Abstract
In this study, sulfur-free expanded graphite (EG) was obtained by using flake graphite as the raw material, and EG/Fe3O4 composites with excellent microwave absorption properties were prepared by a facile one-pot co-precipitation method. The structure and properties of as-prepared EG/Fe3O4 were investigated by scanning electron microscopy (SEM), transmission electron microscopy (TEM), Fourier transform infrared (FT-IR), X-ray diffraction (XRD), Raman, X-ray photoelectron spectrometry (XPS), thermogravimetric (TG), and vibrating sample magnetometry (VSM) characterizations. The Fe3O4 intercalated between the layers of expanded graphite forms a sandwich-like structure which is superparamagnetic and porous. When applied as a microwave absorber, the reflection loss (RL) of EG/Fe3O4 reaches −40.39 dB with a thickness of 3.0 mm (10 wt% loading), and the effective absorption bandwidth (EAB < −10 dB) with RL exceeding −10 dB is 4.76–17.66 GHz with the absorber thickness of 1.5–4.0 mm. Considering its non-toxicity, easy operation, low cost, suitability for large-scale industrial production, and excellent microwave absorbing performance, EG/Fe3O4 is expected to be a promising candidate for industrialized electromagnetic absorbing materials.
1. Introduction
Electromagnetic wave absorbing (EMWA) materials with strong absorption, wide absorption bandwidth, thin thickness, light weight, and high thermal stability are widely used in military and civilian fields [1,2,3,4,5,6,7,8,9,10]. During the past decades, expanded graphite (EG) was widely researched in the area of EMWA considering its wider source, lower cost, larger surface area, high surface activity, rich pore structure, etc., which can be applied in lithium ion batteries [11], biomedicine [12], or electronic heat dissipation [13]. EG also has a wide range of applications in the field of electromagnetic shielding [1,14,15].
However, the preparation of EG by chemical oxidation is mainly carried out by using concentrated sulfuric acid; as a result, the residual sulfur is easy to cause corrosion of metals in applications, especially in high ambient temperature environments [15,16,17,18]. Therefore, the preparation of sulfur-free EG is extremely important for production applications. On the other hand, Fe3O4 has the advantages of superparamagnetism, high saturation magnetic strength, excellent biocompatibility, and excellent magnetic response [19,20]. It has a wide range of applications in new sensing materials [21], medicine [22], and catalysis [23]. At the same time, Fe3O4 is also a vital magnetic medium that can be used in electromagnetic absorbing materials [24,25,26,27,28,29,30,31,32,33,34,35].
Recently, numerous studies reported that graphene/Fe3O4 composites were successfully fabricated with excellent absorbing properties. Zeng et al. [26] synthesized Air@rGO (reduced graphene oxide)/Fe3O4 by a water-in-oil (W/O) emulsion technique followed by a calcination process. The minimum reflection loss (RL) value reaches −52 dB at 10 GHz with a thickness of 2.8 mm. Wang et al. [30] reported a simple hydrothermal method to synthesize Fe3O4@ZnO/rGO composites with a wide effective absorption bandwidth up to 12 GHz with reflection loss RL < −10 dB and a minimal RL (−34 dB) at 6.7 GHz. Huang et al. [32] Through ultrasonic and thermal reduction processed octahedral Fe3O4/rGO composites, the bandwidth of RL exceeded −20 dB (99% absorption) over 5 GHz (12–16 and 17–18 GHz). Wu et al. [33], through a one-pot solvothermal method, synthesized Fe3O4/rGO, obtaining a minimum RL of −22.7 dB at 3.13 GHz. Although graphene/Fe3O4 composites have been successfully fabricated, the traditional method requires at least two or three oxidation processes to synthesize graphene and the use of large quantity organic solvents to obtain the target composite. In addition, there are many other disadvantages, such as poor growth control of Fe3O4 and low yield of composite materials. Obviously, making a high-efficiency microwave-absorbing carbonaceous material in a one-step, non-toxic, cheap, and simple preparation process remains a huge challenge.
Herein, we report the preparation of expanded graphite by the method of mixing acid with nitric acid and phosphoric acid. The expansion rate was up to 300 mL/g which can be proved from Figure S1 in the Supporting Information. The sulfur-free EG intercalation Fe3O4 was synthesized through a facile one-pot co-precipitation method, which was free of additional processing, non-toxic, easy to operate, low in cost, and easy to scale. In addition, the particle size of Fe3O4 prepared by the mechanical stirring method was more uniform, and the particle size distribution was concentrated in the range of 10–20 nm; hence, the growth control of Fe3O4 was good, as shown in Figure S2 in the Supporting Information. The morphology, crystal structure and defects, thermal properties, and absorbing properties of the samples were investigated in detail, and the synthesized material showed effective EMWA absorption.
2. Experimental Section
2.1. Preparation of Sulfur-Free EG
EG was synthesized by chemical oxidation of natural flake graphite. In a typical procedure, 2.0 g graphite was continuously stirred with a combination of 5 mL nitric acid (68% concentration) and 15 mL phosphoric acid (85% concentration) solution under 45 °C. After this, 0.4 g potassium permanganate (KMnO4) was added into the solution when the temperature was raised to 45 °C, and the products was obtained by suction filtration after an 80 min intercalation process. The product was washed with deionized water until the pH was 7.0 and then dried at 60 °C. Finally, sulfur-free EG was obtained by sparking the production in a muffle furnace under 900 °C for 1 min.
