Noble-Gas Chemistry More than Half a Century after the First Report of the Noble-Gas Compound
Abstract
1. Introduction
2. Xenon
2.1. Xenon(II)
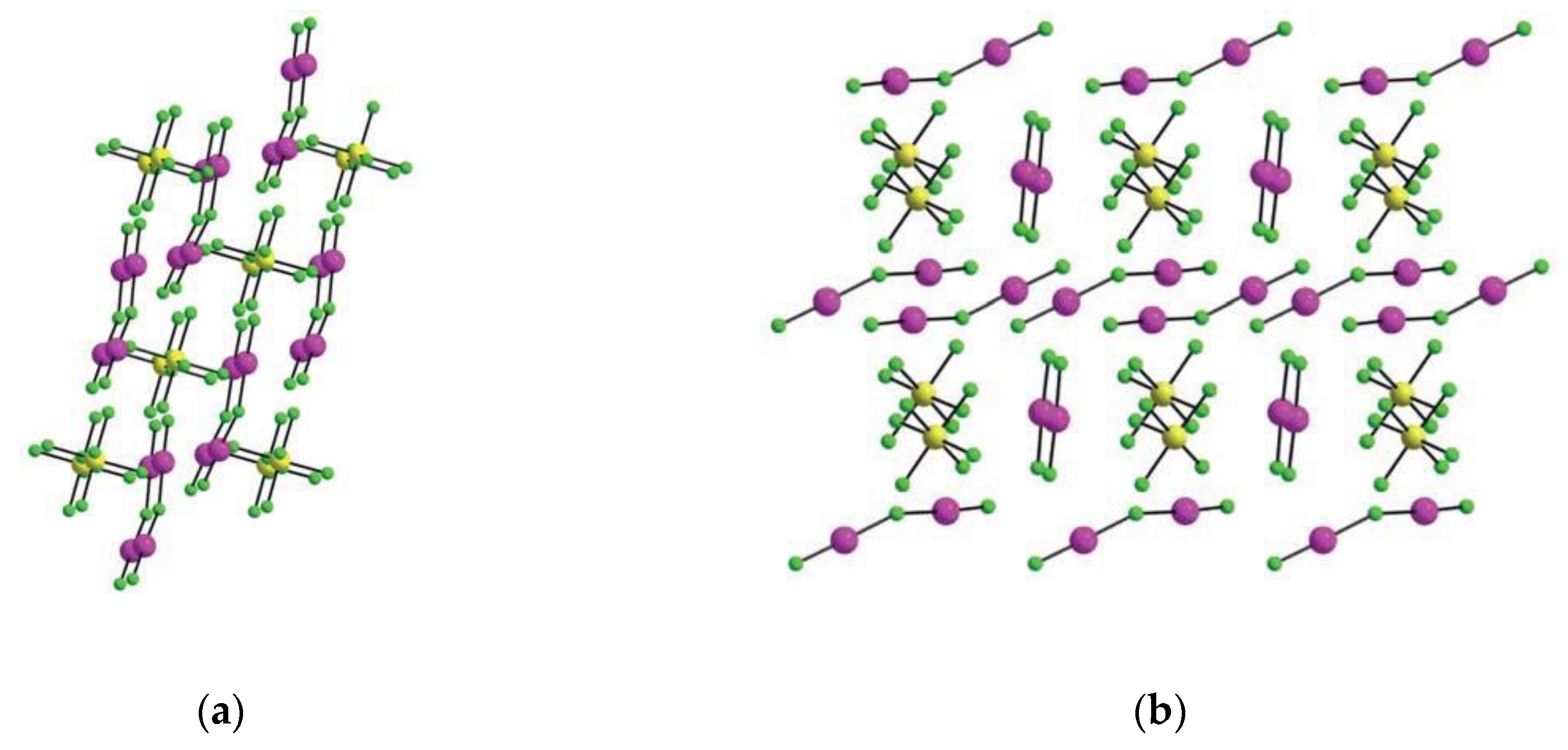
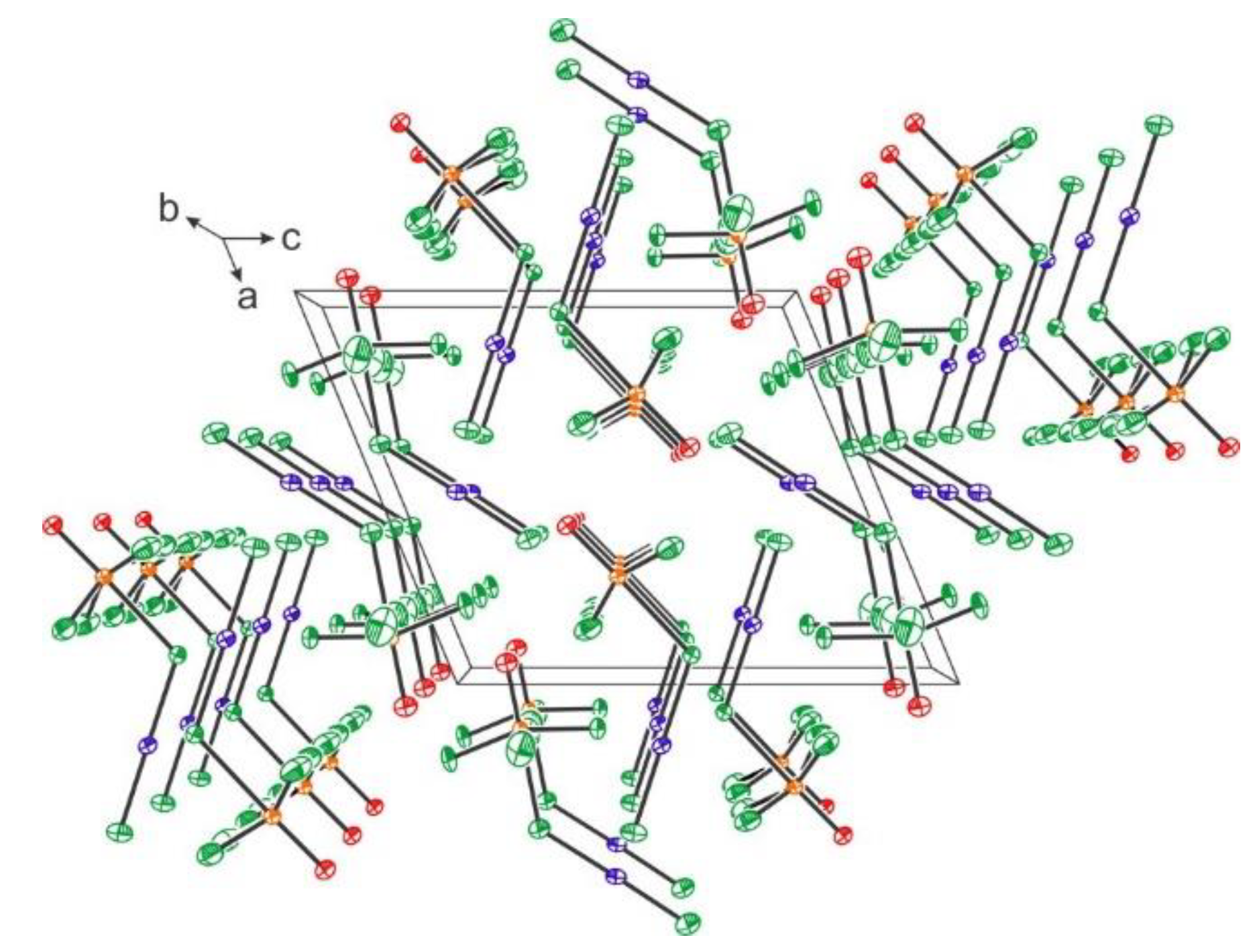
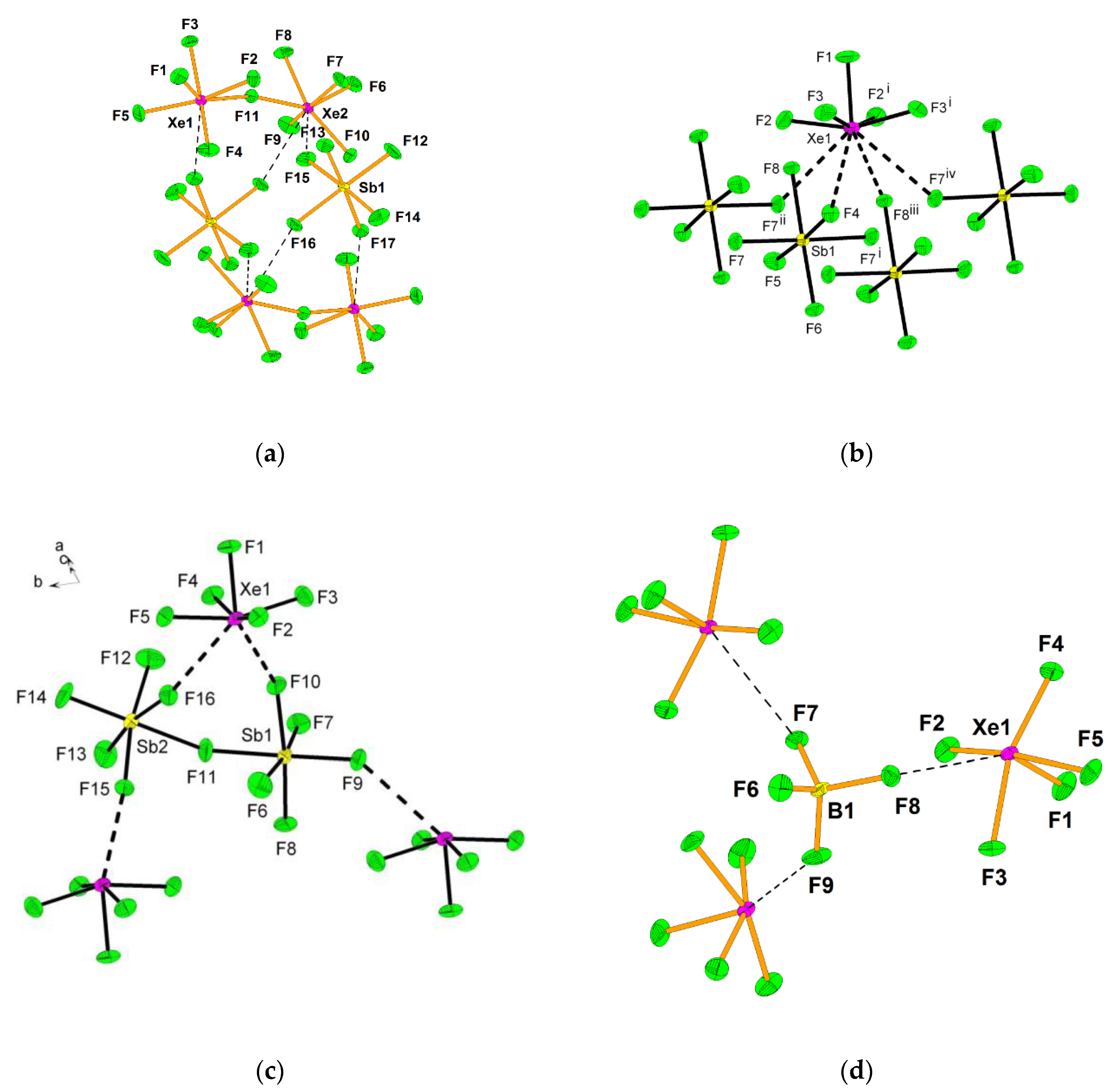
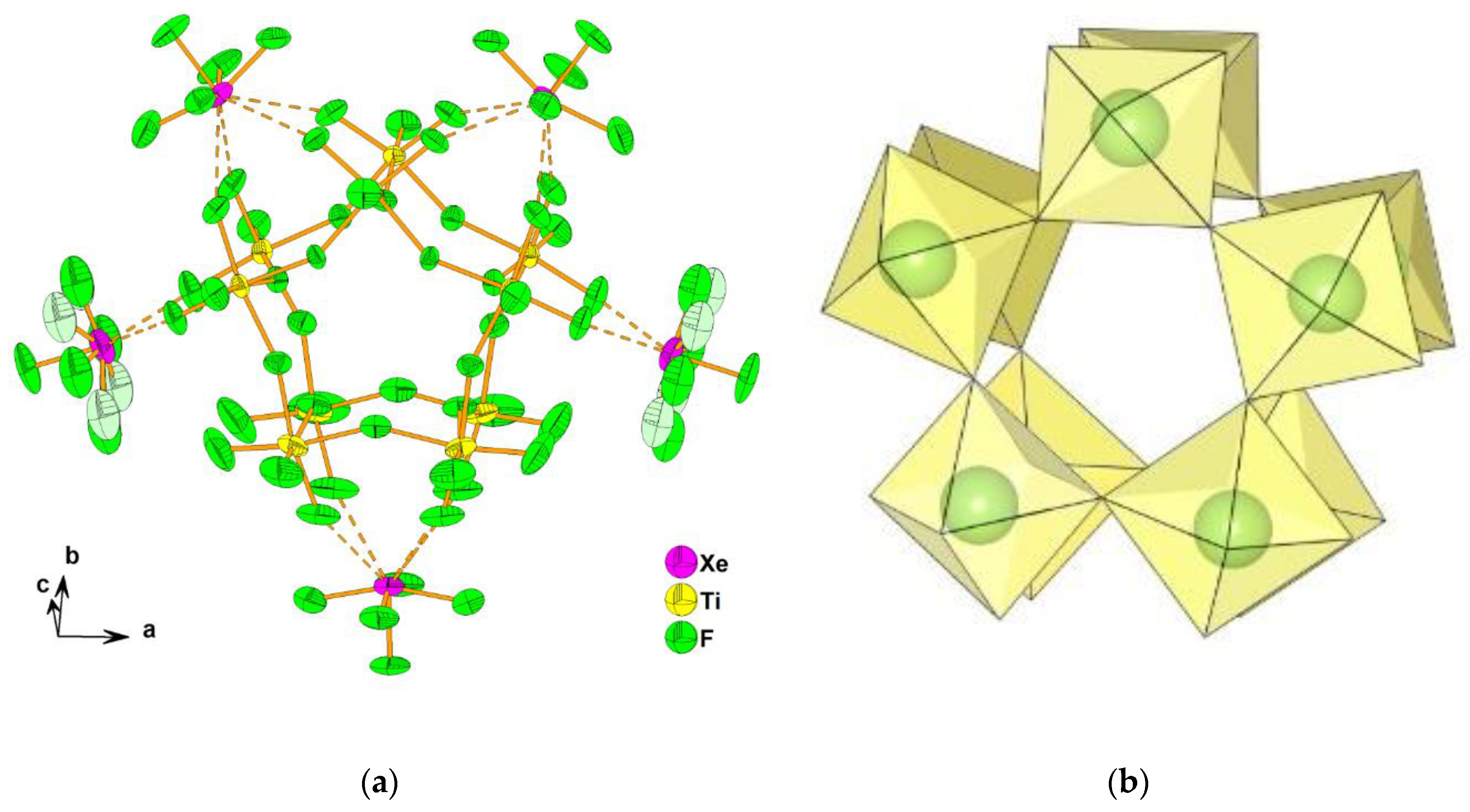
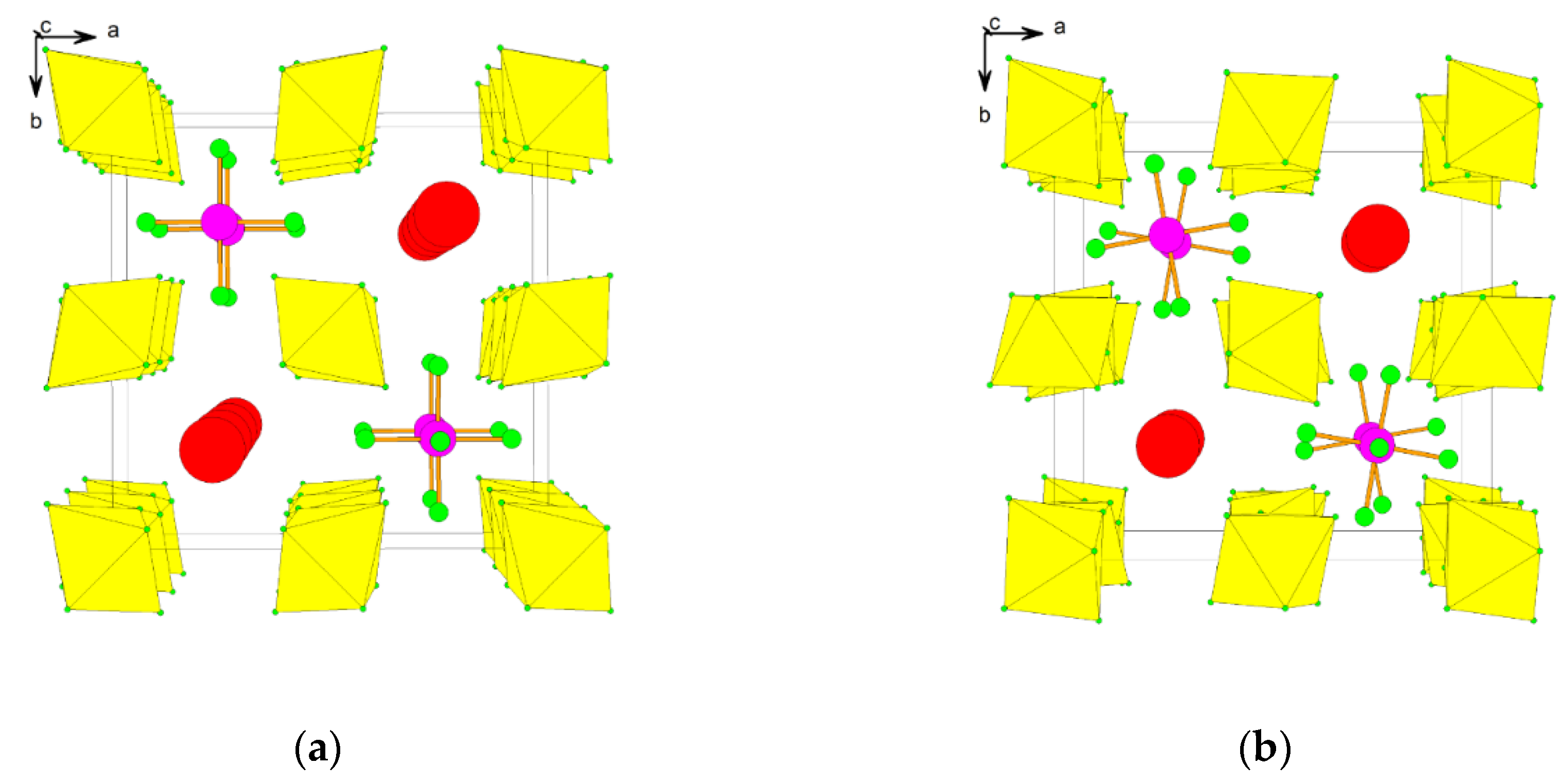
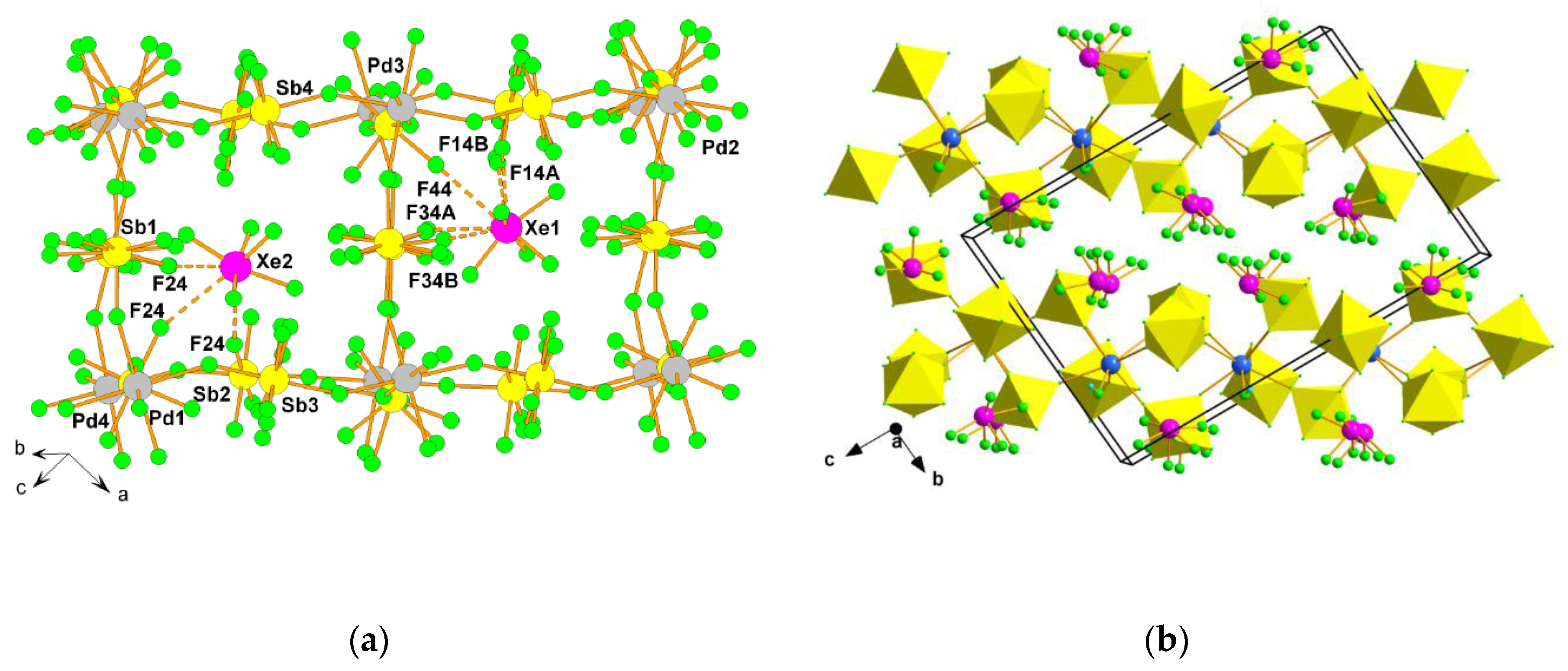
2.2. Xenon(VI) Fluoride and its [XeF5]+ and [Xe2F11]+ Salts
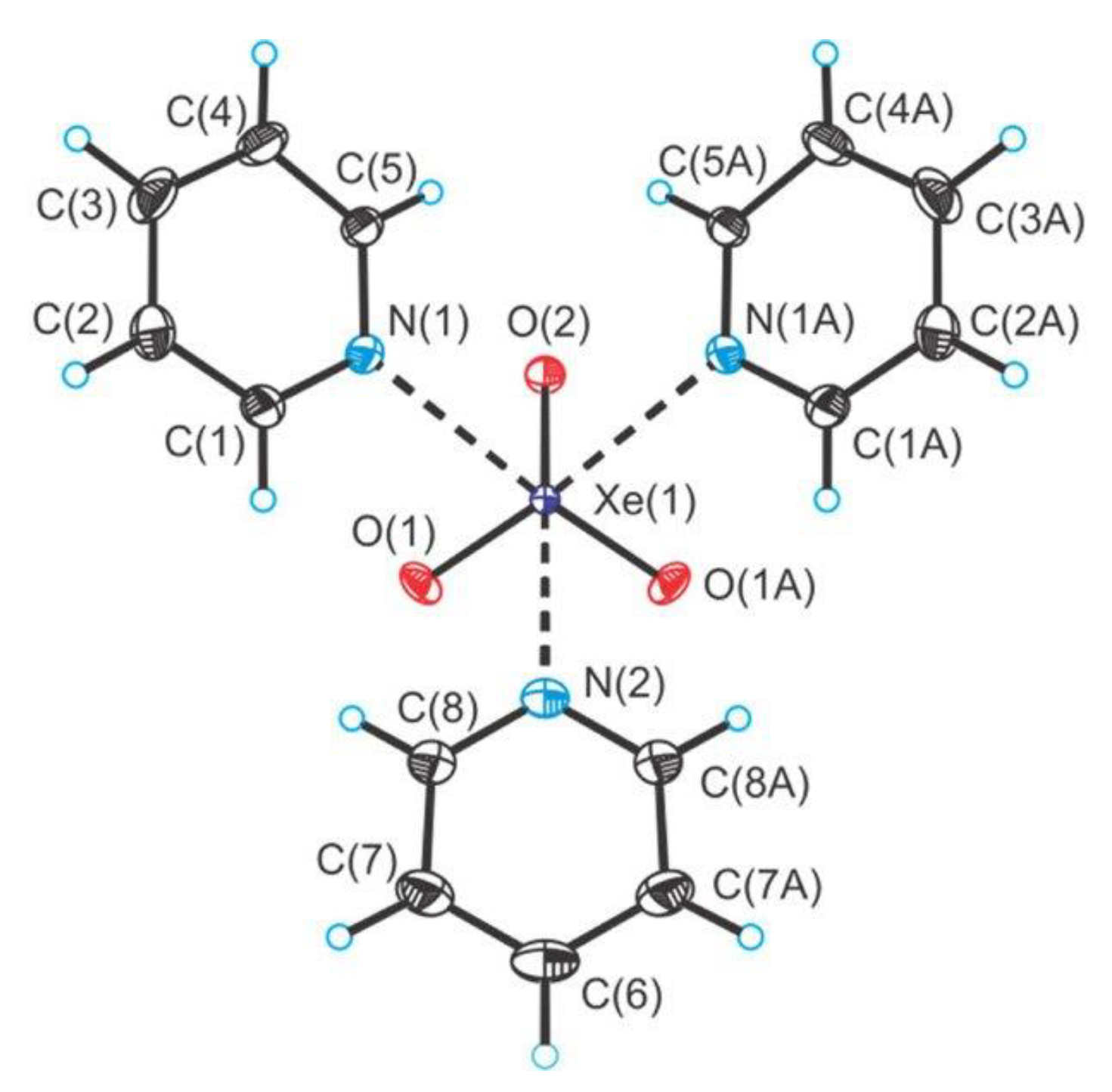
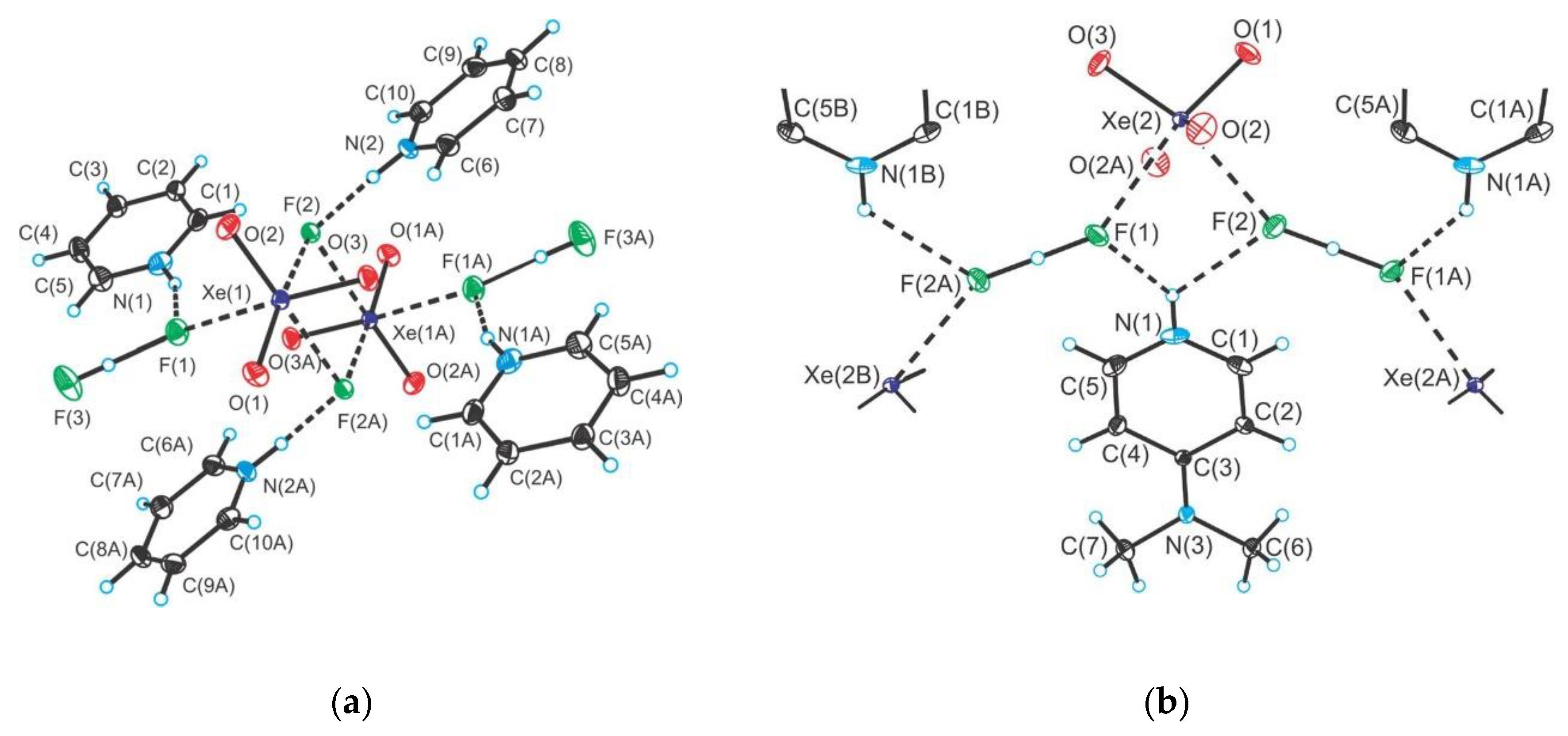
2.3. Xenon(VI) Oxide Compounds
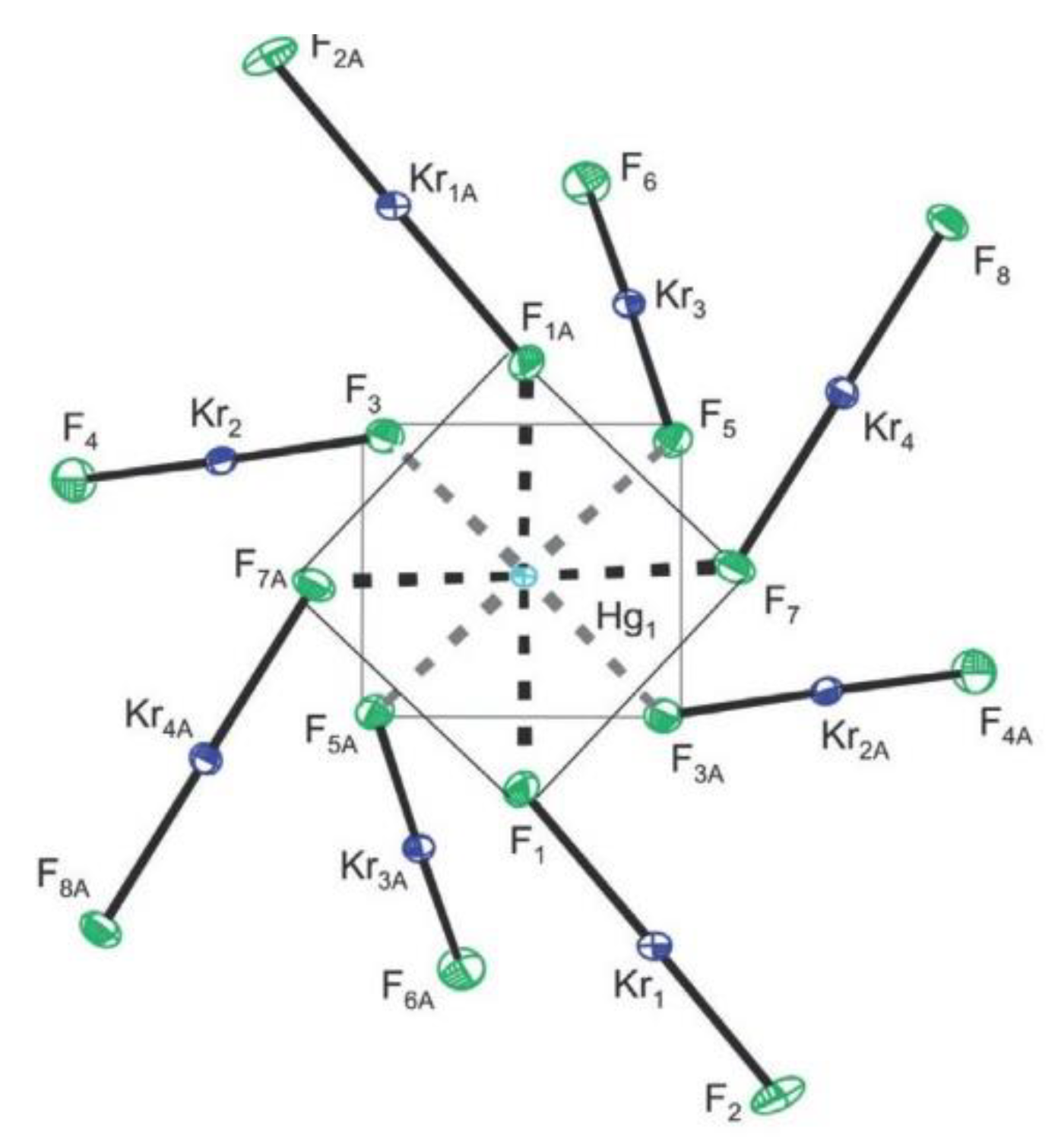
