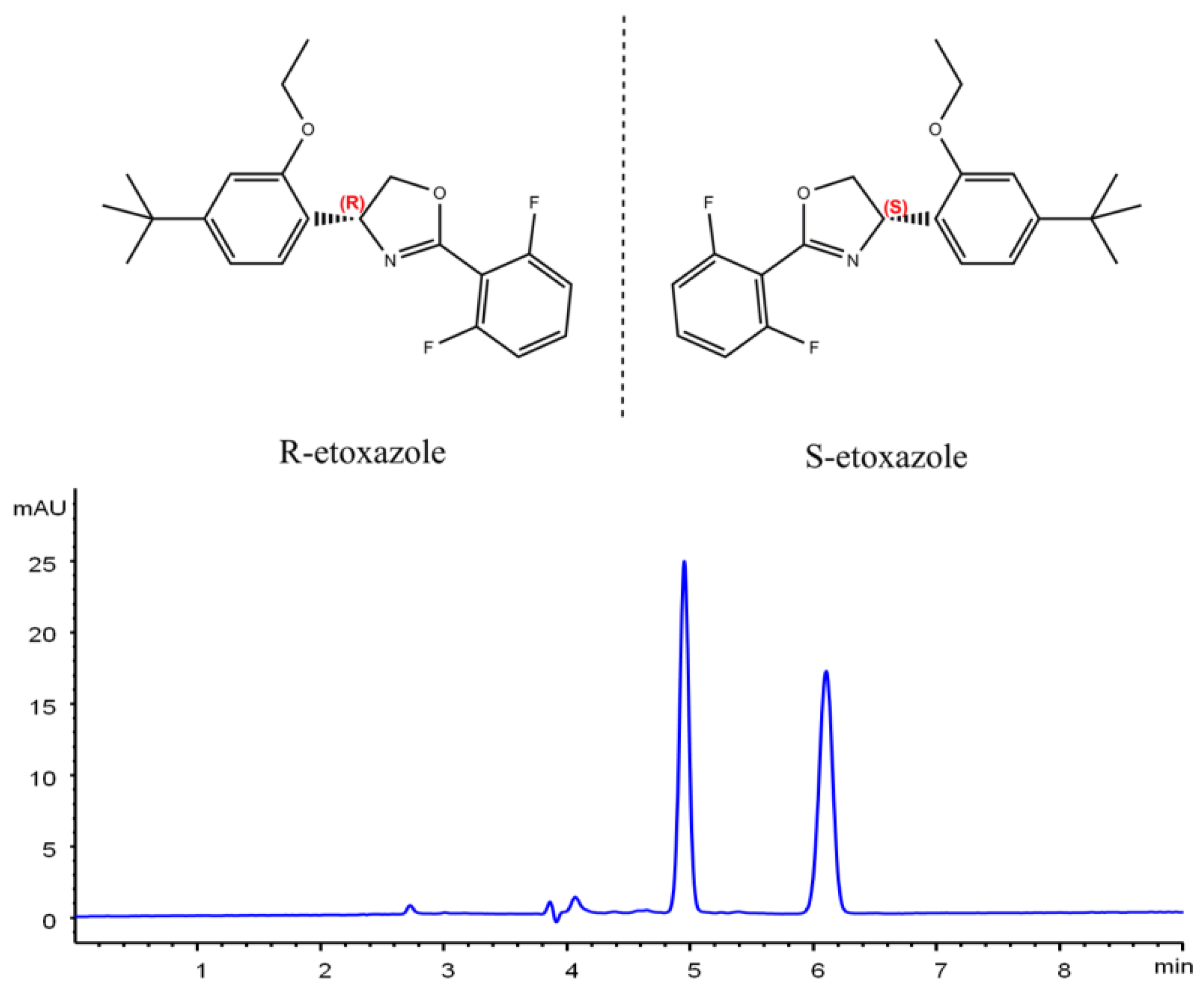
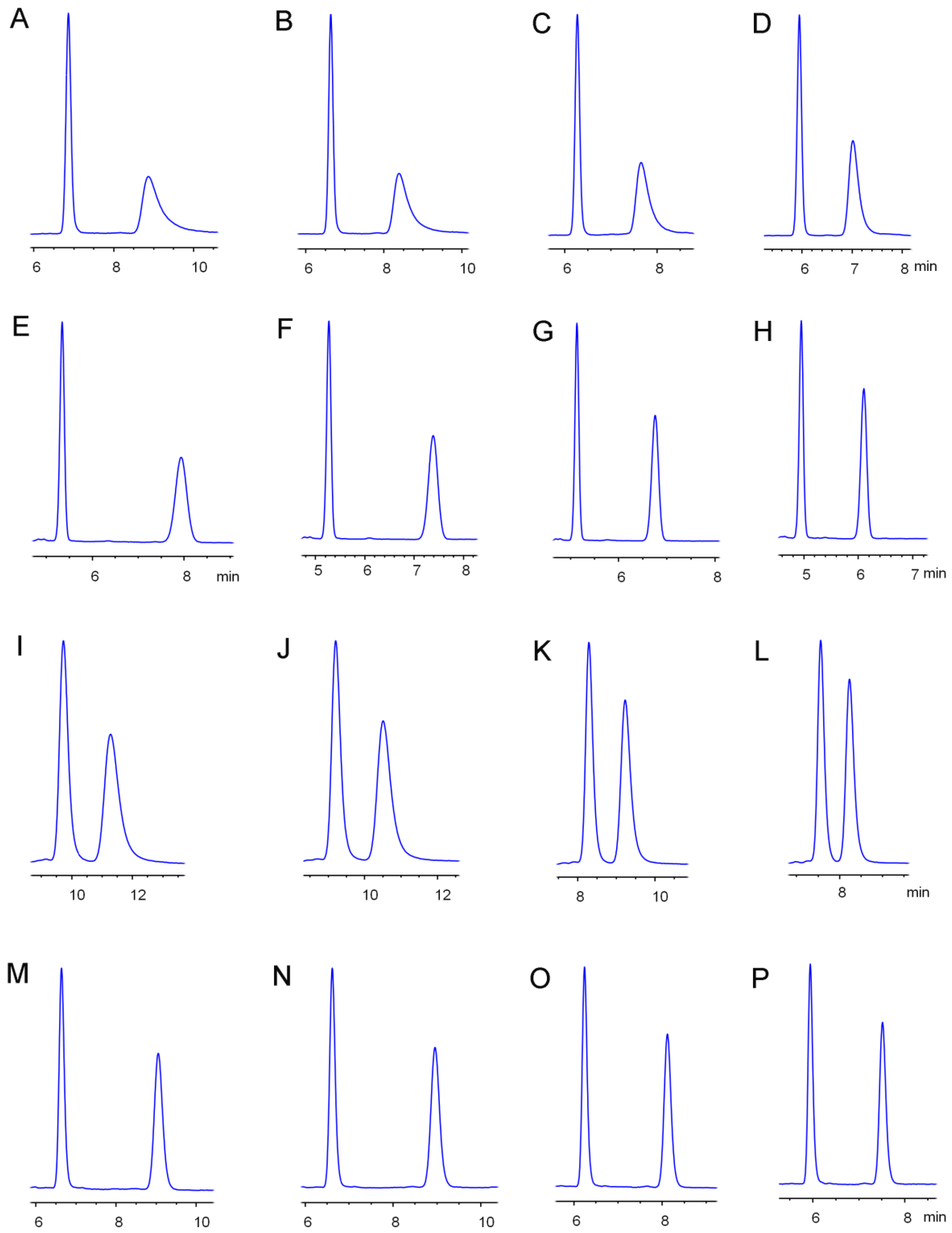
| Column | Mobile Phase | Ratio (v/v) | k1 | k2 | α | Rs | Mobile Phase | Ratio (v/v) | k1 | k2 | α | Rs |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lux Cellulose-1 | HEX/IPA | 98/2 | 1.81 | 3.04 | 1.68 | 4.50 | MEOH/H2O | 100/0 | 0.40 | 0.46 | 1.14 | 0.54 |
| 95/5 | 0.97 | 1.63 | 1.68 | 3.39 | 95/5 | 0.68 | 1.10 | 1.60 | 2.03 | |||
| 90/10 | 0.71 | 1.01 | 1.42 | 2.70 | 90/10 | 1.02 | 1.82 | 1.79 | 2.43 | |||
| 85/15 | 0.60 | 0.77 | 1.29 | 1.83 | 85/15 | 1.47 | 2.69 | 1.83 | 2.68 | |||
| 80/20 | 0.51 | 0.64 | 1.26 | 1.47 | ||||||||
| HEX/BuOH | 98/2 | 1.79 | 2.41 | 1.34 | 2.46 | ACN/H2O | 90/10 | 0.37 | 0.95 | 2.54 | 3.94 | |
| 95/5 | 1.07 | 1.31 | 1.22 | 1.98 | 80/20 | 0.55 | 1.40 | 2.53 | 3.62 | |||
| 90/10 | 0.65 | 0.79 | 1.22 | 1.34 | 70/30 | 0.87 | 2.19 | 2.51 | 3.04 | |||
| 85/15 | 0.49 | 0.58 | 1.19 | 0.97 | 60/40 | 1.46 | 2.73 | 1.87 | 2.00 | |||
| 80/20 | 0.44 | 0.50 | 1.15 | 0.82 | ||||||||
| Lux Cellulose-3 | HEX/IPA | 98/2 | 2.17 | 3.52 | 1.62 | 3.14 | MEOH/H2O | 95/5 | / | / | / | / |
| 95/5 | 1.15 | 1.64 | 1.43 | 2.02 | 90/10 | / | / | / | / | |||
| 90/10 | 0.69 | 0.91 | 1.33 | 1.22 | 85/15 | / | / | / | / | |||
| 85/15 | 0.50 | 0.64 | 1.29 | 0.93 | 80/20 | / | / | / | / | |||
| 80/20 | 0.39 | 0.50 | 1.27 | 0.88 | ||||||||
| HEX/BuOH | 98/2 | 1.06 | 1.92 | 1.81 | 1.18 | ACN/H2O | 90/10 | 0.09 | 0.19 | 2.22 | 1.82 | |
| 95/5 | 0.65 | 1.43 | 2.22 | 1.07 | 80/20 | 0.14 | 0.37 | 2.65 | 3.01 | |||
| 90/10 | 0.42 | 0.80 | 1.92 | 0.97 | 70/30 | 0.27 | 0.76 | 2.83 | 4.88 | |||
