Effects of Pyrrole-Imidazole Polyamides Targeting Human TGF-β1 on the Malignant Phenotypes of Liver Cancer Cells
Abstract
1. Introduction
2. Results
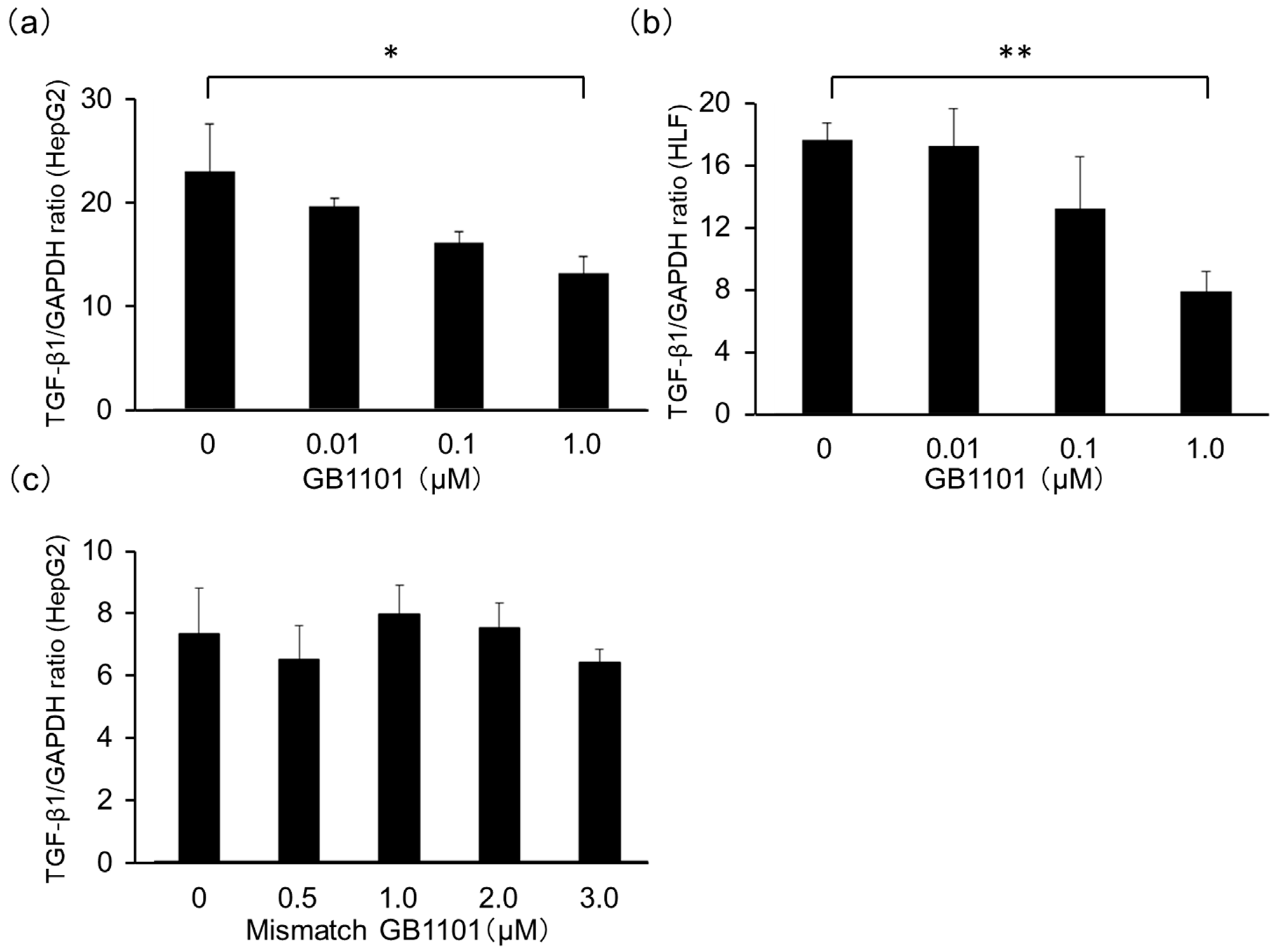
2.1. Effects of TGF-β1-Specific PI Polyamide in HepG2 Cells
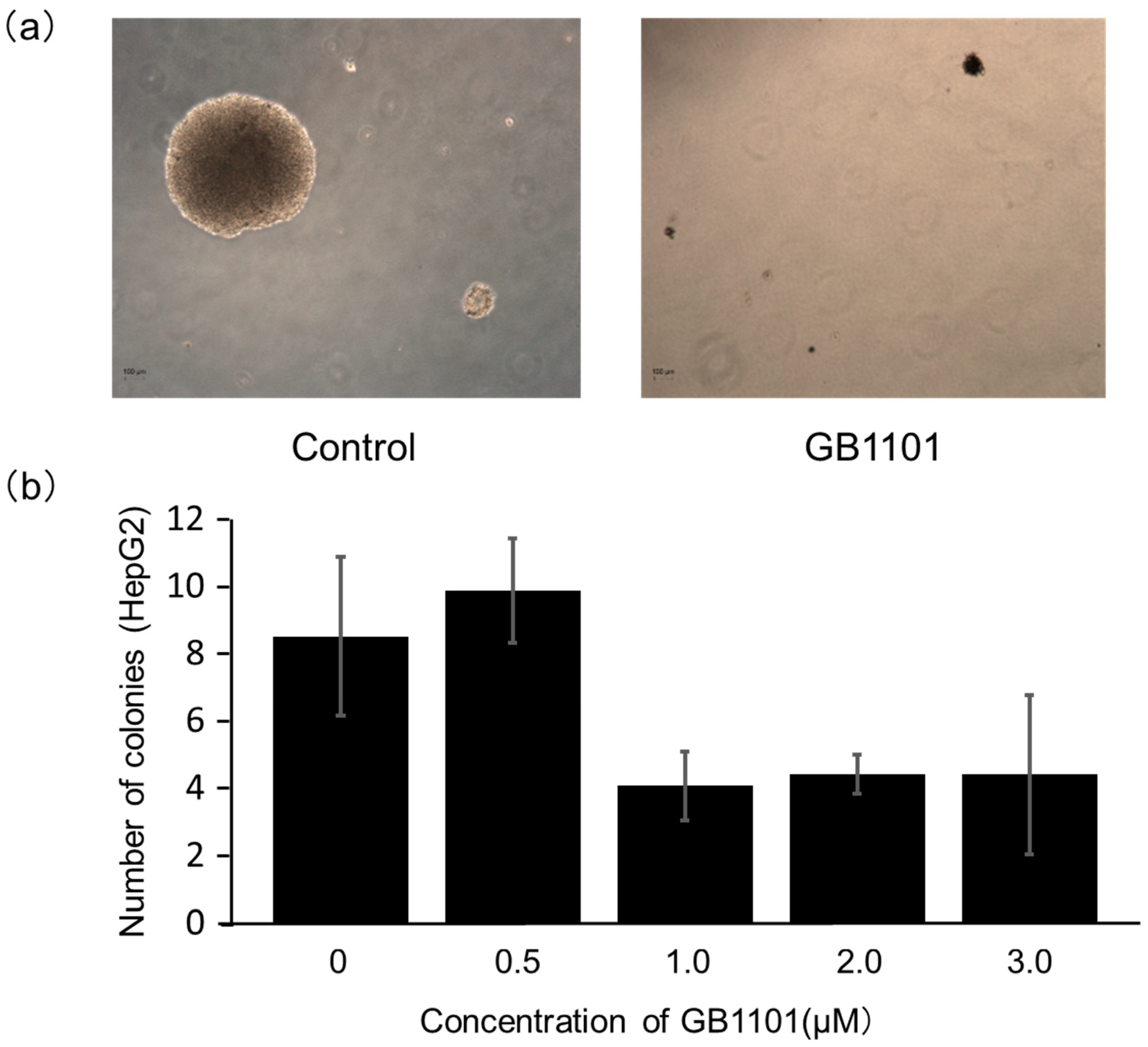
2.2. Effects of PI Polyamide on the Growth of Liver Cancer Cells
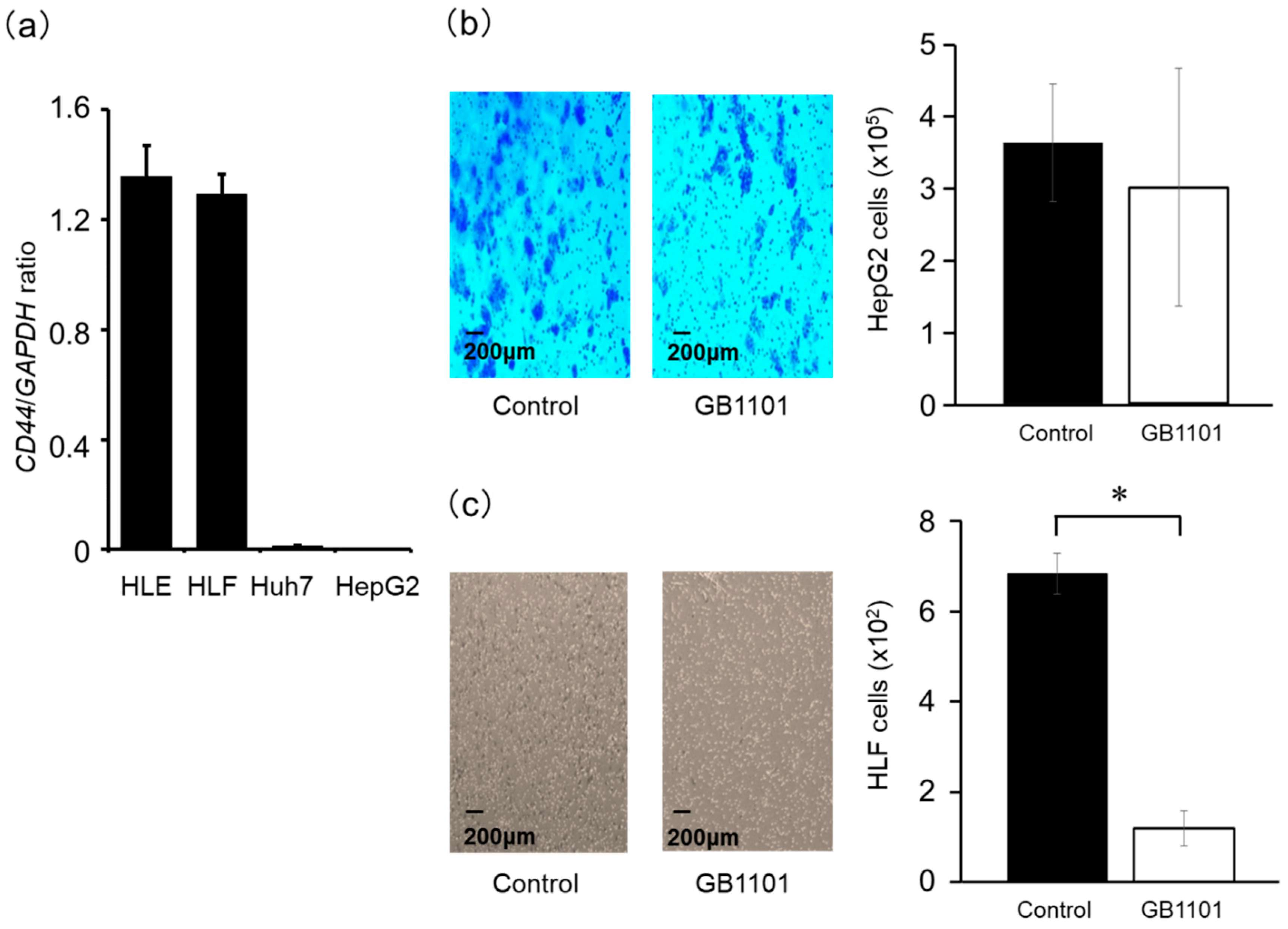
2.3. Effects of TGF-β1 PI Polyamide on the Invasion Capacity of HepG2 and HLF Cells
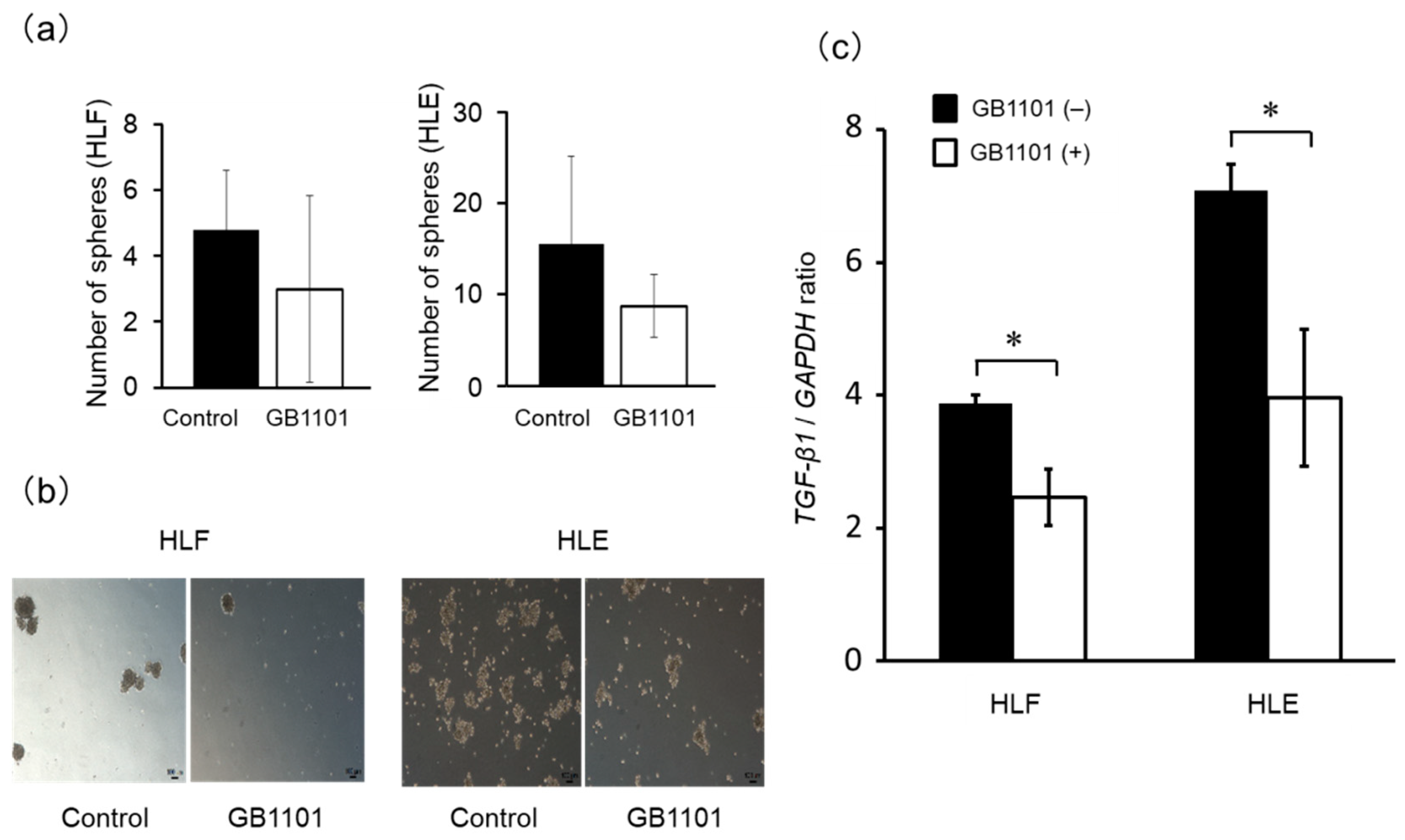
2.4. Effects of TGF-β1 PI Polyamide on Sphere Formation of CSCs
3. Discussion
4. Materials and Methods
4.1. Design and Synthesis of PI Polyamides Targeting the hTGF-β1 Promoter
4.2. Cell Culture
4.3. Assessment of TGF-β1 and CD44 mRNA Expression
4.4. Colony-Formation Assay
4.5. Cell Invasion Assay
4.6. Induction of HLF and HLE Sphere Formation
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fan, Q.-M.; Jing, Y.-Y.; Yu, G.-F.; Kou, X.-R.; Ye, F.; Gao, L.; Li, R.; Zhao, Q.-D.; Yang, Y.; Lu, Z.-H.; et al. Tumor-associated macrophages promote cancer stem cell-like properties via transforming growth factor-beta1-induced epithelial–mesenchymal transition in hepatocellular carcinoma. Cancer Lett. 2014, 352, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.-H.; Yang, F.; Wang, F.; Ma, J.-Z.; Guo, Y.; Tao, Q.-F.; Liu, F.; Pan, W.; Wang, T.-T.; Zhou, C.-C.; et al. A Long Noncoding RNA Activated by TGF-β Promotes the Invasion-Metastasis Cascade in Hepatocellular Carcinoma. Cancer Cell 2014, 25, 666–681. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yin, W.-L.; Ba, Y.-F.; Tian, L.; Gu, Z.-Q.; Zhang, M.-S.; Zhong, C.-N. Transforming growth factor-1 promotes the transcriptional activation of plasminogen activator inhibitor type 1 in carcinoma-associated fibroblasts. Mol. Med. Rep. 2012, 6, 1001–1005. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Giannelli, G.; Bergamini, C.; Fransvea, E.; Sgarra, C.; Antonaci, S. Laminin-5 With Transforming Growth Factor-β1 Induces Epithelial to Mesenchymal Transition in Hepatocellular Carcinoma. Gastroenterology 2005, 129, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Fransvea, E.; Angelotti, U.; Antonaci, S.; Giannelli, G. Blocking transforming growth factor–beta up-regulates E-cadherin and reduces migration and invasion of hepatocellular carcinoma cells. Hepatology 2008, 47, 1557–1566. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Wang, X.W. Cancer stem cells in the development of liver cancer. J. Clin. Investig. 2013, 123, 1911–1918. [Google Scholar] [CrossRef]
- Zöller, M. CD44: Can a cancer-initiating cell profit from an abundantly expressed molecule? Nat. Rev. Cancer 2011, 11, 254–267. [Google Scholar] [CrossRef]
- Mima, K.; Okabe, H.; Ishimoto, T.; Hayashi, H.; Nakagawa, S.; Kuroki, H.; Watanabe, M.; Beppu, T.; Tamada, M.; Nagano, O.; et al. CD44s Regulates the TGF- -Mediated Mesenchymal Phenotype and Is Associated with Poor Prognosis in Patients with Hepatocellular Carcinoma. Cancer Res. 2012, 72, 3414–3423. [Google Scholar] [CrossRef]
- Sugiyama, H.; Lian, C.; Isomura, M.; Saito, I.; Wang, A.H. Distamycin A modulates the sequence specificity of DNA alkylation by duocarmycin A. Proc. Natl. Acad. Sci. USA 1996, 93, 14405–14410. [Google Scholar] [CrossRef]
- Trauger, J.W.; Baird, E.E.; Dervan, P.B. Recognition of DNA by designed ligands at subnanomolar concentrations. Nature 1996, 382, 559–561. [Google Scholar] [CrossRef]
