Synthetic Mono-Rhamnolipids Display Direct Antifungal Effects and Trigger an Innate Immune Response in Tomato against Botrytis Cinerea
Abstract
1. Introduction
2. Results
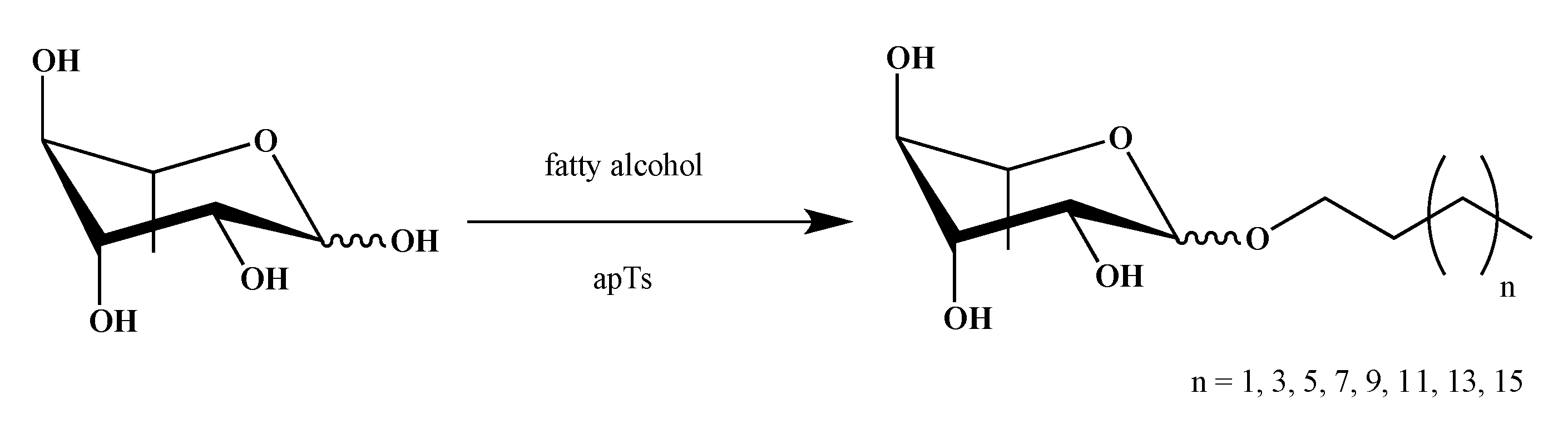
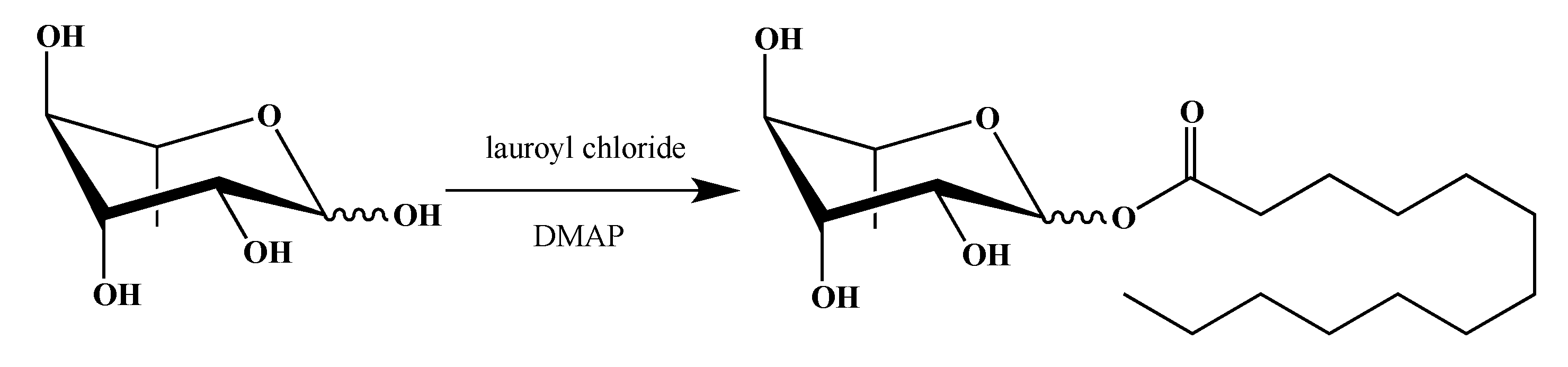
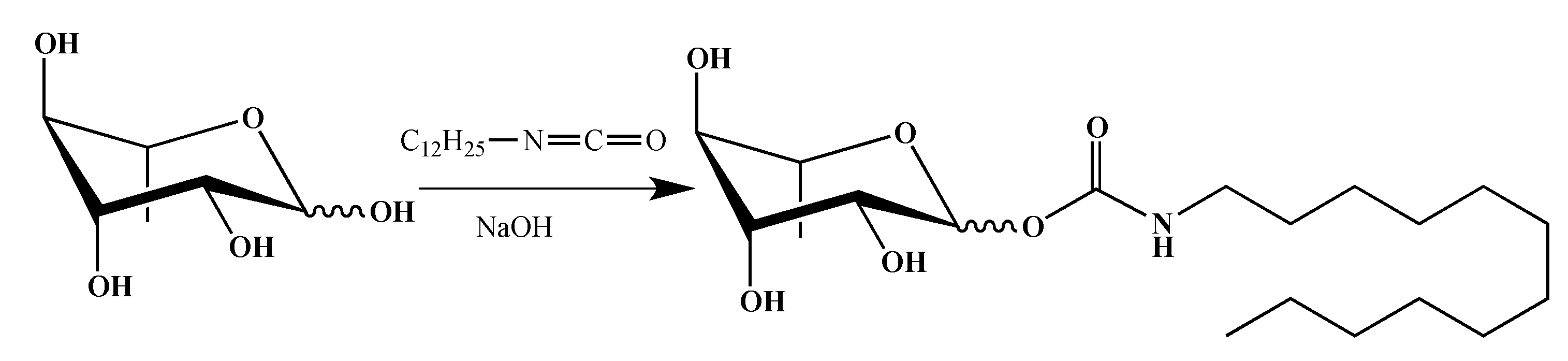
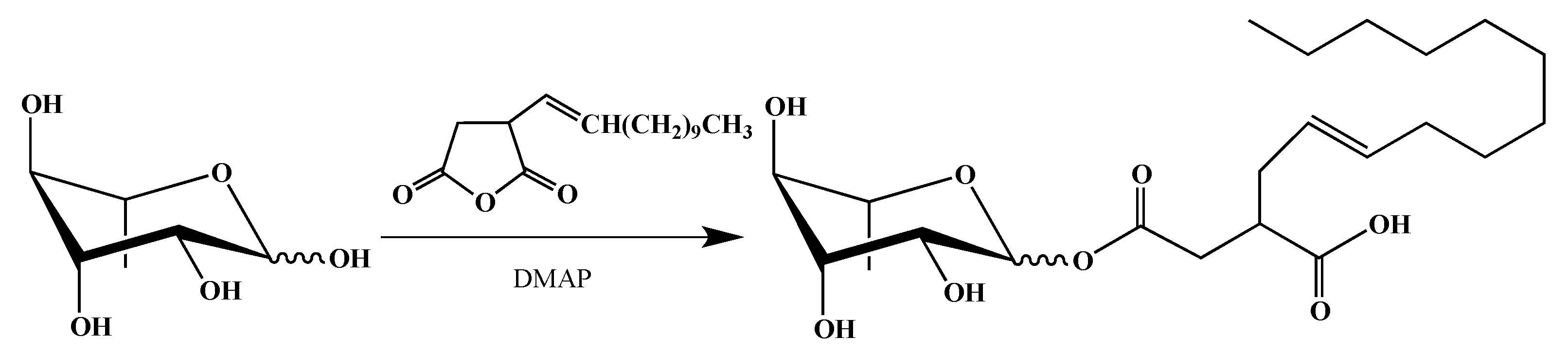
2.1. Synthesis of smRLs
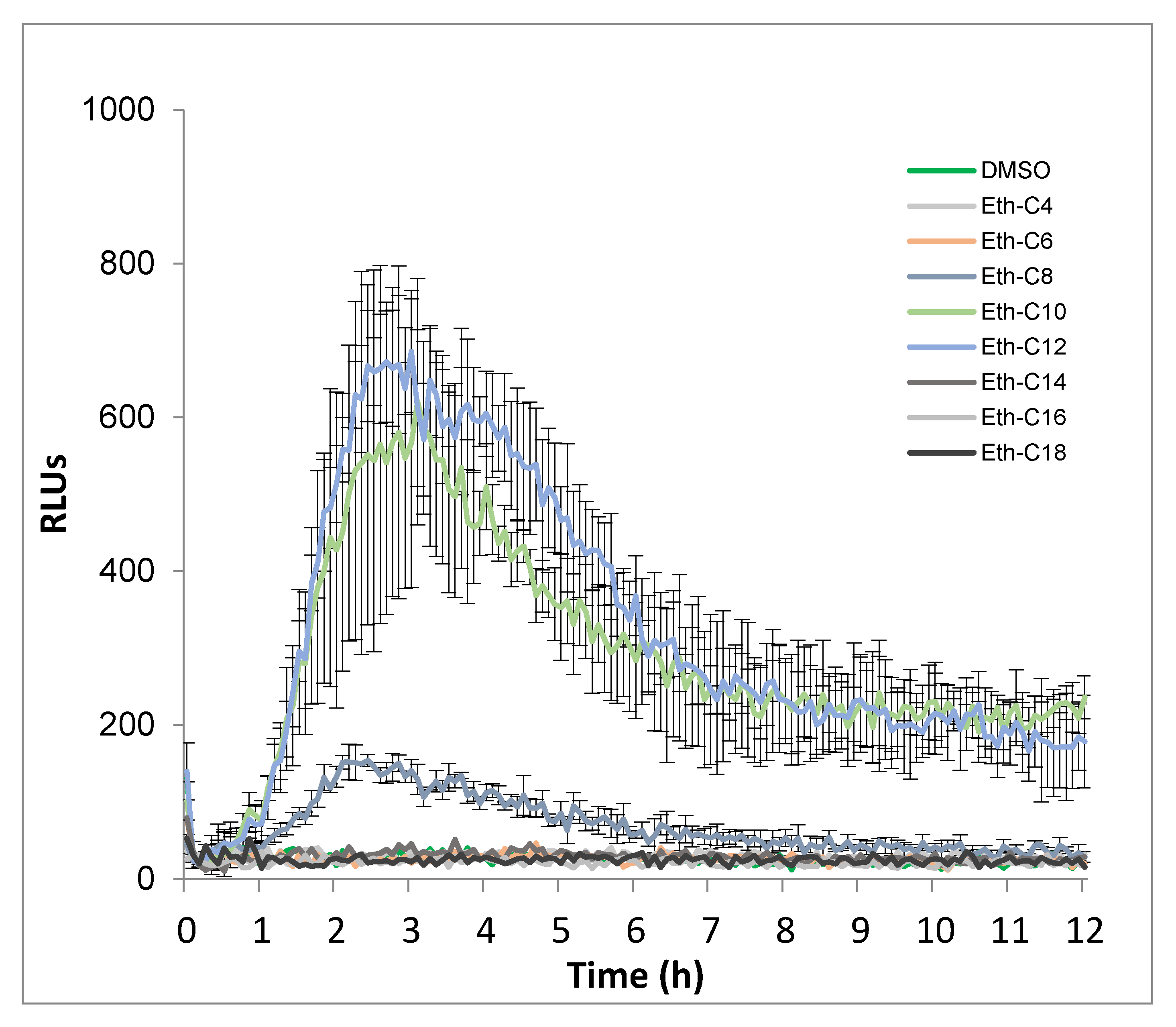
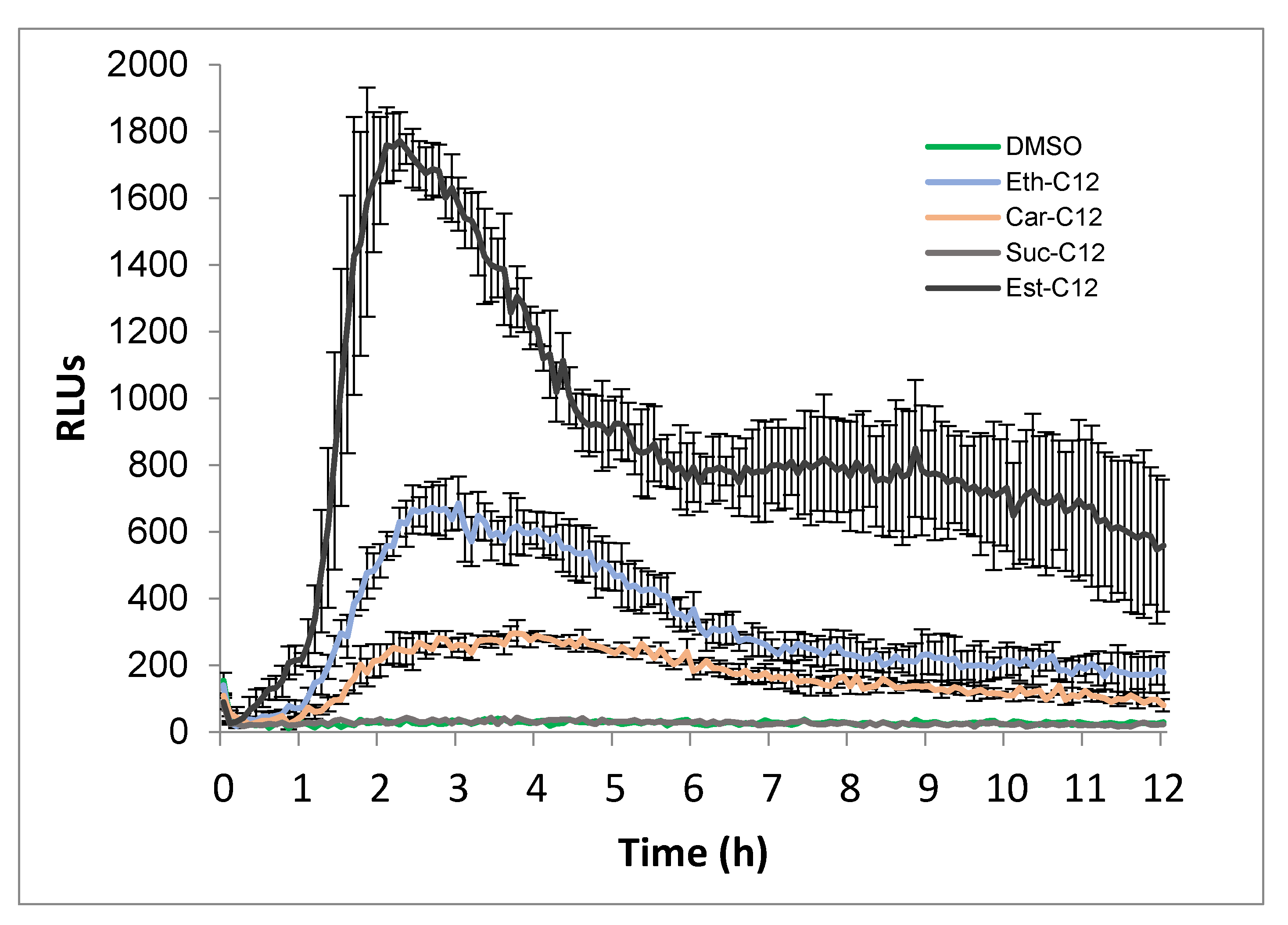
2.2. Ester, Ether, Succinate and Carbamate-Derived smRLs Differentially Activate Plant Immune Signalling in Tomato Leaves
2.3. smRLs Inhibit Conidia Germination and Mycelium Growth of Botrytis Cinerea
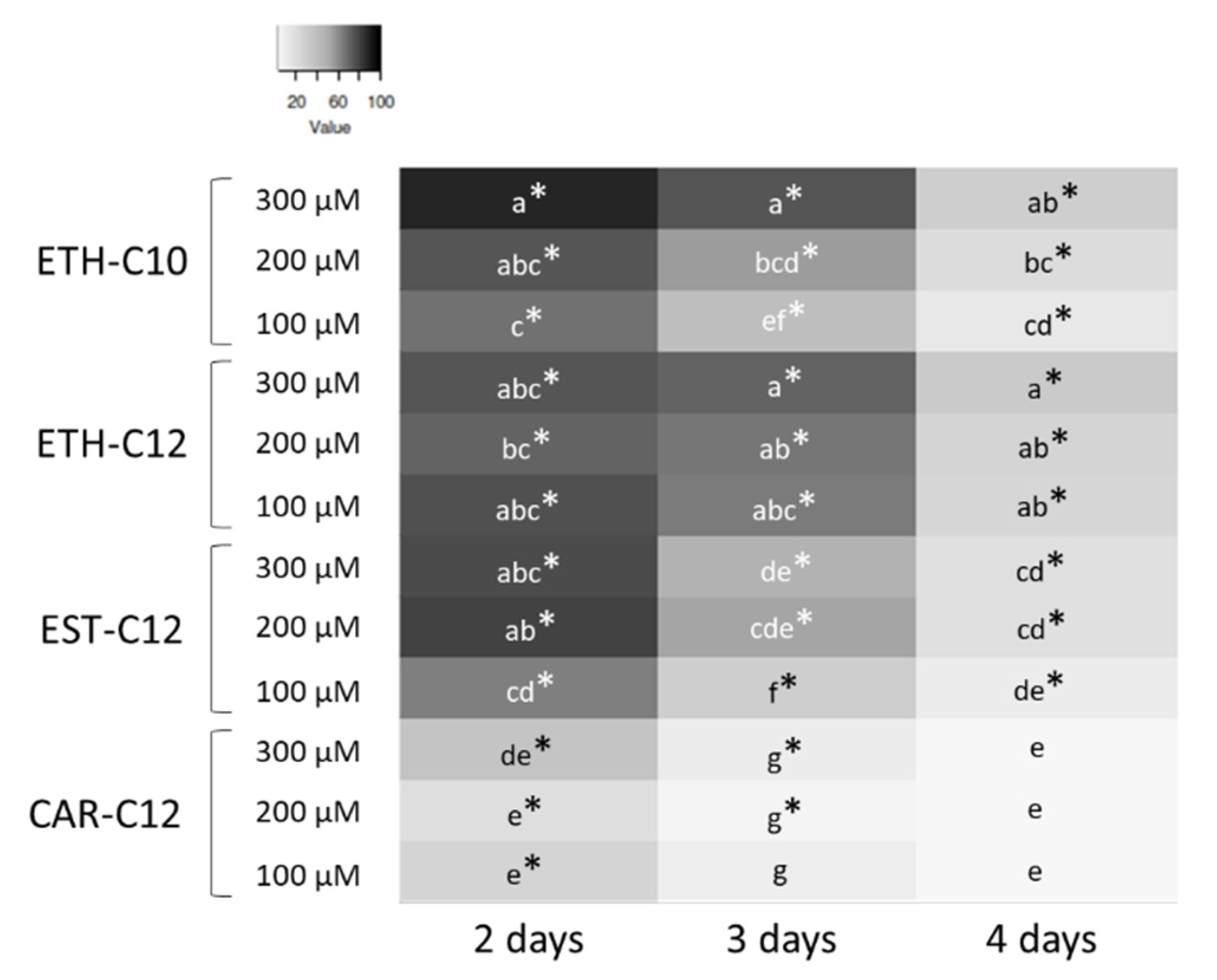
2.3.1. Conidial Germination
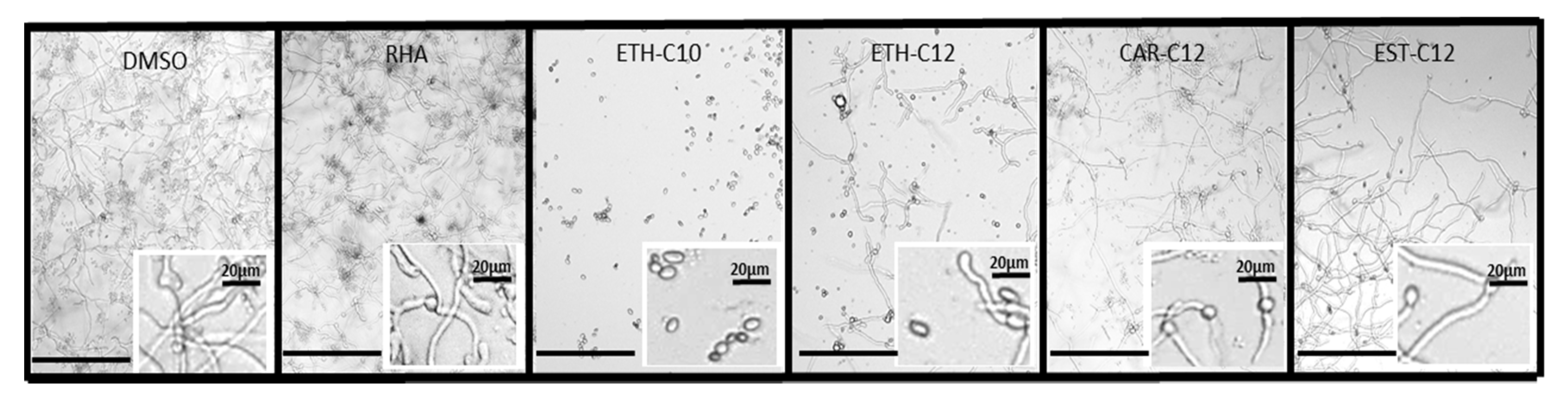
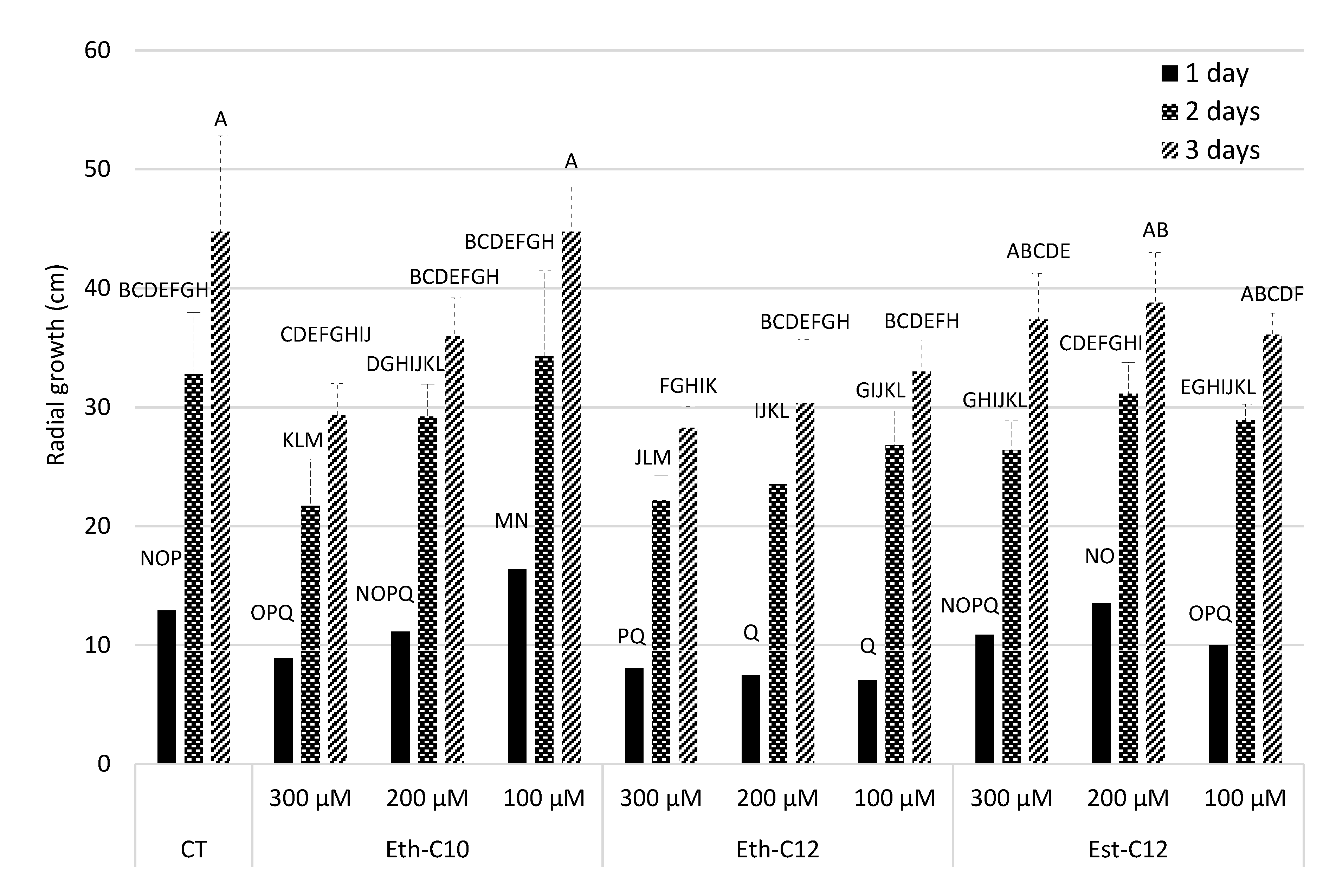
2.3.2. Mycelium Growth
2.4. smRLs Differentially Stimulate Tomato Immunity against Botrytis Cinerea
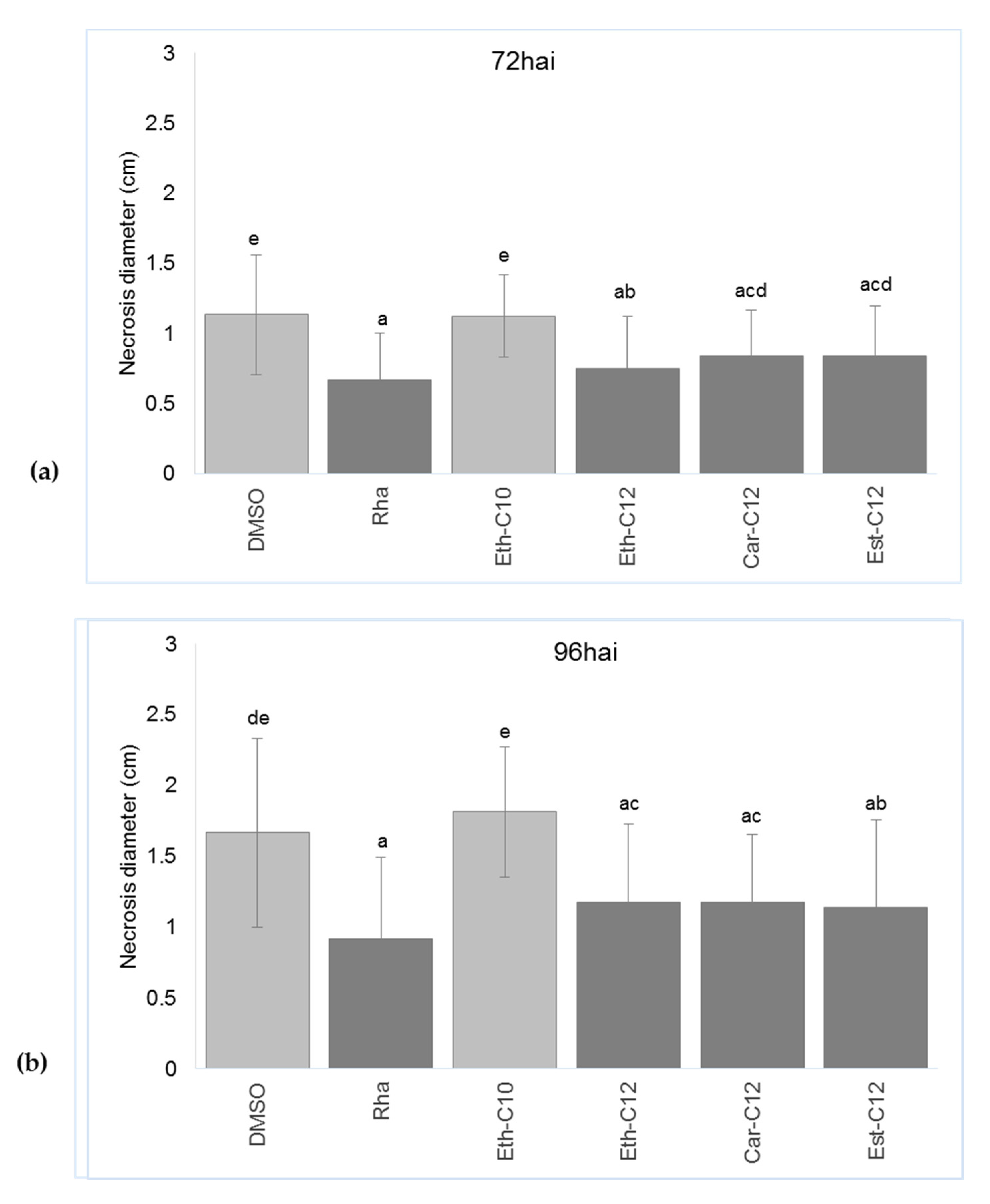
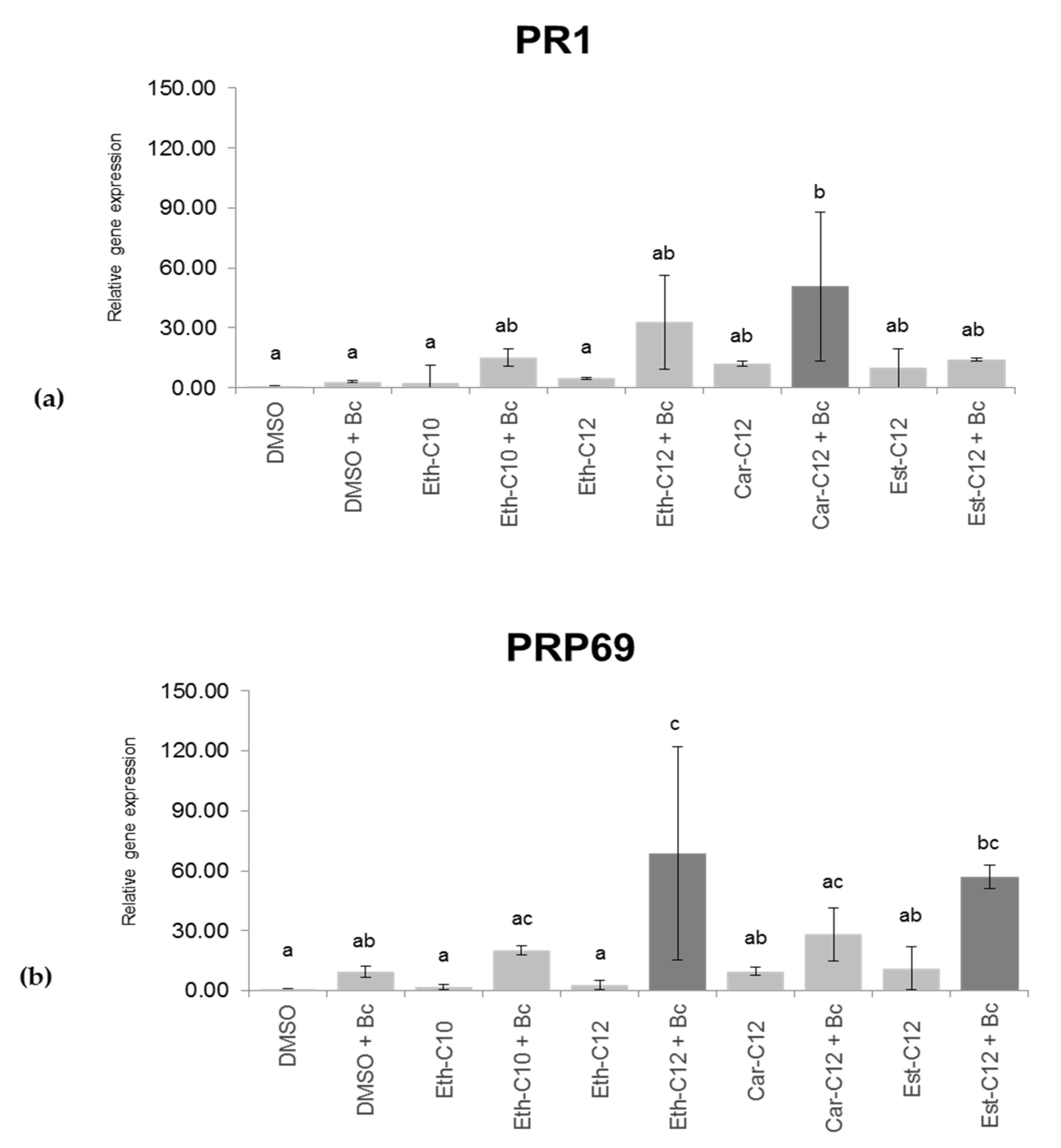
2.5. smRLs that Decrease Symptoms Development by B. cinerea on Detached Tomato Leaves Potentiate Their Defence Responses
3. Discussion
4. Materials and Methods
4.1. smRLs Synthesis
4.1.1. Rhamnose Ethers
Butyl α/β-l-Rhamnopyranoside
Hexyl α/β-l-Rhamnopyranoside
Octyl α/β-l-rhamnopyranoside
Decyl α/β-l-rhamnopyranoside
Dodecyl α/β-l-Rhamnopyranoside
Tetradecyl α/β-l-Rhamnopyranoside
Hexadecyl α/β-l-rhamnopyranoside
4.1.2. Rhamnose Esters
Dodecanoyl α/β-l-Rhamnopyranoside
4.1.3. Rhamnose Carbamates
α/β-l-Rhamnopyranosyl N-Dodecylcarbamate
4.1.4. Mono-rhamnosyl (alkenyl) Succinates
Dodecenylsuccinate α/β-l-Rhamnopyranoside
4.2. Plant Material and Growth Conditions
4.3. Microorganism
4.4. ROS Production
4.5. Antifungal Activity
4.5.1. Conidial Germination Assay
4.5.2. Fungal Development Assay
4.5.3. Dual-Test Assay
4.6. Protection Test
4.7. RNA Extraction, cDNA Synthesis, and Real-Time PCR
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cook, D.E.; Mesarich, C.H.; Thomma, B.P. Understanding plant immunity as a surveillance system to detect invasion. Annu. Rev. Phytopathol. 2015, 53, 541–563. [Google Scholar] [CrossRef] [PubMed]
