The Role of Fucoidans Isolated from the Sporophylls of Undaria pinnatifida against Particulate-Matter-Induced Allergic Airway Inflammation: Evidence of the Attenuation of Oxidative Stress and Inflammatory Responses
Abstract
1. Introduction
2. Results
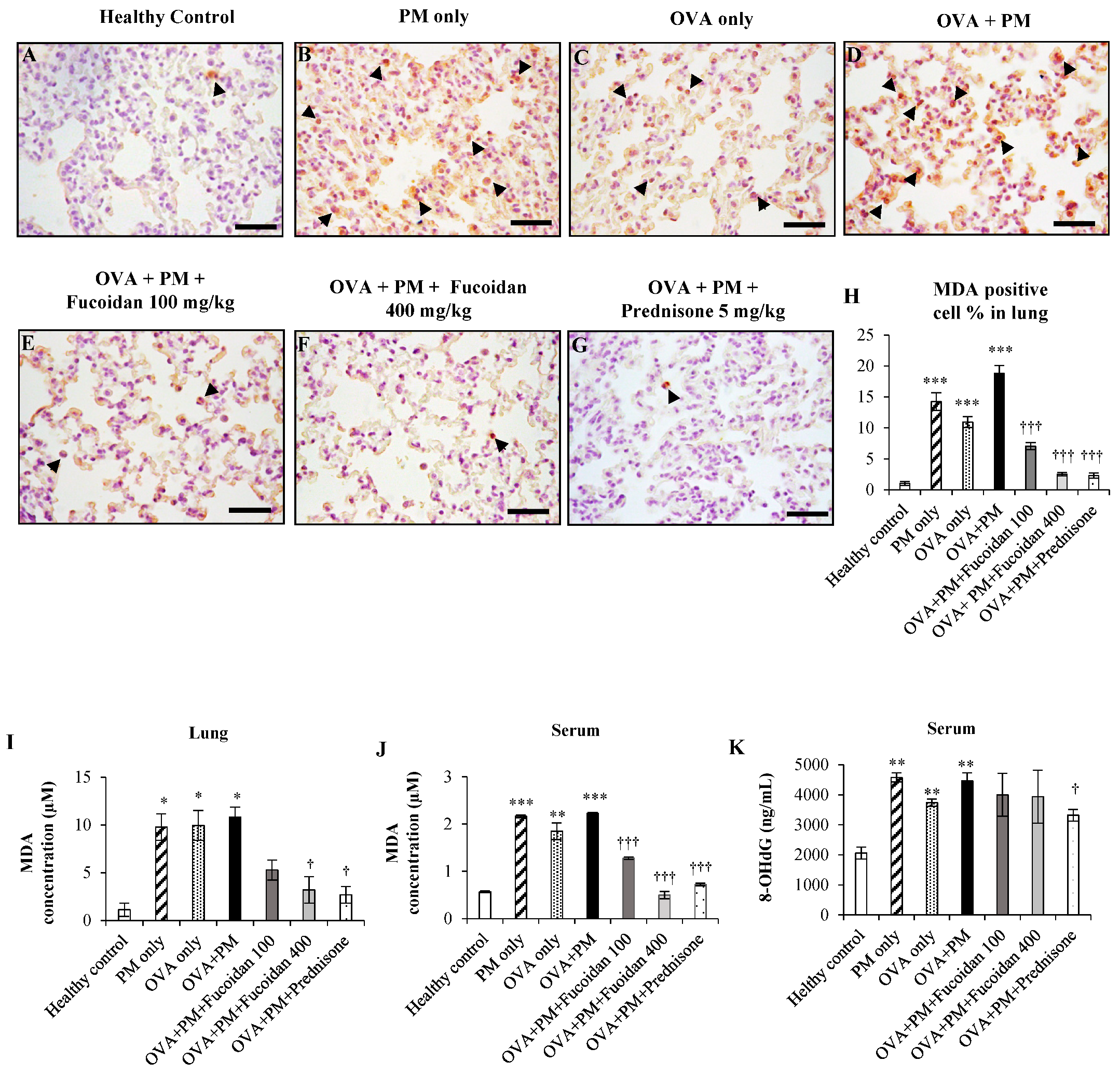
2.1. The Effect of Fucoidan on the Levels of 8-OHdG and Malondialdehyde (MDA) in the Serum and Lungs
