Smilax glabra Roxb. Inhibits Collagen Induced Adhesion and Migration of PC3 and LNCaP Prostate Cancer Cells through the Inhibition of Beta 1 Integrin Expression
Abstract
1. Introduction
2. Results
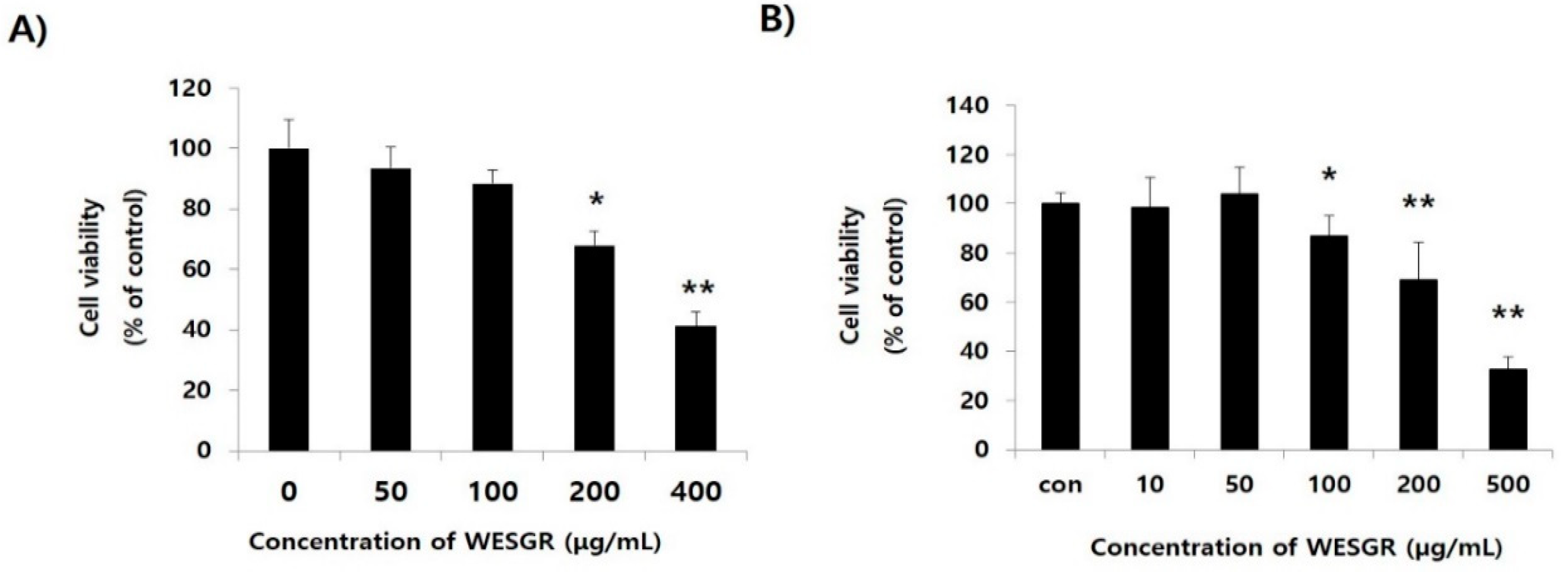
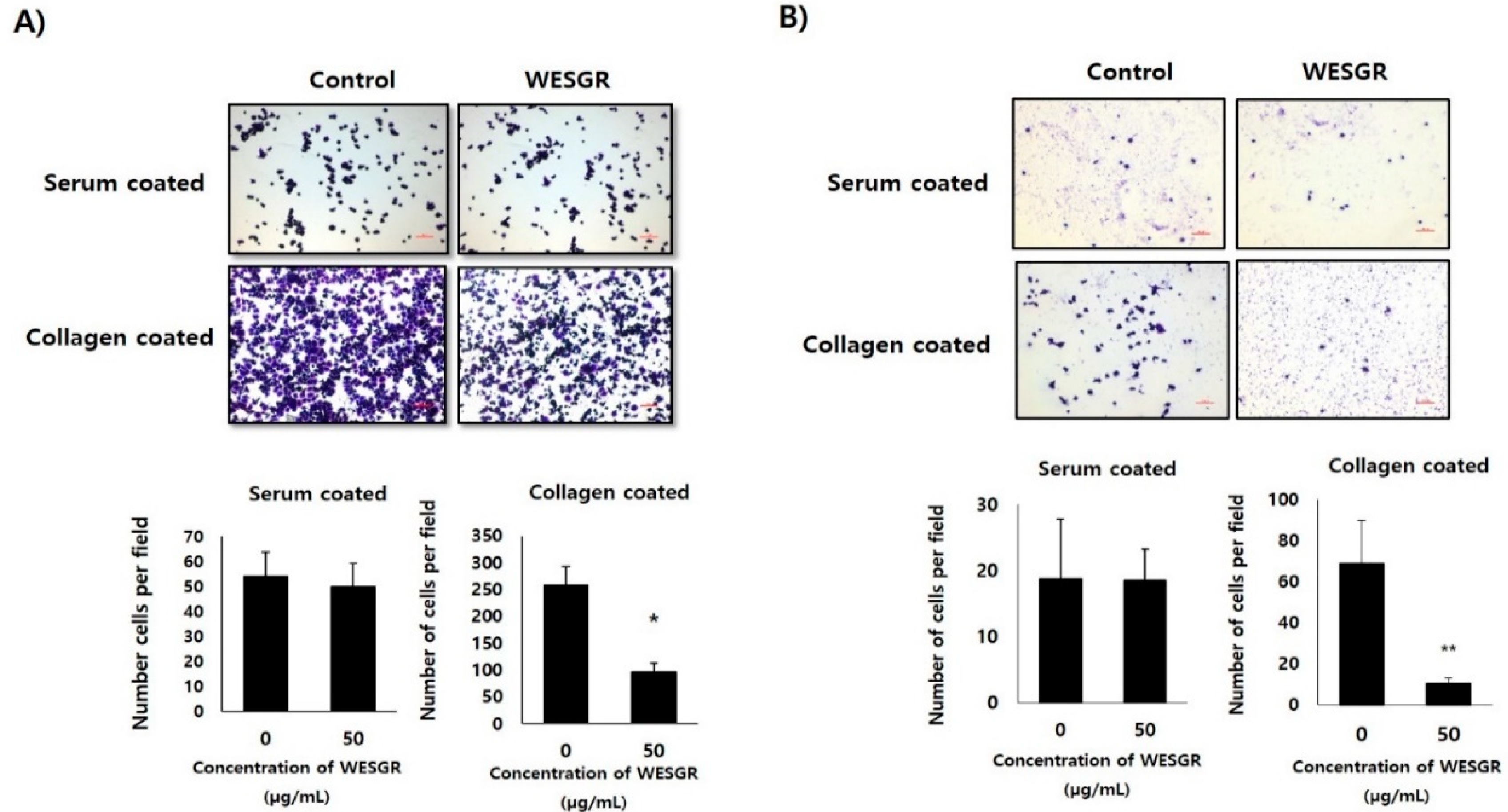
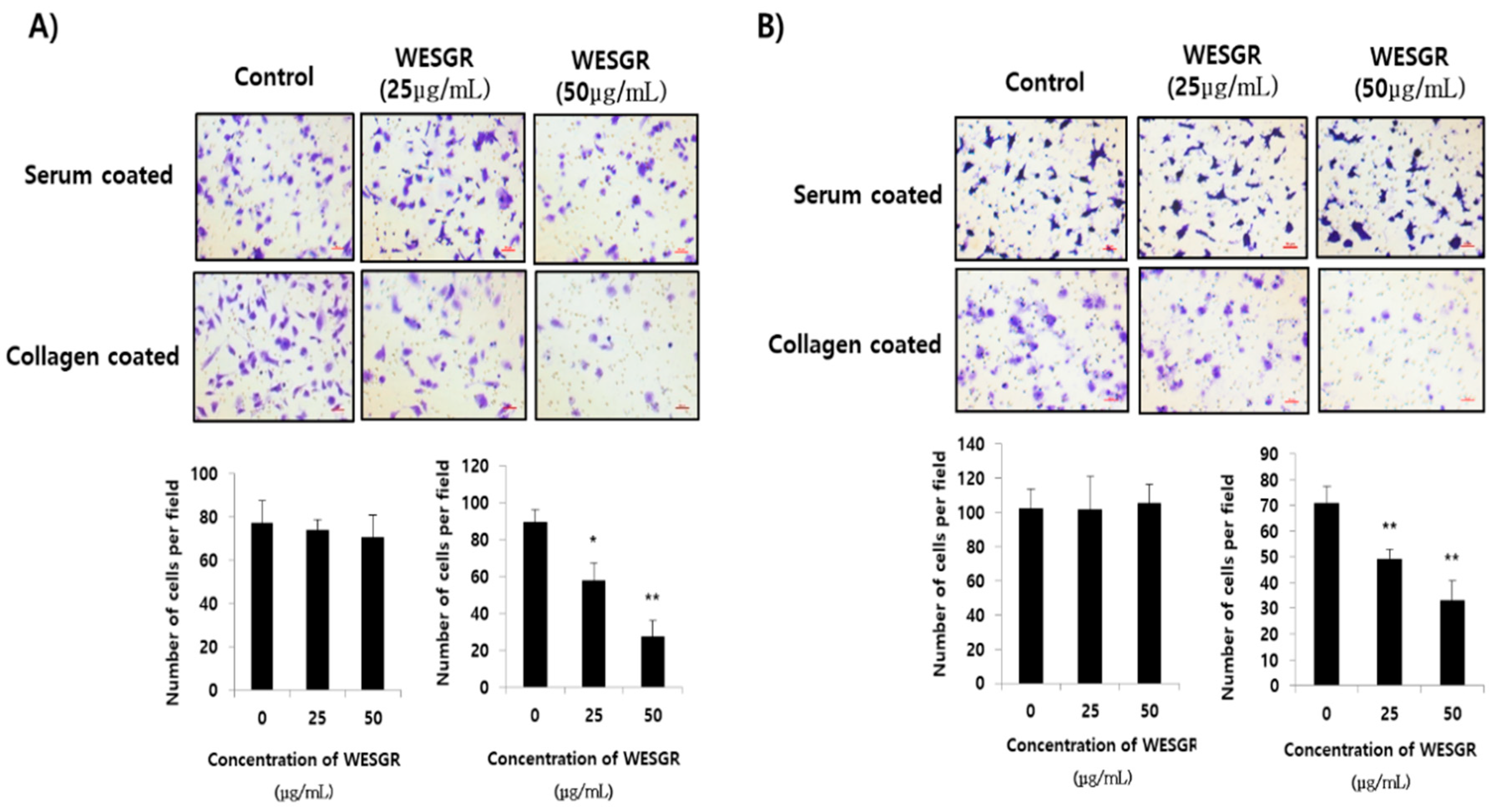
2.1. Collagen-Dependent Adhesion and Migration of PC3 and LNCaP Prostate Cancer Cells are Effectively Attenuated by Treatment with Nontoxicological Levels of WESGR
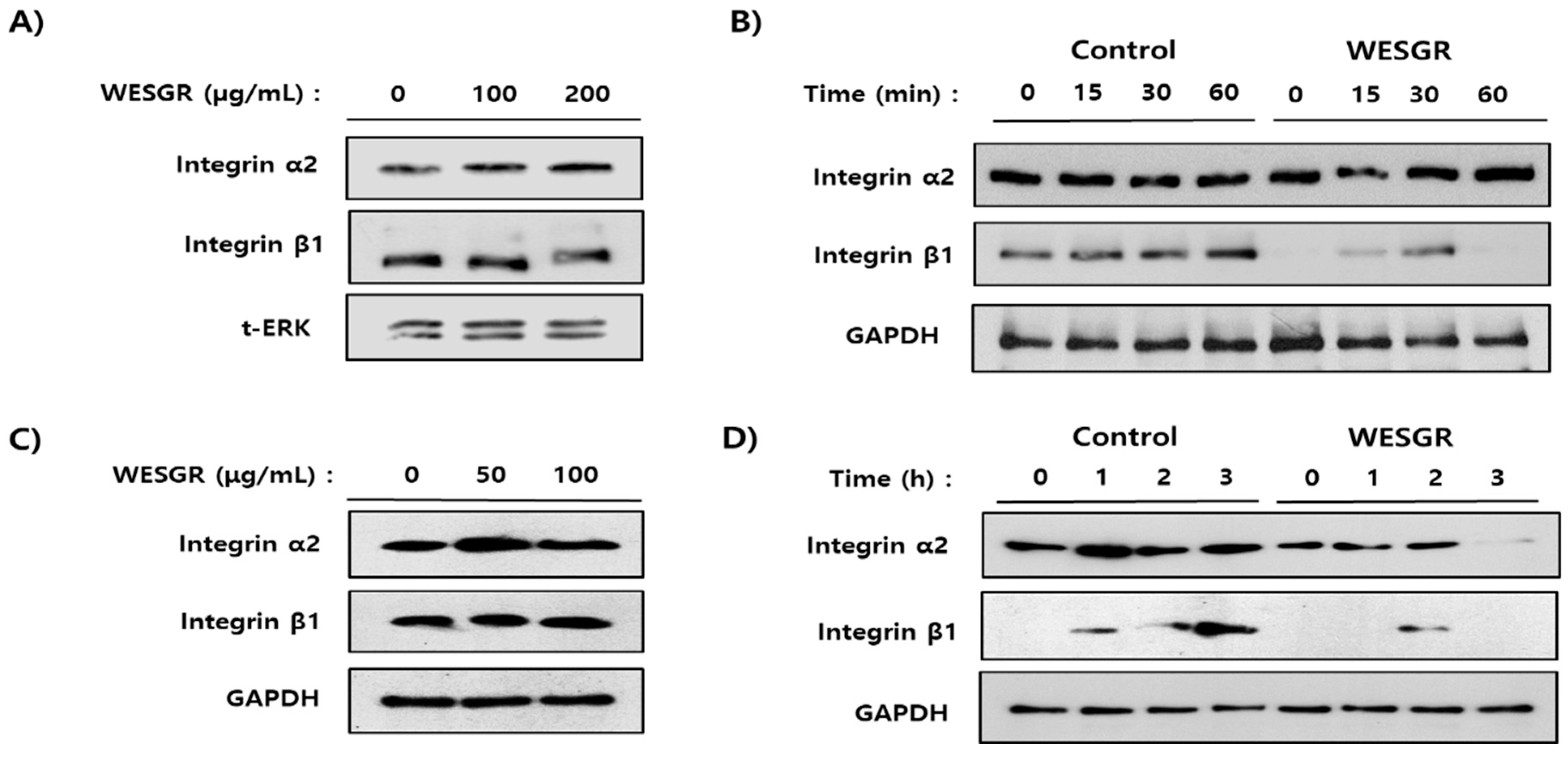
2.2. Collagen-Dependent Expression of β1 Integrin in PC3 and LNCaP Prostate Cancer Cells is Inhibited by Treatment with WESGR
2.3. Collagen-Dependent FAK Signaling of PC3 and LNCaP Prostate Cancer Cells is Attenuated by Treatment with WESGR
2.4. Single Components Were Analyzed by HPLC-MS/MS
2.5. 5-O-Caffeoylquinic Acid is Determined as a Functional Single Component of WESGR
3. Discussion
4. Materials and Methods
4.1. Preparation of WESGR
4.2. Cytotoxicity Assay of WESGR
4.3. Cell Adhesion Assay
4.4. Collagen Against Migration Assay
4.5. Analysis of Collagen for Expression of α2β1 Integrin and Focal Adhesion Kinase (FAK)
4.6. HPLC-MS/MS Analysis
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Daniyal, M.; Siddiqui, Z.A.; Akram, M.; Asif, H.; Sultana, S.; Khan, A. Epidemiology, Etiology, Diagnosis and Treatment of Prostate Cancer. Asian Pac. J. Cancer Prev. 2014, 15, 9575–9578. [Google Scholar] [CrossRef] [PubMed]
