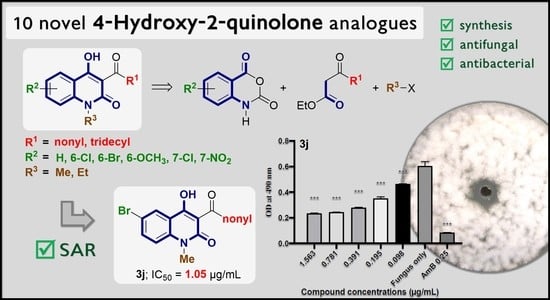
Synthesis and Antimicrobial Activity of Novel 4-Hydroxy-2-quinolone Analogs
Abstract
1. Introduction
2. Results and Discussions
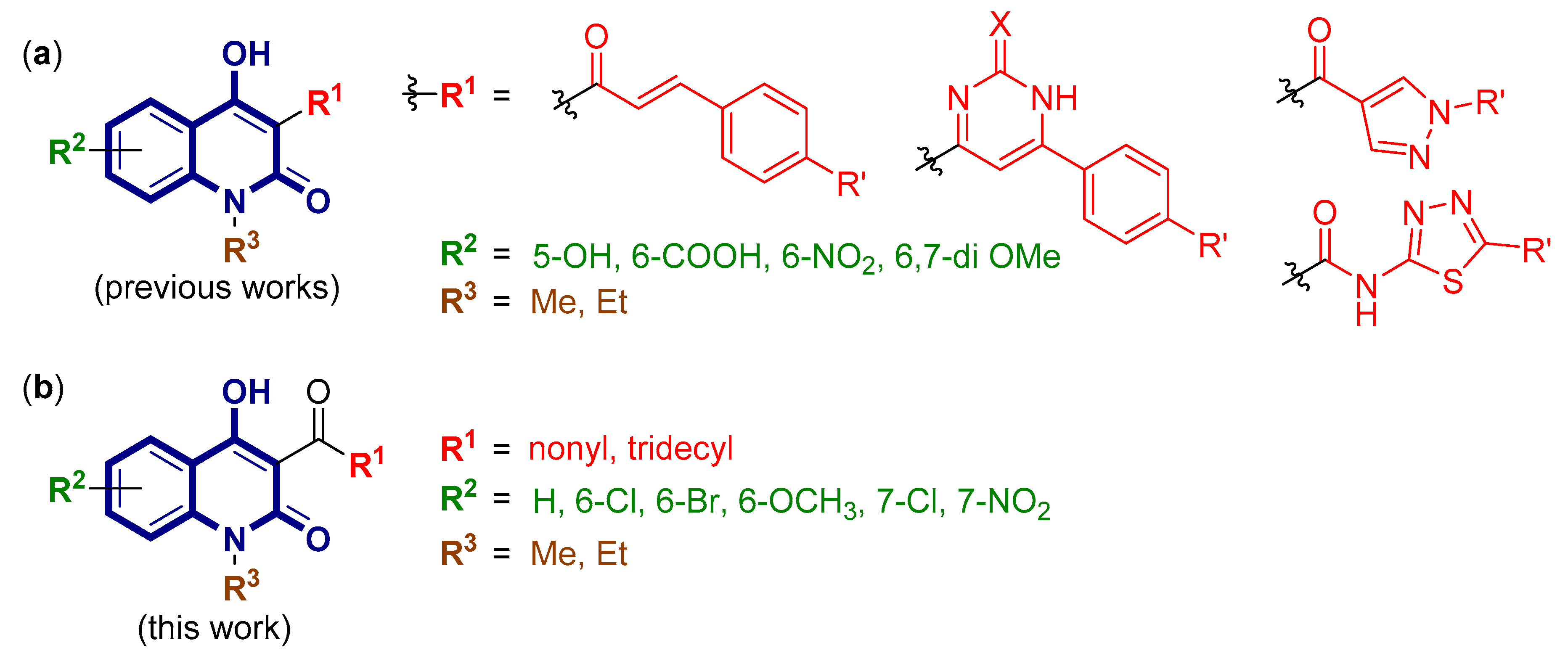
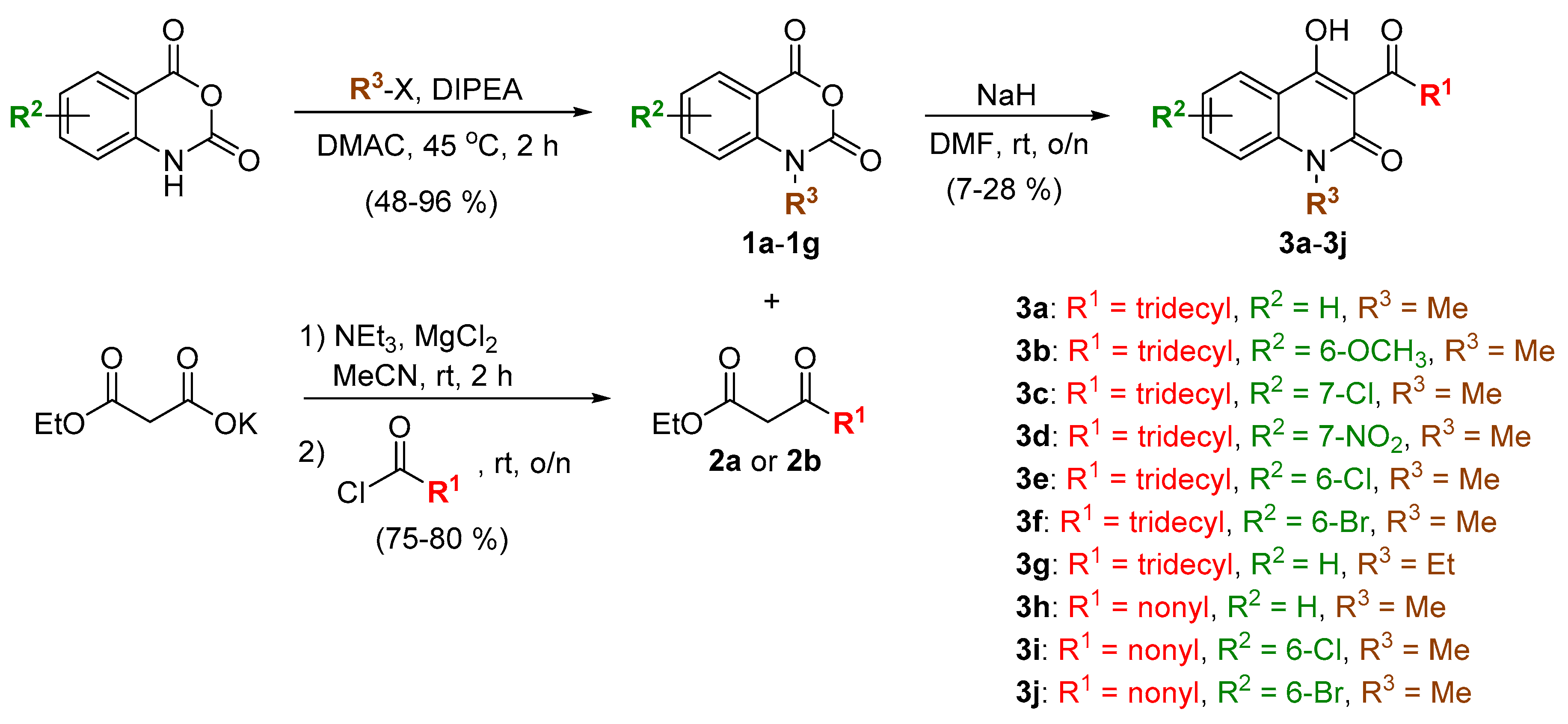
2.1. Chemical Synthesis
2.2. Antimicrobial Activity and Structure–Activity Relationship
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Synthesis of Compounds 1a–1g
3.3. Synthesis of Compounds 2a and 2b
3.4. Synthesis of Compounds 3a–3j, 4d, and 5d
3.5. Evaluation of Antibacterial Activity
3.6. Evaluation of Antifungal Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic Resistance: A Rundown of a Global Crisis. Infect. Drug. Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef] [PubMed]
- Aldo, T.; Rino, R. Changing Priorities in Vaccinology: Antibiotic Resistance’. Front. Immunol. 2018, 9, 1068. [Google Scholar]
- Pekarski, O.; Björk, J.; Hedlund, G.; Andersson, G. The Inhibitory Effect in Experimental Autoimmune Encephalomyelitis by the Immunomodulatory Drug Linomide (PNU-212616) Is Not Mediated via Release of Endogenous Glucocorticoids. Autoimmunity 1998, 28, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Katagi, M.; Bolakatti, G.; Badiger, A.; Satyanarayana, D.; Mamledesai, S.; Sujatha, M. Synthesis, Spectral Characterization and Antimicrobial Activity of Substituted Thiazolyl Derivatives of 2-Quinolones. Drug Res. 2013, 63, 53–59. [Google Scholar] [CrossRef]
- Bolakatti, G.; Katagi, M.S.; Sn, M.; Ml, S.; Dabadi, P.; Miskin, N. Synthesis and Antimicrobial Activity of 4-Hydroxy-1-Methyl/Phenyl-3-(Substituted Anilinoacetyl) Quinolin-2(1H)-One. Rguhs J. Pharm. Sci. 2012, 2, 60–66. [Google Scholar] [CrossRef]
- Hassanin, H.M.; El-Edfawy, S.M. Novel Heterocyclic Derivatives of 2-Quinolinone Associated with Antibacterial and Antitumor Potencies. Heterocycles 2012, 85, 2421. [Google Scholar] [CrossRef]
- Hassan, M.M.; Hassanin, H.M. An Efficient New Route for the Synthesis of Some 3-Hterocyclylquinolinones via Novel 3-(1,2-Dihydro-4-hydroxy-1-methyl-2-oxoquinolin-3-yl)-3-oxopropanal and Their Antioxidant Screening. J. Heterocycl. Chem. 2017, 54, 3321–3330. [Google Scholar] [CrossRef]
