Systematics of Atomic Orbital Hybridization of Coordination Polyhedra: Role of f Orbitals
Abstract
1. Introduction
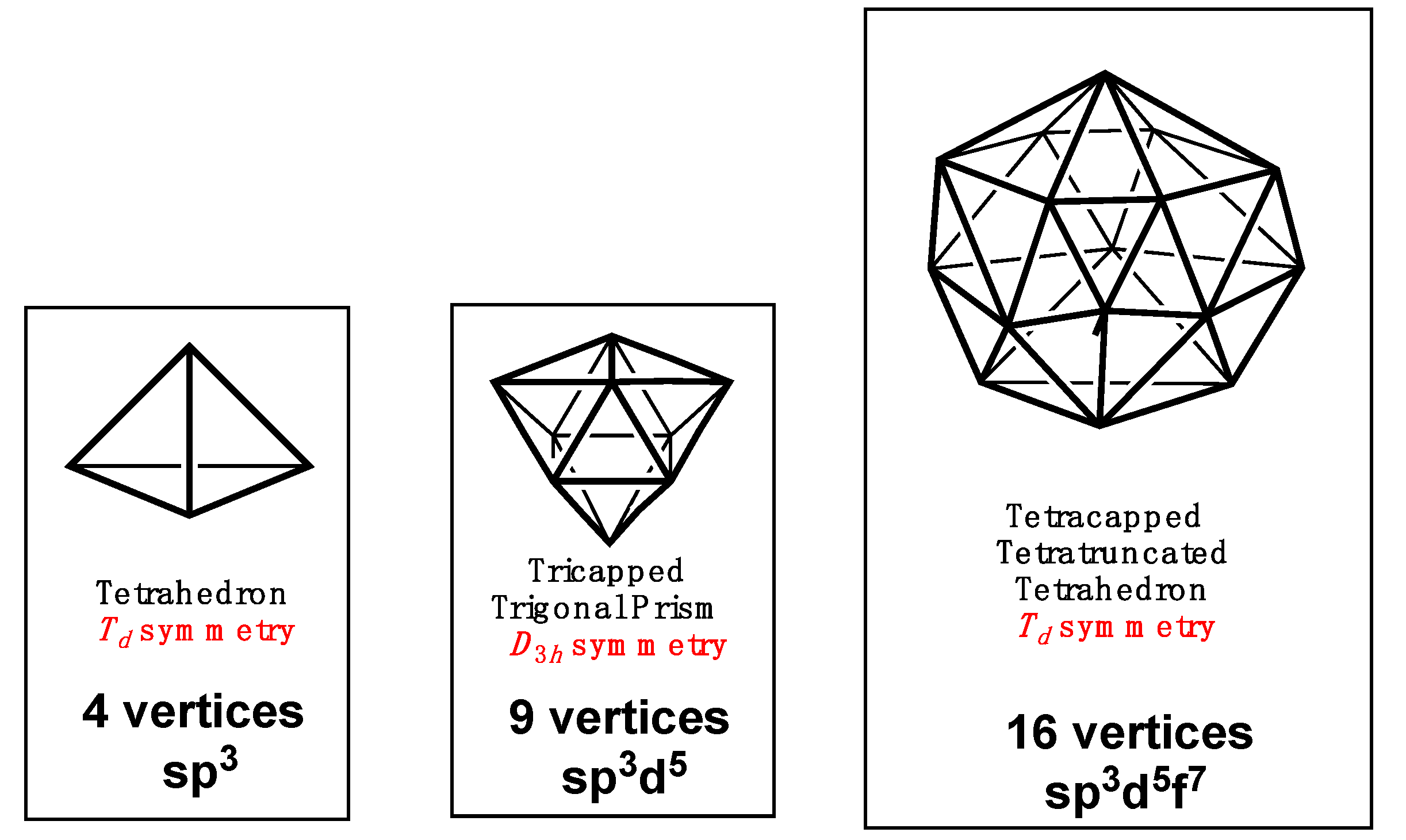
2. Polyhedral Hybridizations
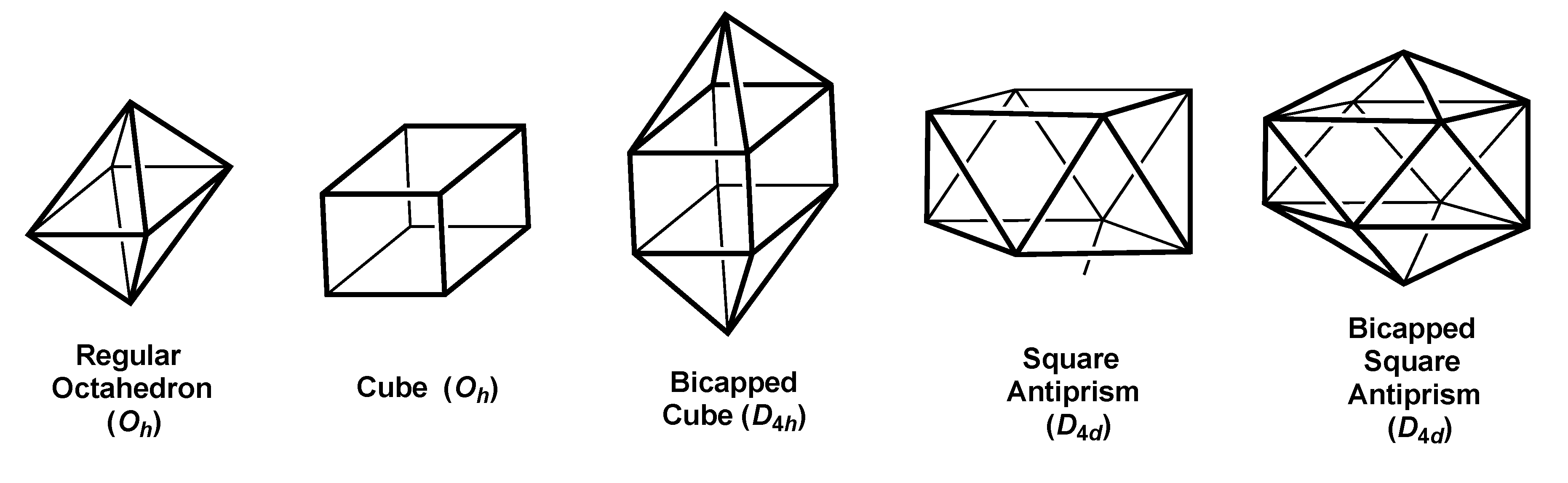
2.1. Polyhedra with Tetragonal Symmetry
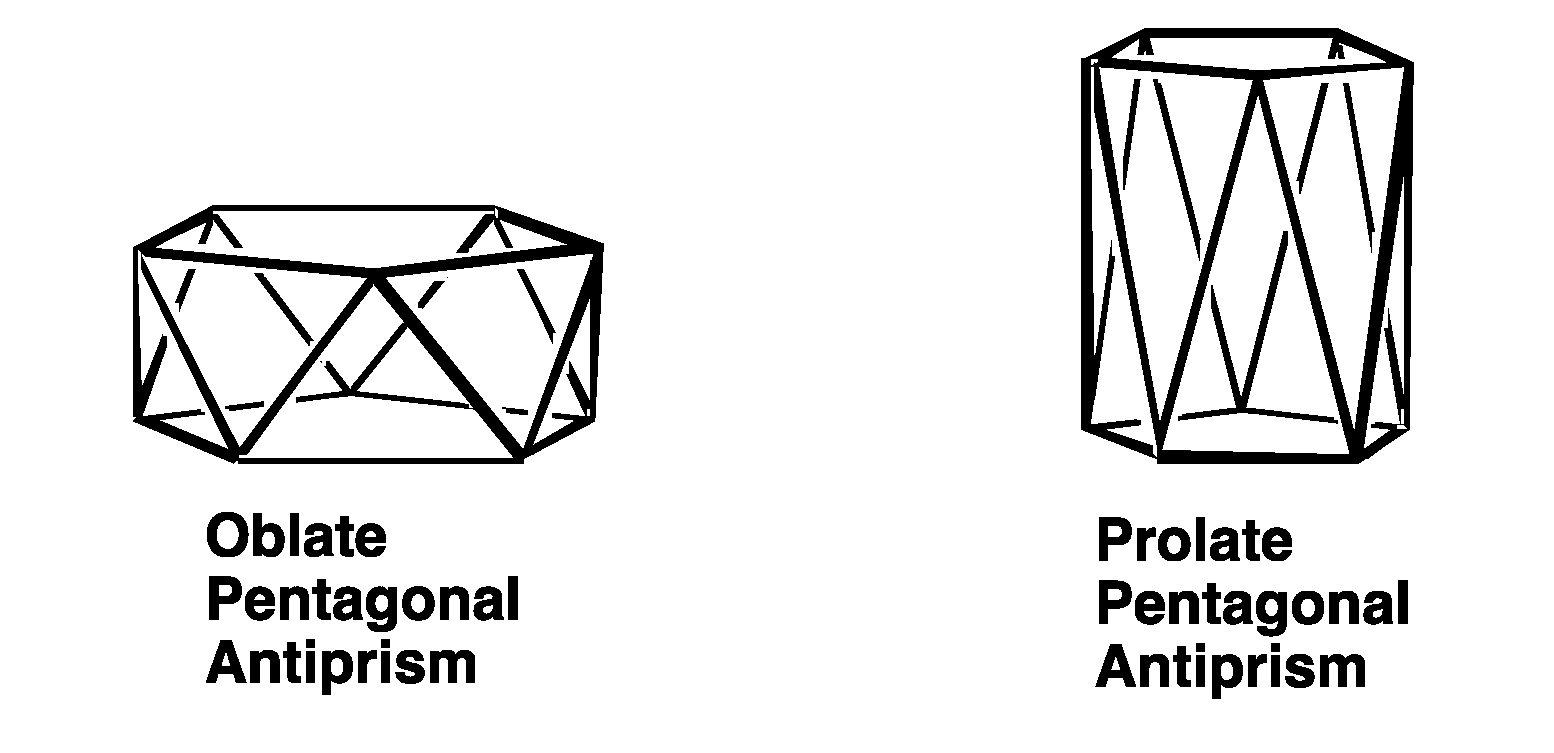
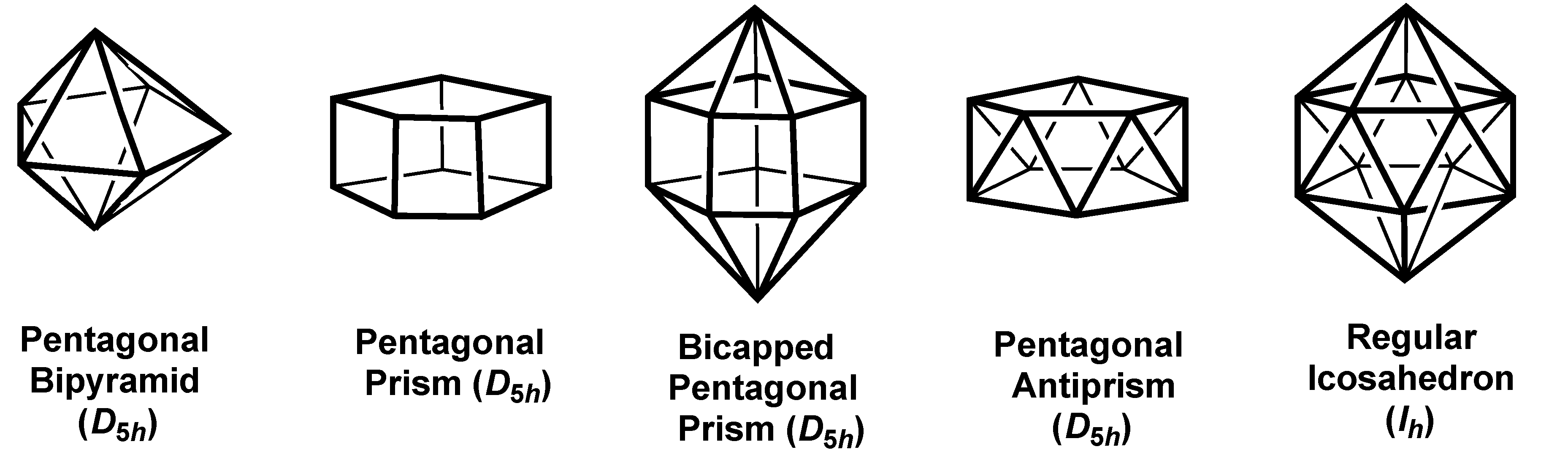
2.2. Polyhedra with Pentagonal Symmetry
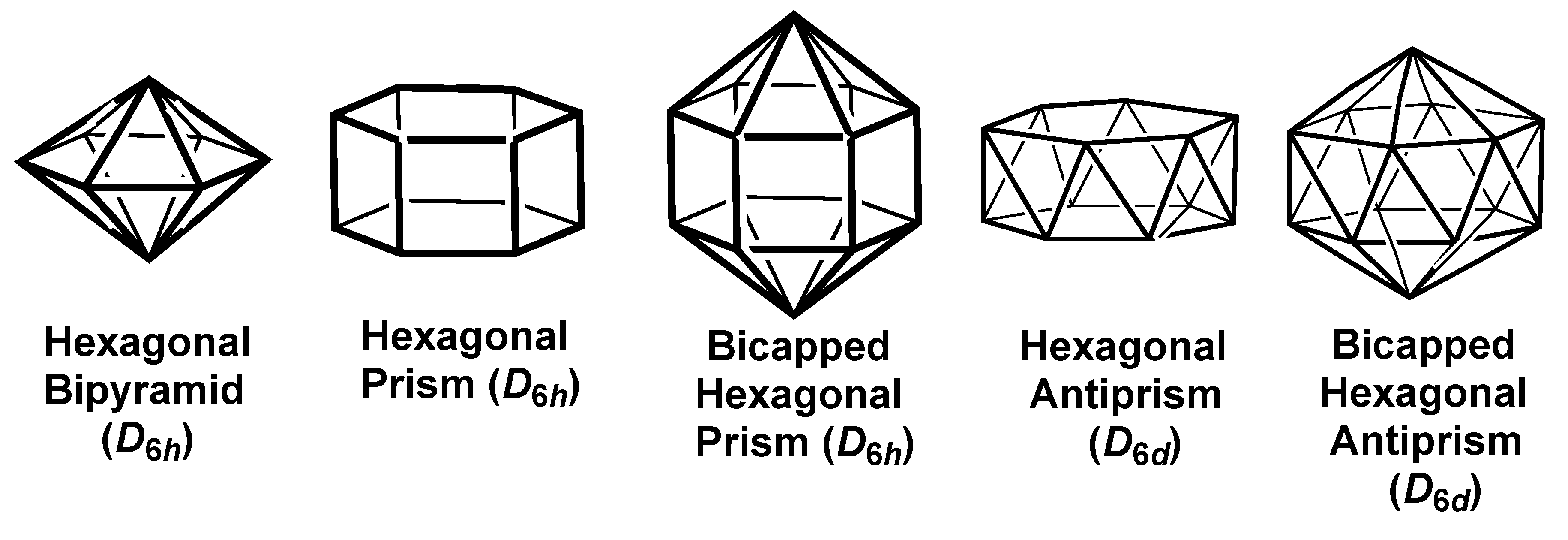
2.3. Polyhedra with Hexagonal Symmetry
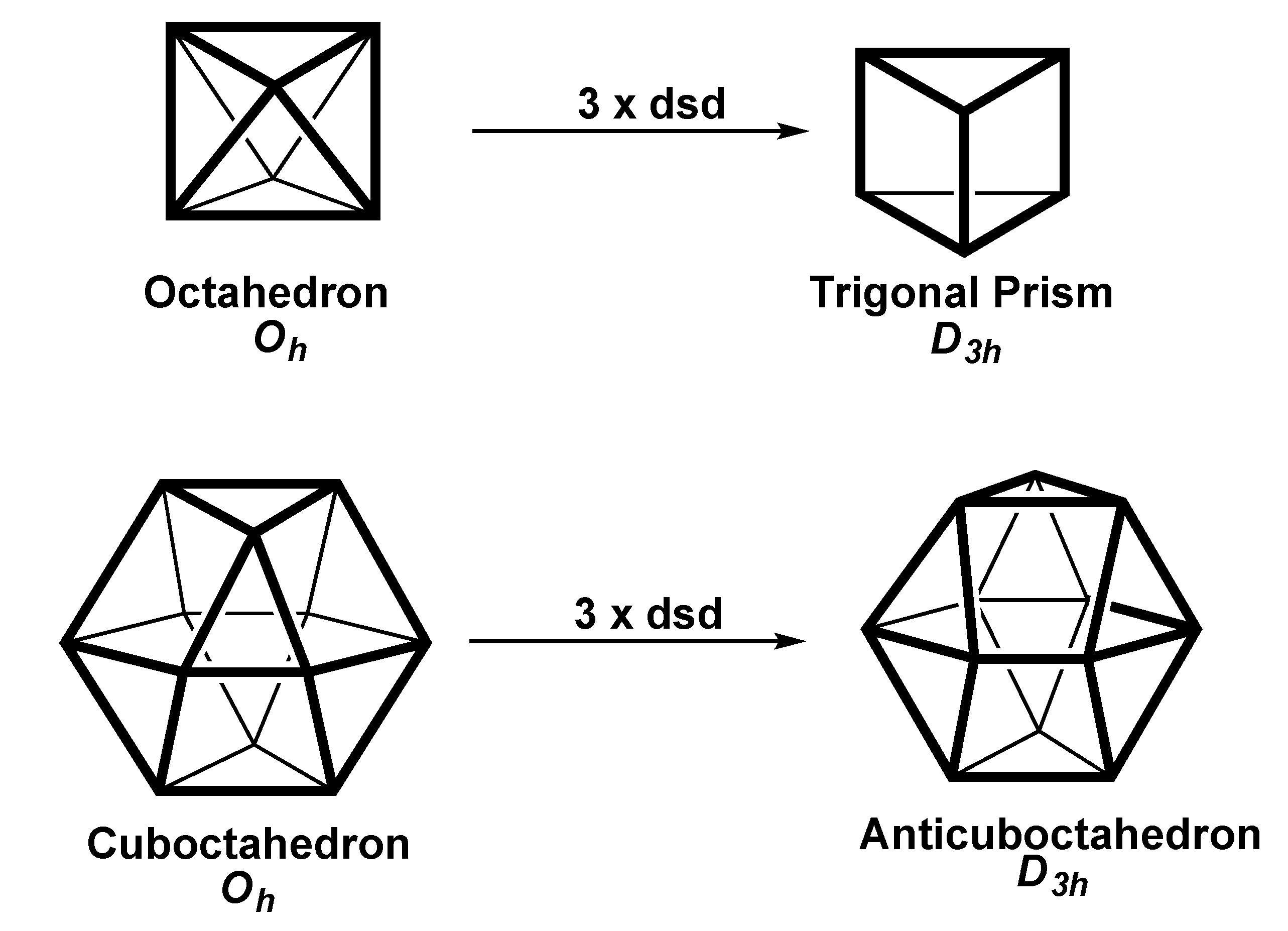
2.4. Trigonal Twist Processes in Polyhedral of Octahedral Symmetry
3. Conclusions
Funding
Conflicts of Interest
References
- King, R.B. Distortions from idealized symmetrical coordination polyhedral by lifting molecular orbital degeneracies. Inorg. Chim. Acta 1998, 270, 68–76. [Google Scholar] [CrossRef]
- Neidig, M.L.; Clark, D.L.; Martin, R.L. Covalency in f-element complexes. Coord. Chem. Rev. 2013, 257, 394–407. [Google Scholar] [CrossRef]
- Kerridge, A. Oxidation state and covalency in f-element metallocenes (M = Ce, Th, Pu): A combined CASSCF and topological study. Dalton Trans. 2013, 42, 16428–16436. [Google Scholar] [CrossRef]