- E Fleshner, N.; Evans, A.; Chadwick, K.; Lawrentschuk, N.; Zlotta, A. Clinical significance of the positive surgical margin based upon location, grade, and stage. Urol. Oncol. Semin. Orig. Investig. 2010, 28, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Dellis, A.; Zagouri, F.; Liontos, M.; Mitropoulos, D.; Bamias, A.; Papatsoris, A.G. Management of advanced prostate cancer: A systematic review of existing guidelines and recommendations. Cancer Treat. Rev. 2019, 73, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Rycaj, K.; Tang, D.G. Molecular determinants of prostate cancer metastasis. Oncotarget 2017, 8, 88211–88231. [Google Scholar] [CrossRef]
- Hynes, R.O. Integrins: Versatility, modulation, and signaling in cell adhesion. Cell 1992, 69, 11–25. [Google Scholar] [CrossRef]
- Rosales, C.; O’Brien, V.; Kornberg, L.; Juliano, R. Signal transduction by cell adhesion receptors. Biochim. et Biophys. Acta (BBA) - Bioenerg. 1995, 1242, 77–98. [Google Scholar] [CrossRef]
- E Hughes, P.; Renshaw, M.W.; Pfaff, M.; Forsyth, J.; Keivens, V.M.; Schwartz, M.A.; Ginsberg, M.H. Suppression of Integrin Activation: A Novel Function of a Ras/Raf-Initiated MAP Kinase Pathway. Cell 1997, 88, 521–530. [Google Scholar] [CrossRef]
- Desgrosellier, J.S.; Cheresh, D.A. Integrins in cancer: biological implications and therapeutic opportunities. Nat. Rev. Cancer 2010, 10, 9–22. [Google Scholar] [CrossRef]
- Mitchell, K.; Svenson, K.B.; Longmate, W.M.; Gkirtzimanaki, K.; Sadej, R.; Wang, X.; Zhao, J.; Eliopoulos, A.; Berditchevski, F.; DiPersio, C.M. Suppression of integrin alpha3beta1 in breast cancer cells reduces cyclooxygenase-2 gene expression and inhibits tumorigenesis, invasion, and cross-talk to endothelial cells. Cancer Res. 2010, 70, 6359–6367. [Google Scholar] [CrossRef]
