Effects of Sesamin, the Major Furofuran Lignan of Sesame Oil, on the Amplitude and Gating of Voltage-Gated Na+ and K+ Currents
Abstract
:1. Introduction
2. Results
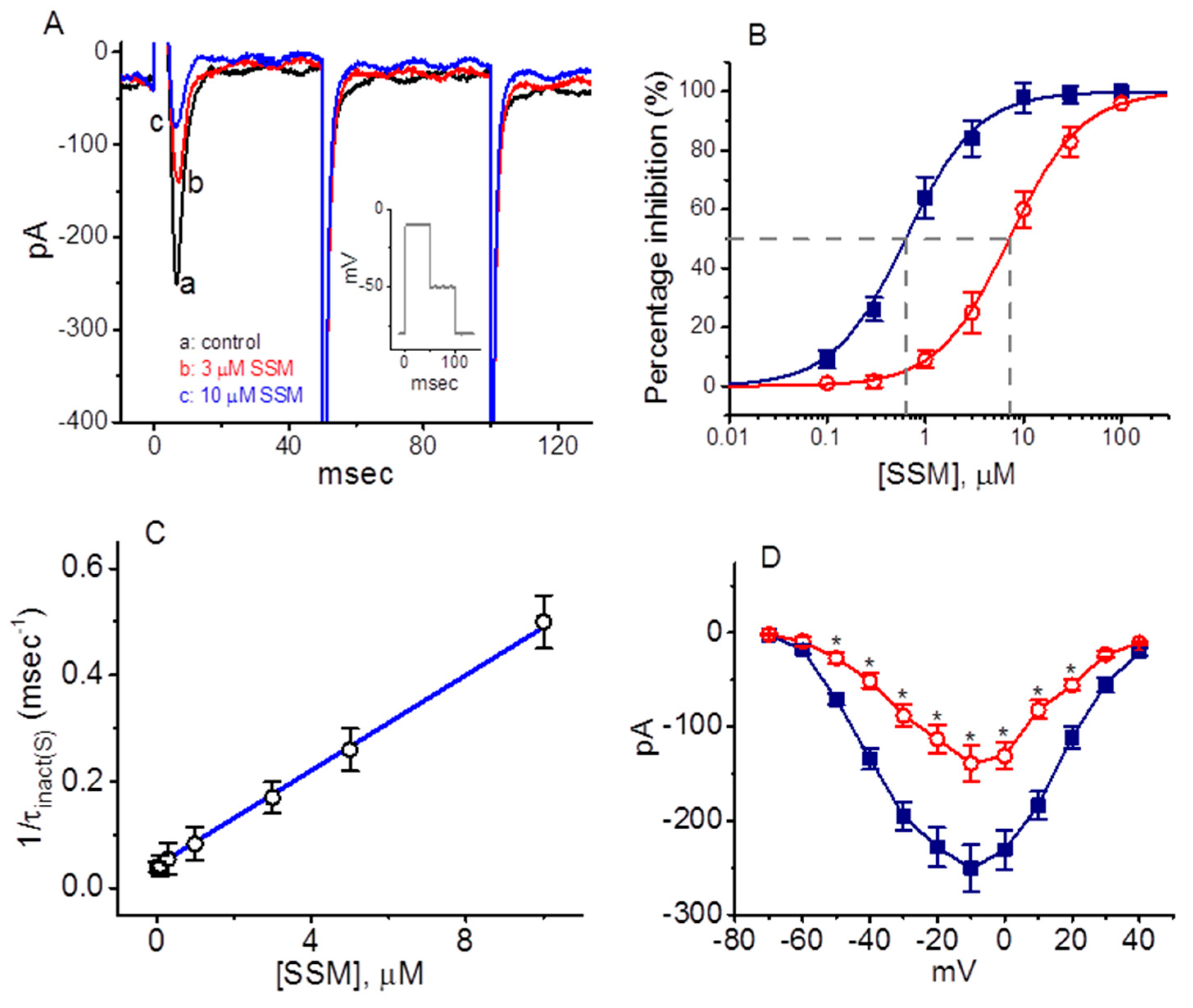
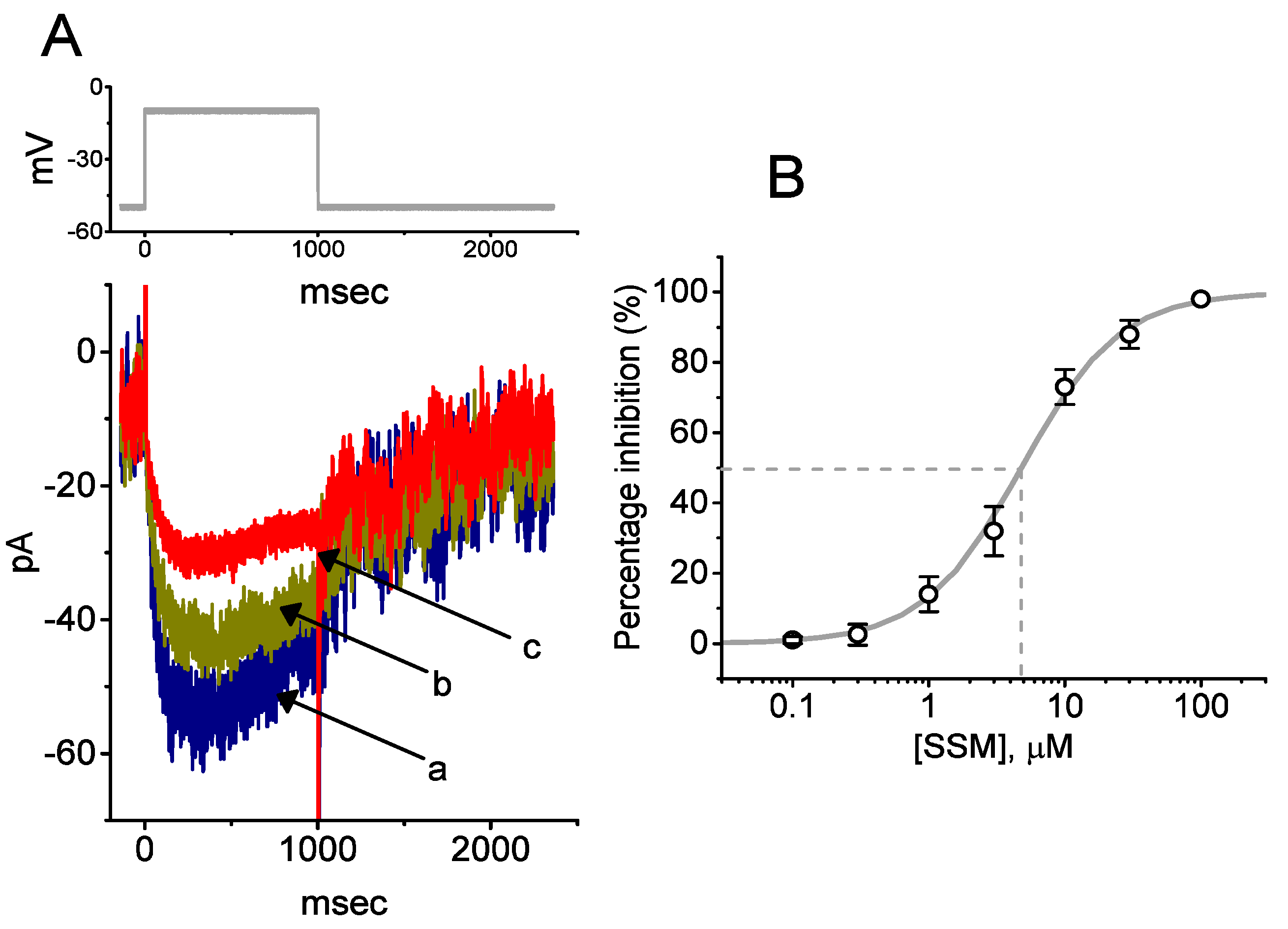
2.1. Inhibitory Effect of Sesamin (SSM) on Voltage-Gated Na+ Currents (INa) Identified in GH3 Cells
2.2. Kinetic Constants of INa Block by SSM
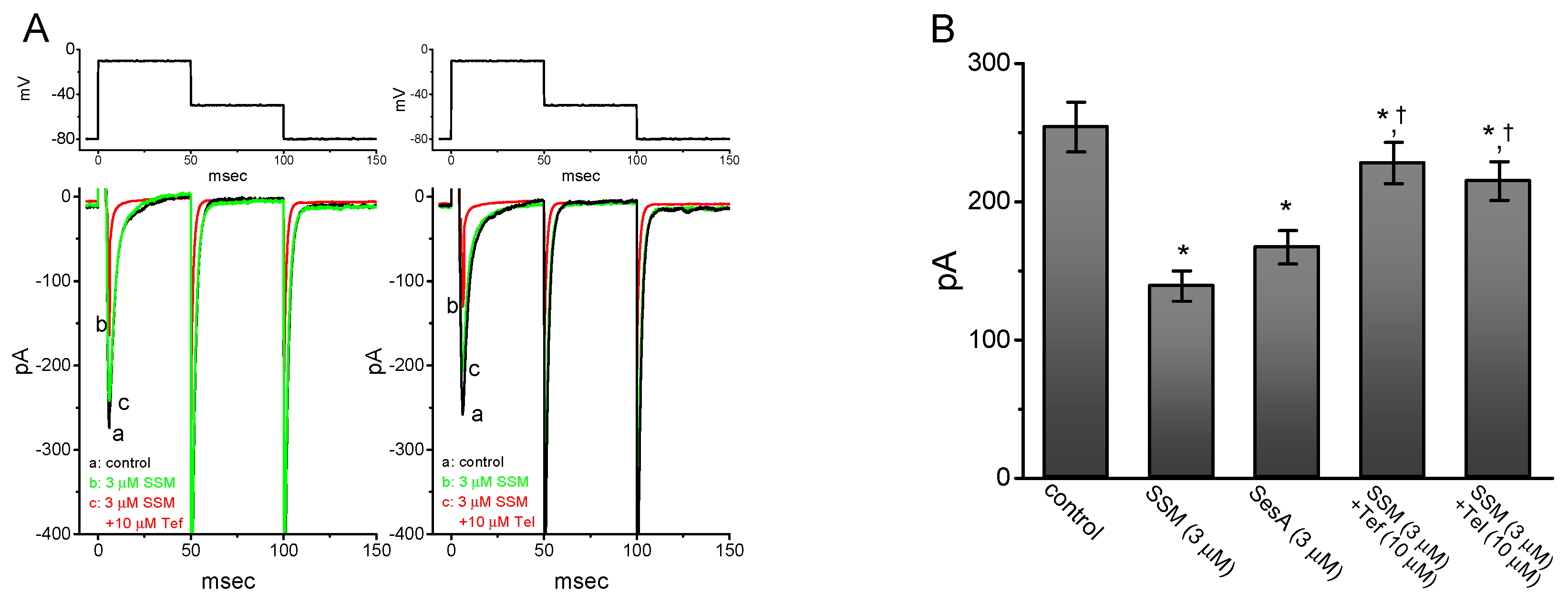
2.3. Comparison Between Effects of SSM, SesA, SSM Plus Tefluthrin, and SSM Plus Telmisartan on Peak INa Identified in GH3 Cells
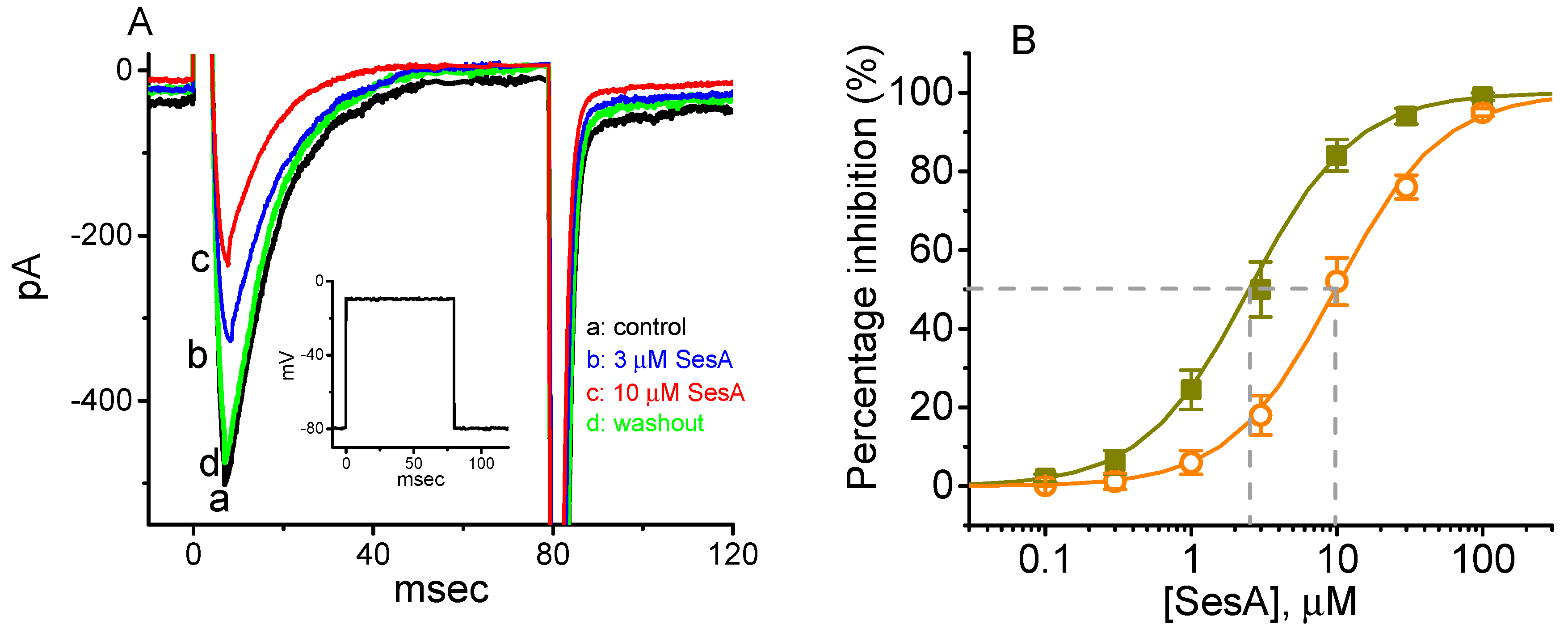
2.4. Concentration-Dependent Inhibition of INa Caused by Sesamolin (SesA)
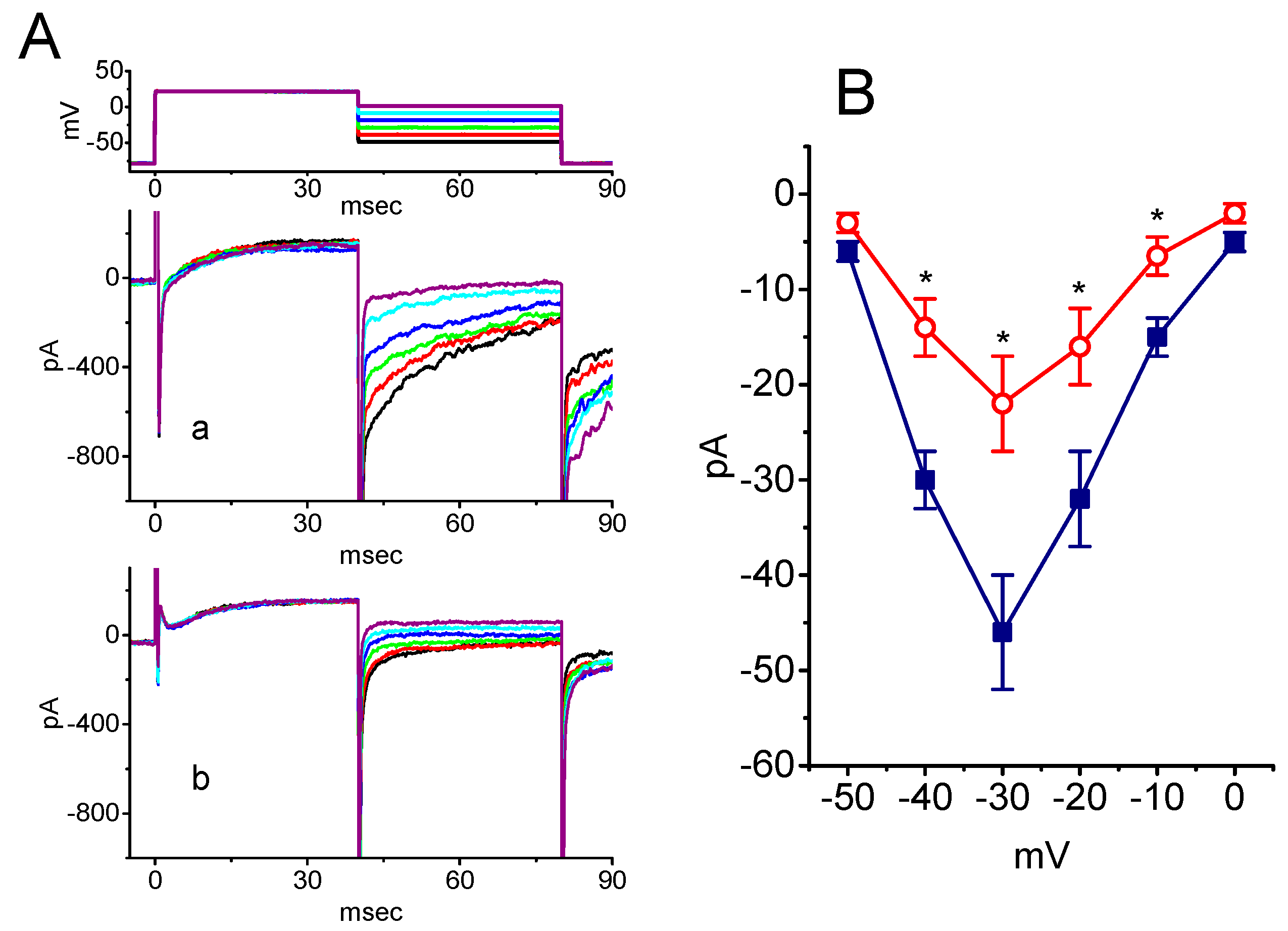
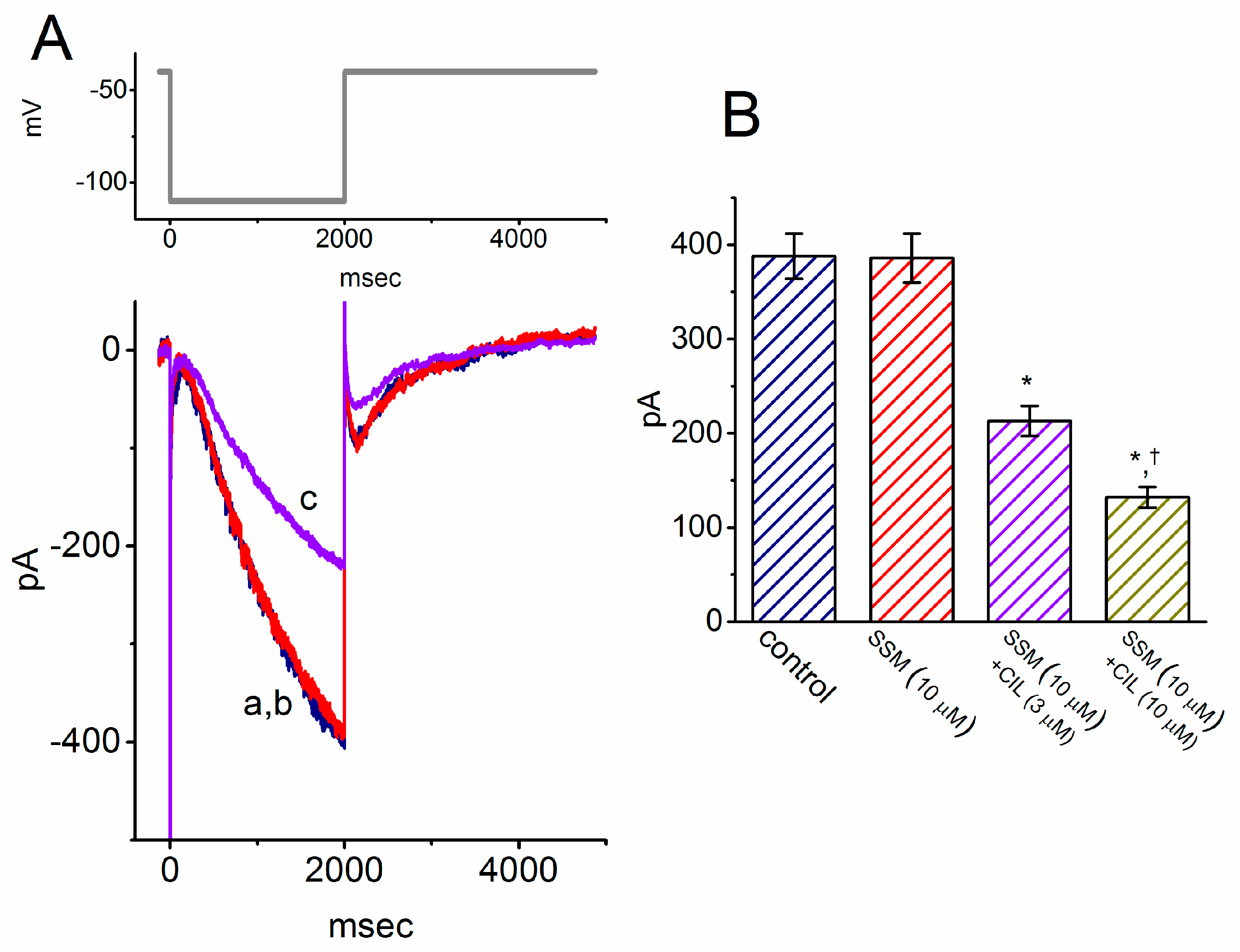
2.5. Inhibitory Effect of SSM on Resurgent INa (INa(R)) in GH3 Cells
2.6. Concentration-Dependent Inhibition of M-Type K+ Currents (IK(M)) Caused by SSM in GH3 Cells
2.7. Inhibitory Effect of SSM on Erg-Mediated K+ Current (IK(erg)) in GH3 Cells
2.8. Mild Inhibitory Effect of SSM on Delayed Rectifier K+ Currents (IK(DR)) in GH3 Cells
2.9. Inability of SSM to Perturb Hyperpolarization-Activated Cation Currents (Ih) in GH3 Cells
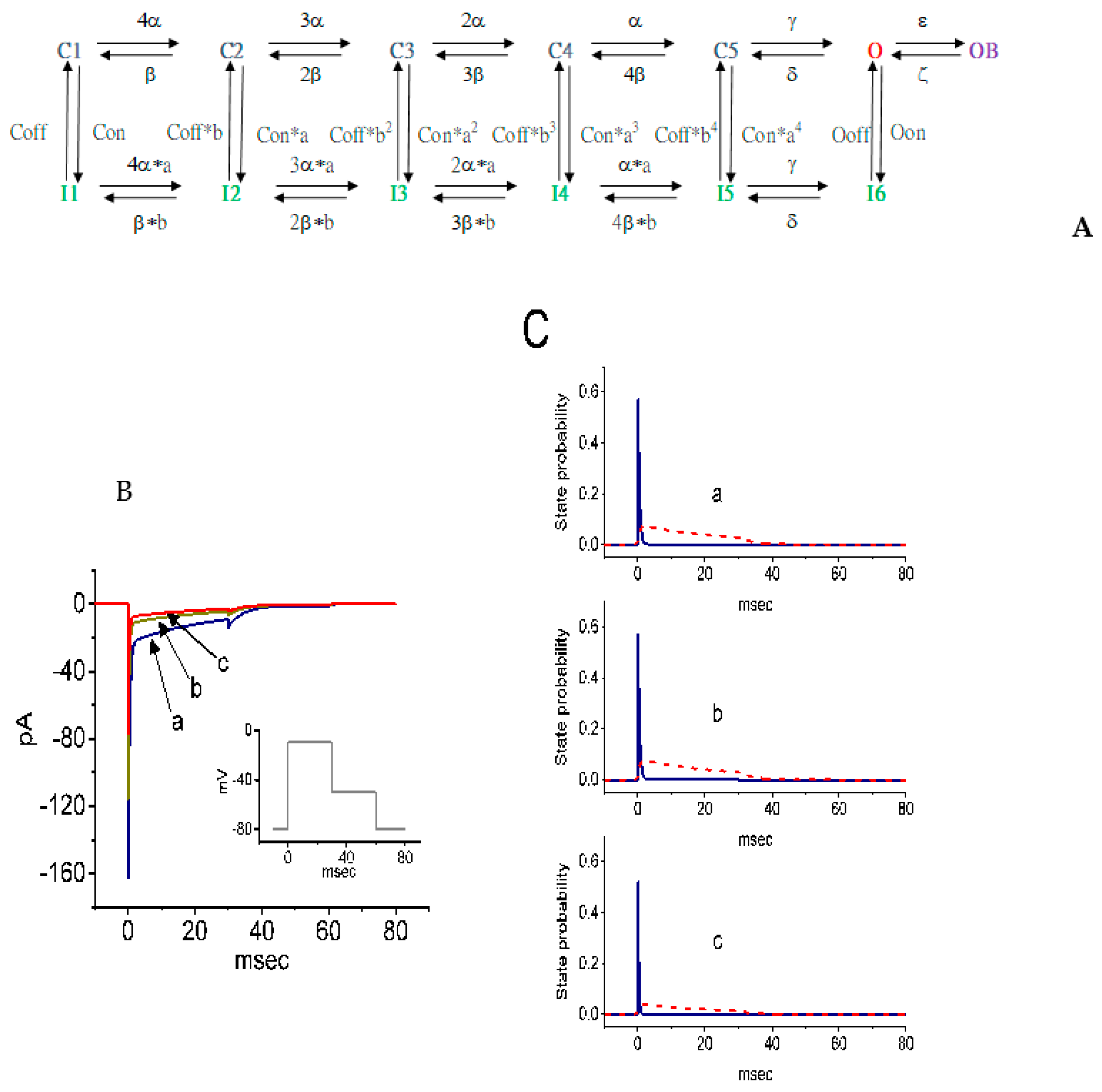
2.10. Simulations of SSM-Mediated Inhibition of INa Derived From a Markov State Model
3. Discussion
4. Materials and Methods
4.1. Chemicals and Solutions
4.2. Cell Culture
4.3. Electrophysiological Measurements
4.4. Data Recordings
4.5. Data Analyses
4.6. Statistical Analyses
4.7. Computer Simulations
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Jayaraj, P.; Narasimhulu, C.A.; Rajagopalan, S.; Parthasarathy, S.; Desikan, R. Sesamol: A powerful functional food ingredient from sesame oil for cardioprotection. Food Funct. 2020, 11, 1198–1210. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Kim, E.Y.; Rodriguez, I.; Nam, Y.H.; Jeong, S.Y.; Hong, B.N.; Choung, S.Y.; Kang, T.H. Sesamum indicum L. and sesamin induce auditory-protective effects through changes in hearing loss-related gene expression. J. Med. Food 2020, 23, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Jeng, K.C.G.; Hou, R.C.W. Sesamin and sesamolin: Nature’s therapeutic lignans. Curr. Enzym. Inhib. 2005, 1, 11–20. [Google Scholar] [CrossRef]
