Anti-Inflammatory and Antioxidant Activities of Terpene- and Polyphenol-Rich Premna odorata Leaves on Alcohol-Inflamed Female Wistar Albino Rat Liver
Abstract
1. Introduction
2. Results
2.1. Metabolomic Analysis
2.2. In Silico ADMET Properties for the Crude Extract of Different Premna odorata Metabolites
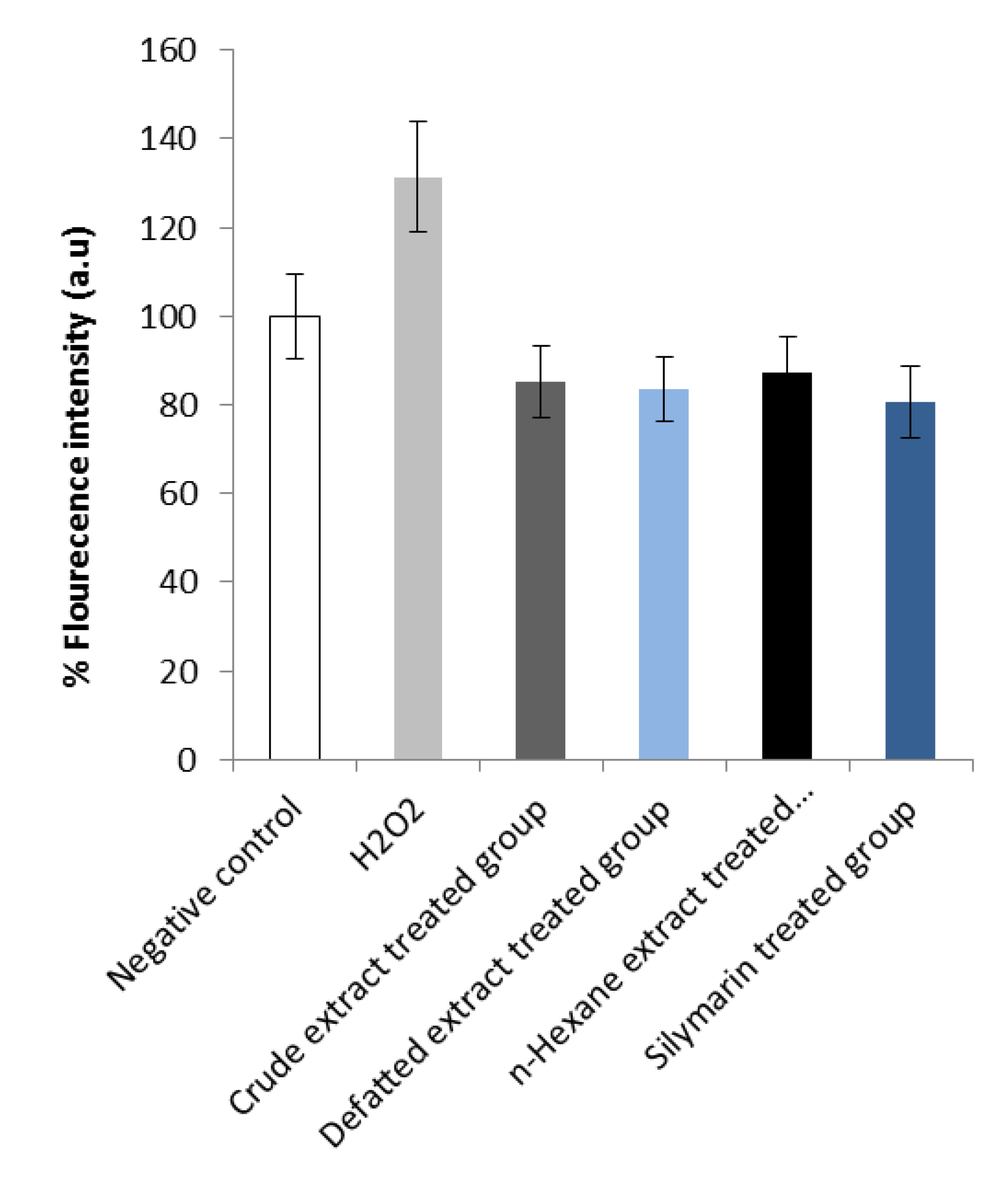
2.3. In Vitro Measurement of Total Reactive Oxygen Species (ROS)
2.4. Acute Toxicity
2.5. Potential Effects of Premna odorata Extracts in Liver Function Tests
2.6. Potential Effects of Premna odorata Extracts on Oxidative Stress Markers, and Antioxidant
2.7. Potential Effects of Premna odorata Extracts on Inflammatory Markers and Adhesion Molecules
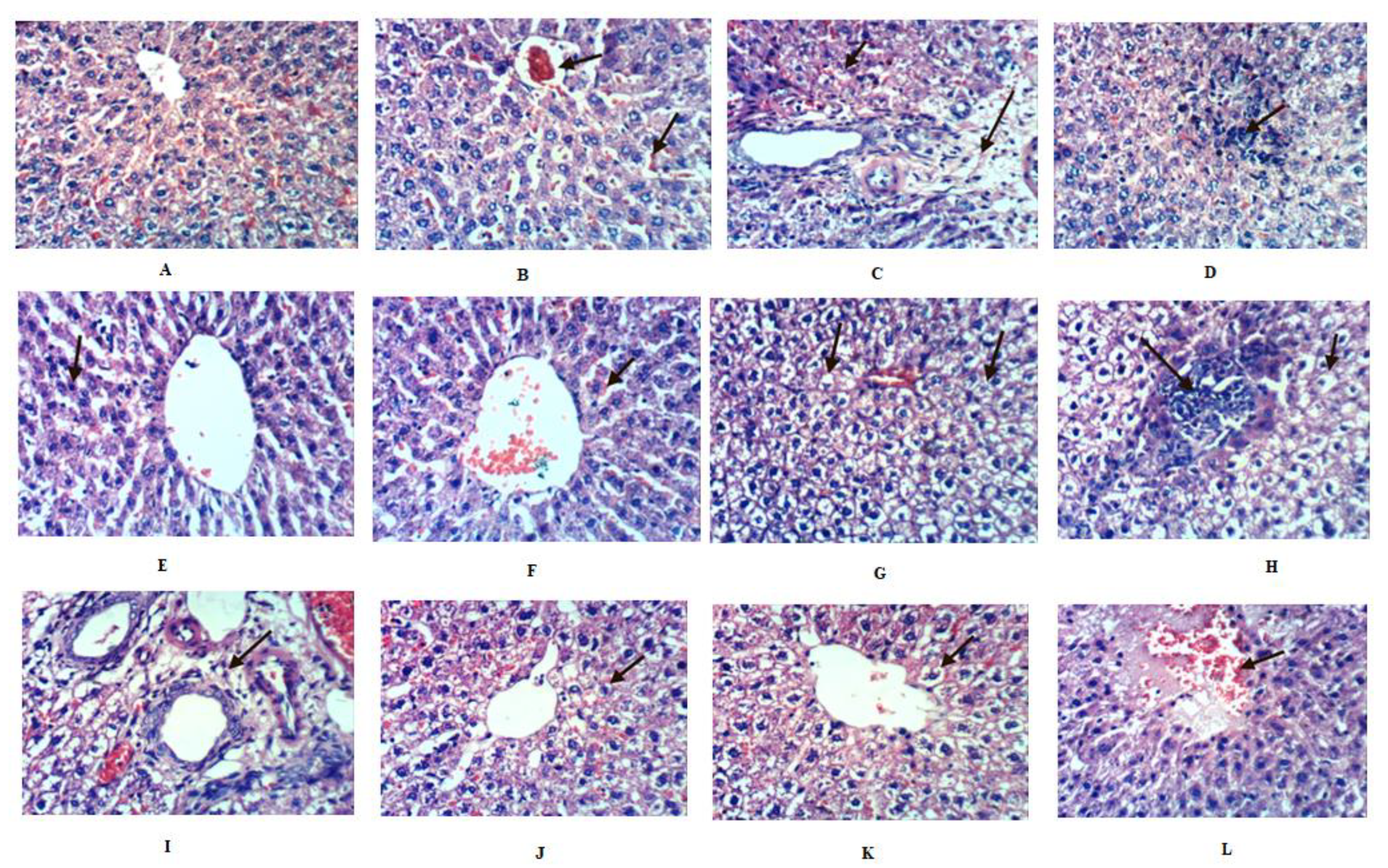
2.8. Histopathological Investigation of Liver
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Material and Extraction
4.3. Metabolomic Analysis Procedure
4.4. In Silico ADMET Properties for the Crude Extract of Different Premna odorata Metabolites
4.5. In Vitro Measurement of Total Reactive Oxygen Species (ROS)
4.5.1. Cell Lines, Culture Conditions
4.5.2. Intracellular ROS Levels Quantification
4.6. Animal Treatment
4.7. Animal Ethical Statement
4.8. Acute Toxicity Test
4.9. Induction of Alcohol Liver Inflammation and Experimental Design
4.9.1. Blood Sampling
4.9.2. Biochemical Analysis
4.10. Statistical Analysis
4.11. Histopathological Examination
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Conti, A.; Gulì, C.; La Torre, D.; Tomasello, C.; Angileri, F.F.; Aguennouz, M.H. Role of inflammation and oxidative stress mediators in gliomas. Cancers 2010, 2, 693–712. [Google Scholar] [CrossRef] [PubMed]
- Marsano, L.S.; Mendez, C.; Hill, D.; Barve, S.; Mcclain, C.J. Diagnosis and treatment of alcoholic liver disease and its complications. Alcohol Res. 2003, 27, 247–256. [Google Scholar]
- Ashley, N.T.; Weil, Z.M.; Nelson, R.J. Inflammation: Mechanisms, costs, and natural variation. Rev. Ecolo. Evol. Syst. 2012, 43, 385–406. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Mukherjee, S. Alcohol metabolism and generation of free radicals: A deep insight. Oa Alcohol. 2014, 2, 10. [Google Scholar]
- Bishayee, A.; Darvesh, A.S.; Politis, T.; McGory, R. Resveratrol and liver disease: From bench to bedside and community. Liver Int. 2010, 30, 1103–1114. [Google Scholar] [CrossRef]
- Abdelhafez, O.H.; Fawzy, M.A.; Fahim, J.R.; Desoukey, S.Y.; Krischke, M.; Mueller, M.J.; Abdelmohsen, U.R. Hepatoprotective potential of Malvaviscus arboreus against carbon tetrachloride-induced liver injury in rats. PLoS ONE 2018, 13, e0202362. [Google Scholar] [CrossRef]
- Pinzon, L.C.; Uy, M.M.; Sze, K.H.; Wang, M.; Chu, I.K. Isolation and characterization of antimicrobial, anti-inflammatory and chemopreventive flavones from Premna odorata Blanco. J. Med. Plant Res. 2011, 5, 2729–2735. [Google Scholar]
- Elmaidomy, A.H.; Mohyeldin, M.M.; Ibrahim, M.M.; Hassan, H.M.; Amin, E.; Rateb, M.E.; Hetta, M.H.; El Sayed, K.A. Acylated iridoids and rhamnopyranoses from premna odorata (lamiaceae) as novel mesenchymal–epithelial transition factor receptor inhibitors for the control of breast cancer. Phytother. Res. 2017, 31, 1546–1556. [Google Scholar] [CrossRef]
- Otsuka, H.; Kashima, N.; Hayashi, T.; Kubo, N.; Yamasaki, K.; Padolina, W.G. Premnaodorosides A, B and C, iridoid glucoside diesters of an acyclic monoterpenediol from leaves of Premna odorata. Phytochemistry 1992, 31, 3129–3133. [Google Scholar] [CrossRef]
- Otsuka, H.; Kubo, N.; Yamasaki, K.; Padolina, W.G. Premnosides A–D: Diacyl 6-O-α-L-rhamnopyranosylcatalpols from Premna odorata. Phytochemistry 1989, 28, 3063–3067. [Google Scholar] [CrossRef]
- Otsuka, H.; Kubo, N.; Yamasaki, K.; Padolina, W.G. Two iridoid glycoside caffeoyl esters from Premna odorata. Phytochemistry 1989, 28, 513–515. [Google Scholar] [CrossRef]
- El-Mudomy, A.H.; Hassan, H.M.; Amin, E.; Mohamed, W.A.; Hetta, M.H. Chemical composition and in vivo anti-inflammatory activity of the lipid extract from Premna odorata Blanco cultivated in Egypt. World J. Pharm. Pharm. Sci. 2015, 5, 129–135. [Google Scholar]