- Kanyuka, K.; Rudd, J.J. Cell surface immune receptors: The guardians of the plant’s extracellular spaces. Curr. Opin. Plant. Biol. 2019, 50, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Schellenberger, R.; Touchard, M.; Clement, C.; Baillieul, F.; Cordelier, S.; Crouzet, J.; Dorey, S. Apoplastic invasion patterns triggering plant immunity: Plasma membrane sensing at the frontline. Mol. Plant. Pathol. 2019, 20, 1602–1616. [Google Scholar] [CrossRef] [PubMed]
- van der Burgh, A.M.; Joosten, M. Plant immunity: Thinking outside and inside the box. Trends Plant. Sci. 2019, 24, 587–601. [Google Scholar] [CrossRef]
- Bigeard, J.; Colcombet, J.; Hirt, H. Signaling mechanisms in pattern-triggered immunity (PTI). Mol. Plant. 2015, 8, 521–539. [Google Scholar] [CrossRef]
- Yip Delormel, T.; Boudsocq, M. Properties and functions of calcium-dependent protein kinases and their relatives in Arabidopsis thaliana. New Phytol. 2019, 224, 585–604. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Brugger, A.; Lamotte, O.; Vandelle, E.; Bourque, S.; Lecourieux, D.; Poinssot, B.; Wendehenne, D.; Pugin, A. Early signaling events induced by elicitors of plant defenses. Mol. Plant. Microbe Interact. 2006, 19, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.M.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C. Hormonal modulation of plant immunity. Annu. Rev. Cell. Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef] [PubMed]
- Boller, T.; Felix, G. A renaissance of elicitors: Perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu. Rev. Plant. Biol. 2009, 60, 379–406. [Google Scholar] [CrossRef] [PubMed]
- Boutrot, F.; Zipfel, C. Function, discovery, and exploitation of plant pattern recognition receptors for broad-spectrum disease resistance. Annu. Rev. Phytopathol. 2017, 55, 257–286. [Google Scholar] [CrossRef]
- Bektas, Y.; Eulgem, T. Synthetic plant defense elicitors. Front. Plant. Sci. 2014, 5, 804. [Google Scholar] [CrossRef]