- Manasian, S.G.; Smith, J.M.; Batista, E.R.; Roland, K.S.; Clark, D.L.; Kozimor, K.A.; Martin, R.L.; Shuh, D.K.; Tyszczak, T. New evidence for 5f covalency in actinocenes determined from carbon K-edge XAS and electronic structure theory. Chem. Sci. 2014, 5, 351–359. [Google Scholar] [CrossRef]
- Kaltsoyannis, N. Does covalency increase or decrease across the actinide series? Implications for minor actinide partitioning. Inorg. Chem. 2013, 52, 3407–3413. [Google Scholar] [CrossRef]
- Xue, D.; Sun, C.; Chen, X. Hybridized valence electrons of 4f0–145d0–16s2: The chemical bonding nature of rare earth elements. J. Rare Earths 2017, 35, 837–843. [Google Scholar] [CrossRef]
- Xue, D.; Sun, C.; Chen, X. Hybridization: A chemical bonding nature of atoms. Chin. J. Chem. 2017, 35, 1452–1458. [Google Scholar] [CrossRef]
- King, R.B. Atomic orbital graphs and the shapes of the g and h orbitals. J. Phys. Chem. 1997, 101, 4653–4656. [Google Scholar] [CrossRef]
- Kimball, G.E. Directed valence. J. Chem. Phys. 1940, 8, 188–198. [Google Scholar] [CrossRef]
- Powell, R.E. Textbook errors 81—5 equivalent d orbitals. J. Chem. Educ. 1968, 45, 45. [Google Scholar] [CrossRef]
