Methylpiperidinopyrazole Attenuates Estrogen-Induced Mitochondrial Energy Production and Subsequent Osteoblast Maturation via an Estrogen Receptor Alpha-Dependent Mechanism
Abstract
1. Introduction
2. Results
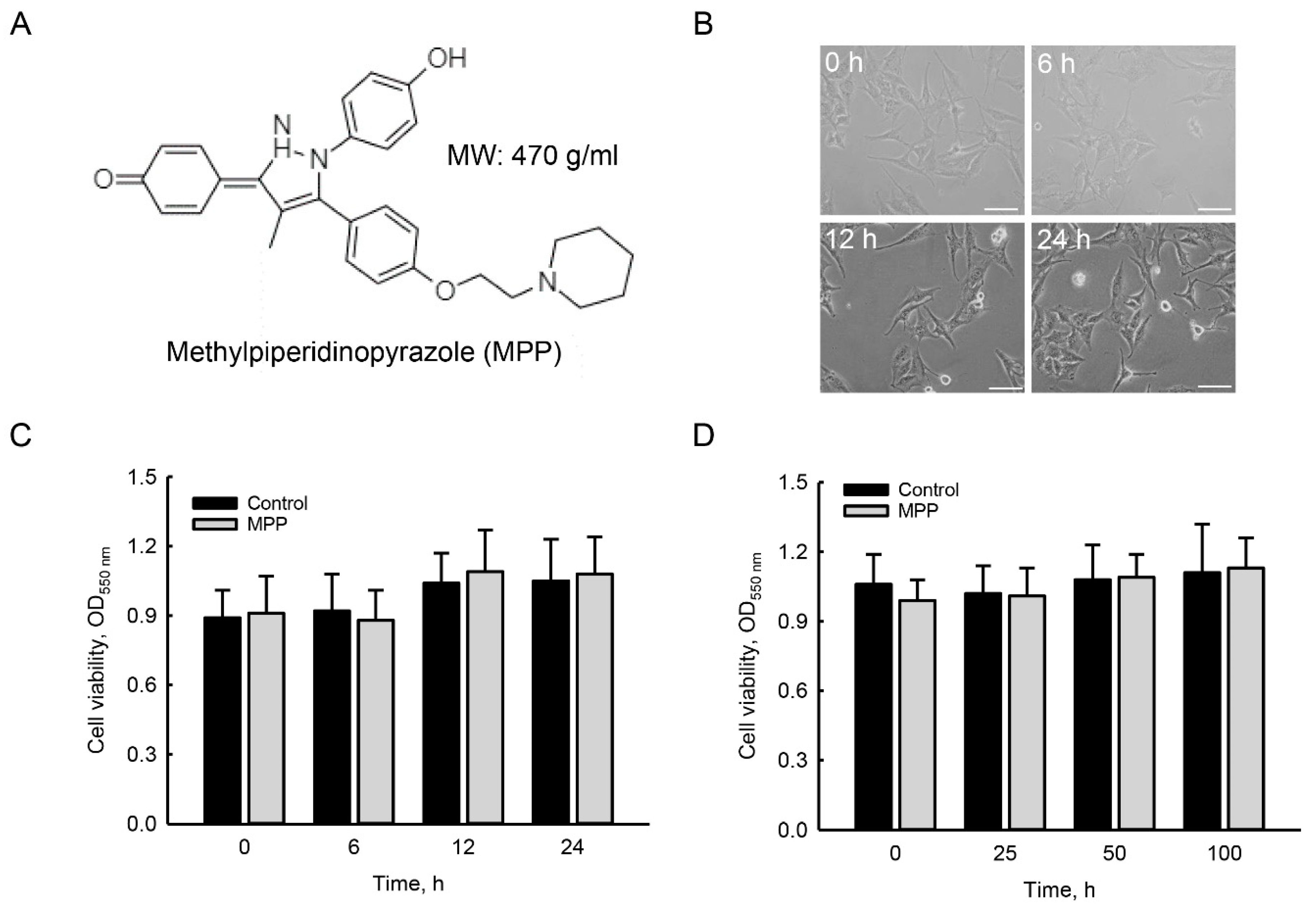
2.1. MPP Did Not Induce Cytotoxicity in Rat Calvarial Osteoblasts
2.2. MPP Diminished Estradiol-Induced Translocation of ERα from the Cytoplasm To Mitochondria in Rat Calvarial Osteoblasts
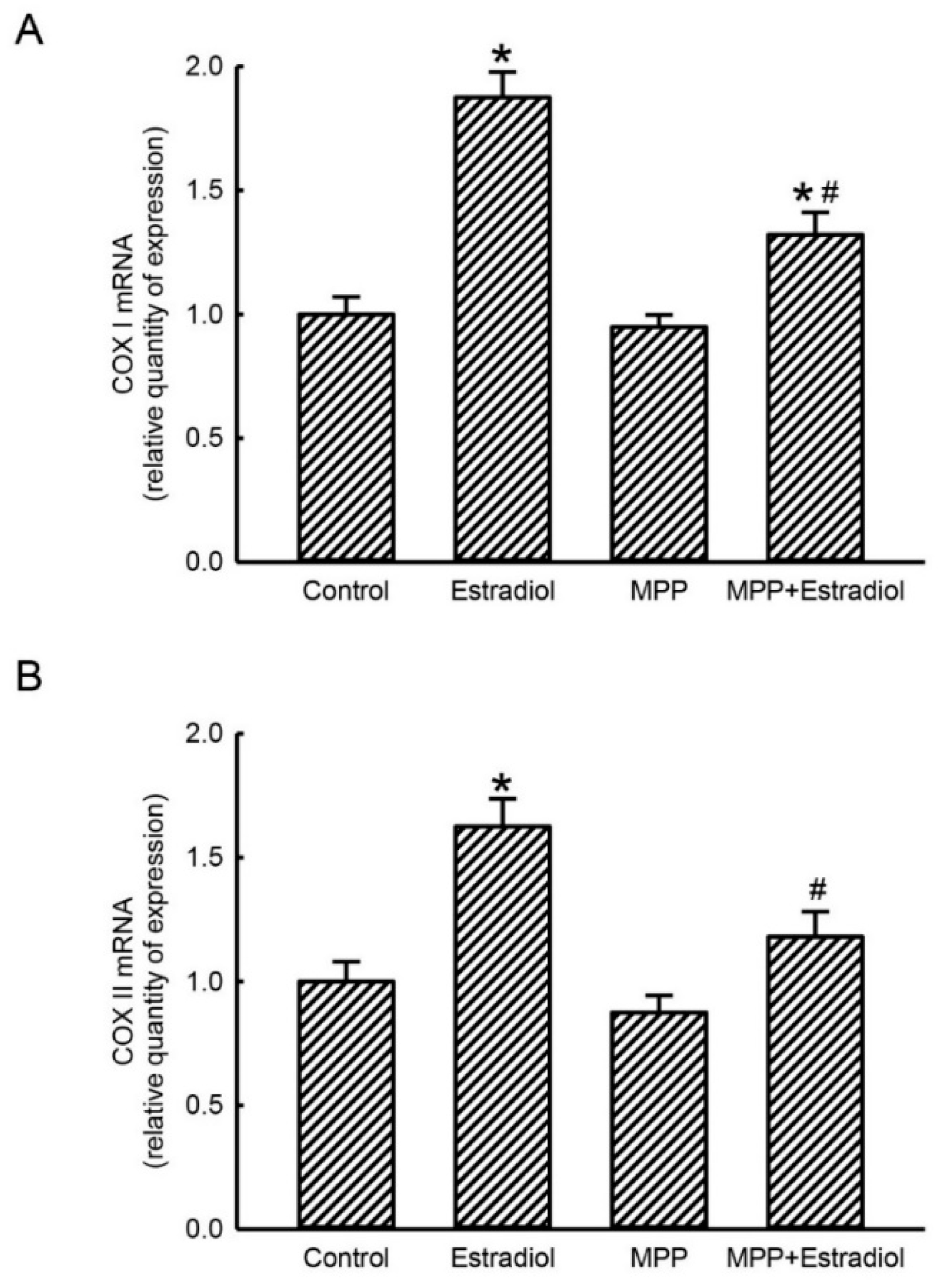
2.3. MPP Concurrently Inhibited Estradiol-Induced Expressions of Mitochondrial Energy Production-Linked COX I and COX II mRNAs in Rat Calvarial Osteoblasts
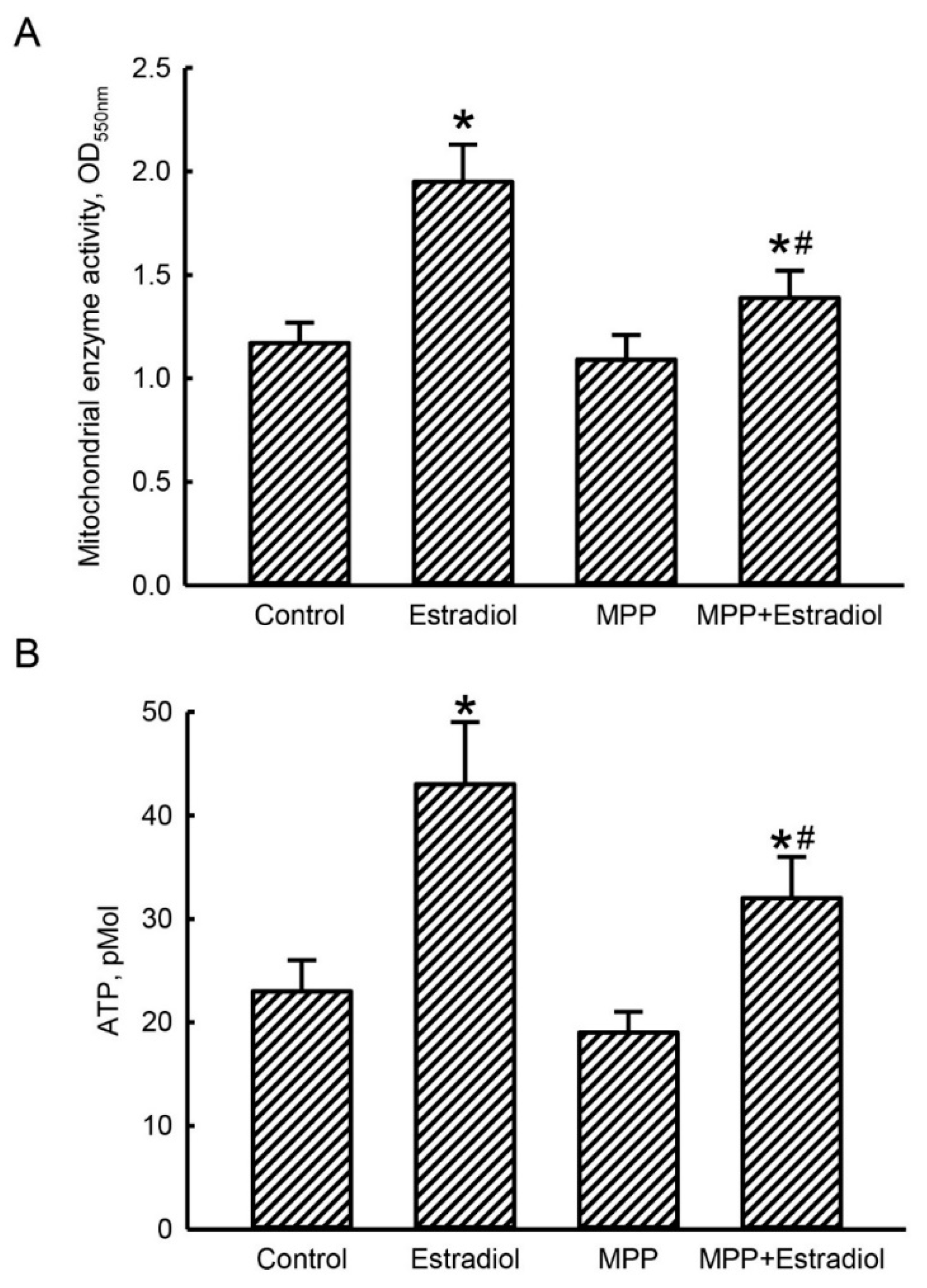
2.4. MPP Subsequently Lowered Estradiol-Triggered Enhancements of Mitochondrial Complex Enzyme Activities and Cellular ATP Levels in Rat Calvarial Osteoblasts
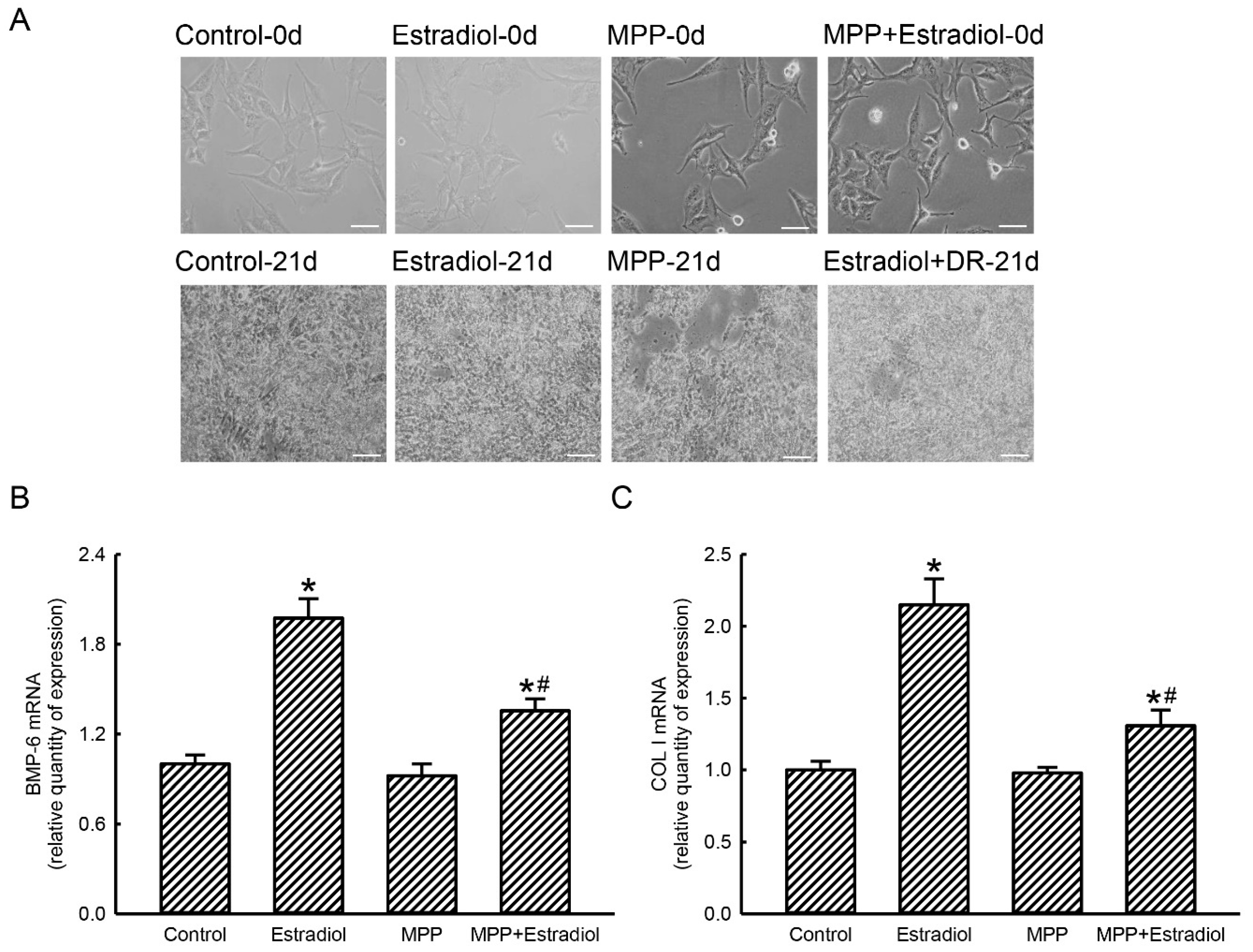
2.5. MPP Accordingly Inhibited Estradiol-Induced Expressions of Osteoblast Maturation-Associated BMP-6 and Type I Collagen mRNAs in Rat Calvarial Osteoblasts
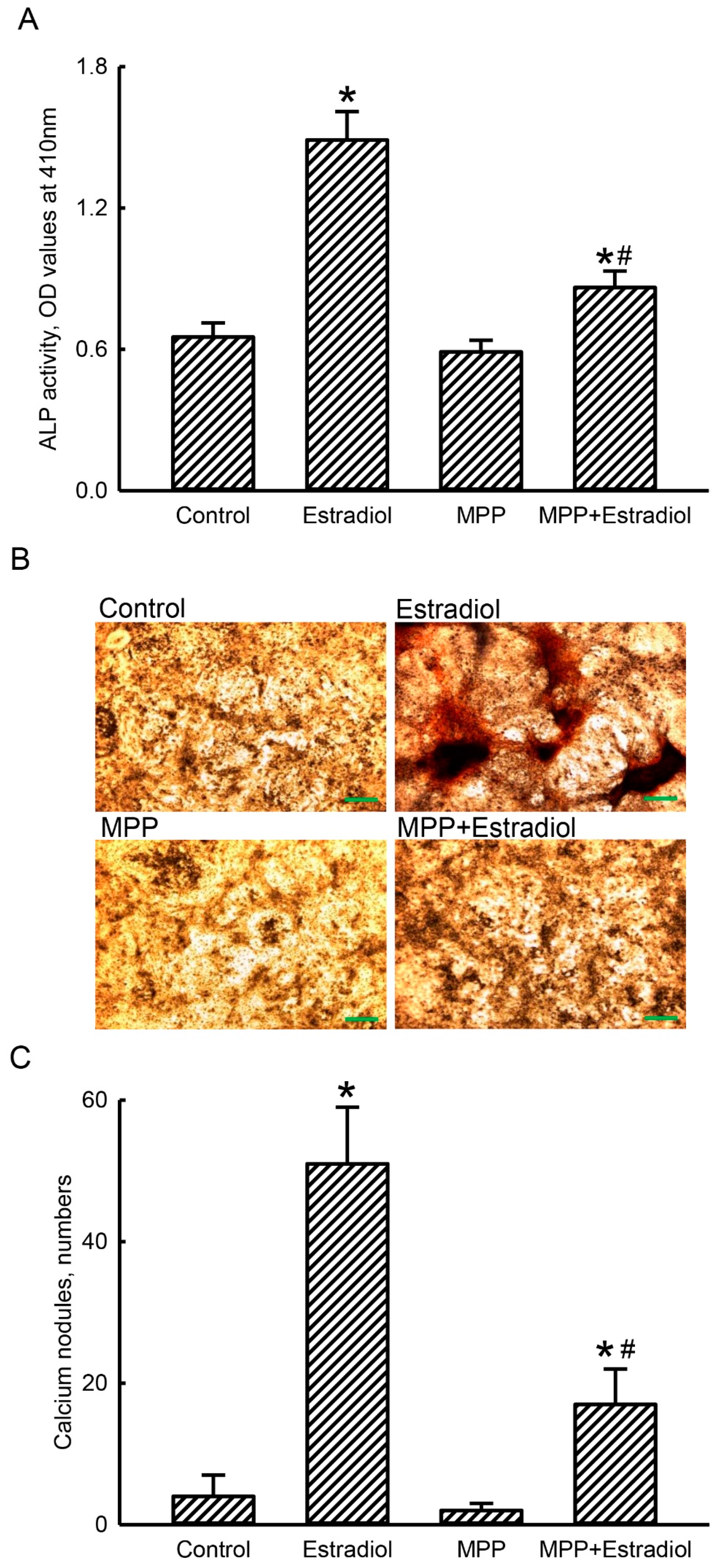
2.6. MPP Lowered Estradiol-Induced Activation and Mineralization of Rat Calvarial Osteoblasts
3. Discussion
4. Materials and Methods
4.1. Preparation of Rat Osteoblasts
4.2. Drug Treatment
4.3. Analyses of Cell Morphology and Cell Survival
4.4. Preparation of Mitochondrial Proteins
4.5. Immunodetection of Mitochondrial ERα Protein
4.6. Real-Time Polymerase Chain Reaction (PCR)
4.7. Assay of Mitochondrial Enzyme Activity
4.8. Measurement of Cellular ATP
4.9. Examination of Osteoblast Mineralization
4.10. Analysis of ALP Activity