- Elmaidomy, A.H.; Hassan, H.M.; Amin, E.; Mohamed, W.; Hetta, M.H. Premna odorata volatile oil as a new mycobacterium tuberculosis growth inhibitor for the control of tuberculosis disease. Eur. J. Med. Plants 2017, 21, 1–11. [Google Scholar] [CrossRef]
- Waleed, A.; Samah, S.; Mona, F.; Abeer, H.; Hossam, M.; Elham, A.; Mona, H. Immunomodulatory effect of Premna odorata volatile oils in Mycobacterium tuberculosis by inhibiting TLR4/NF-κB pathway. J. Herbmed. Pharmacol. 2019, 8, 1–7. [Google Scholar]
- Tantengco, O.A.G.; Jacinto, S.D. Cytotoxic activity of crude extracts and fractions from Premna odorata (Blanco), Artocarpus camansi (Blanco) and Gliricidia sepium (Jacq.) against selected human cancer cell lines. Asian Pac. J. Trop. Biomed. 2015, 5, 1037–1041. [Google Scholar] [CrossRef]
- Harborne, J.B. The flavonoids: Advances in Research Since 1980; CRC press Taylor and Francis Group: New York, NY, USA, 2013. [Google Scholar]
- Otsuka, H.; Kubo, N.; Sasaki, Y.; Yamasaki, K.; Takeda, Y.; Seki, T. Iridoid diglycoside monoacyl esters from stems of Premna japonica. Phytochemistry 1991, 30, 1917–1920. [Google Scholar] [CrossRef]
- Lirio, S.B.; Macabeo, A.P.G.; Paragas, E.M.; Knorn, M.; Kohls, P.; Franzblau, S.G.; Wang, Y.; Aguinaldo, M.A.M. Antitubercular constituents from Premna odorata Blanco. J. Ethnopharmacol. 2014, 154, 471–474. [Google Scholar] [CrossRef]
- Sudo, H.; Takushi, A.; Hirata, E.; Ide, T.; Otsuka, H.; Takeda, Y. Premnaodorosides D–G: Acyclic monoterpenediols iridoid glucoside diesters from leaves of Premna subscandens. Phytochemistry 1999, 52, 1495–1499. [Google Scholar] [CrossRef]
- Otsuka, H.; Watanabe, E.; Yuasa, K.; Ogimi, C.; Takushi, A.; Takeda, Y. verbascoside iridoid glucoside conjugate from Premna corymbosa var. obtusifolia. Phytochem. 1993, 4, 983–986. [Google Scholar] [CrossRef]
- Zhan, Z.J.; Tang, L.; Shan, W.G. A new triterpene glycoside from Premna microphylla. Chem. Nat. Compd. 2009, 45, 197–199. [Google Scholar] [CrossRef]
- Carballo-Villalobos, A.; González-Trujano, M.; López-Muñoz, F. Evidence of mechanism of action of anti-inflammatory/antinociceptive activities of acacetin. Eur. J. Pain 2014, 18, 396–405. [Google Scholar] [CrossRef]
- Singh, S.S. Preclinical pharmacokinetics: An approach towards safer and efficacious drugs. Curr. Drug Metab. 2006, 7, 165–182. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, F.; Wynalda, M. Albumin-lipid interactions: Prostaglandin stability as a probe for characterizing binding sites on vertebrate albumins. Biochemistry 1981, 20, 6129–6134. [Google Scholar] [CrossRef] [PubMed]
- Benson, H.A. Transdermal drug delivery: Penetration enhancement techniques. Curr. Drug Delivery 2005, 2, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Watkins, C.L.; Brennan, P.; Fegan, C.; Takayama, K.; Nakase, I.; Futaki, S.; Jones, A.T. Cellular uptake, distribution and cytotoxicity of the hydrophobic cell penetrating peptide sequence PFVYLI linked to the proapoptotic domain peptide PAD. J. Control. Release 2009, 140, 237–244. [Google Scholar] [CrossRef]
- Zakhari, S. Overview: How is alcohol metabolized by the body? Alcohol Res. 2006, 29, 245. [Google Scholar]
- Edenberg, H.J. The genetics of alcohol metabolism: Role of alcohol dehydrogenase and aldehyde dehydrogenase variants. Alcohol Res. 2007, 30, 5. [Google Scholar]
- Nikfarjam, B.A.; Hajiali, F.; Adineh, M.; Nassiri-Asl, M. Anti-inflammatory Effects of Quercetin and Vitexin on Activated Human Peripheral Blood Neutrophils:-The effects of quercetin and vitexin on human neutrophils. J. Pharmacopunct. 2017, 20, 127. [Google Scholar]
- Lee, J.-H.; Zhou, H.Y.; Cho, S.Y.; Kim, Y.S.; Lee, Y.S.; Jeong, C.S. Anti-inflammatory mechanisms of apigenin: Inhibition of cyclooxygenase-2 expression, adhesion of monocytes to human umbilical vein endothelial cells, and expression of cellular adhesion molecules. Arch. Pharmacal Res. 2007, 30, 1318–1327. [Google Scholar] [CrossRef]
- Yang, Y.; Gong, X.-B.; Huang, L.-G.; Wang, Z.-X.; Wan, R.-Z.; Zhang, P.; Zhang, Q.-Y.; Chen, Z.; Zhang, B.-S. Diosmetin exerts anti-oxidative, anti-inflammatory and anti-apoptotic effects to protect against endotoxin-induced acute hepatic failure in mice. Oncotarget 2017, 8, 30723. [Google Scholar] [CrossRef]