- Saito, Y.; Sekine, W.; Sano, R.; Komatsu, S.; Mizuno, H.; Katabami, K.; Shimada, K.; Oku, T.; Tsuji, T. Potentiation of cell invasion and matrix metalloproteinase production by alpha3beta1 integrin-mediated adhesion of gastric carcinoma cells to laminin-5. Clin. Exp. Metastasis 2010, 27, 197–205. [Google Scholar] [CrossRef]
- Bonaccorsi, L.; Carloni, V.; Muratori, M.; Salvadori, A.; Giannini, A.; Carini, M.; Serio, M.; Forti, G.; Baldi, E. Androgen receptor expression in prostate carcinoma cells suppresses alpha6beta4 integrin-mediated invasive phenotype. Endocrinology 2000, 141, 3172–3182. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Hatakeyama, S.; Yu, S.Y.; Bao, X.; Ohyama, C.; Khoo, K.H.; Fukuda, M.N.; Fukuda, M. Core3 O-glycan synthase suppresses tumor formation and metastasis of prostate carcinoma PC3 and LNCaP cells through down-regulation of alpha2beta1 integrin complex. J. Biol. Chem. 2009, 284, 17157–17169. [Google Scholar] [CrossRef]
- Slambrouck, V.; Van Slambrouck, S.; Jenkins, A.R.; Romero, A.E.; Steelant, W.F. Reorganization of the integrin α2 subunit controls cell adhesion and cancer cell invasion in prostate cancer. Int. J. Oncol. 2009, 34, 1717–1726. [Google Scholar] [CrossRef] [PubMed]
- Bonkhoff, H.; Stein, U.; Remberger, K. Differential expression of α6 and α2 very late antigen integrins in the normal, hyperplastic, and neoplastic prostate: Simultaneous demonstration of cell surface receptors and their extracellular ligands. Hum. Pathol. 1993, 24, 243–248. [Google Scholar] [CrossRef]