- Masuda, T.; Shingai, Y.; Fujimoto, A.; Nakamura, M.; Oyama, Y.; Maekawa, T.; Sone, Y. Identification of cytotoxic dimers in oxidation product from sesamol, a potent antioxidant of sesame oil. J. Agric. Food Chem. 2010, 58, 10880–10885. [Google Scholar] [CrossRef]
- Kuo, P.C.; Lin, M.C.; Chen, G.F.; Yiu, T.J.; Tzen, J.T.C. Identification of methanol-soluble compounds in sesame and evaluation of antioxidant potentials of its lignans. J. Agric. Food Chem. 2011, 59, 3214–3219. [Google Scholar] [CrossRef]
- Baluchnejadmojarad, T.; Mansouri, M.; Ghalami, J.; Mokhtari, Z.; Roghani, M. Sesamin imparts neuroprotection against intrastriatal 6-hydroxydopamine toxicity by inhibition of astroglial activation, apoptosis, and oxidative stress. Biomed. Pharmacother. 2017, 88, 754–761. [Google Scholar] [CrossRef]
- Kim, A.Y.; Yun, C.I.; Lee, J.G.; Kim, Y.J. Determination and daily intake estimation of lignans in sesame seeds and sesame oil products in Korea. Foods 2020, 9, 394. [Google Scholar] [CrossRef] [Green Version]
- Dhar, P.; Chattopadhya, K.; Bhattacharyya, D.; Biswas, A.; Roy, B.; Ghosh, S. Ameliorative influence of sesame lignans on lipid profile and lipid peroxidation in induced diabetic rats. J. Agric. Food Chem. 2007, 55, 5875–5880. [Google Scholar] [CrossRef]
- Liang, Y.T.; Chen, J.; Jiao, R.; Peng, C.; Zuo, Y.; Lei, L.; Liu, Y.; Wang, X.; Ma, K.Y.; Huang, Y.; et al. Cholesterol-lowering activity of sesamin is associated with down-regulation on genes of sterol transporters involved in cholesterol absorption. J. Agric. Food Chem. 2015, 63, 2963–2969. [Google Scholar] [CrossRef]
- Shimizu, S.; Akimoto, K.; Shinmen, Y.; Kawashima, H.; Sugano, M.; Yamada, H. Sesamin is a potent and specific inhibitor of Δ5 desaturase in polyunsaturated fatty acid biosynthesis. Lipids 1991, 26, 512–516. [Google Scholar] [CrossRef]
- Hou, R.C.W.; Huang, H.M.; Tzen, J.T.C.; Jeng, K.C.G. Protective effects of sesamin and sesamolin on hypoxic neuronal and PC12 cells. J. Neurosci. Res. 2003, 74, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Jamarkattel-Pandit, N.; Pandit, N.R.; Kim, M.Y.; Park, S.H.; Kim, K.S.; Choi, H.; Kim, H.; Bu, Y. Neuroprotective effect of defatted