- Jeon, I.H.; Kim, H.S.; Kang, H.J.; Lee, H.S.; Jeong, S.I.; Kim, S.J.; Jang, S.I. Anti-inflammatory and antipruritic effects of luteolin from perilla (p. Frutescens l.) Leaves. Molecules 2014, 19, 6941–6951. [Google Scholar] [CrossRef]
- Viljoen, A.; Mncwangi, N.; Vermaak, I. Anti-inflammatory iridoids of botanical origin. Curr. Med. Chem. 2012, 19, 2104–2127. [Google Scholar] [CrossRef]
- Wang, C.; Gong, X.; Bo, A.; Zhang, L.; Zhang, M.; Zang, E.; Zhang, C.; Li, M. Iridoids: Research advances in their phytochemistry, biological activities, and pharmacokinetics. Molecules 2020, 25, 287. [Google Scholar] [CrossRef]
- Han, N.; Bakovic, M. Biologically active triterpenoids and their cardioprotective and anti-inflammatory effects. J. Bioanal. Biomed. S. 2015, 12, 1948–1955. [Google Scholar]
- Cheon, J.H.; Kim, J.S.; Kim, J.M.; Kim, N.; Jung, H.C.; Song, I.S. Plant sterol guggulsterone inhibits nuclear factor-κB signaling in intestinal epithelial cells by blocking IκB kinase and ameliorates acute murine colitis. Inflamm. Bowel Dis. 2006, 12, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Planavila, A.; Laguna, J.C.; Vázquez-Carrera, M. Nuclear factor-κB activation leads to down-regulation of fatty acid oxidation during cardiac hypertrophy. J. Biol. Chem. 2005, 280, 17464–17471. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Woo, E.R.; Choi, C.Y.; Shin, D.W.; Lee, D.G.; You, H.J.; Jeong, H.G. Protective effect of acteoside on carbon tetrachloride induced hepatotoxicity. Life Sci. 2004, 74, 1051–1064. [Google Scholar] [CrossRef] [PubMed]
- Bachar, S.; Mahmud, Z.; Qais, N. Antioxidant and hepatoprotective activities of ethanolic extracts of leaves of Premna esculenta Roxb. against carbon tetrachloride-induced liver damage in rats. J. Young Pharm. 2012, 4, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, M.; Deepa, K. Hepatoprotective effect of Premna corymbosa (Burm. f.) Rottl. and Willd. leaves extract on CCl4 induced hepatic damage in Wistar albino rats. Asian Pac. J. Trop. Med. 2010, 3, 17–20. [Google Scholar] [CrossRef]
- Vadivu, R.; Suresh, A.J.; Girinath, K.; Kannan, P.B.; Vimala, R.; Kumar, N.S. Evaluation of hepatoprotective and in-vitro cytotoxic activity of leaves of Premna serratifolia Linn. J. Sci. Res. 2009, 1, 145–152. [Google Scholar] [CrossRef]
- Devi, K.P.; Sreepriya, M.; Balakrishna, K.; Devaki, T. Protective effect of Premna tomentosa extract (L. verbanacae) on acetaminophen-induced mitochondrial dysfunction in rats. Mol. Cell. Biochem. 2005, 272, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.; Prakash, C.; Mishra, P.; Tiwari, K.N.; Mishra, S.K.; More, R.S.; Kumar, V.; Singh, J. Hepatoprotective efficacy of Premna integrifolia L. leaves against aflatoxin B1-induced toxicity in mice. Toxicon 2019, 166, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Abdelmohsen, U.R.; Cheng, C.; Viegelmann, C.; Zhang, T.; Grkovic, T.; Ahmed, S.; Quinn, R.J.; Hentschel, U.; Edrada-Ebel, R. Dereplication strategies for targeted isolation of new antitrypanosomal actinosporins A and B from a marine sponge associated Actinokineospora sp. EG49. Mar. Drugs 2014, 12, 1220–1244. [Google Scholar] [CrossRef]
- Elmaidomy, A.H.; Mohammed, R.M.; Hassan, H.I.; Owis, A.E.; Rateb, M.A.; Khanfar, M.; Krischke, M.J.; Mueller, M.; Ramadan Abdelmohsen, U. Metabolomic Profiling and Cytotoxic Tetrahydrofurofuran Lignans Investigations from Premna Odorata Blanco. Metabolites 2019, 9, 223. [Google Scholar]
- Ma, X.-L.; Chen, C.; Yang, J. Predictive model of blood-brain barrier penetration of organic compounds. Acta Pharmacol. Sin. 2005, 26, 500–512. [Google Scholar] [CrossRef]
- Singh, S.; Singh, J. Transdermal drug delivery by passive diffusion and iontophoresis: A review. Med. Res. Rev. 1993, 13, 569–621. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Le, J.; Abraham, M.H.; Hersey, A.; Eddershaw, P.J.; Luscombe, C.N.; Boutina, D.; Beck, G.; Sherborne, B.; Cooper, I. Evaluation of human intestinal absorption data and subsequent derivation of a quantitative structure–activity relationship (QSAR) with the Abraham descriptors. J. Pharm. Sci. 2001, 90, 749–784. [Google Scholar] [CrossRef]