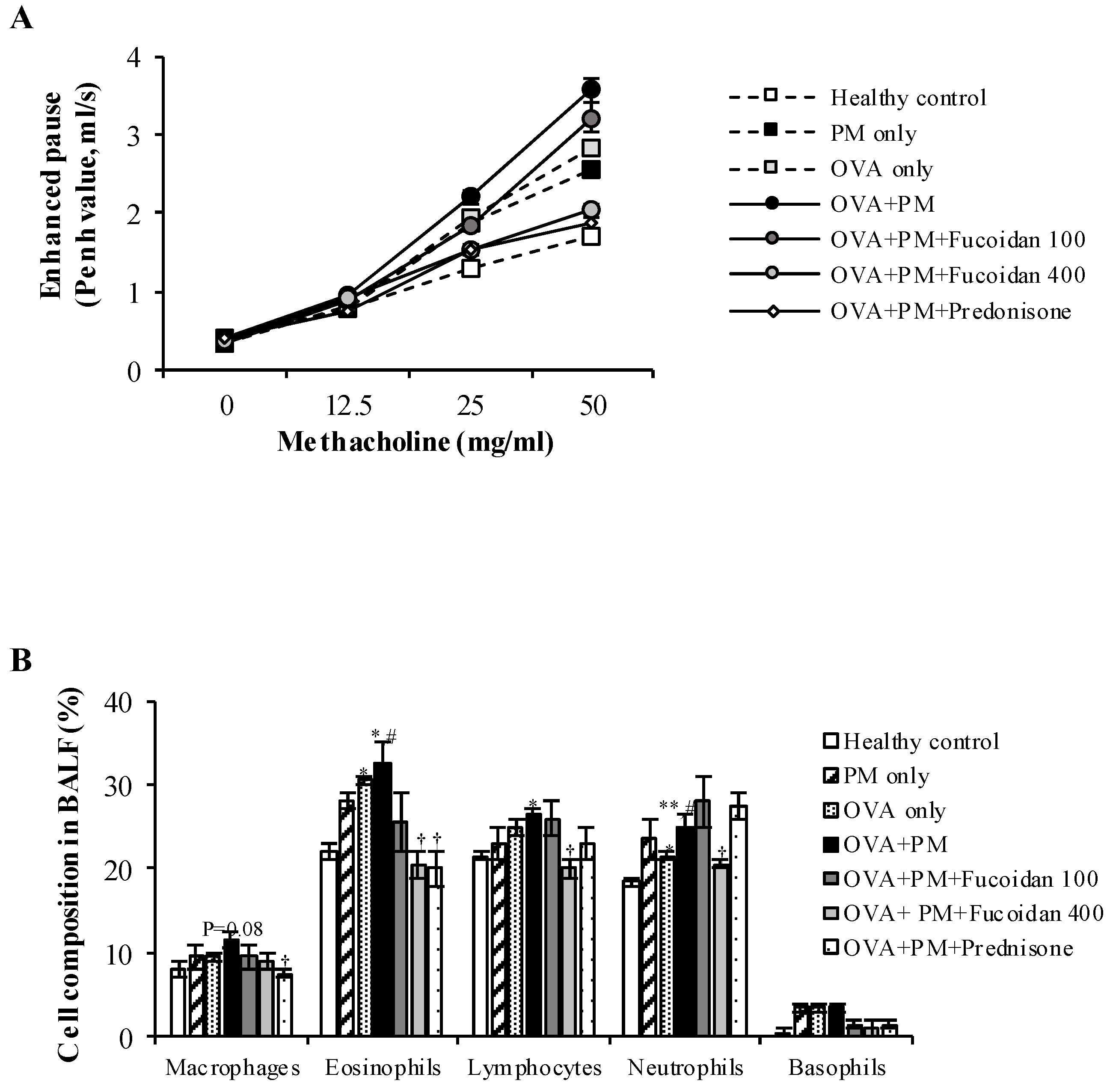
2.2. Fucoidans Attenuated PM-Exacerbated Airway Hyper Responsiveness and Inflammatory Cell Infiltration in Broncho Alveolar Lavage Fluid (BALF)
