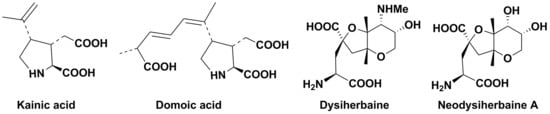
Marine Excitatory Amino Acids: Structure, Properties, Biosynthesis and Recent Approaches to Their Syntheses
Abstract
1. Introduction
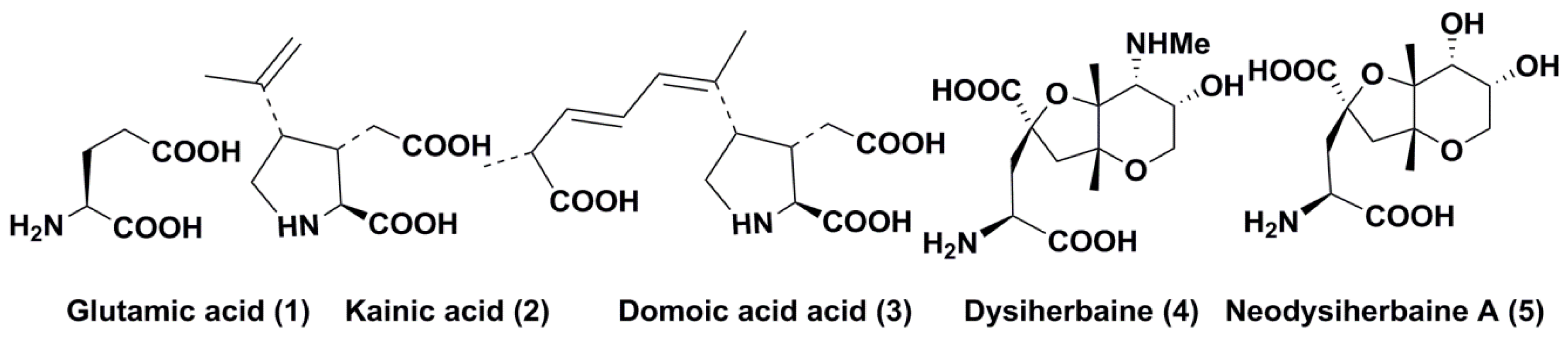
2. Kainic Acid and Related Metabolites
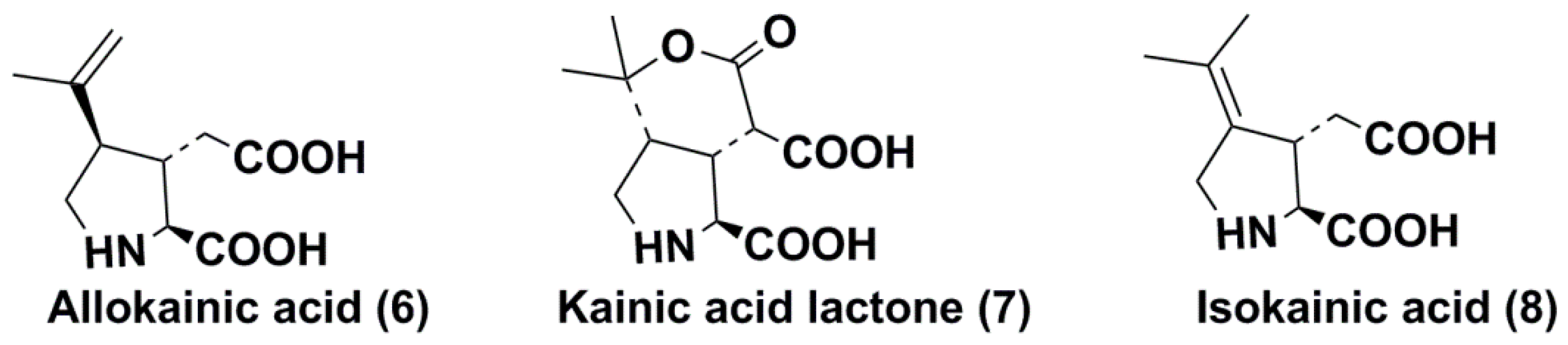
2.1. Discovery, Structure, and Some Properties
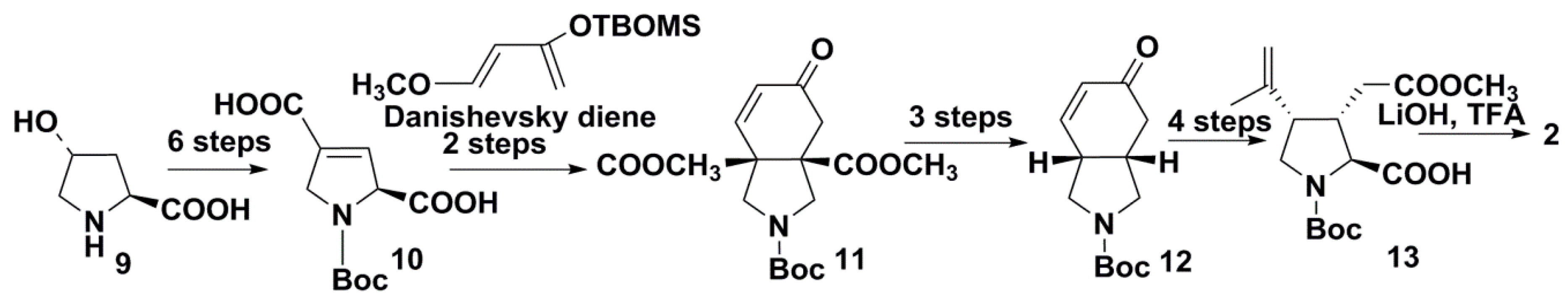
2.2. Recent Syntheses
2.3. Biosynthesis and Application of Kainoids
3. Domoic Acid and Related Compounds
3.1. Discovery, Structure, and Some Properties
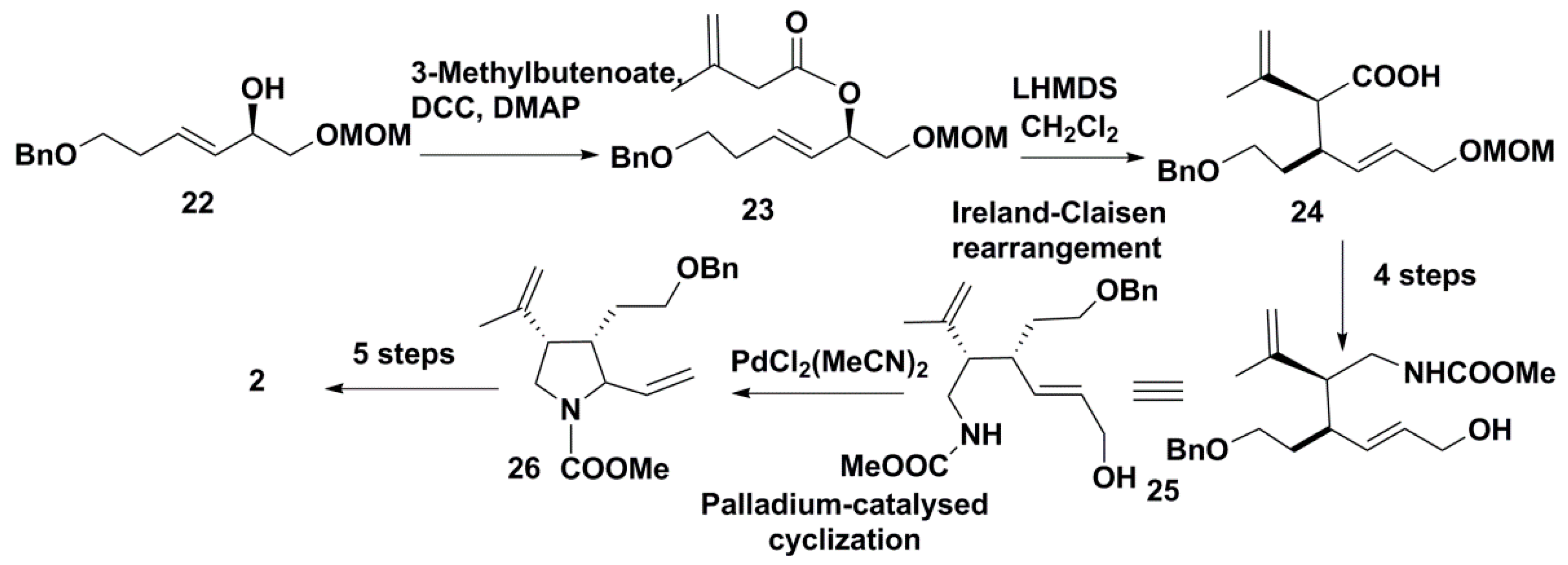
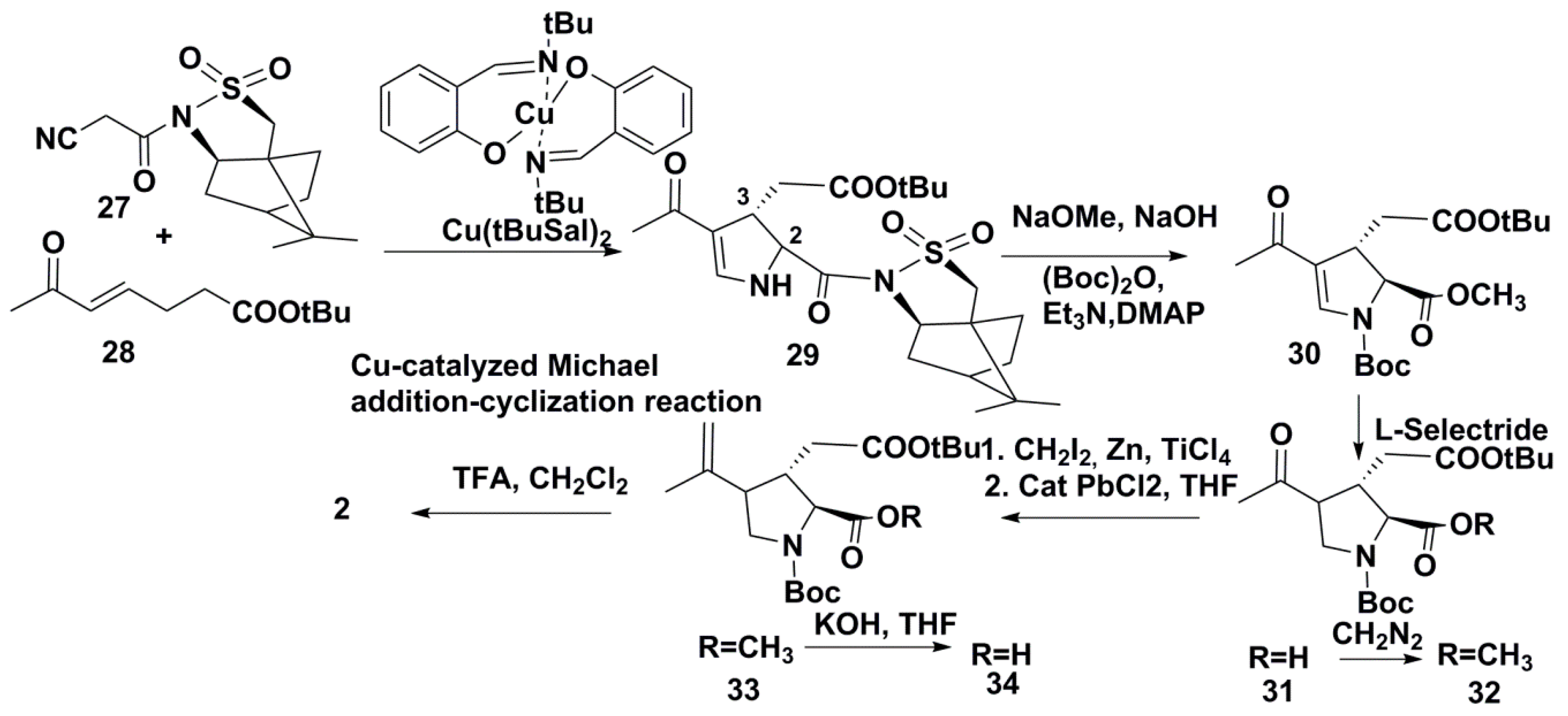
3.2. Synthesis
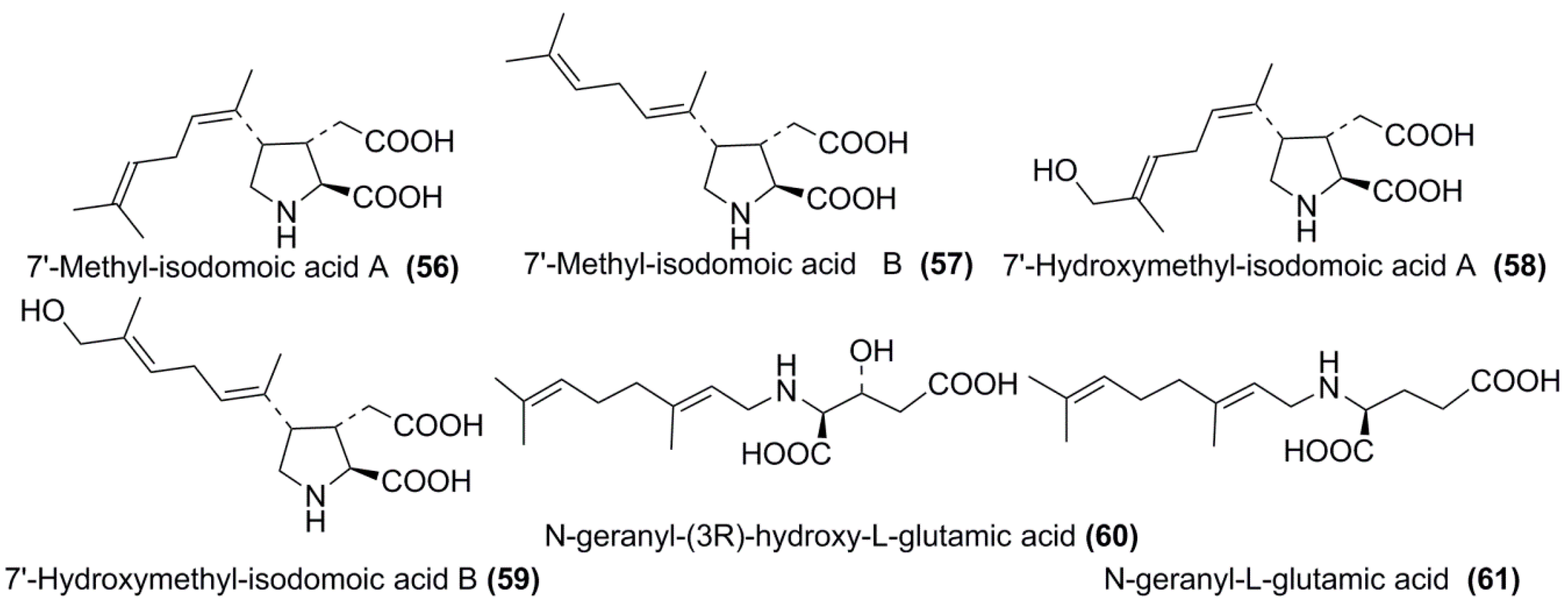
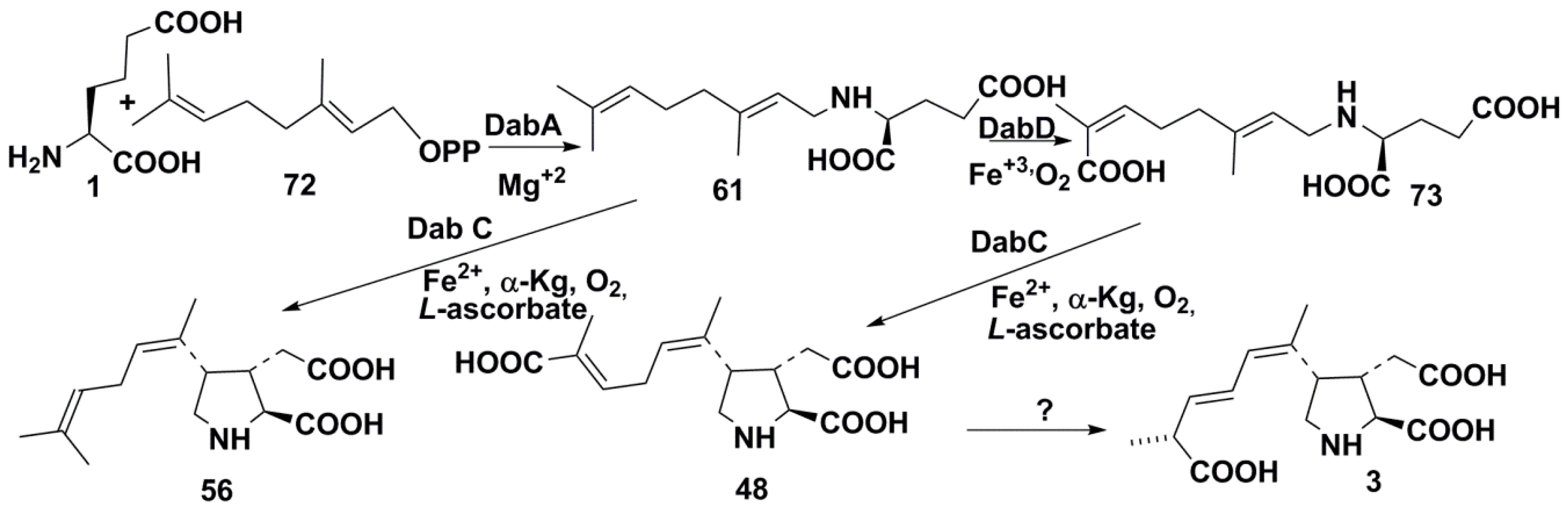
3.3. Biosynthesis, Producers, Biological Action, and Environmental Role
4. Dysiherbaine and Related Compounds
4.1. Discovery and Structures
4.2. Some Recent Approaches to Syntheses
4.3. Probable Origin in Sponges
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Caroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2019, 36, 122–173. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Gupta, S.; Masuda, K.; Moranda, L.; Walker, C.R.; Wang, R. Dinoflagellate and other microalgal toxins; chemistry and biochemistry. Pure Appl. Chem. 1989, 61, 513–516. [Google Scholar] [CrossRef]
- Visciano, P.; Schirone, M.; Berti, M.M.; Milandri, A.; Tofalo, R.; Suzzi, G. Marine biotoxins: Occurrence, toxicity, regulatory limits and reference methods. Front. Microbiol. 2016, 7, 1051. [Google Scholar] [CrossRef] [PubMed]
- Stonik, V.A.; Stonik, I.V. Toxins produced by marine microorganisms: A short review. In Marine and Freshwater Toxins; Gopalakrishnakone, P., Haddad, J.V., Tubaro, A., Kim, E., Kem, W., Eds.; Springer: Dordrecht, The Netherlands, 2016; pp. 3–21. [Google Scholar] [CrossRef]
- Stonik, I.V.; Orlova, T.Y. Domoic acid-producing diatoms of the genus Pseudo-nitzschia H. Peragallo, 1900 (Bacillariophyta) from the North Pacific. Russ. J. Mar. Biol. 2018, 44, 347–354. [Google Scholar] [CrossRef]
- Moloney, M.G. Excitatory amino acids. Nat. Prod. Rep. 1998, 15, 205–219. [Google Scholar] [CrossRef]
- Pei-Gen, X.; Shan-Lin, F. Traditional antiparasitic drugs in China. Parasitol. Today 1986, 2, 353–355. [Google Scholar] [CrossRef]
- Murakami, S.; Takemoto, T.; Shimizu, Z. Studies on the effective principle of Digenea simplex Aq.1, separation of the effective fraction by liquid chromatography. J. Pharm. Soc. Jpn. 1953, 73, 1026–1028. [Google Scholar] [CrossRef]
