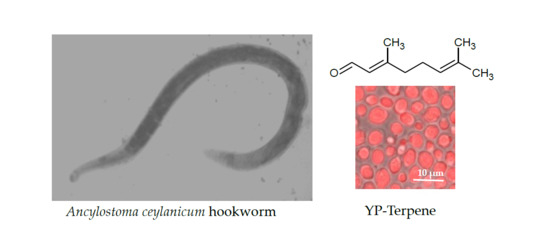
Anthelmintic Activity of Yeast Particle-Encapsulated Terpenes
Abstract
1. Introduction
2. Results
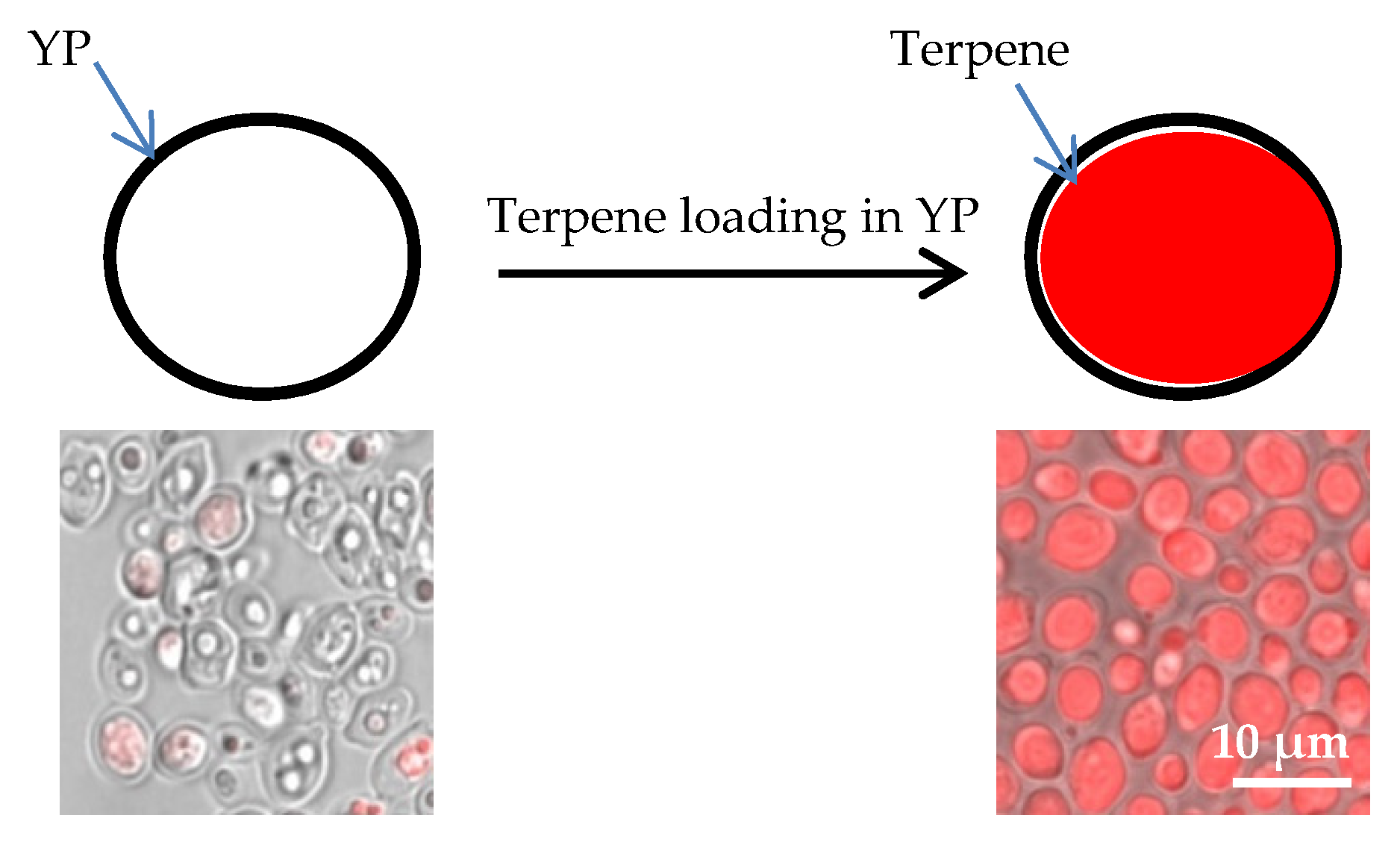
2.1. Yeast Particle Encapsulation of Terpenes
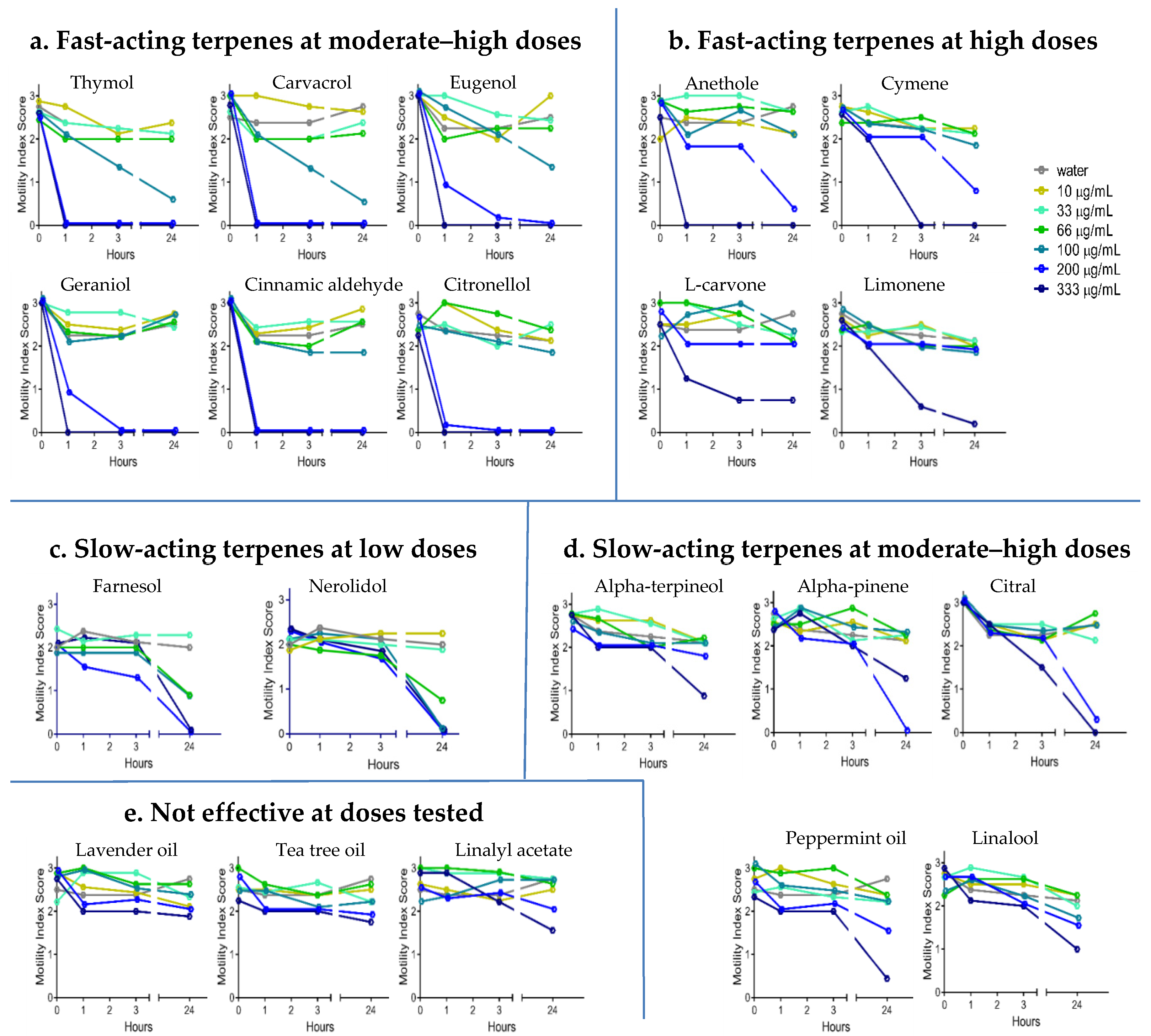
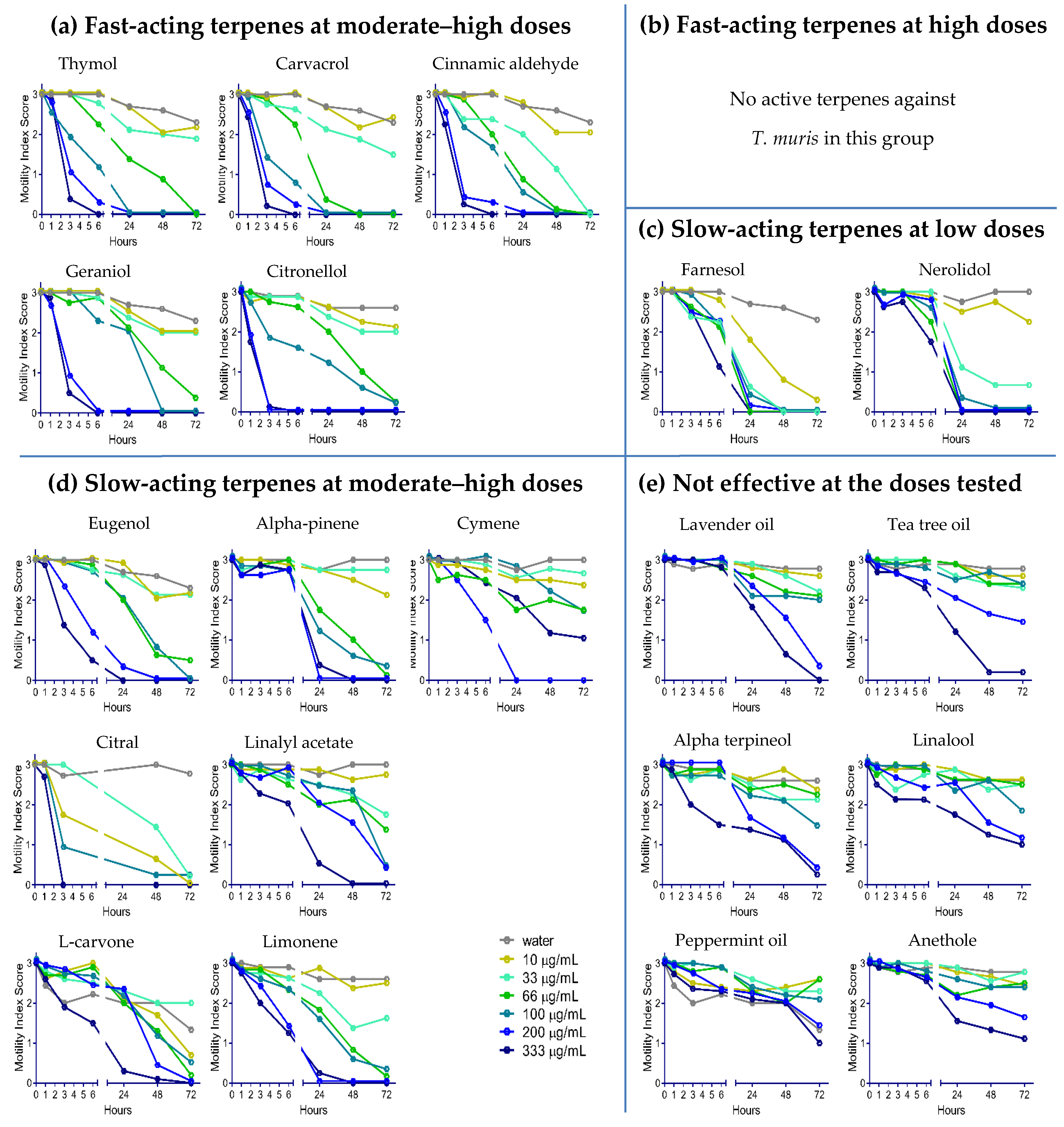
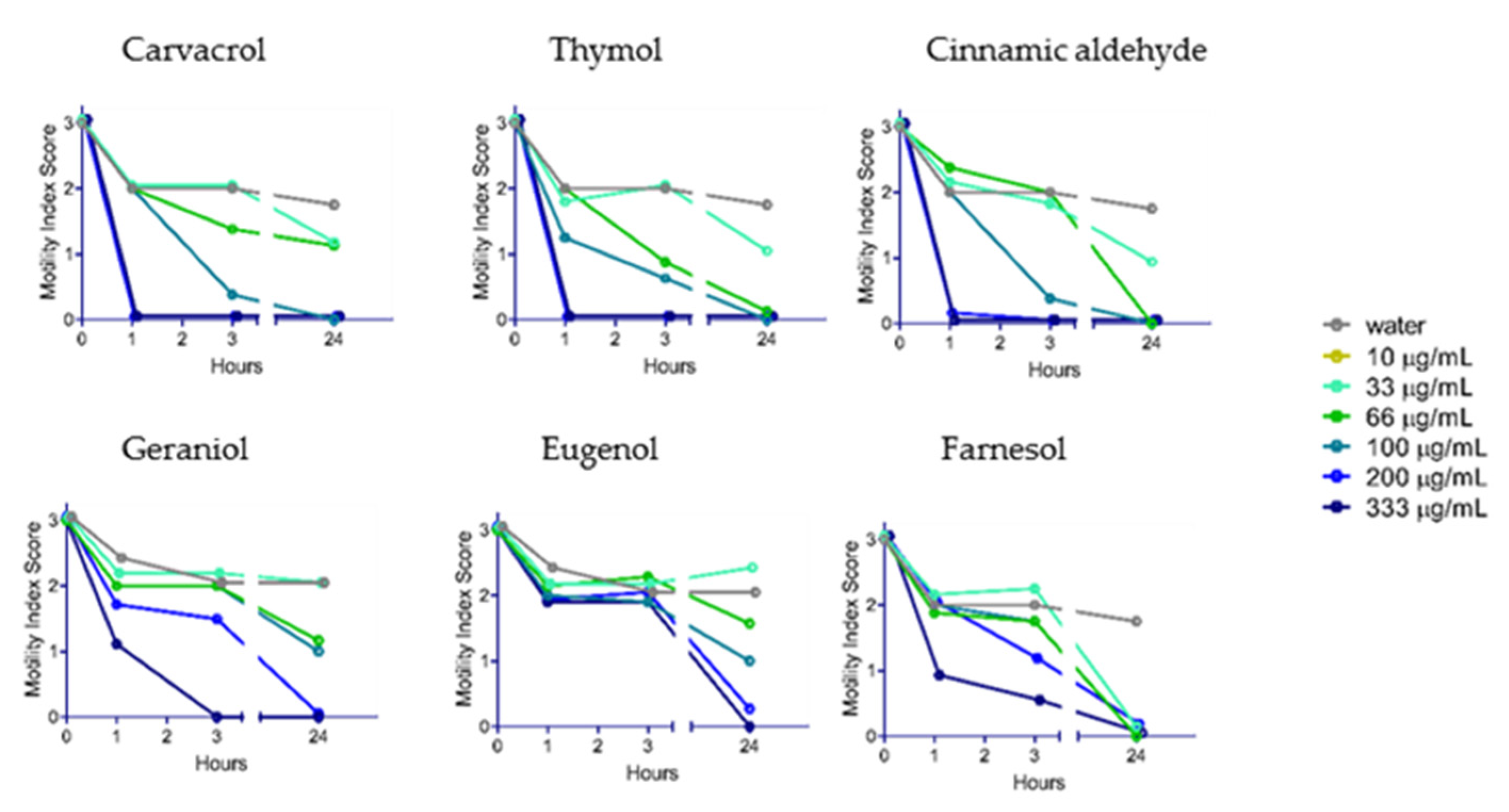
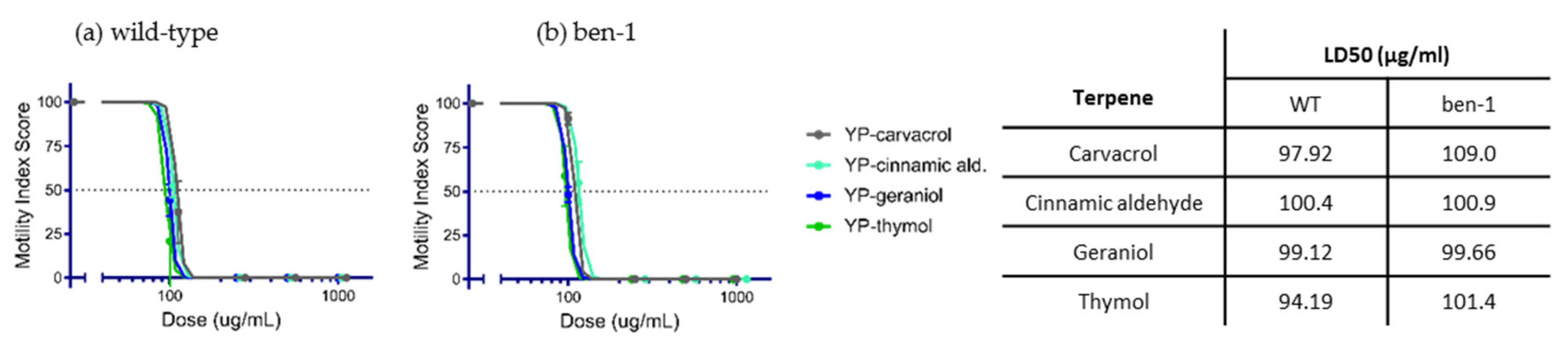
2.2. Anthelmintic Activity of YP–Terpenes In Vitro
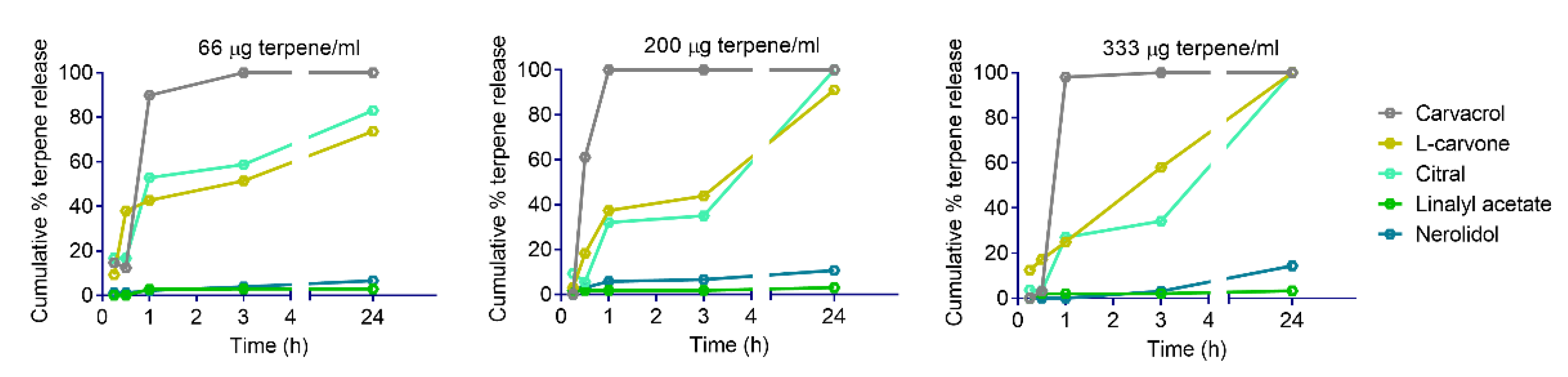
2.3. Kinetics of Terpene Release from YPs
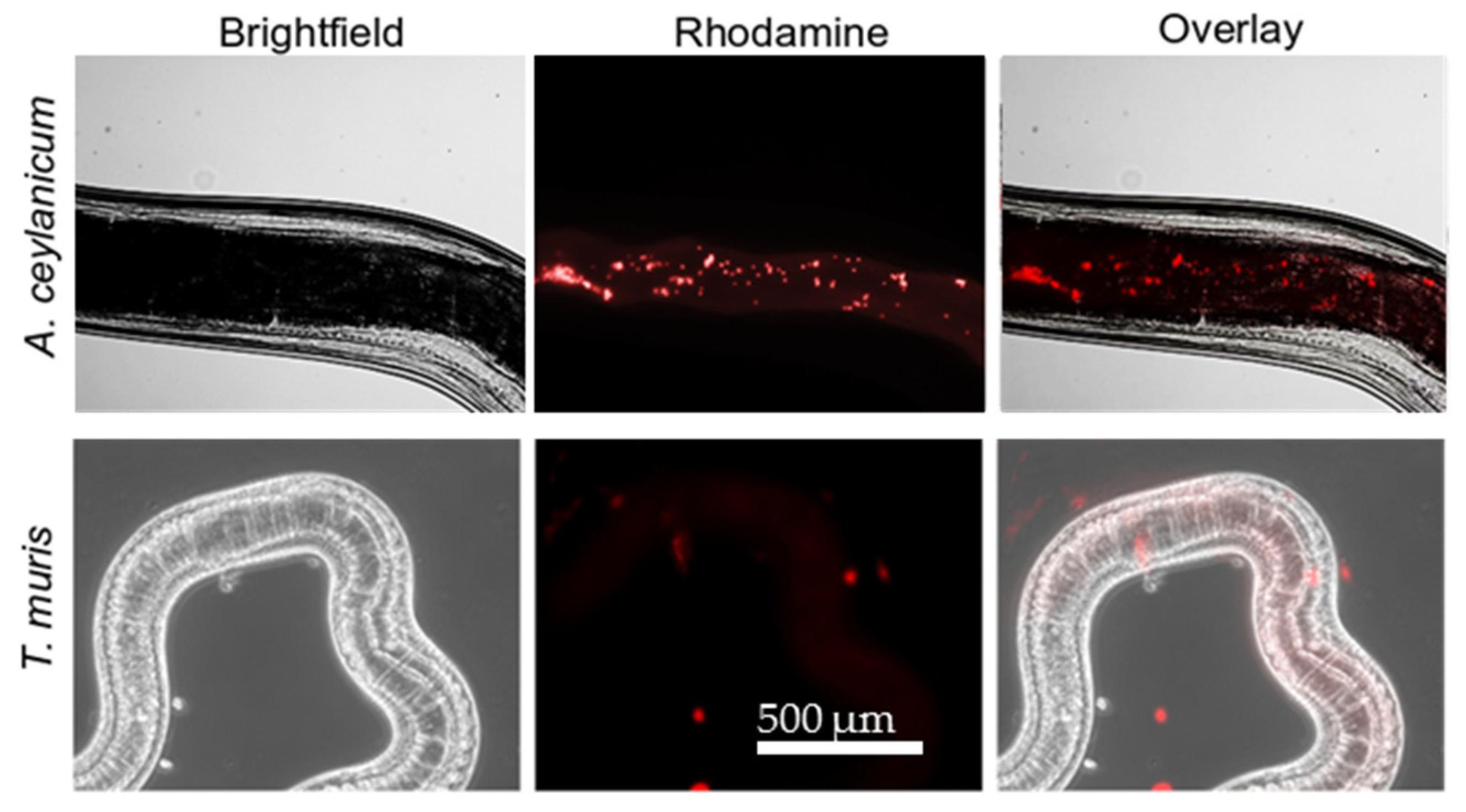
2.4. Difference in Uptake of YP–Terpenes by Hookworms and Whipworms.
3. Discussion
4. Materials and Methods
4.1. YP Loading of Terpenes
4.2. Terpene Release from YP–Terpenes
4.3. Maintenance of Parasites and C. elegans
4.4. Adult Worm In Vitro Screening
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pullan, R.L.; Smith, J.L.; Jasrasaria, R.; Brooker, S.J. Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasites Vectors 2014, 7, 37. [Google Scholar] [CrossRef]
- Barry, M.A.; Simon, G.G.; Mistry, N.; Hotez, P.J. Global trends in neglected tropical disease control and elimination: Impact on child health. Arch. Dis. Child. 