- Fukunaga, T.; Miura, T.; Furuta, K.; Kato, A. Hypoglycemic Effect of the Rhizomes of Smilax glabra in Normal and Diabetic Mice. Boil. Pharm. Bull. 1997, 20, 44–46. [Google Scholar] [CrossRef]
- Xia, D.; Yu, X.; Liao, S.; Shao, Q.; Mou, H.; Ma, W. Protective effect of Smilax glabra extract against lead-induced oxidative stress in rats. J. Ethnopharmacol. 2010, 130, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Ooi, L.S.M.; Sun, S.S.M.; Wang, H.; Ooi, V.E.C. New Mannose-Binding Lectin Isolated from the Rhizome of SarsaparillaSmilax glabraRoxb. (Liliaceae). J. Agric. Food Chem. 2004, 52, 6091–6095. [Google Scholar] [CrossRef]
- Galhena, B.P.; Samarakoon, S.R.; Thabrew, M.I.; Weerasinghe, G.A.K.; Thammitiyagodage, M.G.; Ratnasooriya, W.D.; Tennekoon, K.H. Anti-Inflammatory Activity Is a Possible Mechanism by Which the Polyherbal Formulation Comprised of Nigella sativa (Seeds), Hemidesmus indicus (Root), and Smilax glabra (Rhizome) Mediates Its Antihepatocarcinogenic Effects. Evidence-Based Complement. Altern. Med. 2012, 2012, 1–11. [Google Scholar] [CrossRef]
- Jiang, J.; Xu, Q. Immunomodulatory activity of the aqueous extract from rhizome of Smilax glabra in the later phase of adjuvant-induced arthritis in rats. J. Ethnopharmacol. 2003, 85, 53–59. [Google Scholar] [CrossRef]
- Sa, F.; Gao, J.-L.; Fung, K.-P.; Zheng, Y.; Lee, S.M.Y.; Wang, Y.-T. Anti-proliferative and pro-apoptotic effect of Smilax glabra Roxb. extract on hepatoma cell lines. Chem. Interactions 2008, 171, 1–14. [Google Scholar] [CrossRef]