- Pauling, L.; McClure, V. 5 equivalent d orbitals. J. Chem. Educ. 1970, 47, 15. [Google Scholar] [CrossRef]
- King, R.B. Endohedral nickel and palladium atom in metal clusters: Analogy to endohedral noble gas atoms in fullerenes in polyhedral with five-fold symmetry. Dalton Trans. 2004, 3420–3424. [Google Scholar] [CrossRef]
- Friedman, H.G., Jr.; Choppin, G.R.; Feuerbacher, D.G. The shapes of the f orbitals. J. Chem. Educ. 1964, 41, 354–360. [Google Scholar] [CrossRef]
- Frank, F.C.; Kasper, J.S. Complex alloy structures regarded as sphere packings. I. Definitions and basic principles. Acta Cryst. 1958, 11, 184–190. [Google Scholar] [CrossRef]
- Roy, D.K.; Mondal, R.; Shakhari, P.; Anju, R.S.; Geetharam, K.; Mobin, S.M.; Ghosh, S. Supraicosahedral polyhedra in metallaboranes: Synthesis and structural characterization of 12-, 15-, and 16-vertex rhodaboranes. Inorg. Chem. 2013, 52, 4705–6712. [Google Scholar] [CrossRef] [PubMed]
- Knox, K.; Ginsberg, A.P. X-ray determination of crystal structure of potassium rhenium hydride. Inorg. Chem. 1964, 3, 555–558. [Google Scholar] [CrossRef]