2.2. Preparation of Sulfur-Free EG/Fe3O4 Composites
In order to prevent the oxidation of Fe2+ during the reaction, the molar ratio of Fe3+ to Fe2+ was set to 1:1. EG (100 mg) was weighed into a 100 mL beaker containing 35 mL of water and 10 mL of ethanol. Then, a certain amount of 2.5 mmol of Fe2+ and Fe3+ was directly added to the mixed solution, followed by ultrasonic dispersion for 30 min. The solution was then transferred into a 250 mL three-necked flask and another 30 min of mechanical stirring was done. When the temperature was raised to 80 °C, ammonia water was added dropwise until the solution pH was 11. Subsequently, the black products were washed several times with deionized water and ethanol and dried at 80 °C under vacuum and denoted as S1. For comparison, different molar masses of Fe2+ and Fe3+ (1.00 mmol, 0.75 mmol, 0.50 mmol, 0.25 mmol) being added to the reaction system and the corresponding products were labeled as S2, S3, S4, and S5, respectively. The yield of the composites was roughly obtained by the mass ratio of the actual composite to the theoretical one. Through calculation, we found that the yields were all about 90% according to the thermogravimetric (TG) test.
2.3. Characterization
The morphologies of samples S1–S5 were characterized by scanning electron microscopy (SEM; Hitachi S-4800, 10 kV) and transmission electron microscopy (TEM; FEIF20, 200 kV). X-ray diffraction (XRD) patterns were obtained with a Bruker D8 Advance X-ray diffractometer (XRD, Bruker D8 Advance, Cu Ka radiation, λ = 0.15406 nm, 8°/min). X-ray photoelectron spectrometry (XPS) was measured by K-Alpha Fourier transform infrared (FT-IR, Nicolet FTIR-170SX, 4000–500 cm−1, room temperature) absorption spectra, Raman (HORIBA Lab RAM HR Evolution, 500–2000 cm−1, 514 nm), thermogravimetric analysis (TGA; Shimadzu Corporation DTG-60, from 50 °C to 1050 °C in air). The magnetic properties of samples were characterized by a vibrating sample magnetometer (VSM, Riken Denshi, BHV-525) at room temperature. Electromagnetic (EM) parameters were measured by a vector network analyzer (NA, HP8720ES) over 2–18 GHz, in which powders were pressed to be toroidal samples (outer diameter: 7 mm, inner diameter: 3mm) according to the mass ratio 9:1 of paraffin to EG/Fe3O4 composite.
3. Results and Discussion
XRD measurements were used to investigate the phase composition and the crystalline structure of the samples. Figure 1 shows the XRD spectra of Fe3O4 and EG/Fe3O4 composites. The diffraction peaks located at ~30.4°,~ 35.8°, ~43.4°, ~53.9°, ~57.9°, and ~63.1° can be perfectly indexed to the (220), (311), (400), (422), (511), and (440) planes of Fe3O4. As shown in Figure 1, the XRD patterns of EG show a sharp peak at 20~26.6°, corresponding to the graphitic structure of the short-range order in stacked graphene sheets [36]. No peaks corresponding to any other impurities were detected, suggesting that the EG/Fe3O4 composites were formed via the simplified co-precipitation method.
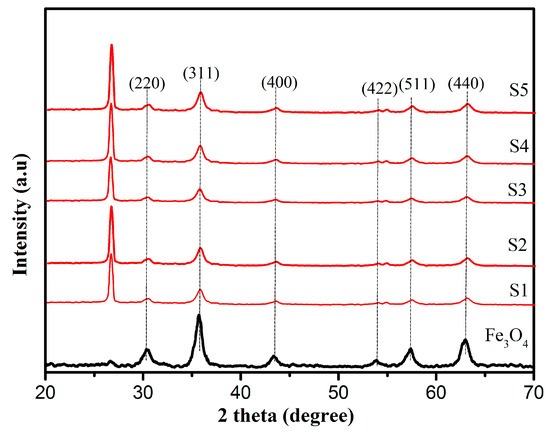
Figure 1.
X-ray diffraction (XRD) patterns of Fe3O4, S1, S2, S3, S4, and S5.
With gradually decreased concentrations of Fe3O4 in EG (Figure 2a–e), it can be clearly seen that Fe3O4 was incorporated into the EG, forming a sandwich-like composite structure. Moreover, there were strong interfacial interactions between the Fe3O4 and EG, and most of the Fe3O4 was attached to the EG sheets (Figure 2a). This indicated the formation of the coordination bonds between Fe3O4 and EG, which is further demonstrated by IR characterization below. In addition, the generated large spaces between the layers of EG also favored multiple reflections to improve the microwave absorption performance.
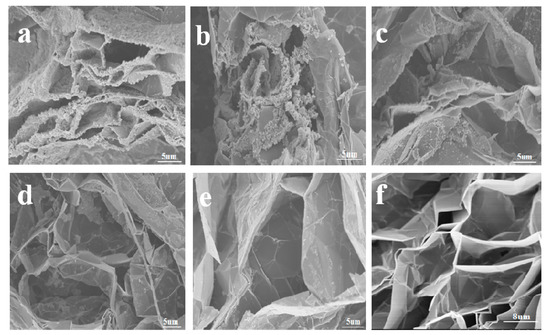
Figure 2.