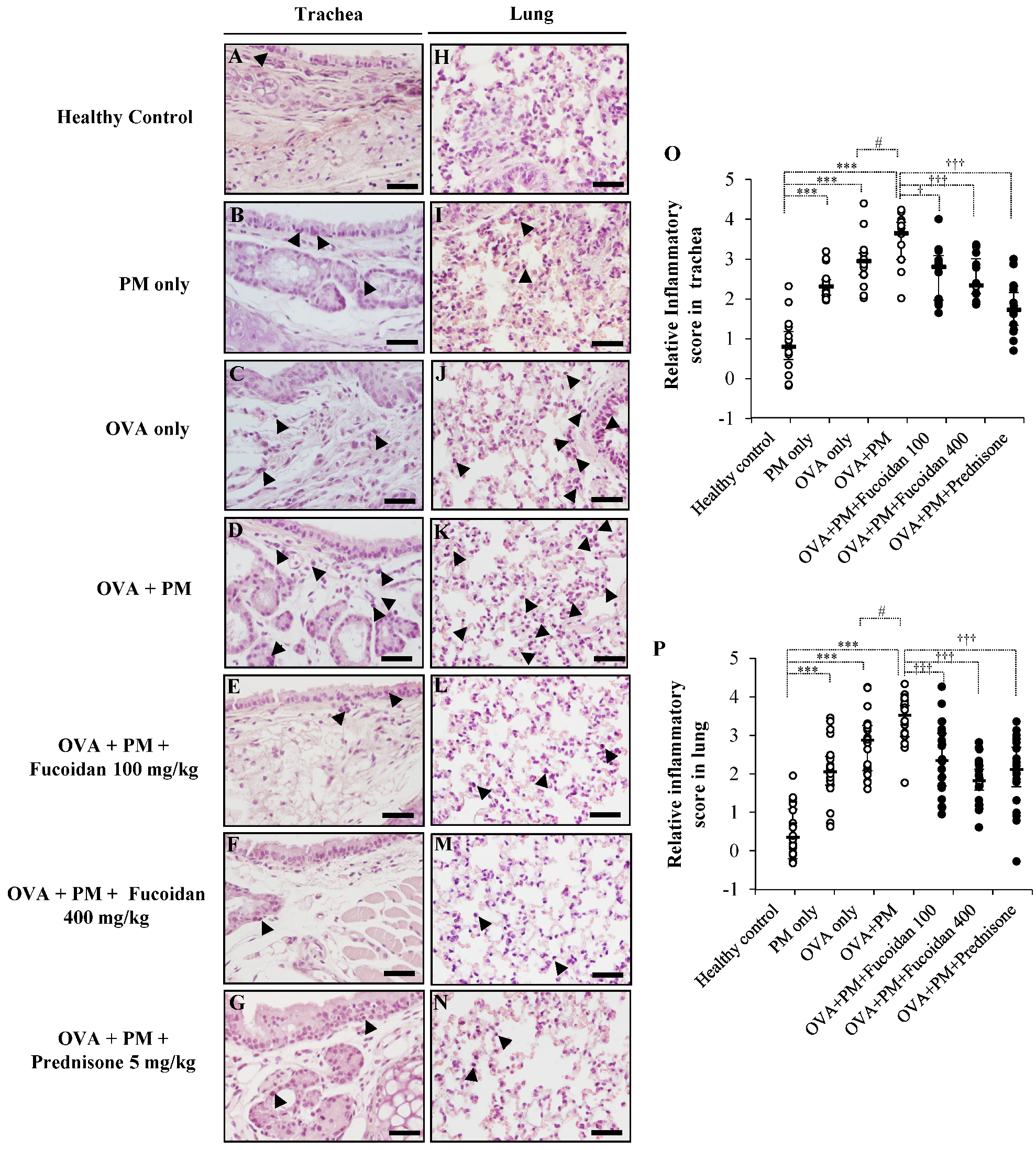
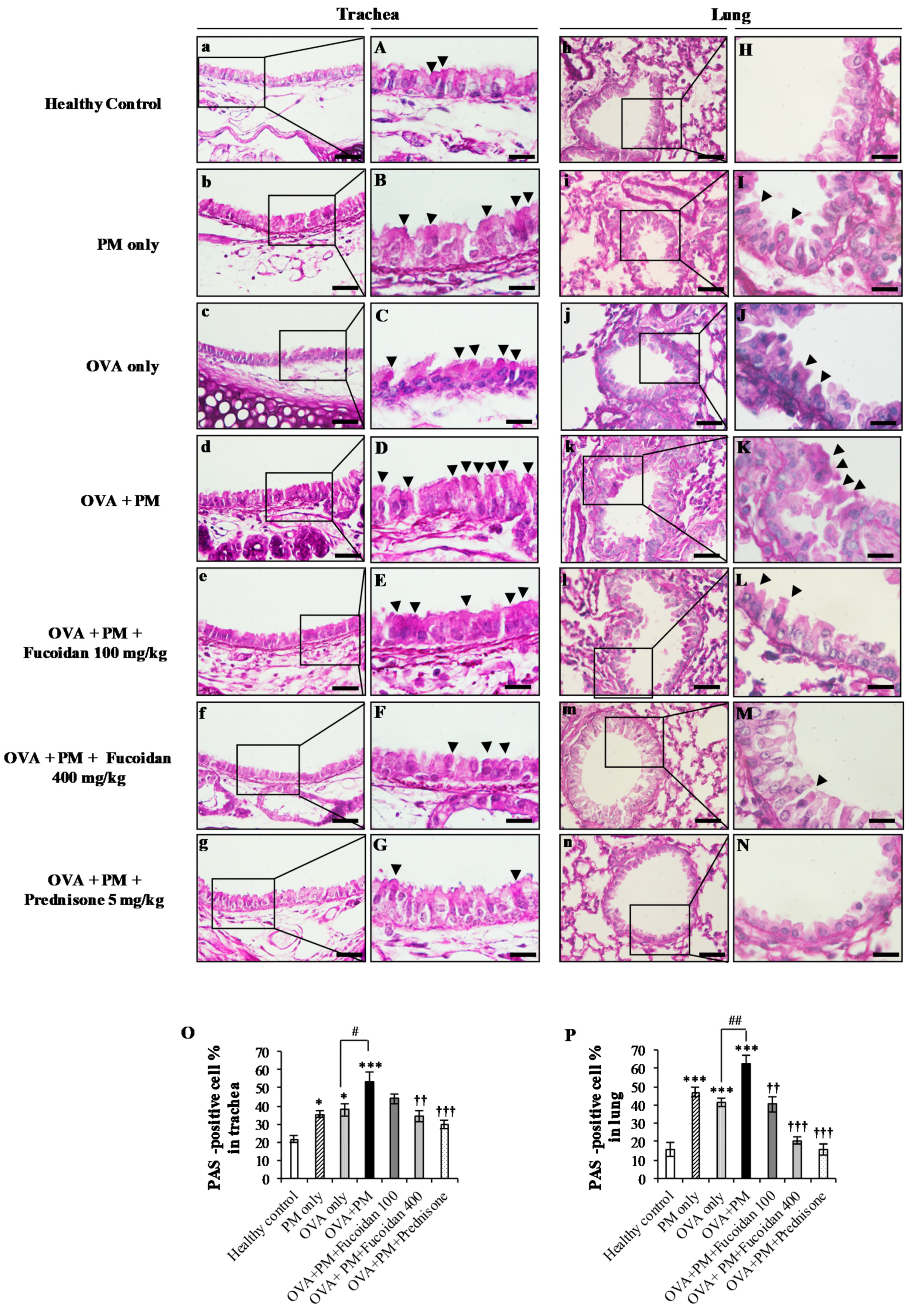
2.3. Pathological Observations in Mouse Trachea and Lungs