- Samarakoon, S.R.; Thabrew, I.; Galhena, B.P.; Tennekoon, K.H. Modulation of apoptosis in human hepatocellular carcinoma (HepG2 cells) by a standardized herbal decoction of Nigella sativa seeds, Hemidesmus indicus roots and Smilax glabra rhizomes with anti- hepatocarcinogenic effects. BMC Complement. Altern. Med. 2012, 12, 25. [Google Scholar] [CrossRef]
- Ooi, L.S.M.; Wong, E.Y.L.; Chiu, L.C.M.; Sun, S.S.M.; Ooi, V.E.C. Antiviral and Anti-proliferative Glycoproteins from the Rhizome of Smilax glabra Roxb (Liliaceae). Am. J. Chin. Med. 2008, 36, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-L.; Zhu, W.; Wang, M.; Xu, X.-J.; Lu, C.-J. Antioxidant and Anti-Inflammatory Activities of Phenolic-Enriched Extracts of Smilax glabra. Evidence-Based Complement. Altern. Med. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- She, T.; Qu, L.; Wang, L.; Yang, X.; Xu, S.; Feng, J.; Gao, Y.; Zhao, C.; Han, Y.; Cai, S.; et al. Sarsaparilla (Smilax Glabra Rhizome) Extract Inhibits Cancer Cell Growth by S Phase Arrest, Apoptosis, and Autophagy via Redox-Dependent ERK1/2 Pathway. Cancer Prev. Res. 2015, 8, 464–474. [Google Scholar] [CrossRef]
- Palmioli, A.; Ciaramelli, C.; Tisi, R.; Spinelli, M.; De Sanctis, G.; Sacco, E.; Airoldi, C. Natural Compounds in Cancer Prevention: Effects of Coffee Extracts and Their Main Polyphenolic Component, 5-O -Caffeoylquinic Acid, on Oncogenic Ras Proteins. Chem. Asian J. 2017, 12, 2457–2466. [Google Scholar] [CrossRef]
- Choi, Y.H.; Kim, H.K.; Linthorst, H.J.; Hollander, J.G.; Lefeber, A.W.; Erkelens, C.; Nuzillard, J.M.; Verpoorte, R. NMR metabolomics to revisit the tobacco mosaic virus infection in Nicotiana tabacum leaves. J. Nat. Prod. 2006, 69, 742–748. [Google Scholar] [CrossRef]