- Yamashita, S.; Furubayashi, T.; Kataoka, M.; Sakane, T.; Sezaki, H.; Tokuda, H. Optimized conditions for prediction of intestinal drug permeability using Caco-2 cells. Eur. J. Pharm. Sci. 2000, 10, 195–204. [Google Scholar] [CrossRef]
- Yee, S. In vitro permeability across Caco-2 cells (colonic) can predict in vivo (small intestinal) absorption in man—Fact or myth. Pharm. Res. 1997, 14, 763–766. [Google Scholar] [CrossRef]
- Chan, L.M.; Lowes, S.; Hirst, B.H. The ABCs of drug transport in intestine and liver: Efflux proteins limiting drug absorption and bioavailability. Eur. J. Pharm. Sc. 2004, 21, 25–51. [Google Scholar] [CrossRef] [PubMed]
- Pelkonen, O.; Turpeinen, M.; Hakkola, J.; Honkakoski, P.; Hukkanen, J.; Raunio, H. Inhibition and induction of human cytochrome P450 enzymes: Current status. Arch. Toxicol. 2008, 82, 667–715. [Google Scholar] [CrossRef] [PubMed]
- Creager, A.N.; Boudia, S.; Jas, N. The political life of mutagens: A history of the Ames test. Identifying Mutat. 2014, 285, 1–19. [Google Scholar]
- Cherubini, A.; Hofmann, G.; Pillozzi, S.; Guasti, L.; Crociani, O.; Cilia, E.; Di Stefano, P.; Degani, S.; Balzi, M.; Olivotto, M. Human ether-a-go-go-related gene 1 channels are physically linked to β1 integrins and modulate adhesion-dependent signaling. Mol. Biol. Cell 2005, 16, 2972–2983. [Google Scholar] [CrossRef]
- Armentano, M.F.; Bisaccia, F.; Miglionico, R.; Russo, D.; Nolfi, N.; Carmosino, M.; Andrade, P.B.; Valentão, P.; Diop, M.S.; Milella, L. Antioxidant and proapoptotic activities of Sclerocarya birrea [(A. Rich.) Hochst.] methanolic root extract on the hepatocellular carcinoma cell line HepG2. Biomed Res. Int. 2015, 2015, 561589. [Google Scholar] [CrossRef] [PubMed]
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.I.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.H.; Soto, D.; Stiassny, M.L. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. 2006, 81, 163–182. [Google Scholar] [CrossRef]
- Keegan, D. Foundations of Distance Education; Routledge Taylor and Francis Group: New York, NY, USA, 2013. [Google Scholar]
- Gangarapu, V.; Gujjala, S.; Korivi, R.; Pala, I. Combined effect of curcumin and vitamin E against CCl4 induced liver injury in rats. Am. J. Life Sci. 2013, 1, 117–124. [Google Scholar] [CrossRef]
- Walter, M.; Gerade, H. A colorimetric method for determination of total bilirubin. Microchem. J. 1970, 15, 231. [Google Scholar]
- Reitman, S.; Frankel, S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957, 28, 56–63. [Google Scholar] [CrossRef]
- Belfield, A.; Goldberg, D.M. Colorimetric determination of alkaline phosphatase activity. Enzyme. 1971, 12, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.T.; Valles, J.; Aznar, J.; Vilches, J. Determination of plasma malondialdehyde like material and its clinical application in stroke patients. Am. J. Clin. Pathol. 1980, 33, 973–976. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, N. A direct colorimetric determination of uric acid in serum and urine with uricase-catalase system. Clin. Chim. Acta. 1971, 31, 421–426. [Google Scholar] [CrossRef]
- McLemore, J.L.; Beeley, P.; Thorton, K.; Morrisroe, K.; Blackwell, W.; Dasgupta, A. Rapid automated determination of lipid hydroperoxide concentrations and total antioxidant status of serum samples from patients infected with HIV: Elevated lipid hydroperoxide concentrations and depleted total antioxidant capacity of serum samples. Am. J. Clin. Pathol. 1998, 109, 268–273. [Google Scholar] [CrossRef][Green Version]
- Erhardt, J.G.; Estes, J.E.; Pfeiffer, C.M.; Biesalski, H.K.; Craft, N.E. Combined measurement of ferritin, soluble transferrin receptor, retinol binding protein, and C-reactive protein by an inexpensive, sensitive, and simple sandwich enzyme-linked immunosorbent assay technique. J. Nut. 2004, 134, 3127–3132. [Google Scholar] [CrossRef] [PubMed]