sesame seeds extract against in vitro and in vivo ischemic neuronal damage. Planta Med. 2010, 76, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Ruankham, W.; Suwanjang, W.; Wongchitrat, P.; Prachayasittikul, V.; Prachayasittikul, S.; Phopin, K. Sesamin and sesamol attenuate H2O2-induced oxidative stress on human neuronal cells via the SIRT1-SIRT3-FOXO3a signaling pathway. Nutr. Neurosci. in press. [CrossRef] [PubMed]
- Nakano, D.; Itoh, C.; Takaoka, M.; Kiso, Y.; Tanaka, T.; Matsumura, Y. Antihypertensive effect of sesamin. IV. Inhibition of vascular superoxide production by sesamin. Biol. Pharm. Bull. 2002, 25, 1247–1249. [Google Scholar] [CrossRef] [Green Version]
- Chiang, H.T.; Wu, S.N. On the mechanism of selective action of probucol on the inwardly rectifying potassium current in GH3 lactotrophs. Drug Dev. Res. 2001, 54, 1–11. [Google Scholar] [CrossRef]
- Chang, W.T.; Gao, Z.H.; Lo, Y.C.; Wu, S.N. Evidence for effective inhibitory actions on hyperpolarization-activated cation current caused by Ganoderma triterpenoids, the main active constituents of Ganoderma spores. Molecules 2019, 24, 4256. [Google Scholar] [CrossRef] [Green Version]
- Waxman, S.G. Channel, neuronal and clinical function in sodium channelopathies: From genotype to phenotype. Nat. Neurosci. 2007, 10, 405–409. [Google Scholar] [CrossRef]
- Lerche, H.; Shah, M.; Beck, H.; Noebels, J.; Johnston, D.; Vincent, A. Ion channels in genetic and acquired forms of epilepsy. J. Physiol. 2013, 591, 753–764. [Google Scholar] [CrossRef]
- Waszkielewicz, A.M.; Gunia, A.; Szkaradek, N.; Sloczyńska, K.; Krupińska, S.; Marona, H. Ion channels as drug targets in central nervous system disorders. Curr. Med. Chem. 2013, 20, 1241–1285. [Google Scholar] [CrossRef] [Green Version]
- Chang, T.T.; Yang, C.J.; Lee, Y.C.; Wu, S.N. Stimulatory action of telmisartan, an antagonist of angiotensin II receptor, on voltage-gated Na+ current: Experimental and theoretical studies. Chin. J. Physiol. 2018, 61, 1–13. [Google Scholar] [CrossRef]