2.4. Fucoidans Attenuated the Infiltration of Eosinophils in the Trachea and Lungs
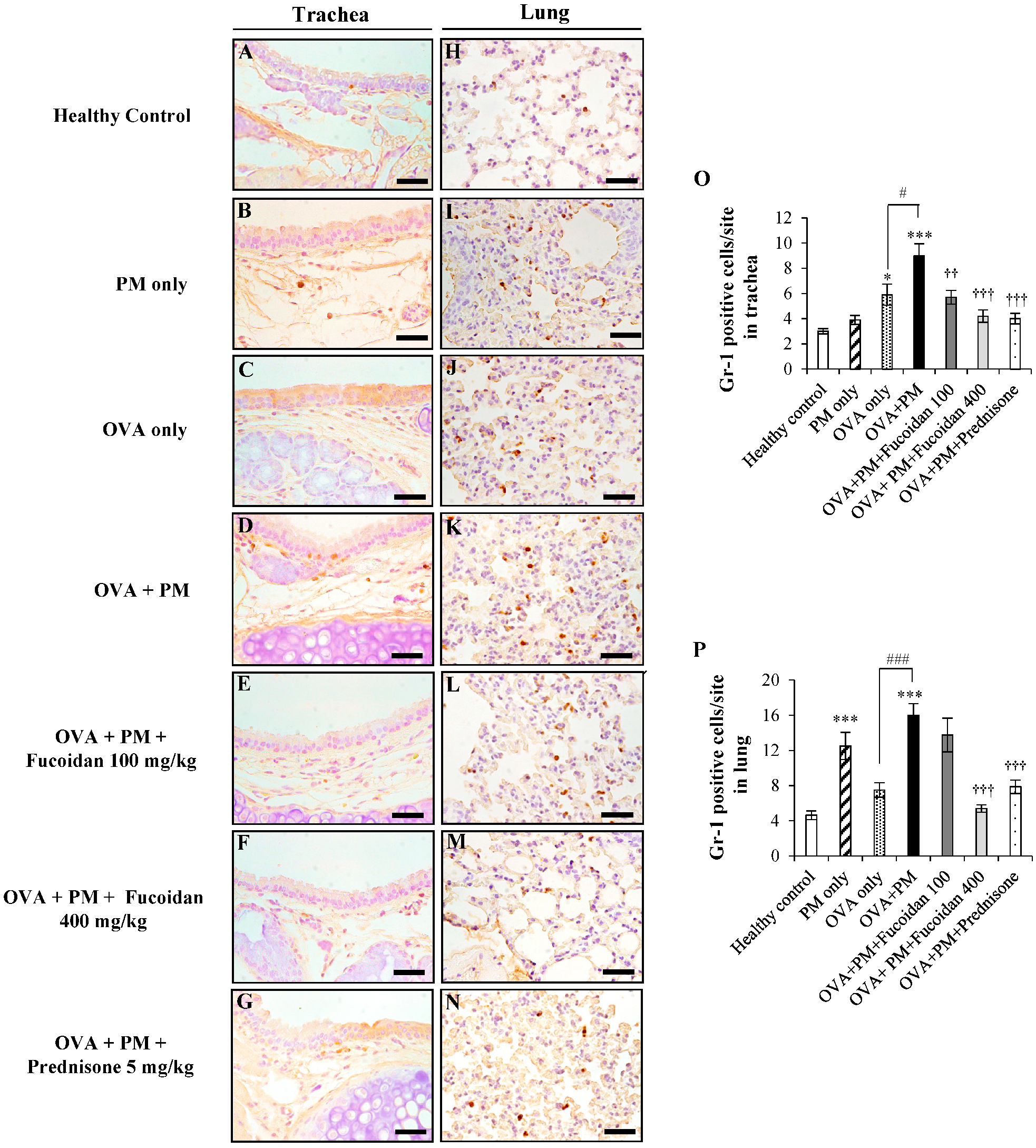
2.5. Fucoidans Attenuated the Infiltration of Gr-1+ Cells in the Trachea and Lungs