- Watase, H.; Nitta, I. The structure of kainic acid, the most active component of Digenea simplex Aq. I. The molecular and crystal structure of zinc kainite dihydrate. Bull. Chem. Soc. Jpn. 1957, 30, 889–900. [Google Scholar] [CrossRef]
- Nitta, I.; Watase, H.; Tomiie, Y. Structure of kainic acid and its isomer, allokainic acid. Nature 1958, 181, 761–762. [Google Scholar] [CrossRef]
- Impellizezeri, G.; Mangiafico, S.; Oriente, G.; Piatelli, M.; Scuit, S.; Fatorusso, E.; Magno, S.; Santacroce, C.; Sica, D. Amino acid and low-molecular-weight carbohydrates of some marine red algae. Phytochemistry 1975, 7, 1549–1557. [Google Scholar] [CrossRef]
- Calaf, R.; Barlatier, A.; Garson, D.; Balansard, G.; Pellegrini, M.; Reynaud, J. Isolation of an unknown kainic peptide from the red alga Alsidium helminthocorton. J. Appl. Phycol. 1989, 1, 257–266. [Google Scholar] [CrossRef]
- Laycook, M.V.; de Freitas, A.S.W.; Wright, J.L.C. Glutamate agonists from marine algae. J. Appl. Phycol. 1989, 1, 113–122. [Google Scholar] [CrossRef]
- Shinozaki, H.; Konishi, S. Action of several anthelmintics and insecticides on rat cortical neurons. Brain Res. 1970, 24, 368–371. [Google Scholar] [CrossRef]
- Ferkany, J.W.; Zaczek, R.; Colyle, J.T. Kainic acid stimulates excitatory amino acid neurotransmitter release at presynaptic receptors. Nature 1982, 298, 757–759. [Google Scholar] [CrossRef]
- Garthwaite, J.; Garthwaite, G. The mechanism of kainic acid neurotoxicity. Nature 1983, 305, 138–140. [Google Scholar] [CrossRef]
- Tremblay, J.-F. Producers strive to bring kainic acid back on the market. Chem. Eng. News 2000, 78, 31. [Google Scholar] [CrossRef]
- Evans, P.A.; Inglesby, P.A. Diastereoselective rhodium-ctalyzed ene-cycloisomerization reactions of alkenylidenecyclopropanes: Total synthesis of (-)-alpha-kainic acid. J. Am. Chem. Soc. 2012, 134, 3635–3638. [Google Scholar] [CrossRef]
- Stathakis, C.I.; Yioti, E.; Gallos, J.K. Total syntheses of (-)-α-kainic acid. Eur. J. Org. Chem. 2012, 25, 4661–4673. [Google Scholar] [CrossRef]
- Orellana, A.; Pandey, S.K.; Carret, S.; Greene, A.E.; Poison, J.-F. A Diels-Alder-based total synthesis of (-) kainic acid. J. Org. Chem. 2012, 77, 5286–5296. [Google Scholar] [CrossRef]
- Reddy, N.K.; Chandrasekhar, S. Total synthesis of (-)-α-kainic acid via chirality transfer through Ireland-Claisen rearrangement. J. Org. Chem. 2013, 78, 3355–3360. [Google Scholar] [CrossRef]