- Abrahams, S.C.; Ginsberg, A.P.; Knox, K. Crystal and molecular structure of potassium rhenium hydride, K2ReH9. Inorg. Chem. 1964, 3, 558–567. [Google Scholar] [CrossRef]
- Bersuker, I.B. Modern aspects of the Jahn-Teller effect theory and applications to molecular problems. Chem. Rev. 2001, 191, 1067–1114. [Google Scholar] [CrossRef]
- Zhou, B.; Denning, M.S.; Kays, D.L.; Goicoechea, J.M. Synthesis and isolation of [Fe@Ge10]3–: A pentagonal prismatic ZIntl ion cage encapsulating an interstitial iron atom. J. Am. Chem. Soc. 2009, 132, 2802–2803. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-Q.; Stegmaier, S.; Fässler, T.F. [Co@Ge10]3–: An intermetalloid cluster with Archimedean pentagonal prismatic structure. Angew Chem. Int. Ed. 2009, 48, 1998–2002. [Google Scholar] [CrossRef]
- Bailar, J.C., Jr. Some problems in the stereochemistry of coordination compounds: Introductory lecture. J. Inorg. Nucl. Chem. 1958, 8, 165–175. [Google Scholar] [CrossRef]
- Amati, M.; Leli, F. Competition between Bailar and Ray-Dutt paths in conformational interconversion of tris-chelated complexes: A DFT study. Theor. Chem. Accts. 2008, 120, 447–457. [Google Scholar] [CrossRef]