- Gorlach, J.; Volrath, S.; Knauf-Beiter, G.; Hengy, G.; Beckhove, U.; Kogel, K.H.; Oostendorp, M.; Staub, T.; Ward, E.; Kessmann, H.; et al. Benzothiadiazole, a novel class of inducers of systemic acquired resistance, activates gene expression and disease resistance in wheat. Plant. Cell 1996, 8, 629–643. [Google Scholar] [CrossRef] [PubMed]
- Lawton, K.A.; Friedrich, L.; Hunt, M.; Weymann, K.; Delaney, T.; Kessmann, H.; Staub, T.; Ryals, J. Benzothiadiazole induces disease resistance in Arabidopsis by activation of the systemic acquired resistance signal transduction pathway. Plant. J. 1996, 10, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Uknes, S.; Mauch-Mani, B.; Moyer, M.; Potter, S.; Williams, S.; Dincher, S.; Chandler, D.; Slusarenko, A.; Ward, E.; Ryals, J. Acquired resistance in Arabidopsis. Plant. Cell 1992, 4, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Ward, E.R.; Uknes, S.J.; Williams, S.C.; Dincher, S.S.; Wiederhold, D.L.; Alexander, D.C.; Ahl-Goy, P.; Metraux, J.P.; Ryals, J.A. Coordinate gene activity in response to agents that induce systemic acquired resistance. Plant. Cell 1991, 3, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Salus, M.; Bektas, Y.; Schroeder, M.; Knoth, C.; Vu, T.; Roberts, P.; Kaloshian, I.; Eulgem, T. The synthetic eicitor 2-(5-bromo-2-hydroxy-phenyl)-thiazolidine-4-carboxylic acid links plant immunity to hormesis. Plant. Physiol. 2016, 170, 444–458. [Google Scholar] [CrossRef] [PubMed]
- Knoth, C.; Salus, M.S.; Girke, T.; Eulgem, T. The synthetic elicitor 3,5-dichloroanthranilic acid induces NPR1-dependent and NPR1-independent mechanisms of disease resistance in Arabidopsis. Plant. Physiol. 2009, 150, 333–347. [Google Scholar] [CrossRef]
- Bektas, Y.; Rodriguez-Salus, M.; Schroeder, M.; Gomez, A.; Kaloshian, I.; Eulgem, T. The synthetic elicitor DPMP (2,4-dichloro-6-{(E)-[(3-methoxyphenyl)imino]methyl}phenol) triggers strong immunity in Arabidopsis thaliana and tomato. Sci. Rep. 2016, 6, 29554. [Google Scholar] [CrossRef]
- Brotman, Y.; Makovitzki, A.; Shai, Y.; Chet, I.; Viterbo, A. Synthetic ultrashort cationic lipopeptides induce systemic plant defense responses against bacterial and fungal pathogens. Appl. Environ. Microbiol. 2009, 75, 5373–5379. [Google Scholar] [CrossRef]