- Fujii, M.; Yokoshima, S.; Fukuyama, T. A unified strategy for kainoid syntheses. Eur. J. Org. Chem. 2014, 22, 4823–4836. [Google Scholar] [CrossRef]
- Oe, K.; Ohfune, Y.; Shinada, T. Short total synthesis of (-)-kainic acid. Org. Lett. 2014, 16, 2550–2553. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Watanabe, K.; Tsukamoto, M.; Shibuya, R.; Morimoto, H.; Ohshima, T. A short scalable route to (-)-α-kainic acid using Pt-catalyzed direct allylic amination. Chem. Eur. J. 2015, 21, 3937–3941. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, J.; Miyano, M.; Yashiro, S.; Umezawa, T.; Matsuda, F. Total synthesis of (-)-kainic acid and (+)-allo-kainic acid through SmI2-mediated intramolecular coupling between allyl chloride and α,β-unsaturated ester. Org. Biomol. Chem. 2017, 15, 6557–6566. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.S.; Li, J.L.; Lui, Y.M.; Du, Z.L.; Huang, Z.Y.; Zhao, N.; Li, N.; Yang, J. Formal total synthesis of (-)-kainic acid. Tetrahedron 2016, 72, 5502–5506. [Google Scholar] [CrossRef]
- Chekan, J.R.; McKinnie, S.M.K.; Moore, M.L.; Poplawski, S.G.; Michael, T.P.; Moore, B.S. Scalable biosynthesis of the seaweed neurochemical, kainic acid. Angew. Chem. Int. Ed. Engl. 2019, 58, 8454–8457. [Google Scholar] [CrossRef]
- Baldwin, J.E.; Moloney, M.G.; Parsons, A.F. Enantioselective synthesis of kainoid analysis by cobalt-mediated cyclization of an amino acid derivative. Tetrahedron 1990, 46, 7263–7282. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, S.; Simonyi, A.; Sun, G.Y.; Sun, A.Y. Kainic acid-mediated excitotoxicity as a model for neurodegeneration. Mol. Neurobiol. 2005, 31, 3–16. [Google Scholar] [CrossRef]
- Zheng, X.Y.; Zhang, H.; Liqang, L.; Luo, Q.; Zhu, J. Kainic acid-induced neurodegenerative model: Potentials and limitations. Biomed. Res. Int. 2011, 2011, 1–10. [Google Scholar] [CrossRef]
- Bertoglio, D.; Amhaoul, H.; Van Eetveldt, A.; Houbrechts, R.; Van De Viver, S.; Ali, I.; Dedeurwaerdere, S. Kainic-acid-induced post-status epilepticus models of temporal lobe epilepsy with diverging seizure phenotype and neuropathology. Front. Neurobiol. 2017, 8, 588. [Google Scholar] [CrossRef]
- Møllerud, S.; Frydenvang, K.; Pickering, D.S.; Kastrup, J.S. Lessons from the crystal structures of kainoid receptors. Neuropharmacol. 2017, 112, 16–28. [Google Scholar] [CrossRef]