2013, 98, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Bethony, J.; Brooker, S.; Albonico, M.; Geiger, S.M.; Loukas, A.; Diemert, D.; Hotez, P.J. Soil-transmitted helminth infections: Ascariasis, trichuriasis, and hookworm. Lancet 2006, 367, 1521–1532. [Google Scholar] [CrossRef]
- Harris, J.B.; Podolsky, M.J.; Bhuiyan, T.R.; Chowdhury, F.; Khan, A.I.; LaRocque, R.C.; Logvinenko, T.; Kendall, J.; Faruque, A.S.; Nagler, C.R.; et al. Immunologic responses to Vibrio cholerae in patients co-infected with intestinal parasites in Bangladesh. PLoS Negl. Trop. Dis. 2009, 3, e403. [Google Scholar] [CrossRef] [PubMed]
- Olds, G.R. Deworming the world. Trans. Am. Clin. Climatol. Assoc. 2013, 124, 265. [Google Scholar]
- Hotez, P. Hookworm and poverty. Ann. N. Y. Acad. Sci. 2008, 1136, 38–44. [Google Scholar] [CrossRef]
- Crompton, D.; Nesheim, M. Nutritional impact of intestinal helminthiasis during the human life cycle. Annu. Rev. Nutr. 2002, 22, 35–59. [Google Scholar] [CrossRef]
- Keiser, J.; Utzinger, J. Efficacy of Current Drugs Against Soil-Transmitted Helminth Infections. JAMA 2008, 16, 1937–1948. [Google Scholar] [CrossRef]
- Keiser, J.; Utzinger, J. The Drugs We Have and the Drugs We Need Against Major Helminth Infections. In Advances in Parasitology; Elsevier Ltd: London, UK, 2010; Volume 73, pp. 197–230. [Google Scholar]