Scanning electron microscopy (SEM) images of expanded graphite (EG)/Fe3O4 prepared from different concentrations of Fe3O4 (gradually decreased from (a–e) in the figure) and EG (f), respectively.
To get further insight into the nanostructure of samples, TEM of EG/Fe3O4 composite was carried out. As can be seen from Figure 3a, the Fe3O4 with an average size of 8–50 nm (can be proved from Figure S3 in the Supporting Information) is strongly attached to the EG sheets even after 30 min ultrasonic treatment with a frequency of 25 khz under room temperature, implying a strong interaction between the EG sheets and Fe3O4 [35], which could be attributed to a Fe-O bond, which was confirmed by FT-IR as below. In Figure 3b, black curved graphite sheets can be easily identified. The crystal plane spacing of 0.34 nm corresponds to the (002) crystal plane of EG. In Figure 3c, Fe3O4 has a lattice fringe of 0.295 nm interplanar spacing, corresponding to the cubic spinel crystal Fe3O4 (220) plane. The corresponding Selected Area Electron Diffraction (SAED) pattern as presented in Figure 3d is well indexed to the Fe3O4 structure with the (220), (311), (400), (511), and (440) plane, identifying the existence of Fe3O4, which is in accordance with the XRD results.
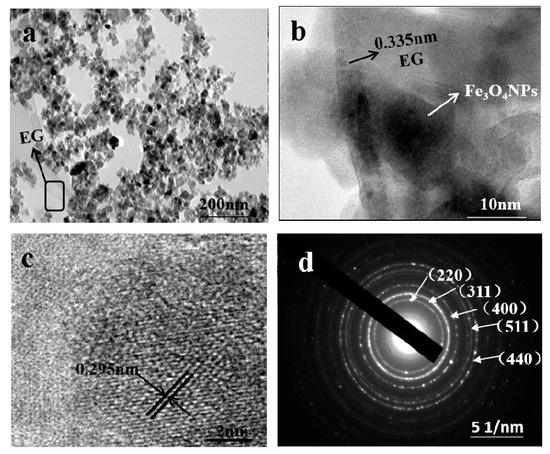
Figure 3.
Transmission electron microscopy (TEM) images of EG/Fe3O4 (S1) composite (a); High Resolution Transmission Electron Microscope (HRTEM) image (b,c) and Selected Area Electron Diffraction (SAED) pattern (d) of EG/ Fe3O4 (S1) composite.
The surface chemical environment of EG and EG/Fe3O4 composites was studied by FT-IR spectroscopy as presented in Figure 4. The intense absorption peaks positioned at ~3428 cm−1 can be ascribed to adsorbed H2O in sample S2. The peaks at ~1047, 1439, 1630, 2922, and 2973 cm−1 can be attributed to C-O stretching vibration of epoxide [30], tertiary C-OH groups stretching [31], C=C skeleton vibration, anti-symmetric stretching vibration of methylene, and stretching vibration absorption peak of a saturated C-H bond, respectively. Moreover, strong bands around 592 cm−1 in the samples can be ascribed to the stretching vibration of Fe-O bond of Fe3O4 [32], meaning that Fe3O4 was chemically bonded to the surface of EG through interaction with the Fe-O bond [33,34,35,37].
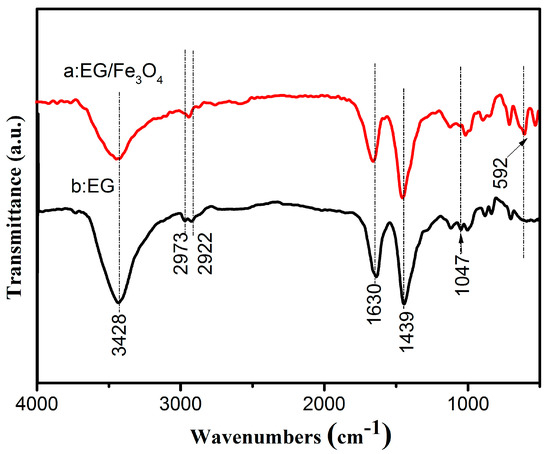
Figure 4.
Fourier transform infrared (FT-IR) spectra of EG and EG/Fe3O4 composites.
From the Raman spectrum of flake graphite, GIC (graphene intercalation compounds), EG, and EG/Fe3O4 in Figure 5, the sharp and strong absorption peak (G peak) at~1581 cm−1 is corresponding to the in-plane vibration of the sp2 carbon atom in graphite and the significant peak at 668 cm−1 in the spectrum of EG/Fe3O4 is corresponding to the characteristic peak of Fe3O4 [12].