| 85/15 | 0.31 | 0.59 | 1.92 | 0.90 | 60/40 | 0.50 | 1.54 | 3.09 | 6.30 | |||
| 80/20 | 0.25 | 0.48 | 1.91 | 0.82 | ||||||||
| Chiralpak IC | HEX/IPA | 90/10 | 1.02 | 4.98 | 4.87 | 13.21 | MEOH/H2O | 100/0 | 0.18 | 0.34 | 1.84 | 2.34 |
| 85/15 | 0.81 | 3.58 | 4.40 | 12.91 | 95/5 | 0.33 | 0.64 | 1.94 | 3.36 | |||
| 80/20 | 0.65 | 2.79 | 4.28 | 12.35 | 90/10 | 0.52 | 1.02 | 1.99 | 4.35 | |||
| 75/25 | 0.54 | 2.30 | 4.23 | 11.55 | 85/15 | 0.80 | 1.64 | 2.06 | 5.26 | |||
| 70/30 | 0.52 | 2.06 | 3.93 | 11.14 | 80/20 | 1.17 | 2.47 | 2.11 | 5.84 | |||
| HEX/BuOH | 95/5 | 1.26 | 4.16 | 3.31 | 14.98 | ACN/H2O | 90/10 | 0.17 | 0.56 | 3.36 | 4.82 | |
| 90/10 | 0.79 | 3.45 | 4.38 | 13.85 | 80/20 | 0.39 | 1.15 | 2.94 | 5.40 | |||
| 85/15 | 0.60 | 2.40 | 4.00 | 12.97 | 70/30 | 0.84 | 2.31 | 2.74 | 6.89 | |||
| 80/20 | 0.48 | 1.80 | 3.79 | 9.33 | 60/40 | 1.89 | 4.98 | 2.64 | 9.40 | |||
| 75/25 | 0.40 | 1.44 | 3.65 | 8.30 | ||||||||
| Chiralpak AD | HEX/IPA | 90/10 | 1.79 | 7.17 | 4.00 | 15.45 | MEOH/H2O | 100/0 | 0.18 | 0.29 | 1.59 | 0.87 |
| 80/20 | 1.05 | 4.32 | 4.12 | 13.73 | 95/5 | 0.37 | 0.58 | 1.55 | 0.92 | |||
| 70/30 | 0.79 | 3.25 | 4.13 | 9.37 | 90/10 | 0.63 | 0.96 | 1.52 | 1.15 | |||
| 60/40 | 0.63 | 2.68 | 4.25 | 9.14 | 85/15 | 0.85 | 1.28 | 1.50 | 1.22 | |||
| 50/50 | 0.59 | 2.51 | 4.25 | 8.69 | ||||||||
| HEX/BuOH | 98/2 | 4.33 | 8.96 | 2.07 | 8.41 | ACN/H2O | 90/10 | 0.23 | 0.29 | 1.30 | 0.76 | |
| 95/5 | 2.26 | 4.92 | 2.18 | 8.04 | 80/20 | 0.41 | 0.52 | 1.27 | 0.87 | |||
| 90/10 | 1.27 | 2.83 | 2.22 | 7.57 | 70/30 | 0.68 | 0.86 | 1.26 | 0.93 | |||
| 85/15 | 0.90 | 2.04 | 2.26 | 7.32 | 60/40 | 1.14 | 1.43 | 1.25 | 1.02 | |||