- Pan, Y.; Cummins, T.R. Distinct functional alterations in SCN8A epilepsy mutant channels. J. Physiol. 2020, 598, 381–401. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A.; Goldin, A.L.; Waxman, S.G. International Union of Pharmacology, XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol. Res. 2005, 57, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.S.; Lo, Y.C.; Peng, H.; Hsu, T.I.; Wu, S.N. Effects of ranolazine, a novel anti-anginal drug, on ion currents and membrane potential in pituitary tumor GH3 cells and NG108-15 neuronal cells. J. Pharmacol. Sci. 2009, 110, 295–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.W.; Chow, J.C.; Tsai, J.J.; Wu, S.N. Characterizing the effects of eugenol on neuronal ionic currents and hyperexcitability. Psychopharmacol. Berl. 2012, 221, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.C.; Tzeng, R.C.; Huang, C.W.; Wu, S.N. The novel direct modulatory effects of perampanel, an antagonist of AMPA receptors, on voltage-gated sodium and M-type potassium currents. Biomolecules 2019, 9, 638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, T.H.; Jin, S.W.; Lee, G.H.; Park, J.S.; Kim, J.Y.; Thai, T.N.; Han, E.H.; Jeong, H.G. Sesamin induces endothelial nitric oxide synthase activation via transient receptor potential vanilloid type 1. J. Agric. Food Chem. 2020, 68, 3474–3484. [Google Scholar] [CrossRef]
- Lei, H.; Han, J.; Wang, Q.; Guo, S.; Sun, H.; Zhang, X. Effects of sesamin on streptozotocin (STZ)-induced NIT-1 pancreatic β-cell damage. Int. J. Mol. Sci. 2012, 13, 16961–16970. [Google Scholar] [CrossRef] [Green Version]
- Kong, X.; Wang, G.D.; Ma, M.Z.; Deng, R.Y.; Guo, L.Q.; Zhang, J.X.; Yang, J.R.; Su, Q. Sesamin ameliorates advanced glycation end products-induced pancreatic β-cell dysfunction and apoptosis. Nutrients 2015, 7, 4689–4704. [Google Scholar] [CrossRef] [Green Version]
- Zheng, S.; Zhao, M.; Ren, Y.; Wu, Y.; Yang, J. Sasamin suppresses STZ induced INS-1 cell apoptosis through inhibition of NF-κB activation and regulation of Bcl-2 family protein expression. Eur. J. Pharmacol. 2015, 750, 52–58. [Google Scholar] [CrossRef]
- So, E.C.; Wu, S.N.; Lo, Y.C.; Su, K. Differential regulation of tefluthrin and telmisartan on the gating charges of INa activation and inactivation as well as on resurgent and persistent INa in a pituitary cell line (GH3). Toxicol. Lett. 2018, 285, 104–112. [Google Scholar] [CrossRef]