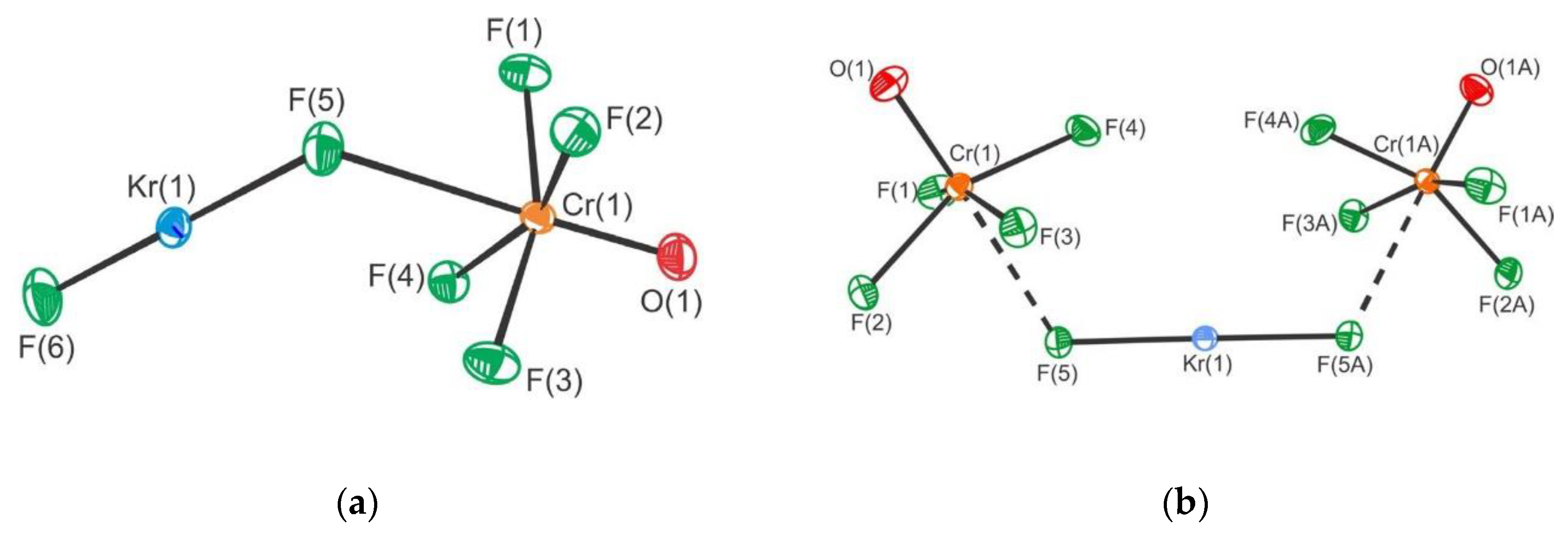
3. Krypton
4. Argon, Neon, Helium
4.1. Chemistry of Argon
4.2. Chemistry of Neon and Helium
5. Bonding Motifs in Noble-Gas Compounds
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Christe, K.O. A renaissance in noble gas chemistry. Angew. Chem. Int. Ed. 2001, 40, 1419–1421. [Google Scholar] [CrossRef]
- Grandinetti, F. Noble Gas Chemistry, Structure, Bonding and Gas-Phase Chemistry, 1st ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2018. [Google Scholar]
- Haner, J.; Schrobilgen, G.J. The chemistry of xenon(IV). Chem. Rev. 2015, 115, 1255–1295. [Google Scholar] [CrossRef]