| Polynomial | Angular Function | Appearance and Orbital Graph | Shape |
|---|---|---|---|
| xy x2 − y2 xz yz | sin2 sin sin2 cos2 sin cos cos sin cos sin | 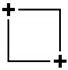 | square |
| 2z2 − r2 (abbreviated as z2) | (3cos2 − 1) | 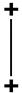 | linear |
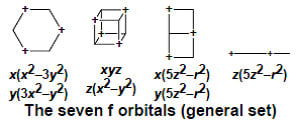
| |m| | Lobes | Shape | Orbital Graph | General Set | Cubic Set |
|---|---|---|---|---|---|
| 3 | 6 | Hexagon | 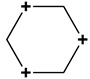 | x(x2 − 3y2) y(3x2 − y2) | none |
| 2 | 8 | Cube |  | xyz z(x2 − y2) | xyz x(z2 − y2) y(z2 − x2) z(x2 − y2) |
| 1 | 6 | Double Square | 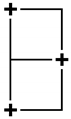 | x(5z2 − r2) y(5z2 − r2) | none |
| 0 | 4 | Linear |  | z(5z2 − r2) | x3 y3 z3 |
| Polyhedron | Coord. | Hybridization | |||
|---|---|---|---|---|---|
| (Symmetry) | No. | Type | s + p | d | f |
| Square (D4h) | 4 | sp2 | A1g + Eu | B1g {x2 − y2} | — |
| Square Bipy (D4h) | 6 | sp3d2 | A1g + A2u + Eu | A1g{z2} + B1g {x2 − y2} | — |
| Octahedron (Oh) | 6 | sp3d2 | A1g + T1u | Eg{x2 − y2,xy} | — |