- Sakai, R.; Minato, S.; Koike, K.; Koike, K.; Jimbo, M.; Kamiya, H. Cellular and subcellular localization of kainic acid in the marine red alga Digenea simplex. Cell Tissue Res. 2005, 322, 491–502. [Google Scholar] [CrossRef] [PubMed]
- De Bortoli, S.; Teardo, E.; Scabo, I.; Morosinotto, T.; Alboresi, A. Evolutionary insight into the ionotropic glutamate receptor superfamily of photosynthetic organisms. Biophys. Chem. 2016, 218, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Wudick, M.M.; Michard, E.; Nunes, C.O.; Feijo, J.A. Comparing plant and animal glutamate receptors: Common traits but different fates? J. Exp. Bot. 2018, 69, 4151–4163. [Google Scholar] [CrossRef] [PubMed]
- Blaskovich, M.A.T. Unusual amino acids in medicinal chemistry. J. Med. Chem. 2016, 59, 10807–10836. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, T.; Daigo, K.; Kondo, Y.; Kondo, K. Studies on the constituents of Chondria armata. VIII. On the structure of domoic acid. Yakugakku Zasshi 1966, 86, 874–877. [Google Scholar] [CrossRef]
- Meda, M.; Kodama, T.; Tanaka, T.; Yoshizumi, H.; Takemoto, T.; Nomoto, K.; Fujita, T. Structures of isodomoic acids A and B, novel insecticidal amino acids from the red alga Chondrus armata. Chem. Parm. Bull. 1986, 34, 4892–4895. [Google Scholar] [CrossRef]
- Wright, J.L.C.; Falk, M.; McInnes, A.G.; Walter, J.A. Identification of isodomoic acid D and two new geometrical isomers of domoic acid in toxic mussels. Can. J. Chem. 1990, 68, 22–25. [Google Scholar] [CrossRef]
- Zaman, L.; Arakawa, O.; Shimosu, A.; Onoue, Y.; Nishio, S.; Shida, Y.; Noguchi, T. Two new isomers of domoic acid from a red alga, Chondria armata. Toxicon 1997, 35, 205–212. [Google Scholar] [CrossRef]
- Clayden, J.; Read, B.; Hebditch, K.R. Chemistry of domoic acid, isodomoic sacid, and their analogues. Tetrahedron 2005, 61, 5713–5724. [Google Scholar] [CrossRef]
- Stonik, V.; Stonik, I. Low-molecular-weight metabolites from diatoms: Structures, biological roles, and biosynthesis. Mar. Drugs 2015, 13, 3672–3709. [Google Scholar] [CrossRef] [PubMed]
- Maeno, Y.; Kotaki, Y.; Terada, R.; Cho, Y.; Konoki, K.; Yotsu-Yamashita, M. Six domoic acids related compounds from the red alga, Chondria armata, and domoic acid biosynthesis by the diatom, Pseudo-nitzchia multiseries. Sci. Rep. 2018, 8, 356. [Google Scholar] [CrossRef]