- Cambiagno, D.A.; Lonez, C.; Ruysschaert, J.M.; Alvarez, M.E. The synthetic cationic lipid diC14 activates a sector of the Arabidopsis defence network requiring endogenous signalling components. Mol. Plant. Pathol. 2015, 16, 963–972. [Google Scholar] [CrossRef]
- Luzuriaga-Loaiza, P.; Schellenberger, R.; De Gaetano, Y.; Obounou Akong, F.; Villaume, S.; Crouzet, J.; Haudrechy, A.; Baillieul, F.; Clément, C.; Lins, L.; et al. Synthetic rhamnolipid bolaforms trigger an innate immune response in Arabidopsis thaliana. Sci. Rep. 2018, 8, 8534. [Google Scholar] [CrossRef]
- Hogan, D.E.; Tian, F.; Malm, S.W.; Olivares, C.; Palos Pacheco, R.; Simonich, M.T.; Hunjan, A.S.; Tanguay, R.L.; Klimecki, W.T.; Polt, R.; et al. Biodegradability and toxicity of monorhamnolipid biosurfactant diastereomers. J. Hazard. Mater. 2019, 364, 600–607. [Google Scholar] [CrossRef]
- Johann, S.; Seiler, T.-B.; Tiso, T.; Bluhm, K.; Blank, L.M.; Hollert, H. Mechanism-specific and whole-organism ecotoxicity of mono-rhamnolipids. Sci. Total Environ. 2016, 548–549, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Mohan, P.K.; Nakhla, G.; Yanful, E.K. Biokinetics of biodegradation of surfactants under aerobic, anoxic and anaerobic conditions. Water Res. 2006, 40, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Borah, S.N.; Goswami, D.; Sarma, H.K.; Cameotra, S.S.; Deka, S. Rhamnolipid biosurfactant against Fusarium verticillioides to control stalk and ear rot disease of maize. Front. Microbiol. 2016, 7, 1505. [Google Scholar] [CrossRef] [PubMed]
- Goswami, D.; Handique, P.J.; Deka, S. Rhamnolipid biosurfactant against Fusarium sacchari the causal organism of pokkah boeng disease of sugarcane. J. Basic Microbiol. 2014, 54, 548–557. [Google Scholar] [CrossRef]
- Kim, B.S.; Lee, J.Y.; Hwang, B.K. In vivo control and in vitro antifungal activity of rhamnolipid B, a glycolipid antibiotic, against Phytophthora capsici and Colletotrichum orbiculare. Pest Manage. Sci. 2000, 56, 1029–1035. [Google Scholar] [CrossRef]