4.11. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reid, I.R. A broader strategy for osteoporosis interventions. Nat. Rev. Endocrinol. 2020, 16, 333–339. [Google Scholar] [CrossRef]
- Kim, B.J.; Koh, J.M. Coupling factors involved in preserving bone balance. Cell. Mol. Life Sci. 2019, 76, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Compston, J.E.; McClung, M.R.; Leslie, W.D. Osteoporosis. Lancet 2019, 393, 364–376. [Google Scholar] [CrossRef]
- Yang, D.H.; Yang, M.Y. The role of macrophage in the pathogenesis of osteoporosis. Int. J. Mol. Sci. 2019, 20, 2093. [Google Scholar] [CrossRef] [PubMed]
- Rharass, T.; Lucas, S. Mechanisms in endocrinology: Bone marrow adiposity and bone, a bad romance? Eur. J. Endocrinol. 2018, 179, R165–R182. [Google Scholar] [CrossRef]
- Hadji, P.; Colli, E.; Regidor, P.A. Bone health in estrogen-free contraception. Osteoporos. Int. 2019, 30, 2391–2400. [Google Scholar] [CrossRef]
- Ensrud, K.E.; Crandall, C.J. Osteoporosis. Ann. Intern. Med. 2017, 167, ITC17–ITC32. [Google Scholar] [CrossRef]
- Curtis, E.M.; Moon, R.J.; Harvey, N.C.; Cooper, C. The impact of fragility fracture and approaches to osteoporosis risk assessment worldwide. Bone 2017, 104, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Kohli, N.; Ho, S.; Brown, S.J.; Sawadkar, P.; Sharma, V.; Snow, M.; García-Gareta, E. Bone remodelling in vitro: Where are we headed?: A review on the current understanding of physiological bone remodelling and inflammation and the strategies for testing biomaterials in vitro. Bone 2018, 110, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Nakashima, T.; Yoshimura, N.; Okamoto, K.; Tanaka, S.; Takayanagi, H. Autoregulation of osteocyte Sema3a orchestrates estrogen action and counteracts bone aging. Cell Metab. 