- Gottesfeld, J.M.; Neely, L.; Trauger, J.W.; Baird, E.E.; Dervan, P.B. Regulation of gene expression by small molecules. Nature 1997, 387, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.-M.; Fukuda, N.; Ueno, T.; Matsuda, H.; Saito, S.; Matsumoto, K.; Ayame, H.; Bando, T.; Sugiyama, H.; Mugishima, H.; et al. Synthetic Pyrrole-Imidazole Polyamide Inhibits Expression of the Human Transforming Growth Factor-β1 Gene. J. Pharmacol. Exp. Ther. 2005, 315, 571–575. [Google Scholar] [CrossRef]
- Matsuda, H.; Fukuda, N.; Ueno, T.; Tahira, Y.; Ayame, H.; Zhang, W.; Bando, T.; Sugiyama, H.; Saito, S.; Matsumoto, K.; et al. Development of Gene Silencing Pyrrole-Imidazole Polyamide Targeting the TGF-β1 Promoter for Treatment of Progressive Renal Diseases. J. Am. Soc. Nephrol. 2005, 17, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Fukuda, N.; Shinohara, K.-I.; Masuhiro, Y.; Hanazawa, S.; Matsuda, H.; Fujiwara, Y.; Ueno, T.; Soma, M. Modulation of the EMT/MET process by pyrrole–imidazole polyamide targeting human transforming growth factor-β1. Int. J. Biochem. Cell Boil. 2015, 66, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, J.; Fukuda, N.; Inoue, T.; Nakai, S.; Saito, K.; Fujiwara, K.; Matsuda, H.; Ueno, T.; Matsumoto, Y.; Watanabe, T.; et al. Preclinical Study of Novel Gene Silencer Pyrrole-Imidazole Polyamide Targeting Human TGF-β1 Promoter for Hypertrophic Scars in a Common Marmoset Primate Model. PLoS ONE 2015, 10, e0125295. [Google Scholar] [CrossRef] [PubMed]
- Dervan, P.B. Molecular recognition of DNA by small molecules. Bioorganic Med. Chem. 2001, 9, 2215–2235. [Google Scholar] [CrossRef]
- Matsuda, H.; Fukuda, N.; Ueno, T.; Katakawa, M.; Wang, X.; Watanabe, T.; Matsui, S.-I.; Aoyama, T.; Saito, K.; Bando, T.; et al. Transcriptional inhibition of progressive renal disease by gene silencing pyrrole–imidazole polyamide targeting of the transforming growth factor-β1 promoter. Kidney Int. 2011, 79, 46–56. [Google Scholar] [CrossRef]