- Monnier, N.; Furlan, A.L.; Buchoux, S.; Deleu, M.; Dauchez, M.; Rippa, S.; Sarazin, C. Exploring the dual interaction of natural rhamnolipids with plant and fungal biomimetic plasma membranes through biophysical studies. Int. J. Mol. Sci. 2019, 20, 1009. [Google Scholar] [CrossRef]
- Stanghellini, M.E.; Miller, R.M. Biosurfactants: Their identity and potential efficacy in the biological control of zoosporic plant pathogen. Plant. Dis. 1997, 81, 4–12. [Google Scholar] [CrossRef]
- Yan, F.; Hu, H.; Lu, L.; Zheng, X. Rhamnolipids induce oxidative stress responses in cherry tomato fruit to Alternaria alternata. Pest. Manage. Sci. 2016, 72, 1500–1507. [Google Scholar] [CrossRef]
- Monnier, N.; Cordier, M.; Dahi, A.; Santoni, V.; Guenin, S.; Clément, C.; Sarazin, C.; Penaud, A.; Dorey, S.; Cordelier, S.; et al. Semipurified rhamnolipid mixes protect Brassica napus against Leptosphaeria maculans early infections. Phytopathology 2020, 110, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Monnier, N.; Furlan, A.; Botcazon, C.; Dahi, A.; Mongelard, G.; Cordelier, S.; Clément, C.; Dorey, S.; Sarazin, C.; Rippa, S. Rhamnolipids from Pseudomonas aeruginosa are elicitors triggering Brassica napus protection against Botrytis cinerea without physiological disorders. Front. Plant. Sci. 2018, 9, 1170. [Google Scholar] [CrossRef]
- Sanchez, L.; Courteaux, B.; Hubert, J.; Kauffmann, S.; Renault, J.H.; Clément, C.; Baillieul, F.; Dorey, S. Rhamnolipids elicit defense responses and induce disease resistance against biotrophic, hemibiotrophic, and necrotrophic pathogens that require different signaling pathways in Arabidopsis and highlight a central role for salicylic acid. Plant. Physiol. 2012, 160, 1630–1641. [Google Scholar] [CrossRef] [PubMed]
- Varnier, A.L.; Sanchez, L.; Vatsa, P.; Boudesocque, L.; Garcia-Brugger, A.; Rabenoelina, F.; Sorokin, A.; Renault, J.H.; Kauffmann, S.; Pugin, A.; et al. Bacterial rhamnolipids are novel MAMPs conferring resistance to Botrytis cinerea in grapevine. Plant. Cell Environ. 2009, 32, 178–193. [Google Scholar] [CrossRef]