- Perrey, C.; Turner, S.J.; Pravica, V.; Howell, W.M.; Hutchinson, I.V. ARMS-PCR methodologies to determine IL-10, TNF-α, TNF-β and TGF-β1 gene polymorphisms. Transplant. Immunol. 1999, 7, 127–128. [Google Scholar] [CrossRef]
- Szarka, A.; Rigo, J.; Lazar, L.; Beko, G.; Molvarec, A. Circulating cytokines, chemokines and adhesion molecules in normal pregnancy and preeclampsia determined by multiplex suspension array. BMC Immunol. 2010, 11, 59. [Google Scholar] [CrossRef]
- Gomori, G. Observations with differential stains on human islets of Langerhans. Am. J. Pathol. 1941, 17, 395. [Google Scholar]
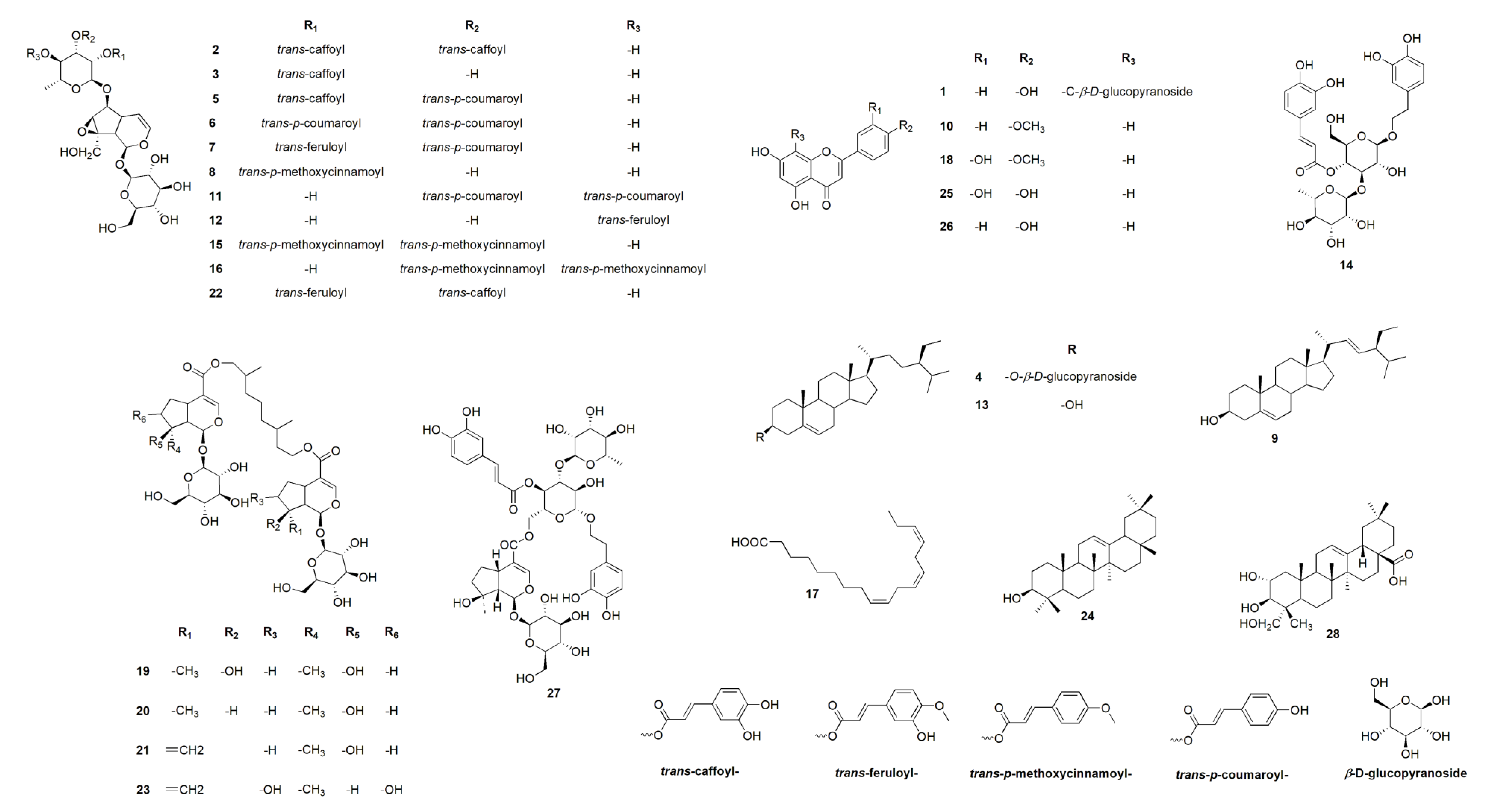
| No. | Identified | Source | MF | tR(min.) | m/z | Adduct | CE | H | DCM | EtOAC | B |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Vitexin | Premna odorata | C21H20O10 | 9.71 | 433.1361 | [M + H]+ | + | + | |||
| 2 | Premnoside A | Premna odorata | C39H44O20 | 11.37 | 833.2746 | [M + H]+ | + | + | + | ||
| 3 | 6- O-α- L-(2’’-O-trans-caffoyl) rhamnopyranosyl catalpol | Premna odorata | C30H38O17 | 11.98 | 671.1910 | [M + H]+ | + | + | + | ||
| 4 | Daucosterol | Premna japonica | C35H60O6 | 12.33 | 577.1969 | [M + H]+ | + | + | |||
| 5 | Premnoside D | Premna odorata | C39H44O19 | 12.61 | 817.2282 | [M + H]+ | + | + | + | ||
| 6 | Premnoside H | Premna odorata | C39H44O18 | 13.00 | 801.2404 | [M + H]+ | + | + | + | ||
| 7 | Premnoside C | Premna odorata | C40H46O19 | 13.09 | 831.2411 | [M + H]+ | + | + | + | ||
| 8 | 6- O-α- L-(2’’-O-trans-p-methoxycinnamoyl) rhamnopyranosyl catalpol | Premna japonica | C31H40O16 | 13.20 | 669.1634 | [M + H]+ | + | + | + | ||
| 9 | Stigmasterol | Premna odorata | C29H48O | 13.33 | 413.2619 | [M + H]+ | + | + | |||
| 10 | Acacetin | Premna odorata | C16H12O5 | 13.60 | 285.1126 | [M + H]+ | + | + | |||
| 11 | Premnoside G | Premna odorata | C39H44O18 | 13.72 | 801.2404 | [M + H]+ | + | + | + | ||
| 12 | 6- O-α- L-(4’’-O-trans-feruloyl) rhamnopyranosyl catalpol | Premna japonica | C31H40O17 | 13.78 | 685.2780 | [M + H]+ | + | + | + | ||
| 13 | β-sitosterol | Premna odorata | C29H50O | 14.19 | 414.1849 | [M + H]+ | + | + | |||
| 14 | Verbascoside | Premna odorata | C29H36O15 | 14.47 | 625.1396 | [M + H]+ | + | + | + | ||
| 15 | Premnoside F | Premna odorata | C41H48O18 | 14.54 | 829.2011 | [M + H]+ | + | + | + | ||
| 16 | Premnoside E | Premna odorata | C41H48O18 | 14.73 | 829.2011 | [M + H]+ | + | + | + | ||
| 17 | Linolenic acid | Premna microphylla | C18H30O2 | 14.81 | 277.1807 | [M - H]+ | + | + | |||
| 18 | Diosmetin | Premna odorata | C16H12O6 | 15.09 | 301.2947 | [M + H]+ | + | + | + | ||
| 19 | Premnaodoroside A | Premna odorata | C42H66O20 | 15.94 | 891.3561 | [M + H]+ | + | + | |||
| 20 | Premnaodoroside B | Premna odorata | C42H66O19 | 16.06 | 875.2437 | [M + H]+ | + | + | |||
| 21 | Premnaodoroside C | Premna odorata | C42H64O19 | 16.12 | 873.3192 | [M + H]+ | + | + | |||
| 22 | Premnoside D | Premna odorata | C40H46O20 | 16.21 | 847.2782 | [M + H]+ | + | + | + | ||
| 23 | Premnaodoroside D | Premna subscandens | C42H64O20 | 17.74 | 889.2297 | [M + H]+ | + | + | |||
| 24 | Β-amyrin | Premna odorata | C30H50O | 18.28 | 465.2018 | [M + K]+ | + | + | |||
| 25 | Luteolin | Premna odorata | C15H10O6 | 18.66 | 309.2349 | [M + Na]+ | + | + | + | ||
| 26 | Apigenin | Premna odorata | C15H10O5 | 20.82 | 293.2147 | [M + Na]+ | + | + | |||
| 27 | Premcoryoside | Premna corymbosa | C45H58O24 | 21.52 | 983.4891 | [M + H]+ | + | + | |||
| 28 | Arjunolic acid | Premna microphylla | C30H48O5 | 21.64 | 489.2793 | [M + H}+ | + | + |
| No. | PPB% | BBB (Cbrain/Cblood) | SP (cm/hour) | HIA% | MDCK (nm/sec) | Caco-2 (nm/sec) | Pgp Inhibition | CYP-2C19 Inhibition | CYP-2C9 Inhibition | CYP-3A4 Inhibition | CYP-3A4 Substrate |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 61.323656 | 0.0385273 | −4.61128 | 31.374153 | 0.5424090 | 5.48785 | No | Inhibitor | Inhibitor | Inhibitor | Weak |
| 2 | 54.348583 | 0.0287162 | −3.02912 | 3.7348170 | 0.0447556 | 13.6259 | Inhibitor | Inhibitor | Inhibitor | Inhibitor | Weak |
| 3 | 33.285061 | 0.0298880 | −4.70662 | 3.3153240 | 0.1538230 | 11.0644 | Inhibitor | Inhibitor | Inhibitor | Inhibitor | Weak |