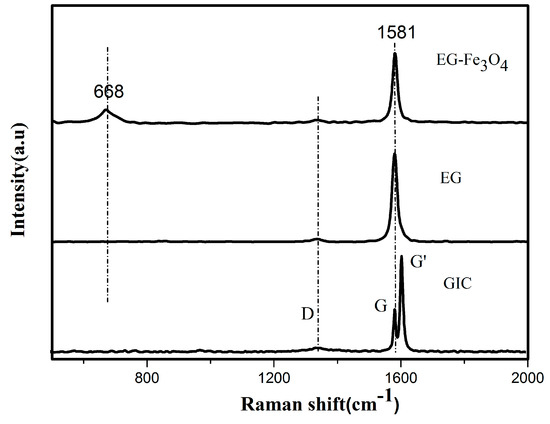
Figure 5.
Raman spectra of graphite, graphene intercalation compounds (GIC), EG and EG/ Fe3O4 composite.
Meanwhile, the small peak (D peak) at 1346 cm−1 and the G’ band at about 1620 cm−1 are associated with defects in the graphite sheet or graphite edge [38,39]. The D’ peak can be clearly seen in GIC, which is obviously higher than the G peak, indicating that the graphite sheet is opened by oxidation in the experiment to facilitate intercalation by the graphite sheet layer. The D peak on the spectrum of EG and EG/Fe3O4 is weak, indicating that the crystal structure of the EG is relatively intact and the degree of damage is low.
X-ray photoelectron spectroscopy was used to determine the composition of the composites (Figure 6). The bands in the wide scan of S2 confirm the presence of C, O, and Fe elements. The observed of O 1s peak in GO at ~531.8 eV is shifted to a lower binding energy (530.6 eV) due to the attachment of the Fe3O4 in the EG/Fe3O4 composites [40]. In Figure 6b, the Fe 2p XPS spectrum exhibits two peaks at ~710.1 and ~724.1 eV, which can be assigned to the Fe 2p 3/2 and Fe 2p 1/2 spin-orbit peaks of Fe3O4, which is consistent with the reported values for Fe3O4 [17,41]. It can be seen that the left front is higher than the right peak, coinciding with the reported Fe3O4 [42], and there are no satellite peaks of iron oxides (such as α-Fe2O3 and γ-Fe2O3), which proves that there is no Fe2O3 phase in the hybrid.
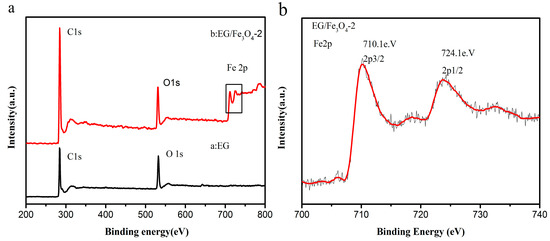
Figure 6.
X-ray photoelectron spectroscopy (XPS): (a) wide scan, (b) Fe 2p spectrum of the EG/Fe3O4 composite.
As shown in Figure 7a, the magnetization curves of the six samples were measured at room temperature. It can be seen that the sample is superparamagnetic and has a small coercive force.
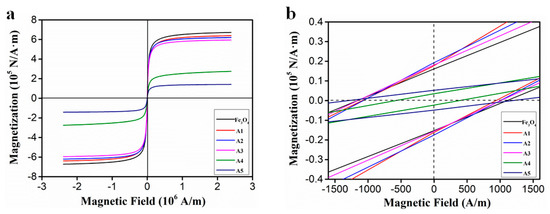
Figure 7.
The magnetization curves of Fe3O4 and EG/ Fe3O4 (a) and an expanded view of the low-field magnetization curves (b).
Table 1 reflects the specific magnetic parameters from Figure 7b, including the coercivity (Hc), remanent magnetization (Mr), and saturation magnetization (Ms). The Ms values decrease with a decrease of Fe3O4 loading amount, and the Ms value of the five samples is lower than that of the pure Fe3O4, which can be attributed to the influence of the nano-sized Fe3O4 and the presence of EG [25].

Table 1.
Magnetic parameters of Fe3O4 nanoparticles and EG/Fe3O4 composites.
From the TG analysis curve, as shown in Figure 8, it can be seen that there are three steps between 0 and 1030 °C for the EG/Fe3O4 composites. Before the temperature rises to 100 °C, a slight weight loss occurs on the curve, which may be caused by the loss of the surface hydroxyl groups or adsorbed water. When the temperature is further increased, the product undergoes a relatively gentle decrease between 100 and 700 °C due to the destruction of the oxygen-containing functional group which is unstable on the surface of the EG, the destruction of the amino group on the surface of Fe3O4, and the evaporation of water vapor. CO2 gas is generated by the combustion of EG between 700 and 850 °C, resulting in a severe weight loss. After 850°C, a mostly steady value is reached corresponding to the mass of Fe2O3. Zong et al. [29,33] found that the oxidation and decomposition of graphene occurred between 350 °C and 510 °C. The EG/Fe3O4 complex prepared by the experiment has better heat resistance than the Reduced Graphene Oxide (RGO)/Fe3O4 complex.
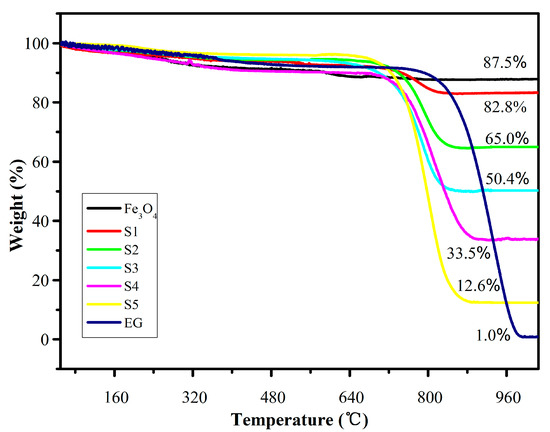
Figure 8.