- Hosui, A.; Tatsumi, T.; Hikita, H.; Saito, Y.; Hiramatsu, N.; Tsujii, M.; Hennighausen, L.; Takehara, T. Signal transducer and activator of transcription 5 plays a crucial role in hepatic lipid metabolism through regulation of CD36 expression. Hepatol. Res. 2016, 47, 813–825. [Google Scholar] [CrossRef]
- Ehley, J.A.; Melander, C.; Herman, D.; Baird, E.E.; Ferguson, H.A.; Goodrich, J.A.; Dervan, P.B.; Gottesfeld, J.M. Promoter Scanning for Transcription Inhibition with DNA-Binding Polyamides. Mol. Cell. Boil. 2002, 22, 1723–1733. [Google Scholar] [CrossRef]
- Otsuki, M.; Fukuda, N.; Inoue, T.; Mineshige, T.; Otsuki, T.; Horikoshi, S.; Endo, M.; Abe, M. Preclinical Study of DNA-Recognized Peptide Compound Pyrrole-Imidazole Polyamide Targeting Human TGF-β1 Promoter for Progressive Renal Diseases in the Common Marmoset. Molecules 2019, 24, 3178. [Google Scholar] [CrossRef]
- Horikoshi, S.; Fukuda, N.; Tsunemi, A.; Okamura, M.; Otsuki, M.; Endo, M.; Abe, M. Contribution of TGF-β1 and Effects of Gene Silencer Pyrrole-Imidazole Polyamides Targeting TGF-β1 in Diabetic Nephropathy. Molecules 2020, 25, 950. [Google Scholar] [CrossRef] [PubMed]
- Yao, E.-H.; Fukuda, N.; Ueno, T.; Matsuda, H.; Nagase, H.; Matsumoto, Y.; Sugiyama, H.; Matsumoto, K. A pyrrole–imidazole polyamide targeting transforming growth factor-β1 inhibits restenosis and preserves endothelialization in the injured artery. Cardiovasc. Res. 2008, 81, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Serie, K.; Fukuda, N.; Nakai, S.; Matsuda, H.; Maruyama, T.; Murayama, Y.; Omata, S. Pyrrole-Imidazole Polyamide Targeting Transforming Growth Factor β1 Ameliorates Encapsulating Peritoneal Sclerosis. Perit. Dial. Int. 2012, 32, 462–472. [Google Scholar] [CrossRef]