- Muhammad, A.; Munawar, A.M.; Makshoof, A. Synthetic and Antibact. Studies of Quinolinylchalcones. J. Appl. Chem. 2007, 7, 1620–1625. [Google Scholar]
- Munawar, A.M.; Muhammad, A.; Hamid, L.S.; Faiz-ul, H.N. Synthesis and Antimicrobial Studies of Some Quinolinylpyrimidine Derivatives. J. Chin. Chem. Soc. 2008, 55, 394–400. [Google Scholar] [CrossRef]
- Magdy, A.I.; Hany, M.H.; Youssef, A.A. Synthesis, Characterization, and Antimicrobial Evaluation of Some Novel 4-Hydroxyquinolin-2(1H)-ones. Synth. Commun. 2014, 44, 3470–3482. [Google Scholar]
- Wenjie, X.; Xueyao, L.; Guixing, M.; Hongmin, Z.; Ya, C.; Johannes, K.; Jie, X.; Song, W. N-thiadiazole-4-hydroxy-2-quinolone-3-carboxamides bearing heteroaromatic rings as novel antibacterial agents: Design, synthesis, biological evaluation and target identification. Eur. J. Med. Chem. 2020, 188, 112022. [Google Scholar]
- Ukita, T.; Mizuno, D.I.; Tamura, T.; Yamakawa, T.; Nojima, S. The Antibacterial Properties of ompounds Containing the Tricarbonylmethane Group. II-III. Yakugaku Zasshi 1951, 71, 234–243. [Google Scholar] [CrossRef][Green Version]
- Rho, T.C.; Bae, E.-A.; Kim, D.-H.; Oh, W.K.; Kim, B.Y.; Ahn, J.S.; Lee, H.S. Anti-Helicobacter Pylori Activity of Quinolone Alkaloids from Evodiae Fructus. Biol. Pharm. Bull. 1999, 22, 1141–1143. [Google Scholar] [CrossRef]
- Hamasaki, N.; Ishii, E.; Tominaga, K.; Tezuka, Y.; Nagaoka, T.; Kadota, S.; Kuroki, T.; Yano, I. Highly Selective Antibacterial Activity of Novel Alkyl Quinolone Alkaloids from a Chinese Herbal Medicine, Gosyuyu (Wu-Chu-Yu), Against Helicobacter Pylori In Vitro. Microbiol. Immunol. 2000, 44, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Guzman, J.D.; Wube, A.; Evangelopoulos, D.; Gupta, A.; Hufner, A.; Basavannacharya, C.; Rahman, M.; Thomaschitz, C.; Bauer, R.; McHugh, T.D.; et al. nteraction of N-methyl-2-alkenyl-4-quinolones with ATP-dependent MurE ligase of Mycobacterium tuberculosis: Antibacterial activity, molecular docking and inhibition kinetics. J. Antimicrob. Chemother. 2011, 66, 1766–1772. [Google Scholar] [CrossRef]