Thermogravimetric (TG) curves of Fe3O4 and composites S1–S5 under air atmosphere.
To investigate the microwave absorption properties and mechanism of EG/Fe3O4, the electromagnetic parameters (relative complex permittivity, εr = ε’ − jε″, and relative complex permeability, μr = μ’ − jμ″) in the range of 2–18 GHz were measured. As is well known, the real permittivity (ε’) and real permeability (μ’) are correlated to the storage ability of electric and magnetic energy, while the imaginary permittivity ε″ and imaginary permeability μ″ symbolize the dissipation of electric and magnetic energy.
As shown in Figure 9a, the values of samples S5, S4, S3, and S2 in the 2–18 GHz range were 15.23–22.58, 9.98–14.22, 10.88–12.20, and 7.28–9.77, respectively, far higher than those of S1 with a high Fe3O4 content which were 2.99–3.31. The values of samples S2–S5 generally decrease with increasing frequency, but the values of the S1 samples are essentially unchanged, demonstrating a frequency-dependent dielectric response [4,29]. Meanwhile, as shown in Figure 9b, the values of ε″ are in ranges of 4.73–9.85, 3.16–6.21, 2.91–4.56, and 1.22–2.71, respectively. Although the values of these four samples fluctuated in the range of 2–18 GHz, they were still much higher than those of sample S1 which were in the range of 0.45–0.18 GHz. It is concluded that the samples with higher EG/Fe3O4 ratios show higher values of ε’ and ε″, which is unrelated to higher conductivities. This is because more EG sheets increase the electric polarization and electric conductivity, since ε’ is an expression of the polarizability of a material at microwave frequencies which consists of dipolar polarization and electric polarization [19,31]. As shown in Figure 9c,d, as for samples S1, S2, S3, S4, and S5, the μ’ and μ″ remain around 1 and 0 in the whole frequency range, respectively.
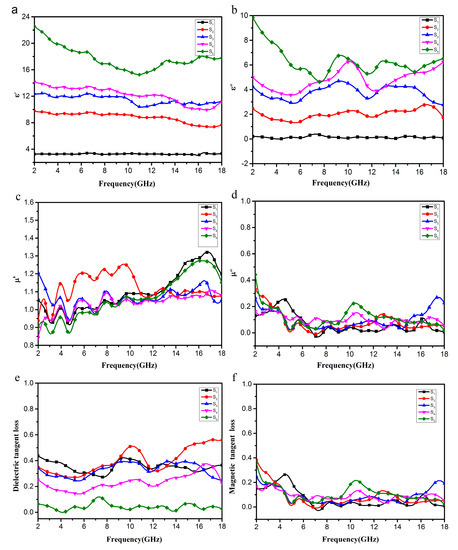
Figure 9.
Frequency dependence on real parts (a) and imaginary parts (b) of the complex permittivity, real parts (c) and imaginary parts (d) of the complex permeability, and the corresponding dielectric loss tangents (e) and magnetic loss tangents (f) of composites S1–S5.
Based on the permeability and permittivity of samples, both the dielectric tangent loss (tan δ = ε″/ε’) and the magnetic tangent loss (tan δM = μ″/μ’) of the EG/Fe3O4 composites can be obtained. The values of tan δ are larger than 0.2 at almost 2–18 GHz for S1, S2, S3, and S4 as shown in Figure 9e, indicating the dielectric loss occurs at the entire frequency range. These results suggest that the EG/Fe3O4 composite has distinct dielectric loss properties. As for S1, the content of Fe3O4 in this sample is the highest, which is almost equal to the pure Fe3O4; hence, tan δM is the highest in these five samples. The resonance peak at about 10.2 GHz for the EG/Fe3O4 is possibly associated with the interfacial interaction between Fe3O4 and EG [26,27].
Based on the transmit-line theory, the reflection loss (RL) can be calculated by the following equations [25].
where Zin is the input impedance of the absorber, μr and εr are respectively the relative complex permeability and permittivity, f is the frequency of microwaves, d is the thickness of the absorber, and c is the velocity of light in free space [43].
As shown in Figure 10a, when the content of Fe3O4 is excessive, the RL absorption peak of sample S1 only appears in the range of 16–16.5 GHz and is relatively sharp, and the range of the absorption peak movement is small with increasing thickness. When the sample thickness is 1.9 mm, the maximum RL absorption can reach at −34.12 dB. The maximum RL reaches −26.9 dB at 16.0 GHz, for S2 composite (Figure 10c) with a thickness of only 1.5 mm. In addition, a bandwidth of RL less than −10 dB (90% absorption) can reach more than 4.1 GHz (from 13.9 to 18.0 GHz). For the composite S3 (Figure 10d), the maximum RL reaches −36.8 dB at 7.7 GHz with a thickness of 2.5 mm, and a bandwidth of RL less than −10 dB can reach 2.0 GHz. As for S4, Figure 10e shows that the maximum RL reaches −40.39 dB at 6.9 GHz with a thickness of 3.0 mm, and EAB: <−10 dB with RL exceeding −10 dB is 4.76–17.66 GHz with an absorber thickness of 1.5–4.0 mm.