- Nakatani, N.; Kayano, S.; Kikuzaki, H.; Sumino, K.; Katagiri, K.; Mitani, T. Identification, quantitative determination, and antioxidative activities of chlorogenic acid isomers in prune (Prunus domestica L.). J. Agric. Food Chem. 2000, 48, 5512–5516. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Park, K.M.; Lee, S.H. Gleditsia sinensis Thorn Attenuates the Collagen-Based Migration of PC3 Prostate Cancer Cells through the Suppression of alpha2beta1 Integrin Expression. Int. J. Mol. Sci. 2016, 17, 328. [Google Scholar] [CrossRef]
- Bubendorf, L.; Schöpfer, A.; Wagner, U.; Sauter, G.; Moch, H.; Willi, N.; Gasser, T.C.; Mihatsch, M.J. Metastatic patterns of prostate cancer: an autopsy study of 1589 patients. Hum. Pathol. 2000, 31, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Seruga, B.; Tannock, I.F. Chemotherapy-Based Treatment for Castration-Resistant Prostate Cancer. J. Clin. Oncol. 2011, 29, 3686–3694. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.-X.; Wang, M.; Yuan, J.-X.; Liu, J.-C. [Effect of ethyl gallate on invasion abilities and its mechanism of breast cancer MDA-MB-231 cells]. Yao xue xue bao = Acta Pharm. Sin. 2015, 50, 45–49. [Google Scholar]
- Lim, J.-C.; Park, J.H.; Buděšínský, M.; Kasal, A.; Han, Y.-H.; Koo, B.-S.; Lee, S.-I.; Lee, D.-U. Antimutagenic constituents from the thorns of Gleditsia sinensis. Chem. Pharm. Bull. 2005, 53, 561–564. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Kinouchi, Y.; Wada, S.-I.; Tokuda, H. Potential Anti-Tumor Promoting Activity of Lupane-Type Triterpenoids from the Stem Bark ofGlochidion zeylanicumandPhyllanthus flexuosus. Planta Med. 2004, 70, 1234–1236. [Google Scholar] [CrossRef]