- Das, R.; Mukhopadhyay, B. Chemical O-Glycosylations: An overview. ChemistryOpen 2016, 5, 401–433. [Google Scholar] [CrossRef] [PubMed]
- Hricovíniová, Z.; Hricovíni, M. An efficient synthesis of novel l-rhamnose based non-ionic surfactants under controlled microwave irradiation. Tetrahedron: Asymmetry 2014, 25, 1008–1014. [Google Scholar] [CrossRef]
- Zheng, H.; Singh, N.; Shetye, G.S.; Jin, Y.; Li, D.; Luk, Y.Y. Synthetic analogs of rhamnolipids modulate structured biofilms formed by rhamnolipid-nonproducing mutant of Pseudomonas aeruginosa. Bioorg. Med. Chem. 2017, 25, 1830–1838. [Google Scholar] [CrossRef]
- Houlmont, J.-P.; Rico-Lattes, I.; Perez, E.; Bordat, P. Medicament Comprising a Reducing Alkyl-Sugar Monomer for the Treatment of Inflammatory Disorders. France Patent WO2005041983A1, 12 May 2005. [Google Scholar]
- Mizutani, T. Protective effects of butylated hydroxyanisole and its analogs on the lung toxicity of butylated hydroxytoluene in mice. Res. Commun. Chem. Pathol. Pharmacol. 1985, 50, 125–133. [Google Scholar]
- Raposo, C.D.; Petrova, K.T.; Barros, M.T.; Calhelha, R.C.; Sokovic, M.; Ferreira, I.C. Synthesis, characterization, antimicrobial and antitumor activities of sucrose Octa(N-ethyl)carbamate. Med. Chem. 2016, 12, 22–29. [Google Scholar] [CrossRef][Green Version]
- Christian, D.; Fitremann, J.; Bouchu, A.; Queneau, Y. Preparation of amphiphilic sucrose carbamates by reaction with alkyl isocyanates in water–alcohol mixtures. Tetrahedron Lett. 2004, 45, 583–586. [Google Scholar] [CrossRef]
- Le Guenic, S.; Chaveriat, L.; Lequart, V.; Joly, N.; Martin, P. Renewable surfactants for biochemical applications and nanotechnology. J. Surfactants Deterg. 2019, 22, 5–21. [Google Scholar] [CrossRef]
- Vatsa, P.; Sanchez, L.; Clément, C.; Baillieul, F.; Dorey, S. Rhamnolipid biosurfactants as new players in animal and plant defense against microbes. Int. J. Mol. Sci. 2010, 11, 5095–5108. [Google Scholar] [CrossRef]
- Wang, H.; Williams, P.A.; Senan, C. Synthesis, characterization and emulsification properties of dodecenyl succinic anhydride derivatives of gum Arabic. Food Hydrocoll. 2014, 37, 143–148. [Google Scholar] [CrossRef]