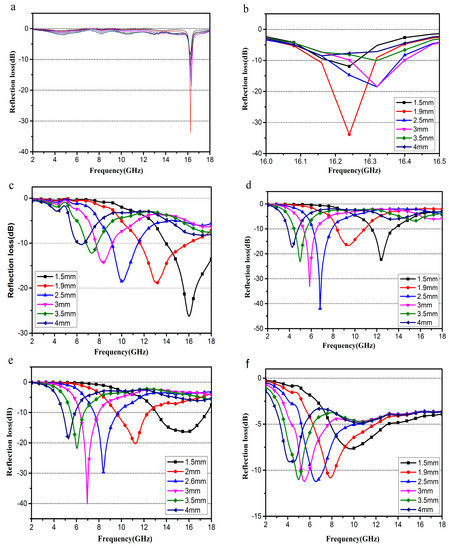
Figure 10.
The calculated RL for S1 (a,b), S2 (c), S3 (d), S4 (e) and S5 (f) with different thicknesses in the frequency range of 2–18 GHz.
It could be found that the RL absorption peak moves towards the low-frequency direction with the increase of thickness, while the RL absorption peak of the sample expands in the low-frequency direction with a decreasing content of Fe3O4.
The excellent microwave absorbing performance of the EG/Fe3O4 nanoparticle composite is mainly attributed to two key factors: impedance matching and electromagnetic wave attenuation [19]. On one hand, the introduction of Fe3O4 has lowered the εr of the EG, and improved the equality of εr and μr, which helps to improve the level of impedance matching [31]. On the other hand, the introduction of Fe3O4 could form more interfaces. The interfaces can act as polarization centers, which are favorable for microwave absorbing. Besides, the enormous aspect ratio, layered structure, and the existence of residual defects and groups of EG/Fe3O4 could cause multiple reflections, which could further enhance the microwave absorption ability of the composite [19,25].
4. Conclusions
In summary, EG/Fe3O4 composites with enhanced microwave absorption properties were prepared by a facile one-pot co-precipitation route, which avoided the usage of any additional chemical agents and inert gas. The preparation process of EG does not contain sulfur, which protects the environment and is easy to operate, low in cost, and easy to scale. In addition, the growth of composite materials could be well controlled by adjusting the molar masses of Fe2+ and Fe3+, and the yield is relatively high. The prepared EG/Fe3O4 sample does not only have better thermal performance than the widely studied RGO/Fe3O4 but also excellent absorbing properties. For the EG/Fe3O4(S4) composite, the maximum RL reached −40.39 dB at 6.9 GHz with a thickness of 3.0 mm, and EAB: <−10 dB with RL exceeding −10 dB is 4.76–17.66 GHz with an absorber thickness of 1.5–4.0 mm. It is believed that such sandwich-like structure composite will find applications in the microwave absorbing area as the base material of smoke bombs for electromagnetic interference, which could play a great role in electromagnetic shielding and heat dissipation.
Supplementary Materials
The following are available online. SEM images of NG, GIC, EG, and Fe3O4; The particle size distribution of the Fe3O4; The picture of 0.50 g EG; Black EG/Fe3O4 composite is rapidly separated under external magnetic field.
Author Contributions
Conceptualization, J.S. and X.M.; methodology, L.L.; software, R.Y.; validation, R.Y., X.M. and J.S.; formal analysis, S.J.; investigation, J.S.; resources, S.C.; data curation, K.C.; writing—original draft preparation, J.S.; writing—review and editing, X.L.; visualization, J.S.; supervision, X.L.; project administration, Q.S.; funding acquisition, Q.S. All authors have read and agreed to the published version of the manuscript. Please turn to the CRediT taxonomy for the term explanation. Authorship must be limited to those who have contributed substantially to the work reported.
Funding
This research was funded by the Fundamental Research Funds for the Central Universities, grant number 2017CX10003” and “The APC was funded by Qinghai Shu”.