| 80/20 | 0.74 | 1.66 | 2.24 | 6.83 |
| Column | Mobile Phase | lnk = −ΔH/RT + ΔS/R+ lnφ | R2 | ΔH (KJ mol−1) | ΔS/R+ lnφ | lnα = −ΔΔH/RT+ ΔΔS/R | R2 | ΔΔH (KJ mol−1) | ΔΔS (J mol−1) |
|---|---|---|---|---|---|---|---|---|---|
| Lux Cellulose-1 | HEX/IPA (85/15) | lnk1 = 857.92/T − 2.8677 | 0.989 | −7.13 | −2.87 | lnα = 588.19/T − 1.5353 | 0.999 | −4.89 | −12.77 |
| lnk2 = 1446.1/T − 4.4031 | 0.995 | −12.02 | −4.78 | ||||||
| HEX/BuOH (60/40) | lnk1 = 229.88/T − 0.7073 | 0.896 | −1.91 | −0.71 | lnα = 995.79/T − 3.1668 | 0.988 | −8.28 | −26.33 | |
| lnk2 = 1225.7/T − 3.8741 | 0.995 | −10.19 | −3.87 | ||||||
| MEOH/H2O (95/5) | lnk1 = 963.97/T − 3.6775 | 0.964 | −8.01 | −3.68 | lnα = 339.91/T − 0.6669 | 0.984 | −2.83 | −5.54 | |
| lnk2 = 1303.9/T − 4.3444 | 0.970 | −10.84 | −4.34 | ||||||
| ACN/H2O (80/20) | lnk1 = 927.83/T − 3.7497 | 0.935 | −7.71 | −3.75 | lnα = 689.72/T − 1.3936 | 0.960 | −5.73 | −11.59 | |
| lnk2 = 1617.5/T − 5.1432 | 0.946 | −13.45 | −5.14 | ||||||
| Lux Cellulose-3 | HEX/IPA (90/10) | lnk1 = 2496.5/T − 7.9557 | 0.978 | −20.76 | −7.96 | lnα = 453.68/T − 1.1271 | 0.985 | −3.77 | −9.37 |
| lnk2 = 2950.1/T − 9.0828 | 0.981 | −24.53 | −9.08 | ||||||
| HEX/BuOH (95/5) | lnk1 = 1596.1/T − 5.8905 | 0.995 | −13.27 | −5.89 | lnα = 1039.6/T − 3.0384 | 0.995 | −8.64 | −25.26 | |
| lnk2 = 2635.7/T − 8.929 | 0.995 | −21.91 | −8.93 | ||||||
| MEOH/H2O (90/10) | / | / | / | / | / | / | / | / | |
| / | / | / | / | ||||||
| ACN/H2O (70/30) | lnk1 = 836/T − 4.1678 | 0.928 | −6.95 | −4.17 | lnα = 970.74/T − 2.2584 | 0.996 | −8.07 | −18.78 | |