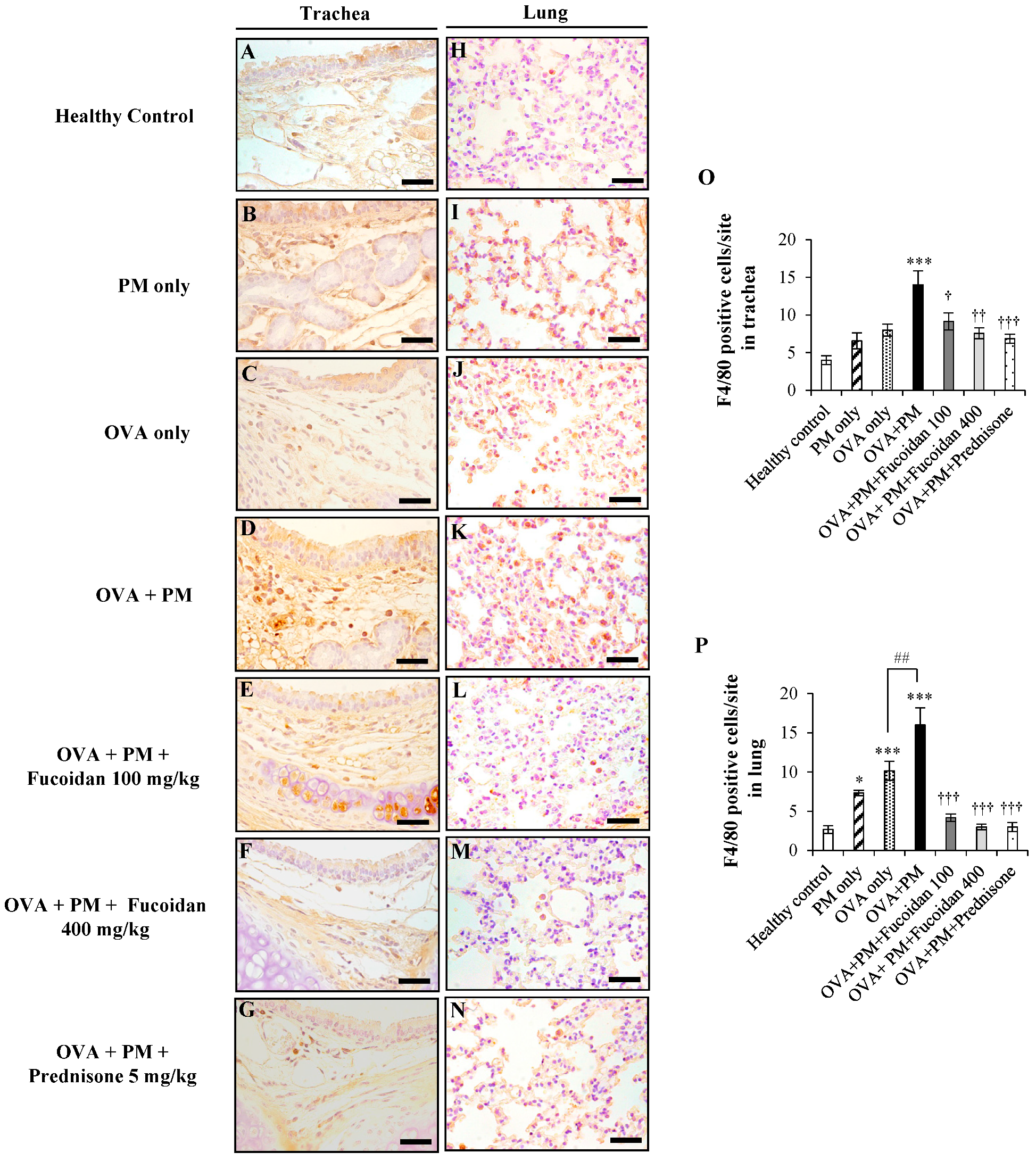
2.6. Fucoidans Attenuated F4/80+ Macrophage Infiltration in the Trachea and Lungs
2.7. Fucoidans Attenuated CD4+ T Cell Infiltration in the Trachea and Lungs