- Wright, J.L.C.; Boyd, R.K.; de Freitas, A.S.W.; Falk, M.; Foxall, R.A.; Jamieson, W.; Pathak, V.P. Identification of domoic acid, a neuroexcitatory amino acids, in toxic mussels from eastern Prince Edward Island. Can. J. Chem. 1989, 67, 481–490. [Google Scholar] [CrossRef]
- Bates, S.; Bird, C.; de Freitas, A.; Foxall, R.; Gilgan, M.; Hanic, L.; Johnson, G.; McCulloch, A.; Odense, P.; Pocklington, R.; et al. Pennate diatom Nitzschia pungens as the primary source of domoic acid, a toxin in shellfish from eastern Prince Edward Island, Canada. Can. J. Fish. Aquat. Sci. 1989, 46, 1203–1215. [Google Scholar] [CrossRef]
- Lefebvre, K.; Bargu, S.; Kieckhefer, T.; Silver, M. From sanddabs to blue whales: The pervasiveness of domoic acid. Toxicon 2002, 40, 971–977. [Google Scholar] [CrossRef]
- Lelong, A.; Hegaret, H.; Soudant, P.; Bates, S. Pseudo-nitzschia (Bacillariophyceae) species, domoic acid and amnesic shellfish poisoning: Revisiting previous paradigms. Phycologia 2012, 51, 168–216. [Google Scholar] [CrossRef]
- Trainer, V.; Bates, S.; Lundholm, N.; Thessen, A.; Cochlan, W.; Adams, N.; Trick, C. Pseudo-nitzschia physiological ecology, phylogeny, toxicity, monitoring and impacts on ecosystem health. Harmful Algae 2012, 14, 271–300. [Google Scholar] [CrossRef]
- Bates, S.; Hubbard, K.; Lundholm, N.; Montresor, M.; Leaw, C. Pseudo-nitzschia, Nitzschia, and domoic acid: New research since 2011. Harmful Algae 2018, 79, 3–43. [Google Scholar] [CrossRef]
- Kumar, K.P.; Kumar, S.P.; Nair, G.A. Risk assessment of the amnesic shellfish poison, domoic acid, on animals and humans. J. Environ. Biol. 2009, 30, 319–325. [Google Scholar] [PubMed]
- Gulland, F.; Haulena, M.; Fauquier, D.; Langlois, G.; Lander, M.; Zabka, T.; Duerr, R. Domoic acid toxicity in Californian sea lions (Zalophus californianus): Clinical signs, treatment and survival. Vet. Rec. 2002, 150, 475–480. [Google Scholar] [CrossRef]
- Hong, Z.; Zhang, Y.; Zuo, Z.; Zhu, R.; Gao, Y. Influences of domoic acid exposure on cardiac development and the expression of cardiovascular relative genes in zebrafish (Danio rerio) embryos. J. Biochem. Mol. Toxicol. 2015, 29, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, K.A.; Frame, E.R.; Gulland, F.; Hansen, J.D.; Kendrick, P.S.; Beyer, R.P.; Bammler, T.K.; Farin, F.M.; Hiolski, E.M.; Smith, D.R.; et al. A novel antibody-based biomarker for chronic algal toxin exposure and sub-acute neurotoxicity. PLoS ONE 2012, 7, e36213. [Google Scholar] [CrossRef] [PubMed]