- Abdel-Ghani, A.A.; Amaal, A.A. Synthesis and study of antimicrobial agents of newly 6-nitro-4-hydroxy-2- quinolone derivatives. Arch. Appl. Sci. Res. 2012, 4, 1339–1344. [Google Scholar]
- Ukrainets, I.V.; Bevz, O.V.; Mospanova, E.V.; Savchenkova, L.V.; Yankovich, S.I. 4-Hydroxy-2-Quinolones. 202*. Synthesis, Chemical and Biological Properties of 4-Hydroxy-6,7-Dimethoxy-2-Oxo-1,2-Dihydroquinoline-3-Carboxylic Acid Alkylamides. Chem. Heterocycl. Compd. 2012, 48, 320–326. [Google Scholar] [CrossRef]
- Jampilek, J.; Musiol, R.; Pesko, M.; Kralova, K.; Vejsova, M.; Carroll, J.; Coffey, A.; Finster, J.; Tabak, D.; Niedbala, H.; et al. Ring-Substituted 4-Hydroxy-1H-Quinolin-2-Ones: Preparation and Biological Activity. Molecules 2009, 14, 1145–1159. [Google Scholar] [CrossRef]
- Appelbaum, P.; Hunter, P. The Fluoroquinolone Antibacterials: Past, Present and Future Perspectives. Int. J. Antimicrob. Agents 2000, 16, 5–15. [Google Scholar] [CrossRef]
- Al-Hiari, Y.; Al-Mazari, I.; Shakya, A.; Darwish, R.; Abu-Dahab, R. Synthesis and Antibacterial Properties of New 8-Nitrofluoroquinolone Derivatives. Molecules 2007, 12, 1240–1258. [Google Scholar] [CrossRef]
- Jönsson, S.; Andersson, G.; Fex, T.; Fristedt, T.; Hedlund, G.; Jansson, K.; Abramo, L.; Fritzson, I.; Pekarski, O.; Runström, A.; et al. Synthesis and Biological Evaluation of New 1,2-Dihydro-4-Hydroxy-2-Oxo-3-Quinolinecarboxamides for Treatment of Autoimmune Disorders: Structure−Activity Relationship. J. Med. Chem. 2004, 47, 2075–2088. [Google Scholar] [CrossRef] [PubMed]
- Tomassoli, I.; Herlem, G.; Picaud, F.; Benchekroun, M.; Bautista-Aguilera, O.M.; Luzet, V.; Jimeno, M.-L.; Gharbi, T.; Refouvelet, B.; Ismaili, L. Synthesis, Regioselectivity, and DFT Analysis of New Antioxidant Pyrazolo[4,3-c]Quinoline-3,4-Diones. Monatsh. Chem. 2016, 147, 1069–1079. [Google Scholar] [CrossRef][Green Version]