- Nabiev, S.S.; Sokolov, V.B.; Chaivanov, B.B. Molecular and crystal structures of noble gas compounds. Russ. Chem. Rev. 2014, 83, 1135–1180. [Google Scholar] [CrossRef]
- Brock, D.S.; Schrobilgen, G.J.; Žemva, B. Noble-Gas Chemistry. In Comprehensive Inorganic Chemistry II, 2nd ed.; Reedijk, J., Poeppelmeier, K., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2013; Volume 1, pp. 755–822. [Google Scholar]
- Hope, E.G. Coordination chemistry of the noble gases and noble gas fluorides. Coord. Chem. Rev. 2013, 257, 902–909. [Google Scholar] [CrossRef]
- Saha, R.; Jana, G.; Pan, S.; Merino, G.; Chattaraj, P.K. How far can one push the noble gases towards bonding? A personal account. Molecules 2019, 24, 2933. [Google Scholar] [CrossRef]
- Grochala, W. On the position of helium and neon in the periodic table of elements. Found. Chem. 2018, 20, 191–207. [Google Scholar] [CrossRef]
- Halpern, D.F.; Tavčar, G.; Tramšek, M. Xenon(II) Fluoride. In e-EROS Encyclopedia of Reagents for Organic Synthesis; John Wiley & Sons: Hoboken, NJ, USA, 2017; Available online: https://onlinelibrary.wiley.com/doi/10.1002/047084289X.rx001.pub2 (accessed on 29 June 2020).