| lnk2 = 1806.7/T − 6.4262 | 0.975 | −15.02 | −6.43 | ||||||
| Chiralpak IC | HEX/IPA (70/30) | lnk1 = 799.27/T − 3.4457 | 0.950 | −6.65 | −3.45 | lnα = 1528.1/T − 3.7584 | 0.991 | −12.70 | −31.25 |
| lnk2 = 2327.3/T − 7.2041 | 0.994 | −19.35 | −7.20 | ||||||
| HEX/BuOH (60/40) | lnk1 = 771.56/T − 3.1208 | 0.964 | −6.41 | −3.12 | lnα = 1217.1/T − 2.7845 | 0.995 | −10.12 | −23.15 | |
| lnk2 = 1988.7/T − 5.9052 | 0.997 | −16.53 | −5.91 | ||||||
| MEOH/H2O (90/10) | lnk1 = 1130.8/T − 4.4897 | 0.969 | −9.40 | −4.49 | lnα = 243.06/T − 0.1384 | 0.969 | −2.02 | −1.15 | |
| lnk2 = 1373.9/T − 4.628 | 0.983 | −11.42 | −4.63 | ||||||
| ACN/H2O (80/20) | lnk1 = 750.13/T − 3.4584 | 0.973 | −6.24 | −3.46 | lnα = 689.69/T − 1.2872 | 0.985 | −5.73 | −10.70 | |
| lnk2 = 1439.8/T − 4.7455 | 0.980 | −11.97 | −4.75 | ||||||
| Chiralpak AD | HEX/IPA (50/50) | lnk1 = 968.4/T − 3.3552 | 0.992 | −8.05 | −3.36 | lnα = 1334.1/T − 3.1136 | 0.990 | −11.09 | −25.89 |
| lnk2 = 2302.5/T − 6.9441 | 0.990 | −19.14 | −6.94 | ||||||
| HEX/BuOH (60/40) | lnk1 = 1225.2/T − 3.3552 | 0.992 | −10.19 | −3.36 | lnα = 751.25/T − 1.7925 | 0.987 | −6.25 | −14.90 | |
| lnk2 = 1976.5/T − 5.1478 | 0.993 | −16.43 | −5.15 | ||||||
| MEOH/H2O (90/10) | lnk1 = 1335.6/T − 5.0511 | 0.966 | −11.10 | −5.05 | lnα = 261.34/T − 0.4704 | 0.972 | −2.17 | −3.91 | |
| lnk2 = 1597/T − 5.5215 | 0.970 | −13.28 | −5.52 | ||||||
| ACN/H2O (60/40) | lnk1 = 1380.7/T − 4.5931 | 0.966 | −11.48 | −4.59 | lnα = 219.06/T − 0.518 | 0.985 | −1.82 | −4.31 | |
| lnk2 = 1599.7/T − 5.1111 | 0.969 | −13.30 | −5.11 |
| Compound | Matrix | Spiked Levels (mg kg−1) | Intraday | Interday | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | ||||||||