- Greef, T.F.A.D.; Nieuwenhuizen, M.M.L.; Stals, P.J.M.; Fitié, C.F.C.; Palmans, A.R.A.; Sijbesma, R.P.; Meijer, E.W. The Influence of Ethylene Glycol Chains on the Thermodynamics of Hydrogen-Bonded Supramolecular Assemblies in Apolar Solvents. Chem. Comm. 2008, 36, 4306. [Google Scholar] [CrossRef] [PubMed]
- Banu, S.; Bollu, R.; Naseema, M.; Gomedhika, P.M.; Nagarapu, L.; Sirisha, K.; Kumar, C.G.; Gundasw, S.K. A Novel Templates of Piperazinyl-1,2-Dihydroquinoline-3-Carboxylates: Synthesis, Anti-Microbial Evaluation and Molecular Docking Studies. Bioorg. Med. Chem. Lett. 2018, 28, 1166–1170. [Google Scholar] [CrossRef]
- Coppola, G.M.; Hardtmann, G.E. The Chemistry of 2H-3,1-Benzoxazine-2,4(1H)Dione (Isatoic Anhydride). 7. Reactions with Anions of Active Methylenes to Form Quinolines. J. Heterocycl. Chem. 1979, 16, 1605–1610. [Google Scholar] [CrossRef]
- Basak, A.; Abouelhassan, Y.; Huigens, R.W. Halogenated quinolines discovered through reductive amination with potent eradication activities against MRSA, MRSE and VRE biofilms. Org. Biomol. Chem. 2015, 13, 10290–10294. [Google Scholar] [CrossRef]
- Luan, G.; Hong, Y.; Drlica, K.; Zhao, X. Suppression of reactive oxygen species accumulation accounts for paradoxical bacterial survival at high quinolone concentration. Antimicrob. Agents Chemother. 2018, 62, e01622-17. [Google Scholar] [CrossRef]
- Hong, Y.; Li, Q.; Gao, Q.; Xie, J.; Huang, H.; Drlica, K.; Zhao, X. Reactive oxygen species play a dominant role in all pathways of rapid quinolone-mediated killing. J Antimicrob Chemother. 2020, 75, 576–585. [Google Scholar] [CrossRef]
- Spiccia, N.; Basutto, J.; Jokisz, P.; Wong, L.S.-M.; Meyer, A.G.; Holmes, A.B.; White, J.M.; Ryan, J.H. 1,3-Dipolar Cycloaddition-Decarboxylation Reactions of an Azomethine Ylide with Isatoic Anhydrides: Formation of Novel Benzodiazepinones. Org. Lett. 2011, 13, 486–489. [Google Scholar] [CrossRef]
- Beutner, G.L.; Kuethe, J.T.; Yasuda, N. A Practical Method for Preparation of 4-Hydroxyquinolinone Esters. J. Org. Chem. 2007, 72, 7058–7061. [Google Scholar] [CrossRef]