Acknowledgments
This work was supported by the Fundamental Research Funds for the Central Universities. We also gratefully acknowledge the support of Ran Liu and Haijiao Xie in Shiyanjia Laboratory.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhao, T.; Jin, W.; Ji, X. Synthesis of sandwich microstructured expanded graphite/barium ferrite connected with carbon nanotube composite and its electromagnetic wave absorbing properties. J. Alloy. Compd. 2017, 712, 59–68. [Google Scholar] [CrossRef]
- Liu, Y.; Du, X.; Wu, C.; Liu, Y.; Liu, Y.; Zhao, G. Reduced graphene oxide decorated with ZnO microrods for efficient electromagnetic wave absorption performance. J. Mater. Sci. Mater. El. 2020, 31, 8637–8648. [Google Scholar] [CrossRef]
- Zhang, K.L.; Zhang, J.Y.; Hou, Z.L. Multifunctional broadband microwave absorption of flexible graphene composites. Carbon 2018, 141, 608–617. [Google Scholar] [CrossRef]
- Liu, G.Z.; Jiang, W.; Wang, Y.P. One-pot synthesis of Ag@Fe3O4/reduced graphene oxide composite with excellent electromagnetic absorption properties. Ceram. Int. 2015, 41, 4982–4988. [Google Scholar] [CrossRef]
- Lian, C.; Wang, Z.; Lin, R. An efficient, controllable and facile two-step synthesis strategy: Fe3O4@RGO composites with various Fe3O4 nanoparticles and their supercapacitance properties. Nano Res. 2017, 10, 3303–3313. [Google Scholar] [CrossRef]
- Lv, H.; Ji, G.; Liu, W. Achieving hierarchical hollow carbon@Fe@Fe3O4 nanospheres with superior microwave absorption properties and lightweight feature. J. Mater. Chem. C 2015, 3, 10232–10241. [Google Scholar] [CrossRef]
- Ning, M.Q.; Li, J.B.; Kuang, B.Y.; Jin, H.B. One-Step Fabrication of N-doped CNTs Encapsulating M nanoparticles (M = Fe, Co, Ni) for Efficient Microwave Absorption. Appl. Surf. Sci. 2018, 447, 244–253. [Google Scholar] [CrossRef]
- Su, H.; Du, Y.; Zhang, J.; Peng, P.; Li, S.; Chen, P.; Gozin, M.; Pang, S. Stabilizing Metastable Polymorphs of Metal-Organic Frameworks via Encapsulation of Graphene Oxide and Mechanistic Studies. ACS Appl. Mater. Interfaces 2018, 10, 32828–32837. [Google Scholar] [CrossRef]
- Kuang, B.; Song, W.; Ning, M.; Li, J.; Zhao, Z.; Guo, D.; Cao, M.; Jin, H. Chemical reduction dependent dielectric properties and dielectric loss mechanism of reduced graphene oxide. Carbon 2018, 127, 209–217. [Google Scholar] [CrossRef]
- Kong, L.; Yin, X.; Yuan, X. Electromagnetic wave absorption properties of graphene modified with carbon nanotube/poly (dimethyl siloxane) composites. Carbon 2014, 73, 185–193. [Google Scholar] [CrossRef]
- Matsumoto, K.; Nakahara, K.; Iwasa, S. SEI Pre-coated graphite anode in lithium ion battery with EMITFSI ionic liquid electrolyte. J. Electrochem. Soc. 2009, 3, 179. [Google Scholar]
- Li, W.; Han, C.; Liu, W. Expanded graphite applied in the catalytic process as a catalyst support. Catal. Today 2007, 125, 278–281. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Wu, Z.G. Heat transfer enhancement of high temperature thermal energy storage using metal foams and expanded graphite. Sol. Energ. Mat. Sol. Cells 2011, 95, 636–643. [Google Scholar] [CrossRef]
- Goyal, R.K. Cost-efficient high performance polyetheretherketone/expanded graphite nanocomposites with high conductivity for EMI shielding application. Mater. Chem. Phys. 2013, 142, 195–198. [Google Scholar] [CrossRef]
- Wang, C.Y.; Li, H.R.; Qin, C.L. Preparation of low-sulfur expanded graphite with different size particles. Adv. Mater. 2013, 652, 745–748. [Google Scholar] [CrossRef]
- Wang, C.Y.; Ma, M.; Qin, C.L. Preparation of expanded graphite with Fe3O4 nanoparticles. Adv. Mater. 2013, 690, 507–510. [Google Scholar]
- Li, J.H.; Da, H.F.; Liu, Q. Preparation of sulfur-free expanded graphite with 320 μm mesh of flake graphite. Mater. Lett. 2006, 60, 3927–3930. [Google Scholar]
- Kumar, R.; Singh, R.K.; Vaz, A.R. Self-assembled and one-step synthesis of Interconnected 3D network of Fe3O4/Reduced graphene oxide nanosheets hybrid for high performance supercapacitor electrode. ACS Appl. Mater. Interface 2017, 9, 8880–8890. [Google Scholar] [CrossRef]
- Chen, D.; Ji, G.; Ma, Y. Graphene-encapsulated hollow Fe3O4 nanoparticle aggregates as a high-performance anode material for lithium ion batteries. ACS Appl. Mater. Interface 2011, 3, 3078–3083. [Google Scholar] [CrossRef]
- Liu, H.; Jia, M.; Zhu, Q. 3D-0D graphene-Fe3O4 quantum dot hybrids as high-performance anode materials for sodium-ion batteries. ACS Appl. Mater. Interface 2016, 8, 26878–26885. [Google Scholar] [CrossRef]