2019, 29, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Vanderschueren, D.; Laurent, M.R.; Claessens, F.; Gielen, E.; Lagerquist, M.K.; Vandenput, L.; Börjesson, A.E.; Ohlsson, C. Sex steroid actions in male bone. Endocr. Rev. 2014, 35, 906–9060. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Olsen, B.R. Osteoblast-derived VEGF regulates osteoblast differentiation and bone formation during bone repair. J. Clin. Invest. 2016, 126, 509–526. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Molecular mechanism of Runx2-dependent bone development. Mol. Cells 2020, 43, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.M.; Lin, Y.L.; Chou, C.W. GATA-3 transduces survival signals in osteoblasts through upregulation of bcl-xL gene expression. J. Bone Min. Res. 2010, 25, 2193–2204. [Google Scholar] [CrossRef]
- Bae, S.J.; Kim, H.J.; Won, H.Y.; Min, Y.K.; Hwang, E.S. Acceleration of osteoblast differentiation by a novel osteogenic compound, DMP-PYT, through activation of both the BMP and Wnt pathways. Sci. Rep. 2017, 7, 8455. [Google Scholar] [CrossRef]
- Shi, S.; Kirk, M.; Kahn, A.J. The role of type I collagen in the regulation of the osteoblast phenotype. J. Bone Miner. Res. 1996, 11, 1139–1145. [Google Scholar] [CrossRef]
- Letts, J.A.; Sazanov, L.A. Clarifying the supercomplex: The higher-order organization of the mitochondrial electron transport chain. Nat. Struct. Mol. Biol. 2017, 24, 800–808. [Google Scholar] [CrossRef]
- Rak, M.; Bénit, P.; Chrétien, D.; Bouchereau, J.; Schiff, M.; El-Khoury, R.; Tzagoloff, A.; Rustin, P. Mitochondrial cytochrome c oxidase deficiency. Clin. Sci. 2016, 130, 393–407. [Google Scholar] [CrossRef]