- Soukhathammavong, P.A.; Sayasone, S.; Phongluxa, K.; Xayaseng, V.; Utzinger, J.; Vounatsou, P.; Hatz, C.; Akkhavong, K.; Keiser, J.; Odermatt, P. Low efficacy of single-dose albendazole and mebendazole against hookworm and effect on concomitant helminth infection in Lao PDR. PLoS Negl. Trop. Dis. 2012, 6, e1417. [Google Scholar] [CrossRef]
- Albonico, M.; Rinaldi, L.; Sciascia, S.; Morgoglione, M.E.; Piemonte, M.; Maurelli, M.P.; Musella, V.; Utzinger, J.; Ali, S.M.; Ame, S.M.; et al. Comparison of three copromicroscopic methods to assess albendazole efficacy against soil-transmitted helminth infections in school-aged children on Pemba Island. Trans. R. Soc. Trop. Med. Hyg. 2013, 107, 493–501. [Google Scholar] [CrossRef]
- Humphries, D.; Mosites, E.; Otchere, J.; Twum, W.A.; Woo, L.; Jones-Sanpei, H.; Harrison, L.M.; Bungiro, R.D.; Benham-Pyle, B.; Bimi, L.; et al. Epidemiology of hookworm infection in Kintampo North Municipality, Ghana: Patterns of malaria coinfection, anemia, and albendazole treatment failure. Am. J. Trop. Med. Hyg. 2011, 84, 792–800. [Google Scholar] [CrossRef]
- Massa, K.; Magnussen, P.; Sheshe, A.; Ntakamulenga, R.; Ndawi, B.; Olsen, A. The combined effect of the Lymphatic Filariasis Elimination Programme and the Schistosomiasis and Soil-transmitted Helminthiasis Control Programme on soil-transmitted helminthiasis in schoolchildren in Tanzania. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Speich, B.; Ali, S.M.; Ame, S.M.; Bogoch II, A.R.; Huwyler, J.; Albonico, M.; Hattendorf, J.; Utzinger, J.; Keiser, J. Efficacy and safety of albendazole plus ivermectin, albendazole plus mebendazole, albendazole plus oxantel pamoate, and mebendazole alone against Trichuris trichiura and concomitant soil-transmitted helminth infections: A four-arm, randomised controlled trial. Lancet Infect. Dis. 2015, 15, 277–284. [Google Scholar] [CrossRef]
- Stothard, J.R.; Rollinson, D.; Imison, E.; Khamis, I.S. A spot-check of the efficacies of albendazole or levamisole, against soil-transmitted helminthiases in young Ungujan children, reveals low frequencies of cure. Ann. Trop. Med. Parasitol. 2009, 103, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, R.M. Drug resistance in nematodes of veterinary importance: A status report. Trends Parasitol. 2004, 20, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Sunish, I.P.; Rajendra, R.; Munirathinam, A.; Kalimuthu, M.; Kumar, V.A.; Nagaraj, J.; Tyagi, B.K. Impact on prevalence of intestinal helminth infection in school children administered with seven annual rounds of diethyl carbamazine (DEC) with albendazole. Indian J. Med. Res. Suppl. 2015, 141, 330. [Google Scholar] [CrossRef]