| Square Prism(D4h) | 8 | sp3d3f | A1g + A2u + Eu | B1g {x2 − y2} + Eg{xz,yz) | B2u{ z(x2 − y2)} |
| Cube(Oh) | 8 | sp3d3f | A1g + T1u | T2g{xy,xz,yz} | A2u{xyz} |
| Bicapped Cube(D4h) | 10 | sp3d4f2 | A1g + A2u + Eu | A1g{z2} + B1g {x2 − y2} + Eg{xz,yz) | A2u(z3} + B2u{ z(x2 − y2)} |
| Square Antiprism(D4d) | 8 | sp3d4 | A1 + B2 + E1 | E2{x2 − y2,xy} + E3{xz,yz) | — |
| Bicap Sq Antipr(D4d) | 10 | sp3d5f | A1 + B2 + E1 | A1{z2} + E2{x2 − y2,xy} + E3{xz,yz) | B2{z3} |
| Polyhedron | Coord. | Hybridization | |||
|---|---|---|---|---|---|
| (Symmetry) | No. | Type | s + p | d | f |
| Pentagon (D5h) | 5 | sp2d2 | A1’ + E1´ | E2´{x2 − y2} | — |
| Pent Bipy (D5h) | 7 | sp3d3 | A1´ + A2˝ + E1´ | A1´{z2} + E2´{x2 − y2,xy} | — |
| Pent Prism (D5h) | 10 | sp3d4f2 | A1´ + A2˝ + E1´ | E2´{x2 − y2} + E1˝{xz,yz} | E2˝{xyz,z(x2 − y2)} |
| Bicap Pent Prism(D5h) | 12 | sp3d5f3 | A1´ + A2˝ + E1´ | A1´{z2} + E2´{x2 − y2,xy} + E1˝{xz,yz} | A2˝{z3} + E2˝{xyz,z(x2 − y2)} |
| Pent Antiprism(D5d) | 10 | sp3d4f2 | A1g + A2u + Eu | E1g{xz,yz} + E2g{x2 − y2,xy} | E2u |
| Bicap Pent Antipr(D5d) | 12 | sp3d5f3 | A1g + A2u + Eu | A1g{z2} + E1g{xz,yz} + E2g{x2 − y2,xy} | A2u{z3} + E2u |