- Lin, P.I.; Tai, Y.T.; Chan, W.P.; Lin, Y.L.; Liao, M.H.; Chen, R.M. Estrogen/ERα signaling axis participates in osteoblast maturation via upregulating chromosomal and mitochondrial complex gene expressions. Oncotarget 2018, 9, 1169–1186. [Google Scholar] [CrossRef]
- Tella, S.H.; Gallagher, J.C. Prevention and treatment of postmenopausal osteoporosis. J. Steroid Biochem. Mol. Biol. 2014, 142, 155–170. [Google Scholar] [CrossRef]
- Rossouw, J.E.; Anderson, G.L.; Prentice, R.L.; LaCroix, A.Z.; Kooperberg, C.; Stefanick, M.L.; Jackson, R.D.; Beresford, S.A.; Howard, B.V.; Johnson, K.C.; et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002, 288, 321–333. [Google Scholar] [CrossRef]
- Sirotkin, A.V.; Harrath, A.H. Phytoestrogens and their effects. Eur. J. Pharmacol. 2014, 741, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Chiavarini, M.; Naldini, G.; Fabiani, R. The role of diet in osteoporotic fracture healing: A systematic review. Curr. Osteoporos. Rep. 2020. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.H.; Tai, Y.T.; Cherng, Y.G.; Liu, S.H.; Chang, Y.A.; Lin, P.I.; Chen, R.M. Genistein induces estrogen receptor-α gene expression in osteoblasts through activation of MAPKs/NF-κB/AP-1 and promotes cell mineralization. Br. J. Nutr. 2014, 111, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Filipović, B.; Šošić-Jurjević, B.; Ajdžanović, V.; Živanović, J.; Manojlović-Stojanoski, M.; Nestorović, N.; Ristić, N.; Trifunović, S.; Milošević, V. The phytoestrogen genistein prevents trabecular bone loss and affects thyroid follicular cells in a male rat model of osteoporosis. J. Anat. 2018, 233, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Huang, Y.R.; Harrington, W.R.; Sheng, S.; Katzenellenbogen, J.A.; Katzenellenbogen, B.S. Antagonists selective for estrogen receptor alpha. Endocrinology 2002, 143, 941–947. [Google Scholar] [CrossRef]