- Wu, S.N.; Wu, Y.H.; Chen, B.S.; Lo, Y.C.; Liu, Y.C. Underlying mechanism of actions of tefluthrin, a pyrethroid insecticide, on voltage-gated ion currents and on action currents in pituitary tumor (GH3) cells and GnRH-secreting (GT1-7) neurons. Toxicology 2009, 258, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.T.; Wu, S.N. Activation of voltage-gated sodium current and inhibition of erg-mediated potassium current caused by telmisartan, an antagonist of angiotensin II type-1 receptor, in HL-1 atrial cardiomyocytes. Clin. Exp. Pharmacol. Physiol. 2018, 45, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.T.; Liu, P.Y.; Lee, K.; Feng, Y.H.; Wu, S.N. Differential inhibitory actions of multitargeted tyrosine kinase inhibitors on different ionic current types in cardiomyocytes. Int. J. Mol. Sci. 2020, 21, 1672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selyanko, A.A.; Hadley, J.K.; Wood, I.C.; Abogadie, F.C.; Delmas, P.; Buckley, N.J.; London, B.; Brown, D.A. Two types of K+ channel subunit, Erg1 and KCNQ2/3, contribute to the M-like current in a mammalian neuronal cell. J. Neurosci. 1999, 19, 7742–7756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.S.; Lai, M.C.; Liu, P.Y.; Lo, Y.C.; Huang, C.W.; Wu, S.N. Characterization of the inhibitory effect of gastrodigenin and gastodin on M-type K+ currents in pituitary cells and hippocampal neurons. Int. J. Mol. Sci. 2019, 21, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillet, C.; Kurth, S.; Kuenzel, T. Muscarinic modulation of M and h currents in gerbil spherical bushy cells. PLoS ONE 2020, 15, e0226954. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.T.; Lo, Y.C.; Wu, S.N. Characterization of convergent suppression by UCL-2077 (3-triphenylmethylaminomethyl)pyridine, known to inhibit slow afterhyperpolarization, of erg-mediated potassium currents and intermediate-conductance calcium-activated potassium channels. Int. J. Mol. Sci. 2020, 21, 1441. [Google Scholar] [CrossRef] [Green Version]
- Kuo, P.C.; Liu, Y.C.; Lo, Y.C.; Wu, S.N. Characterization of inhibitory effectiveness in hyperpolarization-activated cation currents by a group of ent-kaurane-type diterpenoids from Croton tonkinensis. Int. J. Mol. Sci. 2020, 21, 1268. [Google Scholar] [CrossRef] [Green Version]