- Stothard, J.R.; French, M.D.; Khamis, I.S.; Basáñez, M.G.; Rollinson, D. The epidemiology and control of urinary schistosomiasis and soil-transmitted helminthiasis in schoolchildren on Unguja Island, Zanzibar. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 1031–1044. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Rodríguez, A.; Nerín, C.; Batlle, R. New cinnamon-based active paper packaging against Rhizopusstolonifer food spoilage. J. Agric. Food Chem. 2008, 56, 6364–6369. [Google Scholar] [CrossRef]
- Bai, C.Q.; Liu, Z.L.; Liu, Q.Z. Nematicidal Constituents from the Essential Oil of Chenopodium Ambrosioides Aerial Parts. E-Journal Chem. 2011, 8. [Google Scholar] [CrossRef]
- Squires, J.M.; Ferreira, J.F.S.; Lindsay, D.S.; Zajac, A.M. Effects of artemisinin and Artemisia extracts on Haemonchus contortus in gerbils (Meriones unguiculatus). Vet. Parasitol. 2011, 175, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Dai, J.L.; Yang, L.; Qiu, J. In vitro ovicidal and larvicidal activity of the essential oil of Artemisia lancea against Haemonchus contortus (Strongylida). Vet. Parasitol. 2013, 195, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Ferrell, J.A. Hookworm disease: Its ravages, prevention and cure. J. Am. Med. Assoc. 1914, 52. [Google Scholar] [CrossRef]