- Arrieta-Cruz, I.; Librado-Osorio, R.; Flores, A.; Mendoza-Garcés, L.; Chavira, R.; Cárdenas, M.; Gutiérrez-Juárez, R.; Domínguez, R.; Cruz, M.E. Estrogen receptors alpha and beta in POA-AHA region regulate asymmetrically ovulation. Cell. Mol. Neurobiol. 2019, 39, 1139–1149. [Google Scholar] [CrossRef]
- Sen, A.; Iyer, J.; Boddu, S.; Kaul, A.; Kaul, R. Estrogen receptor alpha differentially modulates host immunity in the bladder and kidney in response to urinary tract infection. Am. J. Clin. Exp. Urol. 2019, 7, 110–122. [Google Scholar]
- Lee, W.C.; Guntur, A.R.; Long, F.; Rosen, C.J. Energy Metabolism of the osteoblast: Implications for osteoporosis. Endocr. Rev. 2017, 38, 255–266. [Google Scholar] [CrossRef]
- Shares, B.H.; Busch, M.; White, N.; Shum, L.; Eliseev, R.A. Active mitochondria support osteogenic differentiation by stimulating β-catenin acetylation. J. Biol. Chem. 2018, 293, 16019–16027. [Google Scholar] [CrossRef]
- Cepeda, S.B.; Sandoval, M.J.; Crescitelli, M.C.; Rauschemberger, M.B.; Massheimer, V.L. The isoflavone genistein enhances osteoblastogenesis: Signaling pathways involved. J. Physiol. Biochem. 2020, 76, 99–110. [Google Scholar] [CrossRef]
- Klinge, C.M. Estrogenic control of mitochondrial function and biogenesis. J. Cell. Biochem. 2008, 105, 1342–1351. [Google Scholar] [CrossRef]