- Jafari, N.; Zargar, S.J.; Delnavazi, M.R.; Yassa, N. Cell Cycle Arrest and Apoptosis Induction of Phloroacetophenone Glycosides and Caffeoylquinic Acid Derivatives in Gastric Adenocarcinoma (AGS) Cells. Anti-Cancer Agents Med. Chem. 2018, 18, 610–616. [Google Scholar] [CrossRef]
- In, J.-K.; Kim, J.-K.; Oh, J.S.; Seo, D.-W. 5-Caffeoylquinic acid inhibits invasion of non-small cell lung cancer cells through the inactivation of p70S6K and Akt activity: Involvement of p53 in differential regulation of signaling pathways. Int. J. Oncol. 2016, 48, 1907–1912. [Google Scholar] [CrossRef]

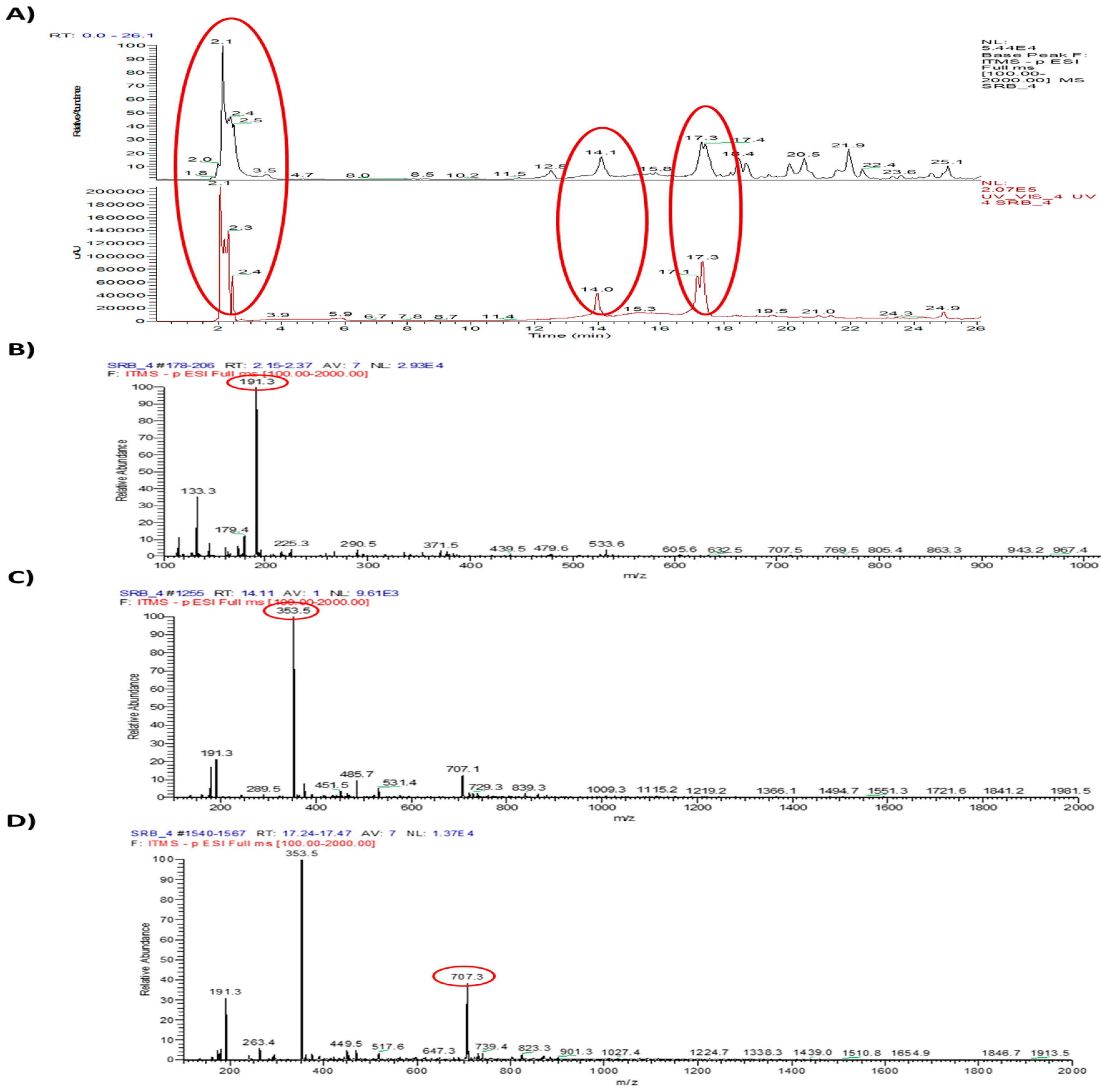

| Number | tR (min) | λmax (nm) | Observed Mass | Main Peak | LC-MS2 of Main Peak | Compounds (MW) | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | 2.1 | 340 | 133.3, 179.4, 191.3, 225.3, 290.5, 371.5, 439.5, 479.6, 533.6, 605.6, 632.5, 707.5, 769.5, 805.4, 863.3, 943.2, 967.4 | 191.3 | 58.9, 70.9, 83.0, 84.9, 92.9, 98.9, 109.0, 110.0, 127.0, 137.0, 153.0, 155.0, 171.0, 173.0, 174.0, 191.1 | N.D. | – |
| 2 | 14.1 | 340 | 191.3, 289.5, 353.5, 451.5, 485.7, 531.4, 707.1, 729.3, 839.3, 1009.3, 1115.2, 1219.2, 1366.1, 1494.7, 1551.3, 1721.6, 1841.2, 1981.5 | 353.5 | 135.0, 160.9, 172.9, 191.0, 192.1, 197.1, 217.2 | Catechin (289.5) | [23,24] |
| Epicatechin (289.5) | [23,24] | ||||||
| 5-O-Caffeoylquinic acid (353.086) | [25] | ||||||
| 4-O-Caffeoylquinic acid (353,086) | [26,27] | ||||||