- Michiels, J.; Missotten, J.; Dierick, N.; Fremaut, D.; Maene, P.; De Smet, S. “In vitro degradation and in vivo passage kinetics of carvacrol, thymol, eugenol and trans-cinnamaldehyde along the gastrointestinal tract of piglets. J. Sci. Food Agric. 2008, 88, 2371–2381. [Google Scholar] [CrossRef]
- Palaniappan, K.; Holley, R.A. Use of natural antimicrobials to increase antibiotic susceptibility of drug resistant bacteria. Int. J. Food Microbiol. 2010, 140, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Shapira, R.; Mimran, E. Isolation and characterization of Escherichia coli mutants exhibiting altered response to thymol. Microb. Drug Resist. 2007, 13, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Gershenzon, J.; Dudareva, N. The function of terpene natural products in the natural world. Nat. Chem. Biol. 2007, 3, 408–414. [Google Scholar] [CrossRef]
- Mukherjee, N.; Mukherjee, S.; Saini, P.; Roy, P.; Sinha Babu, S.P. Phenolics and Terpenoids; the Promising New Search for Anthelmintics: A Critical Review. Mini-Rev. Med. Chem. 2016, 16, 1415–1441. [Google Scholar] [CrossRef]
- Soto, E.R.; Ostroff, G.R. Characterization of multilayered nanoparticles encapsulated in yeast cell wall particles for DNA. Bioconjug. Chem. 2008, 19, 840–848. [Google Scholar] [CrossRef]
- Aouadi, M.; Tesz, G.J.; Nicoloro, S.M.; Wang, M.; Chouinard, M.; Soto, E.; Ostroff, G.R.; Czech, M.P. Orally delivered siRNA targeting macrophage Map4k4 suppresses systemic inflammation. Nature 2009, 458, 1180–1184. [Google Scholar] [CrossRef]
- Soto, E.; Ostroff, G. Glucan particles as carriers of nanoparticles for macrophage-targeted delivery. In Nanomaterials for Biomedicine; American Chemical Society: Washington, DC, USA, 2012; pp. 57–79. [Google Scholar] [CrossRef]
- Mirza, Z.; Soto, E.R.; Dikengil, F.; Levitz, S.M.; Ostroff, G.R. Beta-glucan particles as vaccine adjuvant carriers. In Vaccines for Invasive Fungal Infections; Humana Press: New York, NY, USA, 2017; pp. 143–157. [Google Scholar]
- Soto, E.R.; O’Connell, O.; Dikengil, F.; Peters, P.J.; Clapham, P.R.; Ostroff, G.R. Targeted Delivery of Glucan Particle Encapsulated Gallium Nanoparticles Inhibits HIV Growth in Human Macrophages. J. Drug Deliv. 2016. [Google Scholar] [CrossRef] [PubMed]
- Soto, E.; Rus, F.; Ostroff, G.R. Yeast Cell Wall Particle Encapsulation of Pro-Terpene Payloads. Available online: https://pdfs.semanticscholar.org/5229/afbd113d373534ae75bca45e72af023e6b58.pdf (accessed on 17 June 2019).