- Inami, M.; Fukushima, A.; Ueno, T.; Yamada, T.; Tsunemi, A.; Matsumoto, Y.; Fukuda, N.; Soma, M.; Nakamura, H. Reduction of Dimethylnitrosamine-Induced Liver Fibrosis by the Novel Gene Regulator PI Polyamide Targeting Transforming Growth Factor β1 Gene. Boil. Pharm. Bull. 2015, 38, 1836–1842. [Google Scholar] [CrossRef]
- Armendariz-Borunda, J.; Islas-Carbajal, M.C.; Meza-Garcia, E.; Rincon-Sanchez, A.R.; Lucano, S.; Sandoval, A.S.; Salazar, A.; Berumen, J.; Alvarez, A.; Covarrubias, A.; et al. A pilot study in patients with established advanced liver fibrosis using pirfenidone. Gut 2006, 55, 1663–1665. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Nagase, H.; Watanabe, T.; Nobusue, H.; Suzuki, T.; Asami, Y.; Shinojima, Y.; Kawashima, H.; Takagi, K.; Mishra, R.; et al. Inhibition of MMP-9 transcription and suppression of tumor metastasis by pyrrole-imidazole polyamide. Cancer Sci. 2010, 101, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, F.; Di Marco, R.; Patti, F.; Reggio, E.; Nicoletti, A.; Zaccone, P.; Stivala, F.; Meroni, P.L.; Reggio, A. Blood levels of transforming growth factor-beta 1 (TGF-β1) are elevated in both relapsing remitting and chronic progressive multiple sclerosis (MS) patients and are further augmented by treatment with interferon-beta 1b (IFN-β1b). Clin. Exp. Immunol. 1998, 113, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Petralia, M.C.; Mazzon, E.; Fagone, P.; Basile, M.S.; Lenzo, V.; Quattropani, M.C.; Di Nuovo, S.; Bendtzen, K.; Nicoletti, F. The cytokine network in the pathogenesis of major depressive disorder. Close to translation? Autoimmun. Rev. 2020, 19, 102504. [Google Scholar] [CrossRef] [PubMed]
- Derynck, R.; Zhang, Y.; Feng, X.H. Smads: Transcriptional activators of TGF-beta responses. Cell 1998, 95, 737–740. [Google Scholar] [CrossRef]