- Stelzer, A.C.; Frazee, R.W.; Huis, C.V.; Cleary, J.; Opipari, A.W.; Glick, G.D.; Al-Hashimi, H.M. NMR Studies of an Immunomodulatory Benzodiazepine Binding to Its Molecular Target on the Mitochondrial F1F0-ATPase. Biopolymers 2010, 93, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Goetz, E.H.; Gabor, K.; Oskar, R.P. The chemistry of 2H-3,1-benzoxazine-2,4(1H)dione (isatoic anhydrides) 1. The synthesis of N-substituted 2H-3,1-benzoxazine-2,4(1H)diones. J. Heterocycl. Chem. 1975, 12, 565–572. [Google Scholar]
- Canonne, P.; Boulanger, R.; Chantegrel, B. Reactions of α,ω-Bis(Bromomagnesio)Alkanes with Heterocyclic Anhydrides. Tetrahedron 1987, 43, 663–668. [Google Scholar] [CrossRef]
- Matsuo, K.; Kimura, M.; Kinuta, T.; Takai, N.; Tanaka, K. Syntheses and Antimicrobial Activities of 3-Acyltetramic Acid Derivatives. Chem. Pharm. Bull. 1984, 32, 4197–4204. [Google Scholar] [CrossRef]
- Zou, X.; Jia, X.; Wang, X.; Xie, G. Substitution of Acyl for Acetyl with N-Acylbenzotriazoles Catalyzed by Samarium Triiodide. Synth. Commun. 2007, 37, 1617–1625. [Google Scholar] [CrossRef]
- Liang, Z.; Hua, F.; Renzhong, F.; Yuyang, J.; Yefun, Z. Efficient Copper-Catalyzed Synthesis of N-Alkylanthranilic Acids via an ortho-Substituent Effect of the Carboxyl Group of 2-Halobenzoic Acids at Room Temperature. Adv. Synth. Catal. 2009, 351, 1671–1676. [Google Scholar]
- Cavalieri, S.J.; Harbeck, R.J.; McCarter, Y.S.; Ortez, J.H.; Rankin, I.D.; Sautter, R.L.; Sharp, S.E.; Spiegel, C.A. Manual of Antimicrobial Susceptibility Testing; American Society of Microbiology: Washington, DC, USA, 2005. [Google Scholar]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi, 3rd ed.; Approved Standard; CLSI document M38-A3: Pennsylvania, PA, USA, 2017. [Google Scholar]
- Van de Sande, W.W.; Tavakol, M.; van Vianen, W.; Bakker-Woudenberg, I.A. The effects of antifungal agents to conidial and hyphal forms of Aspergillus fumigatus. Med. Mycol. 2010, 48, 48–55. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 3a–3j and 4d are available from the authors. |

| Compound | Substituents | IC50 1 (µg/mL) | MIC 2 (µg/mL) | |||
|---|---|---|---|---|---|---|