| 4 | 100.000000 | 5.3038700 | −2.20420 | 90.027561 | 0.1220710 | 25.2333 | Inhibitor | No | Inhibitor | Inhibitor | Substrate |
| 5 | 54.577268 | 0.0305549 | −2.97228 | 8.4947690 | 0.0455697 | 14.1947 | Inhibitor | Inhibitor | Inhibitor | Inhibitor | Weak |
| 6 | 56.739888 | 0.0342009 | −2.91786 | 19.057599 | 0.0454732 | 14.7567 | Inhibitor | Inhibitor | Inhibitor | Inhibitor | Substrate |
| 7 | 48.364678 | 0.0424033 | −2.96634 | 16.156072 | 0.0454091 | 14.0366 | Inhibitor | Inhibitor | Inhibitor | Inhibitor | Weak |
| 8 | 33.832287 | 0.06277950 | −4.6442 | 13.170559 | 0.1289870 | 12.3956 | Inhibitor | Inhibitor | Inhibitor | Inhibitor | Weak |
| 9 | 100.000000 | 19.8938000 | −0.717667 | 100.00000 | 3.783450 | 52.3376 | Inhibitor | No | Inhibitor | Inhibitor | Substrate |
| 10 | 90.917451 | 0.15030900 | −3.36001 | 93.042708 | 20.230800 | 12.7923 | No | Inhibitor | Inhibitor | Inhibitor | No |
| 11 | 53.912360 | 0.1319930 | −2.9222 | 52.345314 | 0.0449026 | 15.3124 | Inhibitor | Inhibitor | Inhibitor | Inhibitor | Weak |
| 12 | 32.612262 | 0.0411953 | −4.70477 | 5.9983830 | 0.1105100 | 9.11617 | Inhibitor | Inhibitor | Inhibitor | Inhibitor | Weak |
| 13 | 100.000000 | 19.8883000 | −0.593439 | 100.00000 | 8.8571900 | 52.3734 | Inhibitor | No | Inhibitor | Inhibitor | Substrate |
| 14 | 64.288492 | 0.03167600 | −3.5116 | 7.6711810 | 0.0450549 | 11.1087 | Inhibitor | Inhibitor | Inhibitor | Inhibitor | Weak |
| 15 | 54.629780 | 0.1252660 | −2.92245 | 52.345571 | 0.0451432 | 15.4511 | Inhibitor | Inhibitor | Inhibitor | Inhibitor | Weak |
| 16 | 53.912360 | 0.1319930 | −2.9222 | 52.345314 | 0.0449026 | 15.3124 | Inhibitor | Inhibitor | Inhibitor | Inhibitor | Weak |
| 17 | 100.000000 | 6.16921000 | −0.538273 | 98.273607 | 74.789700 | 27.9738 | Inhibitor | Inhibitor | Inhibitor | Inhibitor | No |
| 18 | 90.160128 | 0.20108600 | −4.13473 | 88.188263 | 23.853100 | 7.02526 | No | Inhibitor | Inhibitor | Inhibitor | No |
| 19 | 34.413157 | 0.0284513 | −3.29001 | 1.9433930 | 0.2328650 | 16.3835 | Inhibitor | Inhibitor | Inhibitor | Inhibitor | Substrate |
| 20 | 40.461576 | 0.0298628 | −3.70182 | 4.3580790 | 0.0841395 | 17.2768 | Inhibitor | Inhibitor | Inhibitor | Inhibitor | Substrate |
| 21 | 41.088270 | 0.0302347 | −3.26196 | 5.1422870 | 0.1599090 | 15.3574 | Inhibitor | Inhibitor | Inhibitor | Inhibitor | Substrate |
| 22 | 45.368220 | 0.0328157 | −3.02594 | 7.1270550 | 0.0447377 | 12.7477 | Inhibitor | Inhibitor | Inhibitor | Inhibitor | Weak |
| 23 | 34.183542 | 0.0283962 | −3.80436 | 2.2958830 | 0.291627 | 17.4161 | Inhibitor | Inhibitor | Inhibitor | Inhibitor | Substrate |
| 24 | 100.000000 | 21.2500000 | −2.22251 | 100.00000 | 0.1749200 | 46.7500 | Inhibitor | No | Inhibitor | Inhibitor | Substrate |
| 25 | 99.717233 | 0.36758200 | −4.28017 | 79.427233 | 36.520500 | 4.53973 | No | Inhibitor | Inhibitor | Inhibitor | No |
| 26 | 97.253409 | 0.56511300 | −4.14570 | 88.122839 | 44.302000 | 10.5468 | No | Inhibitor | Inhibitor | Inhibitor | No |
| 27 | 37.405289 | 0.0273879 | −2.50822 | 0.3453560 | 0.0434853 | 11.7965 | Inhibitor | Inhibitor | Inhibitor | Inhibitor | Substrate |
| 28 | 97.049829 | 0.58860800 | −3.57106 | 91.233319 | 0.0434480 | 20.9815 | Inhibitor | No | Inhibitor | Inhibitor | Substrate |
| No. | Ames Test | TA100-10RLI | TA100-NA | TA1535-10RLI | TA1535-NA | Carcinogenic for Mice | Carcinogenic for Rats | HERG Inhibition |
|---|---|---|---|---|---|---|---|---|
| 1 | Non-mutagenic | − | − | − | − | + | − | High risk |
| 2 | Non-mutagenic | − | − | − | − | + | − | Ambiguous |
| 3 | Non-mutagenic | − | − | − | − | + | − | Ambiguous |
| 4 | Non-mutagenic | − | − | − | − | + | − | Low risk |
| 5 | Non-mutagenic | − | − | − | − | + | − | Ambiguous |
| 6 | Non-mutagenic | − | − | − | − | + | − | Ambiguous |
| 7 | Non-mutagenic | − | − | − | − | + | − | Ambiguous |
| 8 | Non-mutagenic | − | − | − | − | + | − | High risk |
| 9 | Non-mutagenic | − | − | − | − | + | + | Low risk |
| 10 | Mutagenic | + | + | − | − | + | + | Moderate risk |
| 11 | Non-mutagenic | − | − | − | − | + | − | Ambiguous |
| 12 | Non-mutagenic | − | − | − | − | + | − | Ambiguous |