- Gellibert, F.; Woolven, J.; Fouchet, M.H.; Mathews, N.; Goodland, H.; Lovegrove, V.; Laroze, A.; Nguyen, V.L.; Sautet, S.; Wang, R.; et al. Identification of 1,5-naphthyridine derivatives as a novel series of potent and selective TGF-beta type I receptor inhibitors. J. Med. Chem. 2004, 47, 4494–4506. [Google Scholar] [CrossRef]
- Wang, X.-H.; Chen, Z.-G.; Xu, R.-L.; Lv, C.-Q.; Liu, J.; Du, B. TGF-β1 signaling pathway serves a role in HepG2 cell regulation by affecting the protein expression of PCNA, gankyrin, p115, XIAP and survivin. Oncol. Lett. 2017, 13, 3239–3246. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Haraguchi, N.; Utsunomiya, T.; Inoue, H.; Tanaka, F.; Mimori, K.; Barnard, G.F.; Mori, M. Characterization of a Side Population of Cancer Cells from Human Gastrointestinal System. Stem Cells 2006, 24, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Van Pham, P.; Vu, B.T.; Phan, N.L.-C.; Le, H.T.; Phan, N.K. In vitro spontaneous differentiation of human breast cancer stem cells and methods to control this process. Biomed. Res. Ther. 2015, 2, 1253–1261. [Google Scholar] [CrossRef]
- Ma, S.; Lee, T.K.; Zheng, B.-J.; Chan, K.W.; Guan, X.-Y.; Lee, T.K.W. CD133+ HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncogene 2007, 27, 1749–1758. [Google Scholar] [CrossRef]
- Yang, Z.F.; Ho, D.W.; Ng, M.N.; Lau, C.K.; Yu, W.C.; Ngai, P.; Chu, P.W.; Lam, C.T.; Poon, R.T.; Fan, S.T. Significance of CD90+ Cancer Stem Cells in Human Liver Cancer. Cancer Cell 2008, 13, 153–166. [Google Scholar] [CrossRef]
- Endo, K.; Terada, T. Protein expression of CD44 (standard and variant isoforms) in hepatocellular carcinoma: Relationships with tumor grade, clinicopathologic parameters, p53 expression, and patient survival. J. Hepatol. 2000, 32, 78–84. [Google Scholar] [CrossRef]