| Recovery (%) | RSD (%) | Recovery (%) | RSD (%) | Recovery (%) | RSD (%) | Recovery (%) | RSD (%) | |||
| R-etoxazole | Soil | 0.05 | 84.46 | 5.61 | 84.19 | 5.36 | 89.48 | 6.84 | 86.04 | 6.64 |
| 0.5 | 94.45 | 5.15 | 94.05 | 2.57 | 93.99 | 3.30 | 94.16 | 3.84 | ||
| 5 | 97.46 | 5.80 | 97.34 | 1.80 | 99.58 | 4.48 | 98.13 | 4.48 | ||
| Cucumber | 0.05 | 92.33 | 2.51 | 91.02 | 5.53 | 89.56 | 8.02 | 90.97 | 5.89 | |
| 0.5 | 102.66 | 1.30 | 97.30 | 3.25 | 90.99 | 1.47 | 96.98 | 5.39 | ||
| 5 | 98.45 | 1.41 | 96.02 | 3.63 | 93.32 | 1.23 | 95.93 | 3.22 | ||
| Cabbage | 0.05 | 82.75 | 5.00 | 83.80 | 3.28 | 86.71 | 7.31 | 84.42 | 5.85 | |
| 0.5 | 91.99 | 4.03 | 95.04 | 3.23 | 92.02 | 3.23 | 93.02 | 3.83 | ||
| 5 | 95.88 | 2.71 | 97.44 | 2.18 | 94.84 | 3.15 | 96.06 | 2.92 | ||
| Tomato | 0.05 | 82.71 | 4.33 | 82.56 | 6.31 | 84.59 | 6.63 | 83.29 | 5.96 | |
| 0.5 | 92.27 | 3.36 | 89.42 | 2.02 | 94.85 | 4.08 | 92.18 | 4.09 | ||
| 5 | 91.69 | 0.62 | 96.02 | 3.98 | 92.22 | 4.23 | 93.31 | 3.98 | ||
| S-etoxazole | Soil | 0.05 | 85.82 | 5.80 | 88.94 | 4.78 | 87.64 | 4.58 | 87.47 | 5.28 |
| 0.5 | 94.11 | 2.45 | 96.03 | 4.31 | 94.88 | 4.38 | 95.01 | 3.92 | ||
| 5 | 99.48 | 5.45 | 94.91 | 5.80 | 97.59 | 3.73 | 97.33 | 5.42 | ||
| Cucumber | 0.05 | 90.16 | 4.84 | 89.59 | 5.91 | 87.54 | 3.92 | 89.09 | 5.13 | |
| 0.5 | 100.69 | 1.38 | 94.50 | 2.13 | 94.12 | 4.14 | 96.44 | 4.16 | ||
| 5 | 96.12 | 3.69 | 94.96 | 3.35 | 91.75 | 1.77 | 94.28 | 3.65 | ||
| Cabbage | 0.05 | 80.41 | 5.03 | 87.29 | 3.60 | 87.72 | 4.97 | 85.14 | 6.02 | |