- Lu, T.L.; Lu, T.J.; Wu, S.N. Inhibitory effective perturbations of cilobradine (DK-AH269), a blocker of HCN channels, on the amplitude and gating of both hyperpolarization-activated cation and delayed-rectifier potassium currents. Int. J. Mol. Sci. 2020, 21, 2416. [Google Scholar] [CrossRef] [Green Version]
- Vega, A.V.; Espinosa, J.L.; López-Domínguez, A.M.; López-Santiago, L.F.; Navarrete, A.; Cota, G. L-type calcium channel activation up-regulates the mRNAs for two different sodium channel α subunits (Nav1.2 and Nav1.3) in rat pituitary GH3 cells. Mol. Brain Res. 2003, 116, 115–125. [Google Scholar] [CrossRef]
- Stojilkovic, S.S.; Tabak, J.; Bertram, R. Ion channels and signaling in the pituitary gland. Endoc. Rev. 2010, 31, 845–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.; Liu, Y.; Yang, D.; Yuan, F.; Ding, J.; Chen, H.; Tian, H. Sesamin protects SH-SY5Y cells against mechanical stretch injury and promoting cell survival. BMC Neurosci. 2017, 18, 57. [Google Scholar] [CrossRef] [Green Version]
- Hussien, H.M.; Abdou, H.M.; Yousef, M. Cypermethrin induced damage in genomic DNA and histopathological changes in brain and haematotoxicity in rats: The protective effect of sesame oil. Brain Res. Bullet. 2013, 92, 76–83. [Google Scholar] [CrossRef]
- Abdou, H.M.; Hussien, H.M.; Yousef, M.I. Deleterious effects of cypermethrin on rat liver and kidney: Protective role of sesame oil. J. Environ. Sci. Health 2012, 47, 306–314. [Google Scholar] [CrossRef]
- Soliman, M.M.; Attia, H.F.; El-Ella, G.A. Genetic and histopathological alterations induced by cypermethrin in rat kidney and liver: Protection by sesame oil. Int. J. Immunopathol. Pharmacol. 2015, 28, 508–520. [Google Scholar] [CrossRef]
- Ahmad, S.; Elsherbiny, N.M.; Haque, R.; Khan, M.B.; Ishrat, T.; Shah, Z.A.; Khan, M.M.; Ali, M.; Jamal, A.; Katare, D.P.; et al. Sesamin attenuates neurotoxicity in mouse model of ischemic brain stroke. Neurotoxicity 2014, 45, 100–110. [Google Scholar] [CrossRef]
- Park, H.J.; Zhao, T.T.; Lee, K.S.; Lee, S.H.; Shin, K.S.; Park, K.H.; Choi, H.S.; Lee, M.K. Effect of (-)-sesamin on 6-hydroxydopamine-induced neurotoxicity in PC12 cells and dopaminergic neuronal cells of Parkinson’s disease rat models. Neurochem. Int. 2015, 83–84, 19–27. [Google Scholar] [CrossRef]