- Vale, C. Domoic acid: Chemistry and pharmacology. In Seafood and Freshwater Toxins: Pharmacology, Physiology and Detection, 3rd ed.; Botana, L.M., Ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 875–894. [Google Scholar]
- Pulido, O.M. Domoic acid toxicologic pathology: A review. Mar. Drugs 2008, 6, 180–219. [Google Scholar] [CrossRef] [PubMed]
- Stewart, G.; Zorumski, C.; Price, M.; Olney, J. Domoic acid: A dementia-inducing excitotoxic food poison with kainic acid receptor specificity. Exp. Neurol. 1990, 110, 127–138. [Google Scholar] [CrossRef]
- Zabaglo, K.; Chrapusta, E.; Bober, B.; Kaminski, A.; Adamski, M.; Bialczyk, J. Environmental roles and biological activity of domoic acid: A review. Algal Res. 2016, 13, 94–101. [Google Scholar] [CrossRef]
- Holland, P.; Selwood, A.; Mounfort, D.; Wilkins, A.; McNabb, P.; Rhodes, L.; Doucette, G.; Mikulski, C.; King, K. Isodomoic acid C, an unusual amnesic shellfish poisoning toxin from Pseudo-nitzschia australis. Chem. Res. Toxicol. 2005, 18, 814–816. [Google Scholar] [CrossRef]
- Munday, R.; Holland, P.; McNabb, P.; Selwood, A.; Rhodes, L. Comparative toxicity to mice of domoic acid and isodomoic acids A, B and C. Toxicon 2008, 52, 954–956. [Google Scholar] [CrossRef]
- Ohfune, Y.; Tomita, M. Total synthesis of (-)-domoic acid. A revision of the original structure. J. Am. Chem. Soc. 1982, 104, 3511–3513. [Google Scholar] [CrossRef]
- Savage, T.J.; Smith, G.J.; Clark, A.T.; Saucedo, P.N. Condensation of the isoprenoid and amino precursors in the biosynthesis of domoic acid. Toxicon 2012, 59, 25–33. [Google Scholar] [CrossRef]
- Brunson, J.K.; McKinnie, S.M.K.; Chekan, J.R.; McGrow, J.P.; Miles, Z.D.; Bertrand, E.M.; Bielinsky, V.A.; Moore, B.S.; Luhavaya, H.; Obornik, M.; et al. Biosynthesis of the neurotoxin domoic acid in a bloom-forming diatom. Science 2018, 361, 1356–1358. [Google Scholar] [CrossRef]
- McCabe, R.; Hickey, B.; Kudela, R.; Lefebvre, K.; Adams, N.; Bill, B.; Gulland, F.; Thomson, R.; Cochlan, W.; Trainer, V. An unprecedented coastwide toxic algal bloom linked to anomalous ocean conditions. Geophys. Res. Lett. 2016, 43, 10366–10376. [Google Scholar] [CrossRef] [PubMed]