- Zhou, J.; Ren, L.; Tong, J.; Xie, L.; Liu, Z. Surface esterification of corn starch films: Reaction with dodecenyl succinic anhydride. Carbohydr. Polym. 2009, 78, 888–893. [Google Scholar] [CrossRef]
- Issa, A.; Esmaeel, Q.; Sanchez, L.; Courteaux, B.; Guise, J.F.; Gibon, Y.; Ballias, P.; Clement, C.; Jacquard, C.; Vaillant-Gaveau, N.; et al. Impacts of Paraburkholderia phytofirmans strain PsJN on tomato (Lycopersicon esculentum L.) under high temperature. Front. Plant. Sci. 2018, 9, 1397. [Google Scholar] [CrossRef] [PubMed]
- Menhour, B.; Mayon, P.; Plé, K.; Bouquillon, S.; Dorey, S.; Clément, C.; Deleu, M.; Haudrechy, A. A stereocontrolled synthesis of the hydrophobic moiety of rhamnolipids. Tetrahedron Lett. 2015, 56, 1159–1161. [Google Scholar] [CrossRef]
- Felix, G.; Duran, J.D.; Volko, S.; Boller, T. Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant. J. 1999, 18, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Nasir, M.N.; Crowet, J.M.; Lins, L.; Obounou Akong, F.; Haudrechy, A.; Bouquillon, S.; Deleu, M. Interactions of sugar-based bolaamphiphiles with biomimetic systems of plasma membranes. Biochimie 2016, 130, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Henry, G.; Deleu, M.; Jourdan, E.; Thonart, P.; Ongena, M. The bacterial lipopeptide surfactin targets the lipid fraction of the plant plasma membrane to trigger immune-related defence responses. Cell. Microbiol. 2011, 13, 1824–1837. [Google Scholar] [CrossRef]
- Jourdan, E.; Henry, G.; Duby, F.; Dommes, J.; Barthelemy, J.P.; Thonart, P.; Ongena, M. Insights into the defense-related events occurring in plant cells following perception of surfactin-type lipopeptide from Bacillus subtilis. Mol. Plant.-Microbe Interact. 2009, 22, 456–468. [Google Scholar] [CrossRef]
- Steinberg, G.; Schuster, M.; Gurr, S.J.; Schrader, T.A.; Schrader, M.; Wood, M.; Early, A.; Kilaru, S. A lipophilic cation protects crops against fungal pathogens by multiple modes of action. Nature Commun. 2020, 11, 1608. [Google Scholar] [CrossRef] [PubMed]