- Guo, H.L.; Xiao, Y.; Tian, Z.; Li, X.B.; Wang, D.S.; Wang, X.S.; Zhang, Z.W.; Zhao, M.G.; Liu, S.B. Anxiolytic effects of sesamin in mice with chronic inflammatory pain. Nutr. Neurosci. 2016, 19, 231–236. [Google Scholar] [CrossRef]
- Zhao, T.T.; Shin, K.S.; Kim, K.S.; Park, H.J.; Kim, H.J.; Lee, K.E.; Lee, M.K. Effects of (−)-sesamin on motor and memory deficits in an MPTP-lesioned mouse model of Parkinson’s disease treated with l-DOPA. Neuroscience 2016, 339, 644–654. [Google Scholar] [CrossRef]
- Zhao, T.T.; Shin, K.S.; Park, H.J.; Yi, B.R.; Lee, K.E.; Lee, M.K. Effects of (−)-sesamin on chronic stress-induced anxiety disorders in mice. Neurochem. Res. 2017, 42, 1123–1129. [Google Scholar] [CrossRef]
- Liu, Y.L.; Xu, Z.M.; Yang, G.Y.; Yang, D.X.; Ding, J.; Chen, H.; Yuan, F.; Tian, H.L. Sesamin alleviates blood-brain barrier disruption in mice with experimental traumatic brain injury. Acta Pharmacol. Sin. 2017, 38, 1445–1455. [Google Scholar] [CrossRef]
- Farbood, Y.; Ghaderi, S.; Rashno, M.; Khoshnam, S.E.; Khorsandi, L.; Sarkaki, A.; Rashno, M. Sesamin: A promising protective agent against diabetes-associated cognitive decline in rats. Life Sci. 2019, 230, 169–177. [Google Scholar] [CrossRef]
- Shimoyoshi, S.; Takemoto, D.; Ono, Y.; Kitagawa, Y.; Shibata, H.; Tomono, S.; Unno, K.; Wakabayashi, K. Sesame lignans suppress age-related cognitive decline in senescence-accelerated mice. Nutrients 2019, 11, 1582. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, S.M.; Chaurasiya, N.D.; Mohamed, N.M.; Bayoumi, S.A.L.; Tekwani, B.L.; Ross, S.A. Promising selective MAO-B inhibition by sesamin, a lignan from Zanthoxylum flavum stems. Saudi Pharm. J. 2020, 28, 409–413. [Google Scholar] [CrossRef]
- Babaei, P.; Dastras, A.; Tehrani, B.S.; Pourali Roudbaneh, S. The effect of estrogen replacement therapy on visceral fat, serum glucose, lipid profiles and apelin level in ovariectomized rats. J. Menopausal. Med. 2017, 23, 182–189. [Google Scholar] [CrossRef] [Green Version]