| Icosahedron (Ih) | 12 | sp3d5f3 | A1g + T1u | Hg{all d orbitals} | T2u{x3,y3,z3} |
| Polyhedron | Coord. | Hybridization | ||||
|---|---|---|---|---|---|---|
| (Symmetry) | No. | Type | s + p | d | f | g |
| Hexagon (D6h) | 6 | sp2d2f | A1g + E1u | E2g{x2 − y2} | B1u{x(x2 − 3y2)} | — |
| Hexagonal Bipy(D6h) | 8 | sp3d3f | A1g + A2u + E1u | A1u{z2} + E2g{x2 − y2,xy} | B1u{x(x2 − 3y2)} | — |
| Hexagonal Prism(D6h) | 12 | sp3d4f3g | A1g + A2u + E1u | E1g{xz,yz} + E2g{x2 − y2,xy} | B1u + E2u{xyz,z(x2 − y2)} | B2g |
| Bicap Hex Prism(D6h) | 14 | sp3d5f4g | A1g + A2u + E1u | A1u{z2} + E1g{xz,yz}+E2g{x2 − y2,xy} | A2u{z3} + B1u + E2u | B2g |
| Hex Antiprism(D6d) | 12 | sp3d4f4 | A1 + B2 + E1 | E2{x2 − y2,xy} + E5{xz,yz} | E3 + E4{xyz,z(x2 − y2)} | — |
| Bicap Hex Antipr(D5d) | 14 | sp3d5f5 | A1 + B2 + E1 | A1{z2} + E2{x2 − y2,xy} + E5{xz,yz} | B2(z3) + E3 + E4 | — |
| Polyhedron | Coord. | Hybridization | |||
|---|---|---|---|---|---|
| (Symmetry) | No. | Type | s + p | d | f |
| Octahedron (D3d) | 6 | sp3d2 | A1g + A2u + Eu | Eg(x2 − y2,xy}or Eg{xz,yz} | — |
| Trigonal Prism (D3h) | 6 | sp3d2 | A1´ + A2˝ + E1´ | E˝{xz,yz} | — |