| 13 | Non-mutagenic | − | − | − | − | + | − | Low risk |
| 14 | Non-mutagenic | − | − | − | − | + | − | Ambiguous |
| 15 | Non-mutagenic | − | − | − | − | + | − | High risk |
| 16 | Non-mutagenic | − | − | − | − | + | − | High risk |
| 17 | Mutagenic | − | − | − | + | + | + | Moderate risk |
| 18 | Mutagenic | − | + | − | − | + | + | Moderate risk |
| 19 | Mutagenic | − | + | − | − | + | − | Ambiguous |
| 20 | Non-mutagenic | − | − | − | − | + | − | Ambiguous |
| 21 | Mutagenic | − | + | − | − | + | − | Ambiguous |
| 22 | Non-mutagenic | − | − | − | − | + | − | Ambiguous |
| 23 | Mutagenic | − | + | − | − | + | − | Ambiguous |
| 24 | Non-mutagenic | − | − | − | − | + | + | Low risk |
| 25 | Mutagenic | − | + | − | − | + | + | Moderate risk |
| 26 | Mutagenic | + | + | − | − | + | + | Moderate risk |
| 27 | Non-mutagenic | − | − | − | − | + | − | Ambiguous |
| 28 | Non-mutagenic | − | − | − | − | + | + | Low risk |
| Groups | |||||||
|---|---|---|---|---|---|---|---|
| Parameters | 1 | 2 | 3 | 4 | 5 | 6 | |
| Bilirubin (mg/dl) | Mean ± SD | 0.85 ± 0.05 b | 1.41 ± 0.18 a | 0.916 ± 0.07 b | 0.95 ± 0.05 b | 0.916 ± 0.076 b | 0.8 ± 0.05 b |
| % change | 65.88 | 7.76 | 11.76 | 7.76 | 5.88 | ||
| % improvement | 58.11 | 54.11 | 58.11 | 71.76 | |||
| AST (U/I) | Mean ± SD | 39.00 ± 7.81 b | 81.33 ± 21.45 a | 25.47 ± 4.50 d | 28.33 ± 1.53 cd | 32.33 ± 2.08 bc | 31.47 ± 3.51 bcd |
| % change | 108.53 | 34.20 | 27.30 | 17.10 | 19.31 | ||
| % improvement | 143.23 | 133.23 | 125.64 | 127.84 | |||
| ALT (U/I) | Mean ± SD | 108.47 ± 18.85 b | 144.33 ± 25.1 a | 77.33 ± 4.42 bc | 72.00 ± 2.00 bc | 92.47 ± 16.28 b | 93.33 ± 5.77 b |
| % change | 33.05 | 28.71 | 33.62 | 14.75 | 13.96 | ||
| % improvement | 61.76 | 66.68 | 47.81 | 47.02 | |||
| ALP (IU/L) | Mean ± SD | 173.33 ± 29.29 bc | 232.33 ± 2.51 a | 148.33 ± 10.40 bc | 181.47 ± 7.44 b | 185 ± 18.02 b | 141.47 ± 2.51 c |
| % change | 34.04 | 14.42 | 4.69 | 6.73 | 18.27 | ||
| % improvement | 48.47 | 29.32 | 27.29 | 52.42 | |||
| MDA (mmol/l) | Mean ± SD | 2818.85 ± 200.5 c | 4,029.85 ± 200.5 a | 2,026.33 ± 52.50 d | 2,121.47 ± 91.76 d | 2,248.00 ± 141.24 d | 3,250.47 ± 416.41 b |
| % change | 42.97 | 28.11 | 24.73 | 20.22 | 15.33 | ||
| % improvement | 71.07 | 67.21 | 63.20 | 27.60 | |||
| GSH (mg/g tissue used) | Mean ± SD | 412.86 ± 56.94 bc | 299.16 ± 54.98 d | 491.91 ± 27.56 c | 506.88 ± 86.32 b | 495.46 ± 28.99 ab | 411.29 ± 77.11 bc |
| % change | 27.42 | 19.15 | 22.77 | 20.26 | 0.24 | ||
| % improvement | 46.60 | 50.32 | 47.57 | 27.18 | |||
| TAC (mmol/l) | Mean ± SD | 0.26 ± 0.02 a | 0.16 ± 0.02 b | 0.26 ± 0.02 a | 0.26 ± 0.02 a | 0.27 ± 0.03 a | 0.29 ± 0.02 a |
| % change | 38.46 | 0 | 0 | 3.84 | 11.53 | ||
| % improvement | 38.46 | 38.46 | 42.30 | 50.00 | |||
| CRP (ng/mL) | Mean ± SD | 29.30 ± 2.01 b | 54.58 ± 2.18 a | 32.47 ± 2.51 b | 33.33 ± 1.52 b | 29.03 ± 2.74 b | 31.20 ± 1.38 b |
| % change | 86.96 | 10.34 | 13.79 | 1.02 | 6.48 | ||
| % improvement | 75.86 | 72.40 | 86.20 | 80.34 | |||
| TNF-α (pg/mL) | Mean ± SD | 39.33 ± 2.47 bc | 73.03 ± 2.45 a | 43.47 ± 2.76 bc | 33.47 ± 1.53 c | 41.47 ± 2.39 bc | 52.41 ± 1.45 b |
| % change | 98.49 | 10.25 | 15.38 | 5.12 | 33.74 | ||
| % improvement | 76.92 | 102.56 | 82.76 | 53.84 | |||
| ICAM-1 (μg/mL) | Mean ± SD | 5.53 ± 0.47 c | 11.81 ± 1.22 a | 7.46 ± 1.41 b | 6.83 ± 0.77 bc | 6.49 ± 0.41 bc | 6.43 ± 0.56 bc |
| % change | 114.54 | 36.36 | 23.50 | 18.18 | 16.30 | ||
| % improvement | 78.18 | 90.05 | 96.20 | 98.18 | |||
| VCAM-1 (μg/mL) | Mean ± SD | 2.92 ± 0.12 b | 4.46 ± 1.30 a | 3.40 ± 0.20 b | 3.29 ± 0.23 b | 3.22 ± 0.58 b | 2.96 ± 0.11 b |
| % change | 51.72 | 17.24 | 13.79 | 13.79 | 3.45 | ||
| % improvement | 37.93 | 41.37 | 41.37 | 51.72 | |||
| Experiment | Kit Reagents |
|---|---|
| ROS | 2’,7’-dichlorodihydrofluorescein diacetate (H2DCF–DA), Roswell Park Memorial Institute (RPMI) 1640 medium, fetal calf serum, penicillin, and streptomycin |
| Bilirubin | sulfanilic acid, hydrochloric acid, dimethyl sulfoxide |
| AST | phosphate buffer pH 7.5 (100 mmol/L), aspartate (10 0mmol/L), α-ketoglutarate (2 mmol/L) |