- Irwin, R.W.; Yao, J.; Hamilton, R.T.; Cadenas, E.; Brinton, R.D.; Nilsen, J. Progesterone and estrogen regulate oxidative metabolism in brain mitochondria. Endocrinology 2008, 149, 3167–3175. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.D.; Han, W.X.; Liu, Y.X. Suppression of miR-451a accelerates osteogenic differentiation and inhibits bone loss via Bmp6 signaling during osteoporosis. Biomed. Pharmacother. 2019, 120, 109378. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, M.; Fujisawa, R.; Kuboki, Y. Type I collagen-induced osteoblastic differentiation of bone-marrow cells mediated by collagen-alpha2beta1 integrin interaction. J. Cell. Physiol. 2000, 184, 207–213. [Google Scholar] [CrossRef]
- Chen, R.M.; Lin, Y.L.; Jean, W.C.; Chen, J.S.; Wang, J.H.; Liu, H.C. Nitric oxide induces osteoblast apoptosis through the de novol synthesis of Bax protein. J. Orthop. Res. 2002, 20, 295–302. [Google Scholar] [CrossRef]
- Wu, G.J.; Yang, S.T.; Chen, R.M. Major contribution of caspase-9 to honokiol-induced apoptotic insults to human drug-resistant glioblastoma cells. Molecules. 2020, 25, 1450. [Google Scholar] [CrossRef]
- Sun, D.P.; Lee, Y.W.; Chen, J.T.; Lin, Y.W.; Chen, R.M. The bradykinin-BDKRB1 axis regulates aquaporin 4 gene expression and consequential migration and invasion of malignant glioblastoma cells via a Ca2+-MEK1-ERK1/2-NF-κB Mechanism. Cancers 2020, 12, 667. [Google Scholar] [CrossRef]
- Chen, T.G.; Chen, T.L.; Chang, H.C.; Tai, Y.T.; Cherng, Y.G.; Chang, Y.T.; Chen, R.M. Oxidized low-density lipoprotein induces apoptotic insults to mouse cerebral endothelial cells via a Bax-mitochondria-caspase protease pathway. Toxicol. Appl. Pharmacol. 2007, 219, 42–53. [Google Scholar] [CrossRef]