- Specht, C.A.; Lee, C.K.; Huang, H.; Hester, M.M.; Liu, J.; Luckie, B.A.; Santana, M.A.; Mirza, Z.; Khoshkenar, P.; Abraham, A.; et al. Vaccination with Recombinant Cryptococcus Proteins in Glucan Particles Protects Mice against Cryptococcosis in a Manner Dependent upon Mouse Strain and Cryptococcal Species. MBio 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Inpankaew, T.; Schar, F.; Dalsgaard, A.; Khieu, V.; Chimnoi, W.; Chhoun, C.; Sok, D.; Marti, H.; Muth, S.; Odermatt, P.; et al. High prevalence of Ancylostoma ceylanicum hookworm infections in humans, Cambodia, 2012. Emerg. Infect. Dis. 2014, 20, 976. [Google Scholar] [CrossRef] [PubMed]
- Klementowicz, J.E.; Travis, M.A.; Grencis, R.K. Trichuris muris: A model of gastrointestinal parasite infection. Semin. Immunopathol. 2012, 34, 815–828. [Google Scholar] [CrossRef]
- Hu, Y.; Zhan, B.; Keegan, B.; Yiu, Y.Y.; Miller, M.M.; Jones, K.; Aorian, R.V. Mechanistic and Single-Dose In Vivo Therapeutic Studies of Cry5B Anthelmintic Action against Hookworms. PLoS Negl. Trop. Dis. 2012, 6, e1900. [Google Scholar] [CrossRef]
- Hu, Y.; Xiao, S.H.; Aroian, R.V. The new anthelmintic tribendimidine is an L-type (Levamisole and Pyrantel) nicotinic acetylcholine receptor agonist. PLoS Negl. Trop. Dis. 2009, 3, e499. [Google Scholar] [CrossRef]
- Brombacher, T.M.; De Gouveia, K.S.; Cruywagen, L.; Makena, N.; Booley, F.; Tamgue, O.; Brombacher, F. Nippostrongylus brasiliensis infection leads to impaired reference memory and myeloid cell interference. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Hu, Y.; Ellis, B.L.; Yiu, Y.Y.; Miller, M.M.; Urban, J.F.; Shi, L.Z.; Aroian, R.V. An Extensive Comparison of the Effect of Anthelmintic Classes on Diverse Nematodes. PLoS ONE 2013, 8, e70702. [Google Scholar] [CrossRef]
Sample Availability: Samples of the YP-Terpene formulations are available from the authors. |
| A. ceylanicum | T. muris | |||||
| Terpene | 1 h | 3h | 24 h | 1 h | 3 h | 24 h |
| Carvacrol | 200 | 200 | 200 | 333 | 200 | 66 |
| Cinnamic aldehyde | 200 | 200 | 200 | 333 | 200 | 66 |
| Thymol | 200 | 200 | 100 | >333 | 200 | 100 |
| Geraniol | 200 | 200 | 200 | >333 | 200 | 100 |
| Citronellol | 200 | 200 | 200 | >333 | 200 | 200 |
| Eugenol | 200 | 200 | 200 | >333 | >333 | 200 |
| Anethole | 333 | 333 | 200 | >333 | >333 | >333 |
| Cymene | >333 | 333 | 200 | >333 | >333 | 333 |
| Limonene | >333 | 333 | 333 | >333 | >333 | 200 |
| L-carvone | >333 | 333 | 333 | >333 | >333 | 333 |
| Farnesol | >333 | >333 | 66 | >333 | >333 | 33 |
| Nerolidol | >333 | >333 | 66 | >333 | >333 | 66 |
| Alpha pinene | >333 | >333 | 200 | >333 | >333 | 200 |
| Citral | >333 | >333 | 333 | >333 | - | 200 |
| Alpha terpineol | >333 | >333 | 333 | >333 | >333 | >333 |
| Linalool | >333 | >333 | 333 | >333 | >333 | >333 |
| Peppermint oil | >333 | >333 | 333 | >333 | >333 | >333 |
| Linalyl acetate | >333 | >333 | >333 | >333 | >333 | 333 |
| Lavender oil | >333 | >333 | >333 | >333 | >333 | >333 |
| Tea tree oil | >333 | >333 | >333 | >333 | >333 | >333 |
 Fast acting at moderate-high doses
Fast acting at moderate-high doses  Fast acting at high doses
Fast acting at high doses  Slow acting at low doses
Slow acting at low doses  Slow acting at moderate-high doses
Slow acting at moderate-high doses  Not effective at doses tested.
Not effective at doses tested.© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirza, Z.; Soto, E.R.; Hu, Y.; Nguyen, T.-T.; Koch, D.; Aroian, R.V.; Ostroff, G.R. Anthelmintic Activity of Yeast Particle-Encapsulated Terpenes. Molecules 2020, 25, 2958. https://doi.org/10.3390/molecules25132958
Mirza Z, Soto ER, Hu Y, Nguyen T-T, Koch D, Aroian RV, Ostroff GR. Anthelmintic Activity of Yeast Particle-Encapsulated Terpenes. Molecules. 2020; 25(13):2958. https://doi.org/10.3390/molecules25132958
Chicago/Turabian StyleMirza, Zeynep, Ernesto R. Soto, Yan Hu, Thanh-Thanh Nguyen, David Koch, Raffi V. Aroian, and Gary R. Ostroff. 2020. "Anthelmintic Activity of Yeast Particle-Encapsulated Terpenes" Molecules 25, no. 13: 2958. https://doi.org/10.3390/molecules25132958
APA StyleMirza, Z., Soto, E. R., Hu, Y., Nguyen, T.-T., Koch, D., Aroian, R. V., & Ostroff, G. R. (2020). Anthelmintic Activity of Yeast Particle-Encapsulated Terpenes. Molecules, 25(13), 2958. https://doi.org/10.3390/molecules25132958