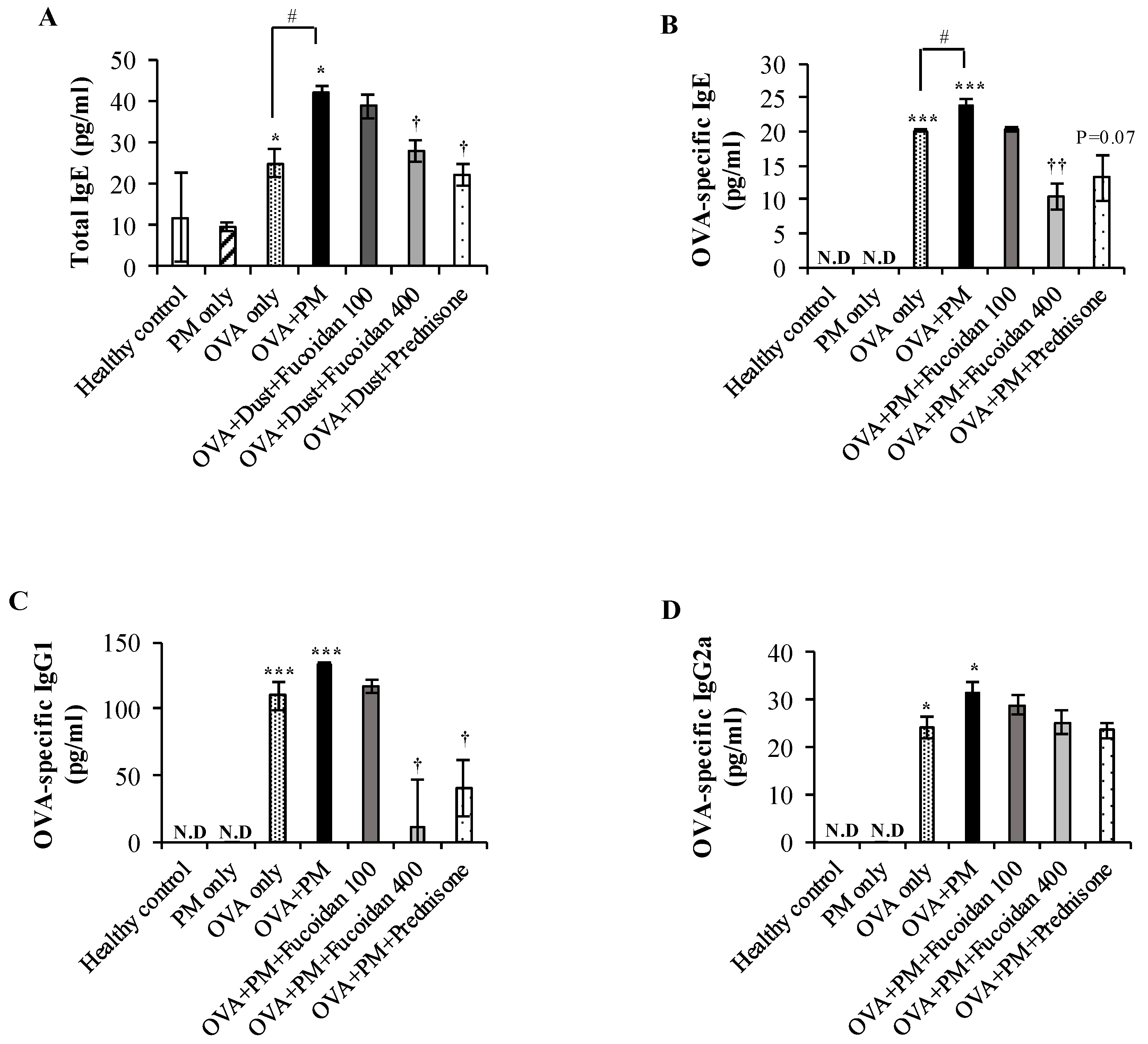
2.8. Fucoidans Attenuated the Serum Level of Total IgE, an Antigen-Specific Antibody, in PM-Exposed and OVA-Sensitized Mice