- Lin, J.W.; Chen, J.T.; Hong, C.Y.; Lin, Y.L.; Wang, K.T.; Yao, C.J.; Lai, G.M.; Chen, R.M. Honokiol traverses the blood-brain barrier and induces apoptosis of neuroblastoma cells via an intrinsic Bax-mitochondrion-cytochrome c-caspase protease pathway. Neuro-Oncol. 2012, 14, 302–314. [Google Scholar] [CrossRef]
- Yeh, P.S.; Wang, W.; Chang, Y.A.; Lin, C.J.; Wang, J.J.; Chen, R.M. Honokiol induces autophagy of neuroblastoma cells through activating the PI3K/Akt/mTOR and endoplasmic reticular stress/ERK1/2 signaling pathways and suppressing cell migration. Cancer Lett. 2016, 370, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Chio, C.C.; Lin, J.W.; Cheng, H.A.; Chiu, W.T.; Wang, Y.H.; Wang, J.J.; Hsing, C.H.; Chen, R.M. MicroRNA-210 targets antiapoptotic Bcl-2 expression and mediates hypoxia-induced apoptosis of neuroblastoma cells. Arch. Toxicol. 2013, 87, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.J.; Chen, T.L.; Tseng, Y.Y.; Wu, G.J.; Hsieh, M.H.; Lin, J.W.; Chen, R.M. Honokiol induces autophagic cell death in malignant glioma through reactive oxygen species-mediated regulation of the p53/PI3K/Akt/mTOR signaling pathway. Toxicol. Appl. Pharmacol. 2016, 304, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.M.; Wu, C.H.; Chang, H.C.; Lin, Y.L.; Sheu, J.R.; Chen, T.L. Propofol suppresses macrophage functions through modulating mitochondrial membrane potential and cellular adenosine triphosphate levels. Anesthesiology 2003, 98, 1178–1185. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.H.; Liao, M.H.; Lin, Y.L.; Lai, C.H.; Lin, P.I.; Chen, R.M. Improving effects of chitosan nanofiber scaffolds on osteoblast proliferation and maturation. Int. J. Nanomed. 2014, 9, 4293–4304. [Google Scholar] [CrossRef]