- Campbell, M.G.; Hoover, A.J.; Ritter, T. Transition Metal-Mediated and Metal-Catalyzed Carbon–Fluorine Bond Formation. In Organometallic Fluorine Chemistry, 1st ed.; Braun, T., Hughes, R.P., Eds.; Springer: Cham, Switzerland, 2015; pp. 1–54. [Google Scholar]
- Xia, D.; Notte, J.; Stern, L.; Goetze, B. Enhancement of XeF2-assisted gallium ion beam etching of silicon layer and endpoint detection from backside in circuit editing. J. Vac. Sci. Technol. B 2015, 33, 06F501. [Google Scholar] [CrossRef]
- Sarkar, D.; Baboly, M.G.; Elahi, M.M.; Abbas, K.; Butner, J.; Piñon, D.; Ward, T.L.; Hieber, T.; Schuberth, A.; Leseman, Z.C. Determination of etching parameters for pulsed XeF2 etching of silicon using chamber pressure data. J. Micromech. Microeng. 2018, 28, 045007. [Google Scholar] [CrossRef]
- Murdzek, J.A.; George, S.M. Effect of crystallinity on thermal atomic layer etching of hafnium oxide, zirconium oxide, and hafnium zirconium oxide. J. Vac. Sci. Technol. A 2020, 38, 022608. [Google Scholar] [CrossRef]
- Johnson, N.R.; Hite, J.K.; Mastro, M.A.; Eddy, C.R., Jr.; George, S.M. Thermal atomic layer etching of crystalline GaN using sequential exposures of XeF2 and BCl3. Appl. Phys. Lett. 2019, 114, 243103. [Google Scholar] [CrossRef]
- Zhang, R.; Drysdale, D.; Koutsos, V.; Cheung, R. Controlled layer thinning and p-type doping of WSe2 by vapor XeF2. Adv. Funct. Mater. 2017, 27, 1702455. [Google Scholar] [CrossRef]
- Alias, M.S.; Yang, Y.; Ng, T.K.; Dursun, I.; Shi, D.; Saidaminov, M.I.; Priante, D.; Bakr, O.M.; Ooi, B.S. Enhanched etching, surface damage recovery, and submicron patterning of hybrid perovskites using a chemically gas-assisted focused-ion beam for subwavelength grating photonic applications. J. Phys. Chem. Lett. 2016, 7, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Bulusheva, L.G.; Okotrub, A.V. Electronic Structure of Fluorinated Graphene. In New Fluorinated Carbons:Fundamentals and Applications, 1st ed.; Boltalina, O.V., Nakajima, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 177–213. [Google Scholar]
- Copetti, G.; Nunes, E.H.; Soares, G.V.; Radtke, C. Mitigating graphene etching on SiO2 during fluorination by XeF2. Mater. Lett. 2019, 252, 11–14. [Google Scholar] [CrossRef]
- Wang, A.; Bok, S.; Mathai, C.J.; Thiruvengadathan, R.; Darr, C.M.; Chen, H.; Zachariah, M.R.; Gangopadhyay, K.; McFarland, J.A.; Maschmann, M.R.; et al. Synthesis, characterization and nanoenergetic utillizations of fluorine, oxygen co-functionalized graphene by one-step XeF2 exposure. Combust. Flame 2020, 215, 324–332. [Google Scholar] [CrossRef]
- Slater, P.; Driscoll, L. Modification of Magnetic and Electronic Properties, in Particular Superconductivity, by Low Temperature Insertion of Fluorine into Oxides. In Photonic and Electronic Properties of Fluoride Materials, 1st ed.; Tressaud, A., Poepelmeier, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 401–421. [Google Scholar]
- Preshlock, S.; Tredwell, M.; Gouverneur, V. 18F-labeling of arenes and heteroarenes for applications in positron emission tomography. Chem. Rev. 2016, 116, 719–766. [Google Scholar] [CrossRef]
- Edel, K.; Ishibashi, J.S.A.; Liu, S.-Y.; Bettinger, H.F. Superelectrophilicity of 1,2-azadiborane: Formation of xenon and carbon monoxide adducts. Angew. Chem. Int. Ed. 2019, 58, 4061–4064. [Google Scholar] [CrossRef]
- Seppelt, K. Metal-xenon complexes. Z. Anorg. Allg. Chem. 2003, 629, 2427–2430. [Google Scholar] [CrossRef]
- Zhong, J.-Q.; Wang, M.; Akter, N.; Stacchiola, D.J.; Lu, D.; Boscoboinik, J.A. Room-temperature in vacuo chemisorption of xenon atoms on Ru(0001) under interface confinement. J. Phys. Chem. C 2019, 123, 13578–13585. [Google Scholar] [CrossRef]
- Boscoboinik, J.A. Chemistry in confined space through the eyes of surface science-2D porous materials. J. Phys. Condens. Matter 2019, 31, 063001. [Google Scholar] [CrossRef]
- Hagiwara, R.; Hollander, F.; Maines, C.; Bartlett, N. The crystal structure of [Ag(XeF2)2]AsF6 formed in the oxidation of Xe by AgFAsF6. Eur. J. Solid State Chem. 1991, 28, 855–866. [Google Scholar]
- Tavčar, G.; Tramšek, M. XeF2 as a ligand to a metal center, an interesting filed of noble gas chemistry. J. Fluorine. Chem. 2015, 174, 14–21. [Google Scholar] [CrossRef]
- Mercier, H.P.A.; Breddemann, U.; Brock, D.S.; Bortolus, M.R.; Schrobilgen, G.J. Syntheses, structures and bonding of NgF2·CrOF4, NgF2·2CrOF4 (Ng = Kr, Xe), and (CrOF4)∞. Chem. Eur. J. 2019, 25, 12105–12119. [Google Scholar] [CrossRef] [PubMed]
- Tramšek, M.; Goreshnik, E.; Tavčar, G. Oxidation of ruthenium and iridium metal by XeF2 and crystal structure determination of [Xe2F3][RuF6]·XeF2 and [Xe2F3][MF6] (M = Ru, Ir). Acta Chim. Slov. 2016, 63, 369–375. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gawrilow, M.; Beckers, H.; Riedel, S.; Cheng, L. Matrix-isolation and quantum-chemical analysis of the C3v conformer of XeF6, XeOF4, and their acetonitrile adducts. J. Phys. Chem. A 2018, 122, 119–129. [Google Scholar] [CrossRef]
- Atwood, D.A. Noble Gases: Inorganic Chemistry. In Encyclopedia of Inorganic and Bioinorganic Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2011; Available online: https://doi.org/10.1002/9781119951438.eibc0150 (accessed on 29 June 2020).