- Bates, S.; Douglas, D.; Doucette, G.; Leger, C. Enhancement of domoic acid production by reintroducing bacteria to axenic cultures of the diatom Pseudo-nitzschia multiseries. Nat. Toxins 1995, 3, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Orlova, T.; Stonik, I.; Aizdaicher, N.; Bates, S.; Leger, C.; Fehling, J. Toxicity, morphology and distribution of Pseudo-nitzschia calliantha, P. multistriata and P. multiseries (Bacillariophyta) from the northwestern Sea of Japan. Bot. Mar. 2008, 51, 297–306. [Google Scholar] [CrossRef]
- Kotaki, Y.; Koike, K.; Yoshida, M.; Thuoc, C.; Huyen, N.; Hoi, N.; Fukuyo, Y.; Kodama, M. Domoic acid production in Nitzschia sp. (Bacillariophyceae) isolated from a shrimp-culture pond in Do Son, Vietnam. J. Phycol. 2000, 36, 1057–1060. [Google Scholar] [CrossRef]
- Olson, M.; Lessard, E.; Wong, C.; Bernhardt, M. Copepod feeding selectivity on microplankton, including the toxigenic diatom, Pseudo-nitzschia spp., in the coastal Pacific Northwest. Mar. Ecol. Prog. Ser. 2006, 326, 207–220. [Google Scholar] [CrossRef]
- Maldonado, M.; Hughes, M.; Rue, E.; Wells, M. The effect of Fe and Cu on growth and domoic acid production by Pseudo-nitzschia multiseries and Pseudo-nitzschia australis. Limnol. Oceanogr. 2002, 47, 515–526. [Google Scholar] [CrossRef]
- Trick, C.; Bill, B.; Cochlan, W.; Wells, M.; Trainer, V.; Pickell, L. Iron enrichment stimulates toxic diatom production in high-nitrate, low-chlorophyll areas. Proc. Natl. Acad. Sci. USA 2010, 107, 5887–5892. [Google Scholar] [CrossRef]
- Sakai, R.; Kamiya, H.; Murata, M.; Shimamoto, K. Dysiherbaine: A new neurotoxic amino acid from the Micronesian marine sponge Dysidea herbaceae. J. Am. Chem. Soc. 1997, 119, 4112–4116. [Google Scholar] [CrossRef]
- Sakai, R.; Swanson, G.T.; Shimamoto, K.; Green, T.; Contractor, A.; Ghetti, A.; Tamura-Horikawa, Y.; Oiwa, C.; Kamiya, H. Pharmacological properties of the potent epileptogenic amino acid dysiherbaine, a novel glutamate receptor agonist isolated from the marine sponge Dysidea herbacea. J. Pharm. Exp. Theurap. 2001, 296, 650–658. [Google Scholar] [PubMed]
- Sakai, R.; Koike, T.; Sasaki, M.; Shimamoto, K.; Oiwa, C.; Yano, A.; Suzuki, K.; Tachibana, K.; Kamiya, H. Isolation, structure determination, and syntheses of neodysiherbaine A, a new excitatory amino acid from a marine sponge. Org. Lett. 2001, 3, 1479–1482. [Google Scholar] [CrossRef]
- Cachet, X.; Poree, F.-H. Total synthesis of dysiherbaine and neodysiherbaine A. RSC Adv. 2013, 3, 12466–12484. [Google Scholar] [CrossRef]
- Rao, M.V.; Naresh, A.; Saketh, G.; Rao, B.V. Formal synthesis of dysiherbaine. Tetr. Lett. 2013, 54, 6931–6933. [Google Scholar] [CrossRef]