- Fang, H.W.; Kao, W.Y.; Lin, P.I.; Chang, G.W.; Hung, Y.J.; Chen, R.M. Effects of polypropylene carbonate/poly(d,l-lactic) acid/tricalcium phosphate elastic composites on improving osteoblast maturation. Ann. Biomed. Eng. 2015, 43, 1999–2009. [Google Scholar] [CrossRef]
Sample Availability: Sample of the compound methylpiperidinopyrazole is available from the authors. |

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeh, P.-S.; Chen, J.-T.; Cherng, Y.-G.; Yang, S.-T.; Tai, Y.-T.; Chen, R.-M. Methylpiperidinopyrazole Attenuates Estrogen-Induced Mitochondrial Energy Production and Subsequent Osteoblast Maturation via an Estrogen Receptor Alpha-Dependent Mechanism. Molecules 2020, 25, 2876. https://doi.org/10.3390/molecules25122876
Yeh P-S, Chen J-T, Cherng Y-G, Yang S-T, Tai Y-T, Chen R-M. Methylpiperidinopyrazole Attenuates Estrogen-Induced Mitochondrial Energy Production and Subsequent Osteoblast Maturation via an Estrogen Receptor Alpha-Dependent Mechanism. Molecules. 2020; 25(12):2876. https://doi.org/10.3390/molecules25122876
Chicago/Turabian StyleYeh, Poh-Shiow, Jui-Tai Chen, Yih-Giun Cherng, Shun-Tai Yang, Yu-Ting Tai, and Ruei-Ming Chen. 2020. "Methylpiperidinopyrazole Attenuates Estrogen-Induced Mitochondrial Energy Production and Subsequent Osteoblast Maturation via an Estrogen Receptor Alpha-Dependent Mechanism" Molecules 25, no. 12: 2876. https://doi.org/10.3390/molecules25122876
APA StyleYeh, P.-S., Chen, J.-T., Cherng, Y.-G., Yang, S.-T., Tai, Y.-T., & Chen, R.-M. (2020). Methylpiperidinopyrazole Attenuates Estrogen-Induced Mitochondrial Energy Production and Subsequent Osteoblast Maturation via an Estrogen Receptor Alpha-Dependent Mechanism. Molecules, 25(12), 2876. https://doi.org/10.3390/molecules25122876