- Gao, Y.; Ruan, B.; Liu, W.; Wang, J.; Yang, X.; Zhang, Z.; Li, X.; Duan, J.; Zhang, F.; Ding, R.; et al. Knockdown of CD44 inhibits the invasion and metastasis of hepatocellular carcinoma both in vitro and in vivo by reversing epithelial-mesenchymal transition. Oncotarget 2015, 6, 7828–7837. [Google Scholar] [CrossRef]
- Kimura, O.; Takahashi, T.; Ishii, N.; Inoue, Y.; Ueno, Y.; Kogure, T.; Fukushima, K.; Shiina, M.; Yamagiwa, Y.; Kondo, Y.; et al. Characterization of the epithelial cell adhesion molecule (EpCAM)+ cell population in hepatocellular carcinoma cell lines. Cancer Sci. 2010, 101, 2145–2155. [Google Scholar] [CrossRef]
- Yamashita, T.; Honda, M.; Nakamoto, Y.; Baba, M.; Nio, K.; Hara, Y.; Zeng, S.S.; Hayashi, T.; Kondo, M.; Takatori, H.; et al. Discrete nature of EpCAM+ and CD90+ cancer stem cells in human hepatocellular carcinoma. Hepatology 2013, 57, 1484–1497. [Google Scholar] [CrossRef]
- Yamashita, T.; Kaneko, S. Orchestration of hepatocellular carcinoma development by diverse liver cancer stem cells. J. Gastroenterol. 2014, 49, 1105–1110. [Google Scholar] [CrossRef]
- Okabe, H.; Ishimoto, T.; Mima, K.; Nakagawa, S.; Hayashi, H.; Kuroki, H.; Imai, K.; Nitta, H.; Saito, S.; Hashimoto, D.; et al. CD44s signals the acquisition of the mesenchymal phenotype required for anchorage-independent cell survival in hepatocellular carcinoma. Br. J. Cancer 2013, 110, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Ding, J.; Chen, C.; Sun, W.; Ning, B.-F.; Wen, W.; Huang, L.; Han, T.; Yang, W.; Wang, C.; et al. Hepatic transforming growth factor beta gives rise to tumor-initiating cells and promotes liver cancer development. Hepatology 2012, 56, 2255–2267. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, N.; Tsunedomi, R.; Yoshimura, K.; Watanabe, Y.; Hazama, S.; Oka, M. Cancer stem-like sphere cells induced from de-differentiated hepatocellular carcinoma-derived cell lines possess the resistance to anti-cancer drugs. BMC Cancer 2014, 14, 722. [Google Scholar] [CrossRef] [PubMed]