- Hoseini, R.; Damirchi, A.; Babaei, P. Vitamin D increases PPARγ expression and promotes beneficial effects of physical activity in metabolic syndrome. Nutrition 2017, 36, 54–59. [Google Scholar] [CrossRef]
- Sayyahi, A.; Jahanshahi, M.; Amini, H.; Sepehri, H. Vitamin E can compensate the density of M1 receptors in the hippocampus of scopolamine-treated rats. Folia Neuropathol. 2018, 56, 215–228. [Google Scholar] [CrossRef]
- McBride, D.W., Jr.; Hamill, O.P. Simplified fast pressure-clamp technique for studying mechanically gated channels. Methods Enzymol. 1999, 294, 482–489. [Google Scholar]
- Raman, I.M.; Bean, B.P. Inactivation and recovery of sodium currents in cerebellar Purkinje neurons: Evidence for two mechanisms. Biophys. J. 2001, 80, 729–737. [Google Scholar] [CrossRef] [Green Version]
- Khaliq, Z.M.; Gouwens, N.W.; Raman, I.M. The contribution of resurgent sodium current to high-frequency firing in Purkinje neurons: An experimental and modeling study. J. Neurosci. 2003, 23, 4899–4912. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.N. Simulations of the cardiac action potential based on the Hodgkin-Huxley kinetics with the use of Microsoft Excel spreadsheet. Chin. J. Physiol. 2004, 47, 15–22. [Google Scholar] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, P.-C.; Kao, Z.-H.; Lee, S.-W.; Wu, S.-N. Effects of Sesamin, the Major Furofuran Lignan of Sesame Oil, on the Amplitude and Gating of Voltage-Gated Na+ and K+ Currents. Molecules 2020, 25, 3062. https://doi.org/10.3390/molecules25133062
Kuo P-C, Kao Z-H, Lee S-W, Wu S-N. Effects of Sesamin, the Major Furofuran Lignan of Sesame Oil, on the Amplitude and Gating of Voltage-Gated Na+ and K+ Currents. Molecules. 2020; 25(13):3062. https://doi.org/10.3390/molecules25133062
Chicago/Turabian StyleKuo, Ping-Chung, Zi-Han Kao, Shih-Wei Lee, and Sheng-Nan Wu. 2020. "Effects of Sesamin, the Major Furofuran Lignan of Sesame Oil, on the Amplitude and Gating of Voltage-Gated Na+ and K+ Currents" Molecules 25, no. 13: 3062. https://doi.org/10.3390/molecules25133062
APA StyleKuo, P.-C., Kao, Z.-H., Lee, S.-W., & Wu, S.-N. (2020). Effects of Sesamin, the Major Furofuran Lignan of Sesame Oil, on the Amplitude and Gating of Voltage-Gated Na+ and K+ Currents. Molecules, 25(13), 3062. https://doi.org/10.3390/molecules25133062