| R 1 | R 2 | R 3 | A. flavus | S. aureus | E. coli | |
| 3a | tridecyl | H | Me | 70.97 ± 3.71 | >1000 | >1000 |
| 3b | tridecyl | 6-OCH3 | Me | 42.15 ± 18.61 | >1000 | >1000 |
| 3c | tridecyl | 7-Cl | Me | 14.72 ± 2.35 | >1000 | >1000 |
| 3d | tridecyl | 7-NO2 | Me | 38.45 ± 5.51 | >1000 | >1000 |
| 3e | tridecyl | 6-Cl | Me | 37.08 ± 1.52 | >1000 | >1000 |
| 3f | tridecyl | 6-Br | Me | 23.19 ± 3.19 | >1000 | >1000 |
| 3g | tridecyl | H | Et | 9.48 ± 6.17 | >1000 | >1000 |
| 3h | nonyl | H | Me | 8.45 ± 1.59 | >1000 | >1000 |
| 3i | nonyl | 6-Cl | Me | 4.60 ± 1.14 | * | >1000 |
| 3j | nonyl | 6-Br | Me | 1.05 ± 1.31 | ** | >1000 |
| 4d3 | tridecyl 3 | 7-NO2 3 | Me 3 | 23.44 ± 1.63 | >1000 | >1000 |
| AMB 4 | – | – | – | 1.93 ± 1.04 | ND | ND |
| CIP 5 | – | – | – | ND | 0.835 | 0.0288 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khamkhenshorngphanuch, T.; Kulkraisri, K.; Janjamratsaeng, A.; Plabutong, N.; Thammahong, A.; Manadee, K.; Na Pombejra, S.; Khotavivattana, T. Synthesis and Antimicrobial Activity of Novel 4-Hydroxy-2-quinolone Analogs. Molecules 2020, 25, 3059. https://doi.org/10.3390/molecules25133059
Khamkhenshorngphanuch T, Kulkraisri K, Janjamratsaeng A, Plabutong N, Thammahong A, Manadee K, Na Pombejra S, Khotavivattana T. Synthesis and Antimicrobial Activity of Novel 4-Hydroxy-2-quinolone Analogs. Molecules. 2020; 25(13):3059. https://doi.org/10.3390/molecules25133059
Chicago/Turabian StyleKhamkhenshorngphanuch, Thitiphong, Kittipat Kulkraisri, Alongkorn Janjamratsaeng, Napasawan Plabutong, Arsa Thammahong, Kanitta Manadee, Sarisa Na Pombejra, and Tanatorn Khotavivattana. 2020. "Synthesis and Antimicrobial Activity of Novel 4-Hydroxy-2-quinolone Analogs" Molecules 25, no. 13: 3059. https://doi.org/10.3390/molecules25133059
APA StyleKhamkhenshorngphanuch, T., Kulkraisri, K., Janjamratsaeng, A., Plabutong, N., Thammahong, A., Manadee, K., Na Pombejra, S., & Khotavivattana, T. (2020). Synthesis and Antimicrobial Activity of Novel 4-Hydroxy-2-quinolone Analogs. Molecules, 25(13), 3059. https://doi.org/10.3390/molecules25133059