- Wurtz, N.R.; Turner, J.M.; Baird, E.E.; Dervan, P.B. Fmoc solid phase synthesis of polyamides containing pyrrole and imidazole amino acids. Org. Lett. 2001, 3, 1201–1203. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds GB1101 are available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takagi, K.; Midorikawa, Y.; Takayama, T.; Abe, H.; Fujiwara, K.; Soma, M.; Nagase, H.; Miki, T.; Fukuda, N. Effects of Pyrrole-Imidazole Polyamides Targeting Human TGF-β1 on the Malignant Phenotypes of Liver Cancer Cells. Molecules 2020, 25, 2883. https://doi.org/10.3390/molecules25122883
Takagi K, Midorikawa Y, Takayama T, Abe H, Fujiwara K, Soma M, Nagase H, Miki T, Fukuda N. Effects of Pyrrole-Imidazole Polyamides Targeting Human TGF-β1 on the Malignant Phenotypes of Liver Cancer Cells. Molecules. 2020; 25(12):2883. https://doi.org/10.3390/molecules25122883
Chicago/Turabian StyleTakagi, Keiko, Yutaka Midorikawa, Tadatoshi Takayama, Hayato Abe, Kyoko Fujiwara, Masayoshi Soma, Hiroki Nagase, Toshio Miki, and Noboru Fukuda. 2020. "Effects of Pyrrole-Imidazole Polyamides Targeting Human TGF-β1 on the Malignant Phenotypes of Liver Cancer Cells" Molecules 25, no. 12: 2883. https://doi.org/10.3390/molecules25122883
APA StyleTakagi, K., Midorikawa, Y., Takayama, T., Abe, H., Fujiwara, K., Soma, M., Nagase, H., Miki, T., & Fukuda, N. (2020). Effects of Pyrrole-Imidazole Polyamides Targeting Human TGF-β1 on the Malignant Phenotypes of Liver Cancer Cells. Molecules, 25(12), 2883. https://doi.org/10.3390/molecules25122883