| 0.5 | 94.22 | 4.28 | 94.14 | 3.80 | 89.89 | 3.14 | 92.75 | 4.37 | ||
| 5 | 97.27 | 6.07 | 98.65 | 3.38 | 96.89 | 3.49 | 97.61 | 4.55 | ||
| Tomato | 0.05 | 80.27 | 3.14 | 83.52 | 5.66 | 88.19 | 6.26 | 84.00 | 6.55 | |
| 0.5 | 91.21 | 3.02 | 93.16 | 2.23 | 95.13 | 5.25 | 93.17 | 4.14 | ||
| 5 | 92.01 | 1.62 | 97.17 | 4.62 | 95.30 | 4.82 | 94.83 | 4.60 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; He, Y.; Wang, S.; Shi, D.; Xu, Y.; Yang, F.; Wang, J.; He, L. Chiral Separation and Determination of Etoxazole Enantiomers in Vegetables by Normal-Phase and Reverse-Phase High Performance Liquid Chromatography. Molecules 2020, 25, 3134. https://doi.org/10.3390/molecules25143134
Zhang P, He Y, Wang S, Shi D, Xu Y, Yang F, Wang J, He L. Chiral Separation and Determination of Etoxazole Enantiomers in Vegetables by Normal-Phase and Reverse-Phase High Performance Liquid Chromatography. Molecules. 2020; 25(14):3134. https://doi.org/10.3390/molecules25143134
Chicago/Turabian StyleZhang, Ping, Yuhan He, Sheng Wang, Dongmei Shi, Yangyang Xu, Furong Yang, Jianhao Wang, and Lin He. 2020. "Chiral Separation and Determination of Etoxazole Enantiomers in Vegetables by Normal-Phase and Reverse-Phase High Performance Liquid Chromatography" Molecules 25, no. 14: 3134. https://doi.org/10.3390/molecules25143134
APA StyleZhang, P., He, Y., Wang, S., Shi, D., Xu, Y., Yang, F., Wang, J., & He, L. (2020). Chiral Separation and Determination of Etoxazole Enantiomers in Vegetables by Normal-Phase and Reverse-Phase High Performance Liquid Chromatography. Molecules, 25(14), 3134. https://doi.org/10.3390/molecules25143134