- Do, H.; Kang, C.W.; Cho, J.H.C.; Gilbertson, S.R. Enantioselective synthesis of (-) -dysiherbaine. Org. Lett. 2015, 17, 3972–3974. [Google Scholar] [CrossRef] [PubMed]
- Hirai, T.; Shibata, K.; Niwano, Y.; Shiozuki, M.; Hashimoto, Y.; Morita, N.; Ban, S.; Tamura, O. Total synthesis of neodysiherbaine A via 1,3-dipolar cycloaddition of a chiral nitrone template. Org. Lett. 2017, 19, 6320–6323. [Google Scholar] [CrossRef]
- Sakai, R.; Yoshida, K.; Kimura, A.; Koike, K.; Jimbo, M.; Koike, K.; Kobiyama, A.; Kamiya, H. Cellular origin of dysiherbaine, an excitatory amino acid derived from a marine sponge. ChemBioChe 2008, 9, 543–551. [Google Scholar] [CrossRef]
Sample Availability: Not available. |








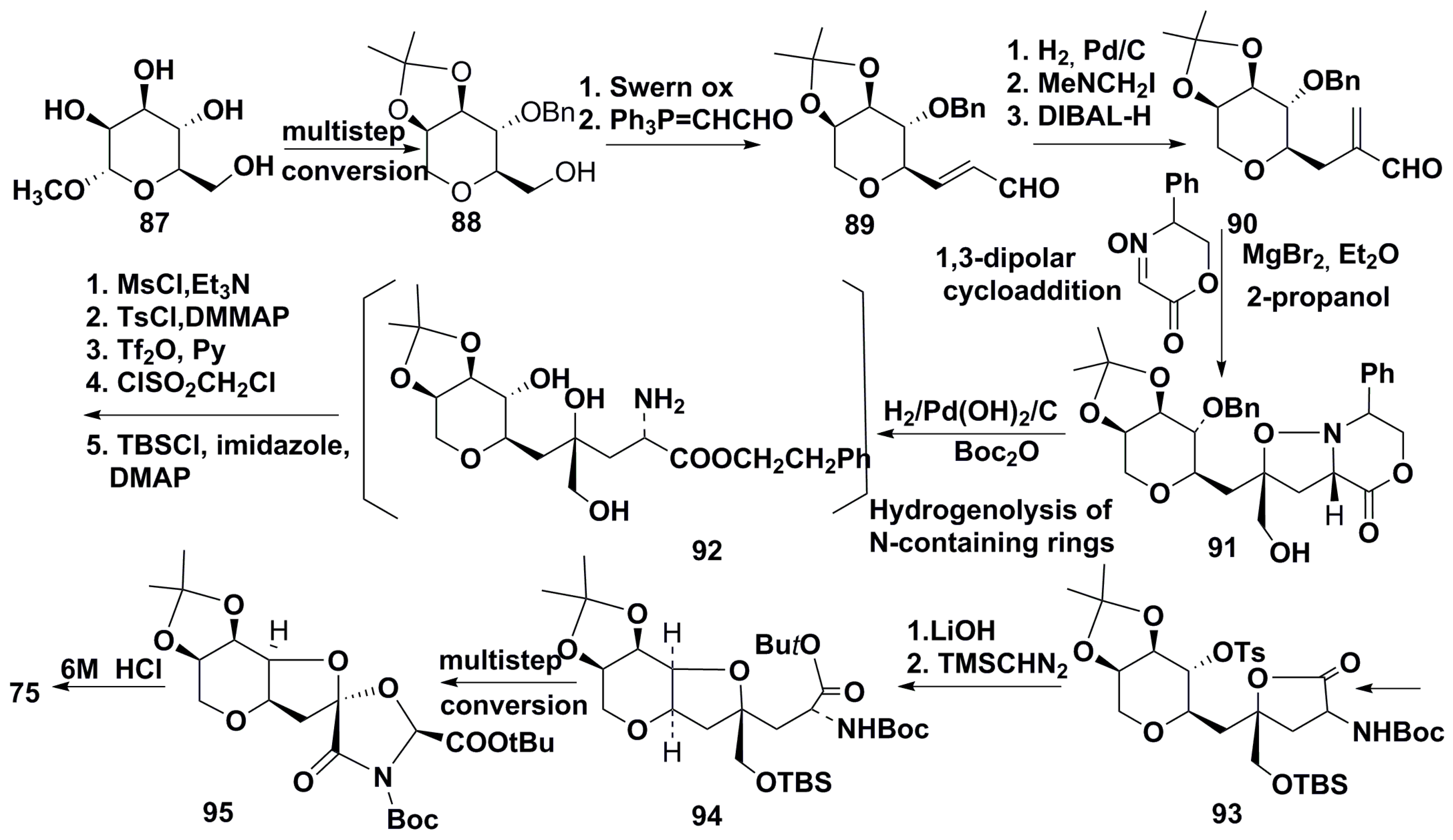
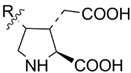
| Compound | R (Side Chain)= | Name | KARs, μM | References |
|---|---|---|---|---|
| 3 | 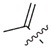 | Domoic acid | 2.2 | [38,44,45] |
| 48 |  | Isodomoic acid A | 4.4 | [39,42] |
| 49 |  | Isodomoic acid B | 4990 | [39,42] |
| 50 | 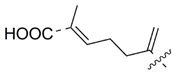 | Isodomoic acid C | 171 | [39,42] |
| 51 | 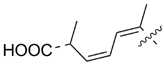 | Isodomoic acid D | 600 | [40,41] |
| 52 | 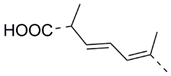 | Isodomoic acid E | 600 | [42] |
| 53 | 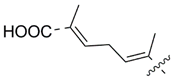 | Isodomoic acid F | 67 | [42] |
| 54 |  | Isodomoic acid G | [41,42] | |
| 55 | 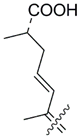 | Isodomoic acid H | [41,42] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stonik, V.A.; Stonik, I.V. Marine Excitatory Amino Acids: Structure, Properties, Biosynthesis and Recent Approaches to Their Syntheses. Molecules 2020, 25, 3049. https://doi.org/10.3390/molecules25133049
Stonik VA, Stonik IV. Marine Excitatory Amino Acids: Structure, Properties, Biosynthesis and Recent Approaches to Their Syntheses. Molecules. 2020; 25(13):3049. https://doi.org/10.3390/molecules25133049
Chicago/Turabian StyleStonik, Valentin A., and Inna V. Stonik. 2020. "Marine Excitatory Amino Acids: Structure, Properties, Biosynthesis and Recent Approaches to Their Syntheses" Molecules 25, no. 13: 3049. https://doi.org/10.3390/molecules25133049
APA StyleStonik, V. A., & Stonik, I. V. (2020). Marine Excitatory Amino Acids: Structure, Properties, Biosynthesis and Recent Approaches to Their Syntheses. Molecules, 25(13), 3049. https://doi.org/10.3390/molecules25133049