| Cuboctahedron (Oh) | 12 | sp3d5f3 | A1g + T1u | Eg(x2 − y2,xy} + T2g{xy,xz,yz} | T2u{x(z2 − y2),y(z2 − x2),z(x2 − y2)} |
| Cuboctahedron (D3d) | 12 | sp3d5f3 | A1g + A2u + Eu | A1g{z2} + Eg(x2 − y2,xy} + Eg{xz,yz} | A2u{z3} + Eu |
| Anticuboctahedron(D3h) | 12 | sp3d5f3 | A1´ + A2˝ + E1´ | A1´{z2} + E´(x2 − y2,xy} + E˝{xz,yz} | A1´{x(x2 − 3y2} + E´{xz2,yz2} |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
King, R.B. Systematics of Atomic Orbital Hybridization of Coordination Polyhedra: Role of f Orbitals. Molecules 2020, 25, 3113. https://doi.org/10.3390/molecules25143113
King RB. Systematics of Atomic Orbital Hybridization of Coordination Polyhedra: Role of f Orbitals. Molecules. 2020; 25(14):3113. https://doi.org/10.3390/molecules25143113
Chicago/Turabian StyleKing, R. Bruce. 2020. "Systematics of Atomic Orbital Hybridization of Coordination Polyhedra: Role of f Orbitals" Molecules 25, no. 14: 3113. https://doi.org/10.3390/molecules25143113
APA StyleKing, R. B. (2020). Systematics of Atomic Orbital Hybridization of Coordination Polyhedra: Role of f Orbitals. Molecules, 25(14), 3113. https://doi.org/10.3390/molecules25143113