- Mazej, Z.; Goreshnik, E. Crystal growth and characterization of the mixed-cation Rb+/[XeF5]+ and Cs+/[XeF5]+ salts. Eur. J. Inorg. Chem. 2017, 2017, 2800–2807. [Google Scholar] [CrossRef]
- Mazej, Z.; Goreshnik, E.A. Single-crystal structure determination of NO2SbF6, XeF5SbF6 and XeF5Sb2F11. J. Fluorine Chem. 2015, 175, 47–50. [Google Scholar] [CrossRef]
- Mazej, Z. Photochemical Syntheses of Fluorides in Liquid Anhydrous Hydrogen Fluoride. In Modern Synthesis Processes and Reactivity of Fluorinated Compounds, 1st ed.; Groult, H., Leroux, F., Tressaud, A., Eds.; Elsevier: London, UK, 2017; pp. 587–607. [Google Scholar]
- Mazej, Z.; Goreshnik, E.A. Largest perfluorometallate [Ti10F45]− oligomer and polymeric ([Ti3F13]−)∞ and ([TiF5]−)∞ anions prepared as [XeF5]+ salts. New. J. Chem. 2016, 40, 7320–7325. [Google Scholar] [CrossRef]
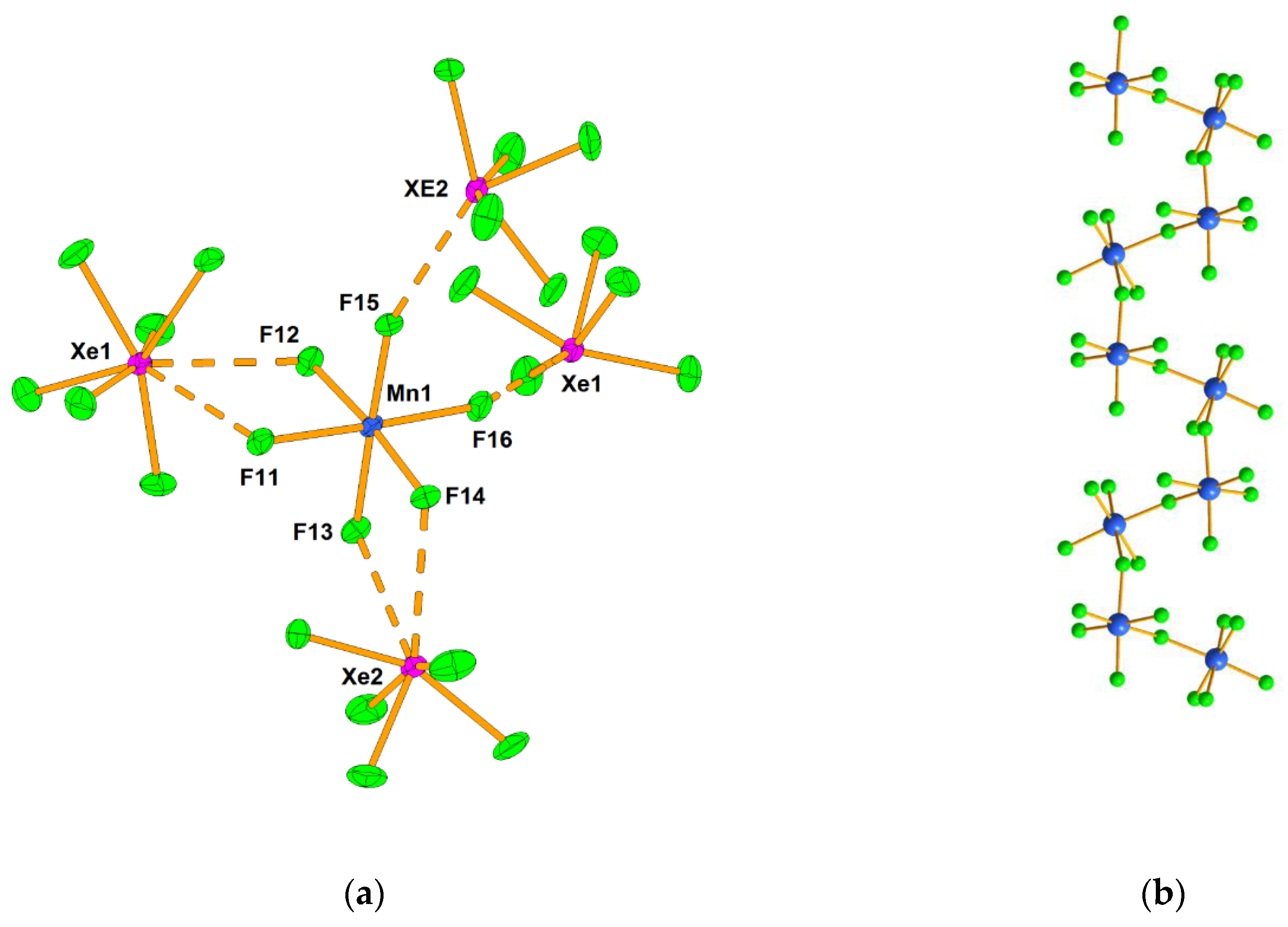
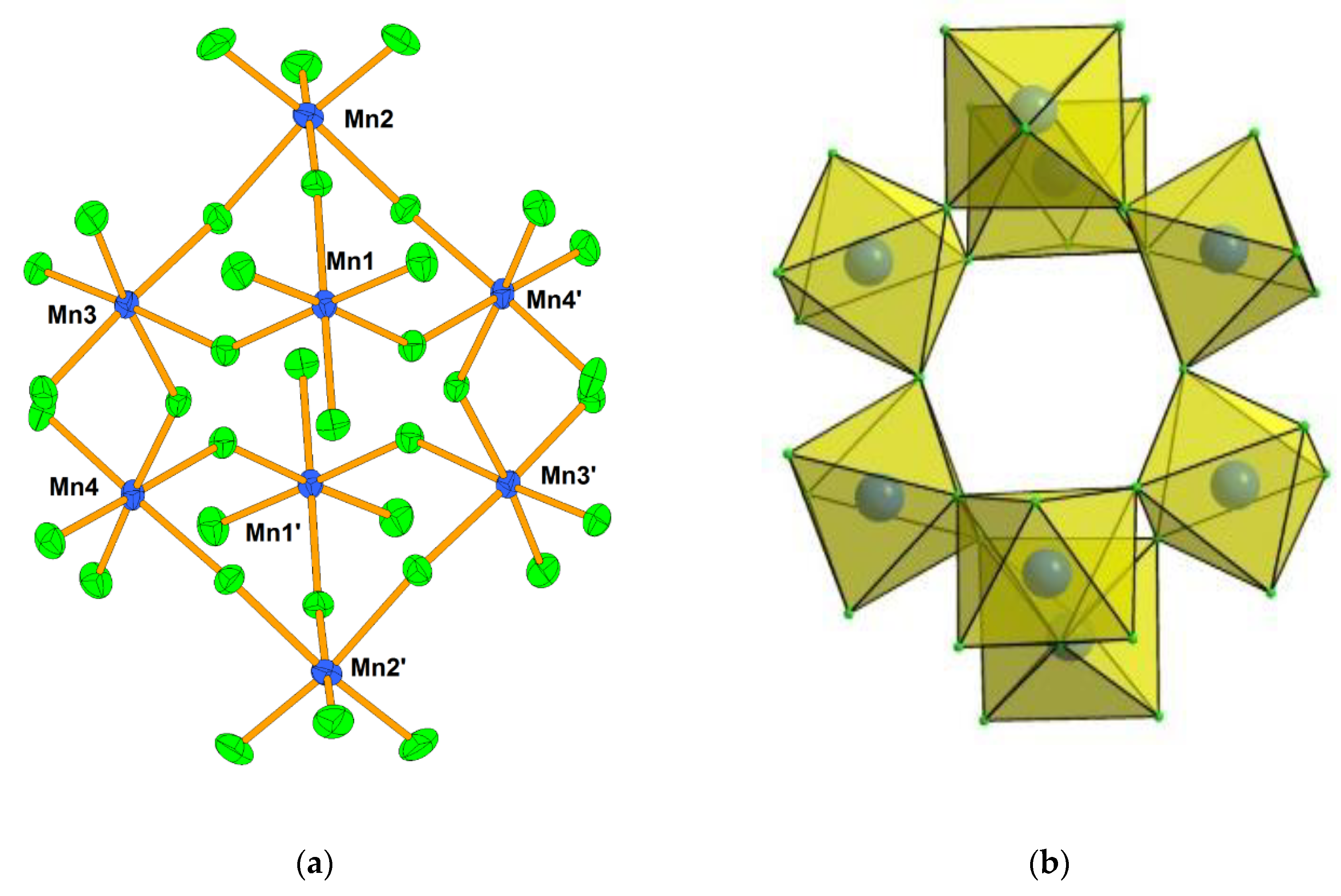
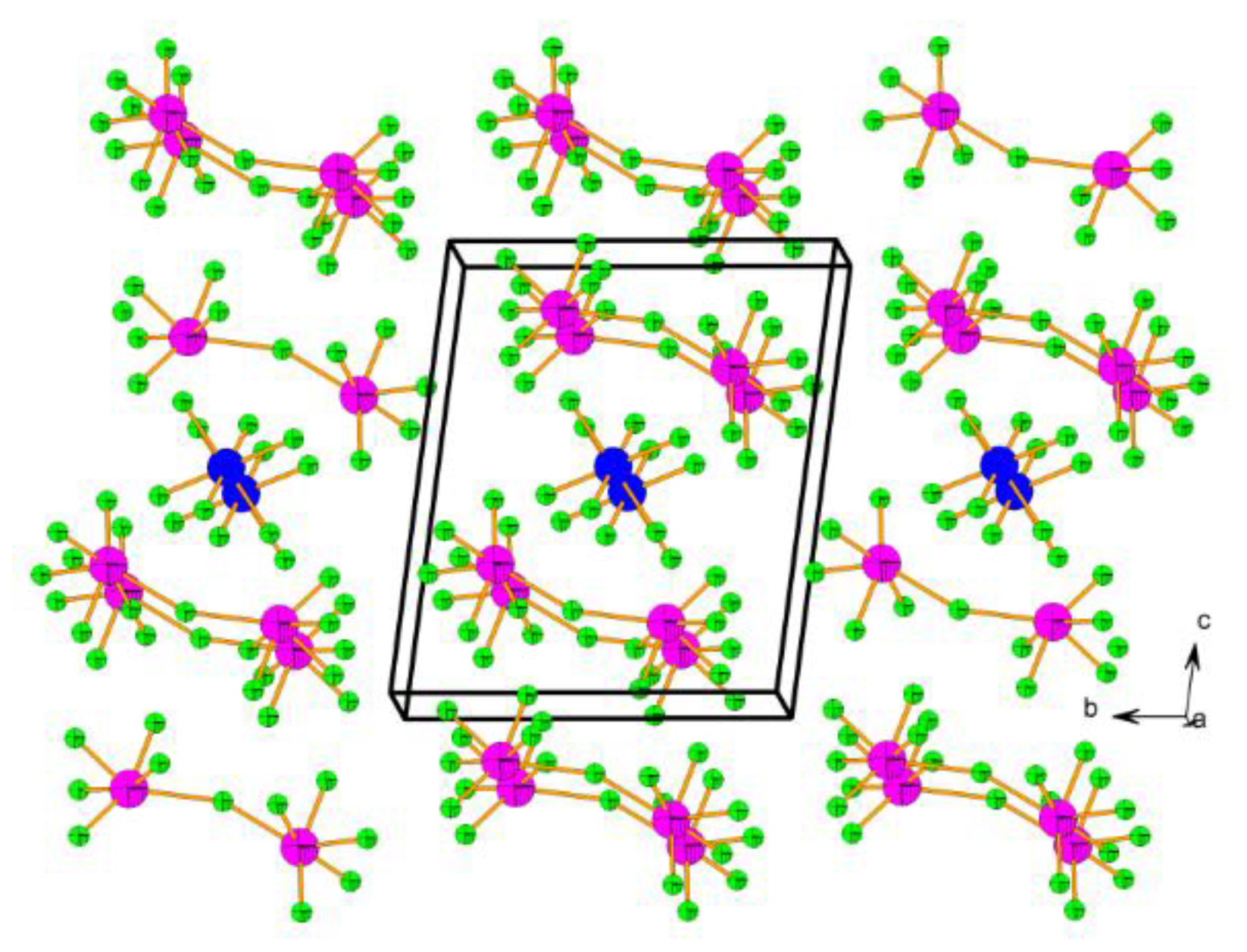
- Mazej, Z.; Goreshnik, E.; Jagličić, Z.; Filinchuk, Y.; Tumanov, N.; Akslerud, L.G. Photochemical synthesis and characterization of xenon(VI) hexafluoridomanaganates(IV). Eur. J. Inorg. Chem. 2017, 2017, 2130–2137. [Google Scholar] [CrossRef]
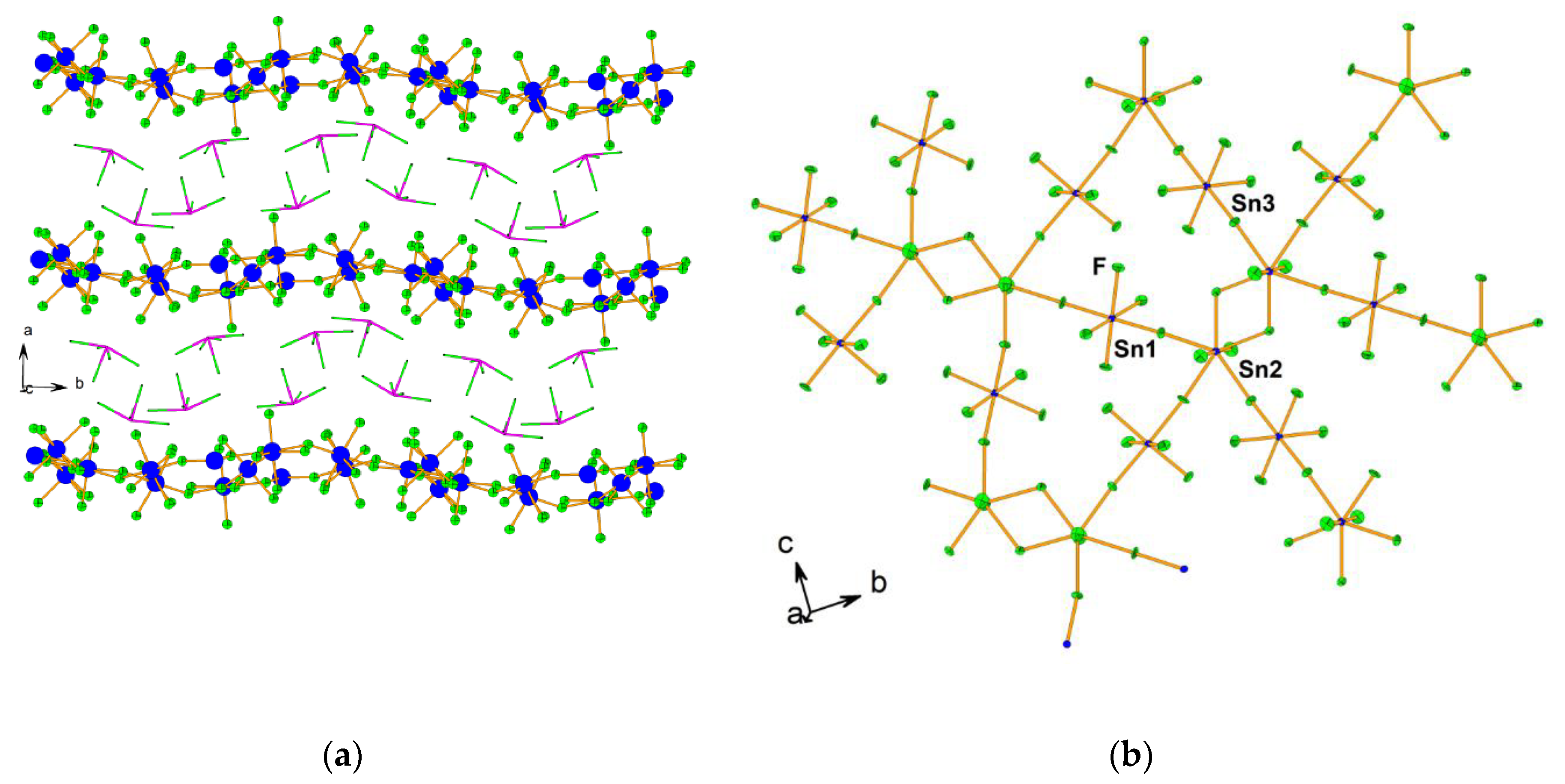
- Mazej, Z.; Goreshnik, E. Crystal structures of photochemically prepared (Xe2F11)2(MF6) (M = Sn, Pb) and (XeF5)4(Sn5F24) containing six- and seven-coordinated tin(IV). Eur. J. Inorg. Chem. 2019, 2019, 1265–1272. [Google Scholar] [CrossRef]
- Goettel, J.T.; Bortolus, M.R.; Stuart, D.G.; Mercier, H.P.A.; Schrobilgen, G.J. Chromium oxide tetrafluoride and its reactions with xenon hexafluoride; the [XeF5]+ and [Xe2F11]+ salts of the [CrVIOF5]−, [CrVOF5]2−, [CrV2O2F8]2−, and [CrIVF6]2− anions. Chem. Eur. J. 2019, 25, 15815–15829. [Google Scholar] [CrossRef] [PubMed]
- Mazej, Z.; Goreshnik, E. [XeF5]+/metal and [XeF5]+/non-metal mixed-cation salts of hexafluoridoantimonate(V). Eur. J. Inorg. Chem. 2015, 2015, 1453–1456. [Google Scholar] [CrossRef]
- Mazej, Z.; Goreshnik, E. Influence of the increasing size of the M2+ cation on the crystal structures of XeF5M(SbF6)3 (M = Ni, Mg, Cu, Zn, Co, Mn, Pd) and (XeF5)3[Hg(HF)2](SbF6)7. Eur. J. Inorg. Chem. 2016, 2016, 3356–3364. [Google Scholar] [CrossRef]
- Marczenko, K.M.; Mercier, H.P.A.; Schrobilgen, G.J. A stable crown ether complex with a noble-gas compound. Angew. Chem. Int. Ed. 2018, 57, 12448–12452. [Google Scholar] [CrossRef] [PubMed]
- Marczenko, K.M.; Goettel, J.T.; Mercier, H.P.A.; Schrobilgen, G.J. Xenon trioxide adducts of O-donor ligands; [(CH3)2CO]3XeO3, [(CH3)2SO]3(XeO3)2, (C2H5NO)3(XeO3)2, and [(C6H5)3PO]2XeO3. Chem. Eur. J. 2019, 25, 12357–12366. [Google Scholar] [CrossRef] [PubMed]
- Goettel, J.T.; Mercier, H.P.A.; Schrobilgen, G.J. XeO3 as adducts of pyridine, 4-dimethylaminopyriridine, and their pyridinium salts. J. Fluorine Chem. 2018, 211, 60–69. [Google Scholar] [CrossRef]
- Lehmann, J.F.; Mercier, H.P.A.; Schrobilgen, G.J. The chemistry of krypton. Coord. Chem. Rev. 2002, 233–234, 1–39. [Google Scholar] [CrossRef]
- DeBackere, J.R.; Schrobilgen, G.J. A homoleptic KrF2 complex, [Hg(KrF2)8][AsF6]2·2HF. Angew. Chem. Int. Ed. 2018, 57, 13167–13171. [Google Scholar] [CrossRef]
- Linnartz, H.; Verdes, D.; Maier, J.P. Rotationally resolved infrared spectrum of the charge transfer complex [Ar–N2]+. Science 2002, 297, 116–1167. [Google Scholar] [CrossRef][Green Version]
- Ascenzi, D.; Tosi, P.; Roithova, J.; Ricketts, C.L.; Schröder, D.; Lockyear, J.F.; Parkes, M.A.; Price, S.D. Generation of the organo-rare gas dications HCCRg2+ (Rg = Ar and Kr) in the reaction of acetylene dications with rare gases. Phys. Chem. Chem. Phys. 2008, 10, 7121–7128. [Google Scholar] [CrossRef]
- Lockyear, J.F.; Douglas, K.; Price, S.D.; Karwowska, M.; Fijalkowski, K.J.; Grochala, W.; Remeš, M.; Roithova, J.; Schröeder, D. Generation of the ArCF22+ dication. J. Phys. Chem. Lett. 2010, 1, 358–362. [Google Scholar] [CrossRef]