| ALT | alanine 200 mmol/L, 2,4-dinitrophenyl hydrazine (1 mmol/L) |
| ALP | standard phenol (1.59 mmol/L), buffer pH 10 (50 mmol/L), phenyl phosphate (5 mmol/L), EDTA (100 mmol/L), 4-aminophenazone (50 mmol/L), potassium ferricyanide (200 mmol/L) |
| MDA | standard MDA (10 mmol/mL), thiobarbituric acid (25 mmol/L), detergent (3 mmol/L), stabilizer (15 mmol/L) |
| GSH | DTNB (1 mmol/L) |
| TAC | sulfuric acid, sodium phosphate, ammonium molybdate |
| CRP | capture antibody-coated microplate: one plate of 96 wells coated with a rabbit anti-rat CRP antibody detection antibody/enzyme conjugates (100 x): concentrated horseradish peroxidase (HRP) conjugated to a rabbit anti-rat CRP antibody containing stabilizers and preservative standard (10 x): rat serum with elevated levels of CRP, wash buffer: powdered phosphate-buffered saline (PBS) with 0.05% Tween-20, TMB substrate: solution containing 3, 3’, 5, 5’-tetramethylbenzidine (TMB) stop solution: diluted phosphoric acid |
| TNF-α | Rat TNF-α microplates – 96-well polystyrene microplates (12 strips of 8 wells) coated with a monoclonal antibody specific to rat TNF-α Rat TNF-α conjugate – 23 mL/vial of a polyclonal antibody against the rat TNF-α conjugated to horseradish peroxidase with preservatives Rat TNF-α standard – 1.5 ng/vial of the recombinant rat TNF-α in a buffered protein base with preservatives, lyophilized Rat TNF-α control – the recombinant rat TNF-α in a buffered protein base with preservatives. lyophilized The concentration range of the rat TNF-α after reconstitution. The assay value of the control should be within the range specified on the label, assay diluent RD1-41 – 12.5 mL/vial of the buffered protein base with preservatives, calibrator diluent RDS-17 – 21 mL/vial of the buffered protein base with preservatives, wash buffer concentrate – 50 mL/vial of a 25-fold concentrated solution of a buffered surfactant with preservatives, color reagent A – 12.5 mL/vial of the stabilized hydrogen peroxidase, color reagent B – 12.5 mL/vial of the stabilized chromogen (tetramethylbenzidine) Stop solution – 23 mL/vial of a diluted hydrochloric acid solution, plate covers – adhesive strips |
| VCAM-1 | Pre-coated, ready-to-use 96-well strip plate, plate sealer for 96 wells, standard diluent, assay diluent A, assay diluent B, stop solution, standard, detection reagent A, detection reagent B, TMB substrate, wash buffer (30 x concentrate); |
| ICAM-1 | Pre-coated 96-well strip microplate, wash buffer, stop solution, assay diluent(s), lyophilized standard, biotinylated detection antibody, streptavidin-conjugated HRP, TMB One-Step Substrate |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elmaidomy, A.H.; Alhadrami, H.A.; Amin, E.; Aly, H.F.; Othman, A.M.; Rateb, M.E.; Hetta, M.H.; Abdelmohsen, U.R.; M. Hassan, H. Anti-Inflammatory and Antioxidant Activities of Terpene- and Polyphenol-Rich Premna odorata Leaves on Alcohol-Inflamed Female Wistar Albino Rat Liver. Molecules 2020, 25, 3116. https://doi.org/10.3390/molecules25143116
Elmaidomy AH, Alhadrami HA, Amin E, Aly HF, Othman AM, Rateb ME, Hetta MH, Abdelmohsen UR, M. Hassan H. Anti-Inflammatory and Antioxidant Activities of Terpene- and Polyphenol-Rich Premna odorata Leaves on Alcohol-Inflamed Female Wistar Albino Rat Liver. Molecules. 2020; 25(14):3116. https://doi.org/10.3390/molecules25143116
Chicago/Turabian StyleElmaidomy, Abeer H., Hani A. Alhadrami, Elham Amin, Hanan F. Aly, Asmaa M. Othman, Mostafa E. Rateb, Mona H. Hetta, Usama Ramadan Abdelmohsen, and Hossam M. Hassan. 2020. "Anti-Inflammatory and Antioxidant Activities of Terpene- and Polyphenol-Rich Premna odorata Leaves on Alcohol-Inflamed Female Wistar Albino Rat Liver" Molecules 25, no. 14: 3116. https://doi.org/10.3390/molecules25143116
APA StyleElmaidomy, A. H., Alhadrami, H. A., Amin, E., Aly, H. F., Othman, A. M., Rateb, M. E., Hetta, M. H., Abdelmohsen, U. R., & M. Hassan, H. (2020). Anti-Inflammatory and Antioxidant Activities of Terpene- and Polyphenol-Rich Premna odorata Leaves on Alcohol-Inflamed Female Wistar Albino Rat Liver. Molecules, 25(14), 3116. https://doi.org/10.3390/molecules25143116