- Erogul, S.; Bas, S.Z.; Ozmen, M. A new electrochemical sensor based on Fe3O4 functionalized graphene oxide-gold nanoparticle composite film for simultaneous determination of catechol and hydroquinone. Electrochim. Acta 2015, 186, 302–313. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, W.; Wang, Y. Fabrication of cluster/shell Fe3O4/Au nanoparticles and application in protein detection via a SERS method. J. Mater. Chem. C 2010, 114, 19607–19613. [Google Scholar] [CrossRef]
- Liu, R.; Guo, Y.; Odusote, G. Core–shell Fe3O4 polydopamine nanoparticles serve multipurpose as drug carrier, catalyst support and carbon adsorbent. ACS Appl. Mater. Interface 2013, 5, 9167–9171. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Chao, G. Supraparamagnetic, conductive, and processable multifunctional graphene nanosheets coated with high-density Fe3O4 nanoparticle. ACS Appl. Mater. Interface 2010, 2, 3201–3210. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jia, X.; Li, Y. Synthesis and microwave absorption property of flexible magnetic film based on graphene oxide/carbon nanotubes and Fe3O4 nanoparticles. J. Mater. Chem. A 2014, 2, 14940–14946. [Google Scholar] [CrossRef]
- Zeng, Q.; Xiong, X.; Chen, P. Air@rGO€Fe3O4 microspheres with spongy shell: Self-assembly and microwave absorption performance. J. Mater. Chem. C 2016, 4, 10518–10528. [Google Scholar] [CrossRef]
- Qu, B.; Zhu, C.; Li, C. Coupling hollow Fe3O4-Fe nanoparticles with graphene sheets for high-performance electromagnetic wave absorbing material. ACS Appl. Mater. Interface 2016, 8, 3730–3735. [Google Scholar] [CrossRef]
- Huo, X.; Liu, J.; Wang, B. A one-step method to produce graphene-Fe3O4 composites and their excellent catalytic activities for three-component coupling of aldehyde, alkyne and amine. J. Mater. Chem. A 2012, 1, 651–656. [Google Scholar] [CrossRef]
- Zong, M.; Huang, Y.; Zhao, Y. Facile preparation, high microwave absorption and microwave absorbing mechanism of RGO-Fe3O4 composites. Rsc. Adv. 2013, 3, 23638–23648. [Google Scholar] [CrossRef]
- Yang, X.; Niu, Y.; Li, Q. The influence of fabrication methods on structure and microwave absorption property of Fe3O4/rGO Composites. J. Supercond. Nov. Magn. 2018, 3, 1–9. [Google Scholar] [CrossRef]
- Zong, M.; Huang, Y.; Wu, H. Facile synthesis of RGO/Fe3O4/Ag composite with high microwave absorption capacity. Mater. Lett. 2013, 111, 188–191. [Google Scholar] [CrossRef]
- Huang, Y.; Qi, Q.; Pan, H. Facile preparation of octahedral Fe3O4/RGO composites and its microwave electromagnetic properties. J. Mater. Sci. Mater. El. 2016, 27, 9577–9583. [Google Scholar] [CrossRef]
- Wu, J.; Ye, Z.; Liu, W. The effect of GO loading on electromagnetic wave absorption properties of Fe3O4/reduced graphene oxide hybrids. Ceram. Int. 2017, 43, 13146–13153. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, L.; Jiang, K. Distinctly enhanced permeability and excellent microwave absorption of expanded graphite/Fe3O4 nanoring composites. RSC Adv. 2017, 7, 11561–11567. [Google Scholar] [CrossRef]
- Zhao, L.; Gao, M.; Yue, W. Sandwich-structured graphene-Fe3O4@carbon nanocomposites for high-performance lithium-ion batteries. ACS Appl. Mater. Interface 2015, 7, 9709–9715. [Google Scholar] [CrossRef]
- Zhou, G.; Xu, X.; Zhu, W. Dispersedly embedded loading of Fe3O4 nanoparticles into graphene nanosheets for highly efficient and recyclable removal of heavy metal ions. New J. Chem. 2015, 39, 7355–7362. [Google Scholar] [CrossRef]
- Mahmoudian, M.R.; Alias, Y.; Basirun, W.J. Synthesis and characterization of Fe3O4, rose like and spherical/reduced graphene oxide nanosheet composites for lead (II) sensor. Electrochim. Acta 2015, 169, 126–133. [Google Scholar] [CrossRef]
- Su, J.; Cao, M.; Ren, L. Fe3O4-grapheme nanocomposites with improved lithium storage and magnetism properties. J. Phy. Chem. C 2011, 115, 14469–14477. [Google Scholar] [CrossRef]
- Rarani, E.M.; Etesami, N.; Esfahany, M.N. Influence of the uniform electric field on viscosity of magnetic nanofluid (Fe3O4-EG). J. Appl. Phys. 2012, 112, 567–720. [Google Scholar]
- Kim, Y.K.; Min, D.H. Durable large-area thin films of graphene/carbon nanotube double layers as a transparent electrode. Langmuir 2009, 25, 11302–11306. [Google Scholar] [CrossRef]
- Ren, Y.L.; Wu, H.Y.; Lu, M.M. Quaternary nanocomposites consisting of graphene, Fe3O4@Fe core@Shell, and ZnO nanoparticles: Synthesis and excellent electromagnetic absorption properties. ACS Appl. Mater. Interface 2012, 4, 6436–6442. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zeng, H.; Robinson, D.B. Monodisperse MFe2O4 (M = Fe, Co, Mn) Nanoparticles. J. Am. Chem. Soc. 2004, 126, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Hu, X.; Bai, X. Synthesis and electromagnetic, microwave absorbing properties of core-shell Fe3O4-poly (3, 4-ethylenedioxythiophene) microspheres. ACS Appl. Mater. Interface 2011, 3, 3839–3845. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).