- Ram, R.S.; Bernath, P.F. Fourier transform emission spectroscopy of NeH+. J. Mol. Spectrosc. 1985, 113, 451–457. [Google Scholar] [CrossRef]
- Barlow, M.J.; Swinyard, B.M.; Owen, P.J.; Cernicharo, J.; Gomez, H.L.; Ivison, R.J.; Krause, O.; Lim, T.L.; Matsuura, M.; Olofsson, G.; et al. Detection of a noble gas molecular ion, 36ArH+, in the Crab Nebula. Science 2013, 342, 1343–1344. [Google Scholar] [CrossRef] [PubMed]
- Tsuge, M.; Kalinowski, J.; Gerber, R.B.; Lee, Y.-P. Infrared identification of proton-bound rare-gas dimers (XeHXe)+, (KrHKr)+, and (KrHXe)+ and their deuterated species in solid hydrogen. J. Phys. Chem. A. 2015, 119, 2651–2660. [Google Scholar] [CrossRef] [PubMed]
- Kriachtchev, L.; Petterson, M.; Runeberg, N.; Lundell, J.; Räsänen, M. A stable argon compound. Nature 2000, 406, 874–876. [Google Scholar] [CrossRef] [PubMed]
- Fortenberry, R.C.; Ascenzi, D. ArCH2+: A detectable noble gas molecule. ChemPhysChem 2018, 19, 3388–3392. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.P.; McDonald II, D.C.; Duncan, M.A. An argon-oxygen covalent bond in the ArOH+ molecular ion. Angew. Chem. Int. Ed. 2018, 57, 5081–5085. [Google Scholar] [CrossRef]
- Jin, J.; Li, W.; Liu, Y.; Wang, G.; Zhou, M. Preparation and characterization of chemically bonded argon- boroxol ring cation complexes. Chem. Sci. 2017, 8, 6594–6600. [Google Scholar] [CrossRef]
- Mayer, M.; van Lessen, V.; Rohdenburg, M.; Hou, G.-L.; Yang, Z.; Exner, R.M.; Aprà, E.; Azov, V.A.; Grabowsky, S.; Xantheas, S.S.; et al. Rational design of an argon- binding superelectrophilic anion. Proc. Natl. Acad. Sci. USA 2019, 116, 8167–8172. [Google Scholar] [CrossRef]
- Güsten, R.; Wiesemeyer, H.; Neufeld, D.; Menten, K.M.; Graf, U.U.; Jacobs, K.; Klein, B.; Ricken, O.; Risacher, C.; Strutzki, J. Astrophysical detection of the helium hydride ion HeH+. Nature 2019, 568, 357–360. [Google Scholar] [CrossRef]
- Croswell, K. Space is the place for impossible molecules. Knowable Mag. Annu. Rev. 2019. Available online: https://www.knowablemagazine.org/article/physical-world/2019/noble-gas-molecules-in-space (accessed on 29 June 2020). [CrossRef]
- Vos, W.L.; Finger, L.W.; Hemley, R.J.; Hu, J.Z.; Mao, H.K.; Schouten, J.A. A high-pressure van der Walls compound in solid nitrogen-helium mixtures. Nature 1992, 358, 46–48. [Google Scholar] [CrossRef]
- Dong, X.; Oganov, A.R.; Goncharov, A.F.; Stavrou, E.; Lobanov, S.; Saleh, G.; Qian, G.-R.; Zhu, Q.; Gatti, C.; Deringer, V.L.; et al. A stable compound of helium and sodium at high pressure. Nat. Chem. 2017, 9, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Hester, B.R.; dos Santos, A.M.; Molaison, J.J.; Hancock, J.C.; Wilkinson, A.P. Synthesis of defect perovskites (He2-x□x)(CaZr)F6 by inserting helium into the negative thermal expansion material CaZrF6. J. Amer. Chem. Soc. 2017, 139, 13284–13287. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.; Rohdenburg, M.; van Lessen, V.; Nierstenhöfer, M.C.; Aprà, E.; Grabowsky, S.; Asmis, K.R.; Jenne, C.; Warneke, J. First steps towards a stable neon compound: Observation and bonding analysis of [B12(CN)11Ne]−. Chem. Comun. 2020, 56, 4591–4594. [Google Scholar] [CrossRef] [PubMed]
- Grochala, W.; Khriachtchev, L.; Räsänen, M. Noble-Gas Chemistry. In Physics and Chemistry at Low Temperatures; Khriachtchev, L., Ed.; CRC Press: Boca Raton, Florida, USA, 2011; pp. 419–446. [Google Scholar]
- Braïda, B.; Hiberty, P.C. The essential role of charge-shift bonding in hypervalent prototype XeF2. Nature Chem. 2013, 5, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Kurzydłowski, D.; Zaleski-Ejgierd, P.; Grochala, W.; Hoffmann, R. Freezing in resonance structures for better packing: XeF2 becomes (XeF+)(F−) at large compression. Inorg. Chem. 2011, 50, 3832–3840. [Google Scholar] [CrossRef] [PubMed]