| 3-O-Caffeoylquinic acid (353.086) | [26,27] | ||||||
| Cinchonain Ib (451.5) | [23,24] | ||||||
| 3 | 17.3 | 340 | 191.3, 263.4, 353.5, 449.5, 517.6, 647.3, 707.3, 739.4, 823.3, 901.3, 1027.4, 1224.7, 1338.3, 1439.0, 1510.8, 1654.9, 1846.7, 1913.5 | 707.1 | 307.0, 351.1, 353.1, 353.7, 452.3, 514.3, 555.2, 617.1, 658.1, 689.3 | Neoastilbin (449.5) | [23,24] |
| Astilbin (449.5) | [23,24] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, O.Y.; Ryu, S.; Choi, J.K.; Lee, S.H. Smilax glabra Roxb. Inhibits Collagen Induced Adhesion and Migration of PC3 and LNCaP Prostate Cancer Cells through the Inhibition of Beta 1 Integrin Expression. Molecules 2020, 25, 3006. https://doi.org/10.3390/molecules25133006
Kwon OY, Ryu S, Choi JK, Lee SH. Smilax glabra Roxb. Inhibits Collagen Induced Adhesion and Migration of PC3 and LNCaP Prostate Cancer Cells through the Inhibition of Beta 1 Integrin Expression. Molecules. 2020; 25(13):3006. https://doi.org/10.3390/molecules25133006
Chicago/Turabian StyleKwon, Oh Yun, Sujin Ryu, Jong Kyu Choi, and Seung Ho Lee. 2020. "Smilax glabra Roxb. Inhibits Collagen Induced Adhesion and Migration of PC3 and LNCaP Prostate Cancer Cells through the Inhibition of Beta 1 Integrin Expression" Molecules 25, no. 13: 3006. https://doi.org/10.3390/molecules25133006
APA StyleKwon, O. Y., Ryu, S., Choi, J. K., & Lee, S. H. (2020). Smilax glabra Roxb. Inhibits Collagen Induced Adhesion and Migration of PC3 and LNCaP Prostate Cancer Cells through the Inhibition of Beta 1 Integrin Expression. Molecules, 25(13), 3006. https://doi.org/10.3390/molecules25133006