- Goswami, D.; Borah, S.N.; Lahkar, J.; Handique, P.J.; Deka, S. Antifungal properties of rhamnolipid produced by Pseudomonas aeruginosa DS9 against Colletotrichum falcatum. J. Basic Microbiol. 2015, 55, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.I.; Gudina, E.J.; Teixeira, J.A.; Rodrigues, L.R. Sodium chloride effect on the aggregation behaviour of rhamnolipids and their antifungal activity. Sci. Rep. 2017, 7, 12907. [Google Scholar] [CrossRef]
- Sha, R.; Meng, Q. Antifungal activity of rhamnolipids against dimorphic fungi. J. Gen. Appl. Microbiol. 2016, 62, 233–239. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robineau, M.; Le Guenic, S.; Sanchez, L.; Chaveriat, L.; Lequart, V.; Joly, N.; Calonne, M.; Jacquard, C.; Declerck, S.; Martin, P.; et al. Synthetic Mono-Rhamnolipids Display Direct Antifungal Effects and Trigger an Innate Immune Response in Tomato against Botrytis Cinerea. Molecules 2020, 25, 3108. https://doi.org/10.3390/molecules25143108
Robineau M, Le Guenic S, Sanchez L, Chaveriat L, Lequart V, Joly N, Calonne M, Jacquard C, Declerck S, Martin P, et al. Synthetic Mono-Rhamnolipids Display Direct Antifungal Effects and Trigger an Innate Immune Response in Tomato against Botrytis Cinerea. Molecules. 2020; 25(14):3108. https://doi.org/10.3390/molecules25143108
Chicago/Turabian StyleRobineau, Mathilde, Sarah Le Guenic, Lisa Sanchez, Ludovic Chaveriat, Vincent Lequart, Nicolas Joly, Maryline Calonne, Cédric Jacquard, Stéphane Declerck, Patrick Martin, and et al. 2020. "Synthetic Mono-Rhamnolipids Display Direct Antifungal Effects and Trigger an Innate Immune Response in Tomato against Botrytis Cinerea" Molecules 25, no. 14: 3108. https://doi.org/10.3390/molecules25143108
APA StyleRobineau, M., Le Guenic, S., Sanchez, L., Chaveriat, L., Lequart, V., Joly, N., Calonne, M., Jacquard, C., Declerck, S., Martin, P., Dorey, S., & Ait Barka, E. (2020). Synthetic Mono-Rhamnolipids Display Direct Antifungal Effects and Trigger an Innate Immune Response in Tomato against Botrytis Cinerea. Molecules, 25(14), 3108. https://doi.org/10.3390/molecules25143108