- Moran, M.D.; Brock, D.S.; Mercier, H.P.A.; Schrobilgen, G.J. Xe3OF3+, a precursor to a noble-gas nitrate; syntheses and structural characterizations of FXeONO2, XeF2·HNO3, and XeF2·N2O4. J. Am. Chem. Soc. 2010, 132, 13823–13839. [Google Scholar] [CrossRef]
- Tavčar, G.; Tramšek, M.; Bunič, T.; Benkič, P.; Žemva, B. New class of coordination compounds with noble gas fluorides as ligands to metal ions. J. Fluorine Chem. 2004, 125, 1579–1584. [Google Scholar]
- Žemva, B.; Jesih, A.; Templeton, D.H.; Zalkin, A.; Cheetham, A.K.; Bartlett, N. Phases in the system XeF2/XeF5AsF6 and structural and vibrational evidence for the following ionization pathway: XeF2 → XeF+ + F−. J. Am. Chem. Soc. 1987, 109, 7420–7427. [Google Scholar] [CrossRef]
- Kaupp, M.; van Wüllen, C.; Franke, R.; Schmitz, F.; Kutzelnigg, W. The structure of XeF6 and of compounds isoelectronic with it. A challenge to computational chemistry and to the qualitative theory of the chemical bond. J. Am. Chem. Soc. 1996, 118, 11939–11950. [Google Scholar] [CrossRef]
- Dixon, D.A.; de Jong, W.A.; Peterson, K.A.; Christe, K.O.; Schrobilgen, G.J. Heats of formation of xenon fluorides and the fluxionality of XeF6 from high level electronic structure calculations. J. Am. Chem. Soc. 2005, 127, 8327–8634. [Google Scholar] [CrossRef] [PubMed]
- Frenking, G.; Lein, M. Electronic Structure of Main-group Compounds. In Encyclopedia of Inorganic and Bioinorganic Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2011; Available online: https://doi.org/10.1002/9781119951438.eibc0067 (accessed on 6 June 2020).
- Gillespie, R.J.; Hargittai, I. The VSEPR Model of Molecular Geometry, 1st ed.; Allyn and Bacon: Boston, MA, USA, 1991. [Google Scholar]
- Hughes, M.J.; Mercier, H.P.A.; Schrobilgen, G.J. Syntheses, Raman spectra and X-ray crystal structures of [XeF5][μ-F(OsO3F2)2] and [M][OsO3F3] (M = XeF5+, Xe2F11+). Inorg. Chem. 2010, 49, 3501–3515. [Google Scholar] [CrossRef] [PubMed]
- Hogness, T.R.; Lunn, E.G. The ionization of hydrogen by electron impact as interpreted by positive ray analysis. Phys. Rev. 1925, 26, 44–55. [Google Scholar] [CrossRef]
- Adeleke, A.A.; Kunz, M.; Greenberg, E.; Prakapenka, V.B.; Yao, Y.; Stavrou, E. A high-pressure compound of argon and nickel: Noble gas in the Earth’s core? ACS Earth Space Chem. 2019, 3, 2517–2524. [Google Scholar] [CrossRef]
- Pan, S.; Jana, G.; Merino, G.; Chattaraj, P.K. Noble-noble strong union: Gold at its best to make a bond with a noble gas atom. ChemistryOpen 2019, 8, 173–187. [Google Scholar] [CrossRef]
- Bauzá, A.; Frontera, A. Aerogen Bonding Interaction: A New Supramolecular Force? Angew. Chem. Int. Ed. 2015, 54, 7340–7343. [Google Scholar] [CrossRef]
- Gomila, R.M.; Frontera, A. Covalent and non-covalent noble gas bonding interactions in XeFn derivatives (n = 2-6): A combined theoretical and ICSD analysis. Front. Chem. 2020, 8, 395. [Google Scholar] [CrossRef]
- Britvin, S.N. Xenon in oxide frameworks: At the crossroads between inorganic chemistry and planetary science. Dalton Trans. 2020, 49, 5778–5782. [Google Scholar] [CrossRef]
- Bauzá, A.; Frontera, A. σ/π hole noble gas bonding interactions: Insights from theory and experiment. Coord. Chem. Rev. 2020, 404, 213112. [Google Scholar] [CrossRef]







© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazej, Z. Noble-Gas Chemistry More than Half a Century after the First Report of the Noble-Gas Compound. Molecules 2020, 25, 3014. https://doi.org/10.3390/molecules25133014
Mazej Z. Noble-Gas Chemistry More than Half a Century after the First Report of the Noble-Gas Compound. Molecules. 2020; 25(13):3014. https://doi.org/10.3390/molecules25133014
Chicago/Turabian StyleMazej, Zoran. 2020. "Noble-Gas Chemistry More than Half a Century after the First Report of the Noble-Gas Compound" Molecules 25, no. 13: 3014. https://doi.org/10.3390/molecules25133014
APA StyleMazej, Z. (2020). Noble-Gas Chemistry More than Half a Century after the First Report of the Noble-Gas Compound. Molecules, 25(13), 3014. https://doi.org/10.3390/molecules25133014