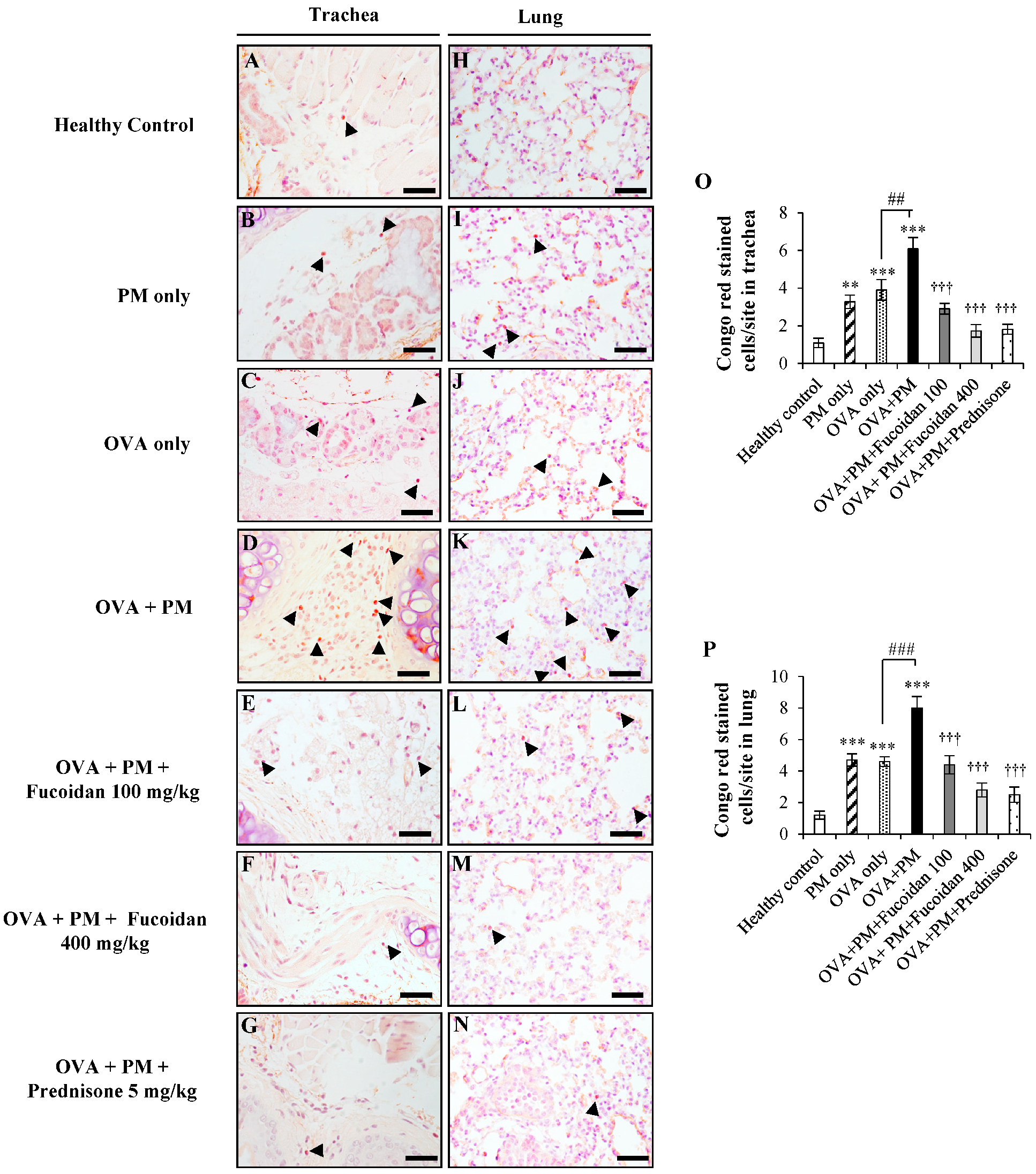
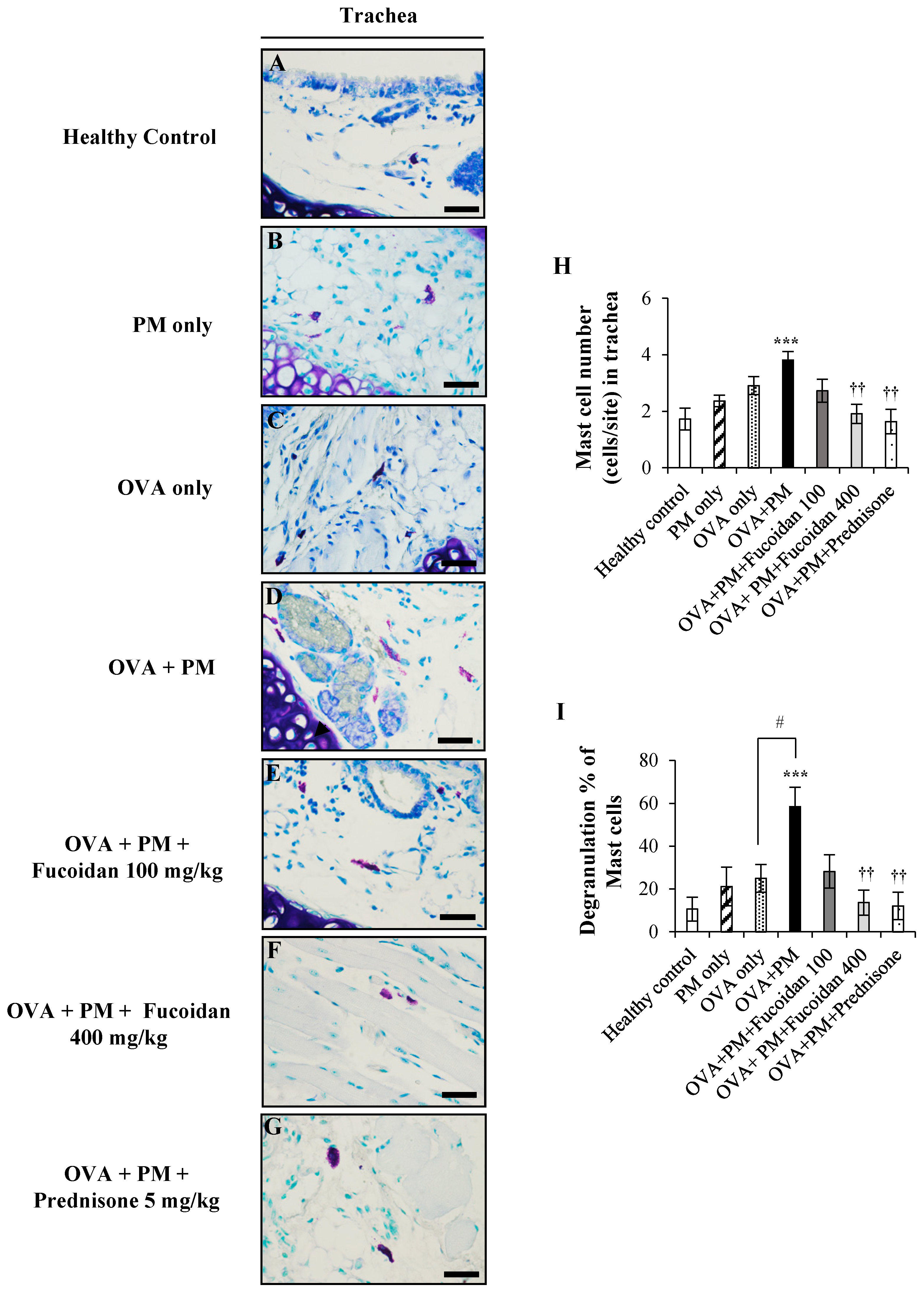
2.9. Fucoidans Attenuated the Activation and Degranulation of Mast Cells in PM-Exposed and OVA-Sensitized Mice
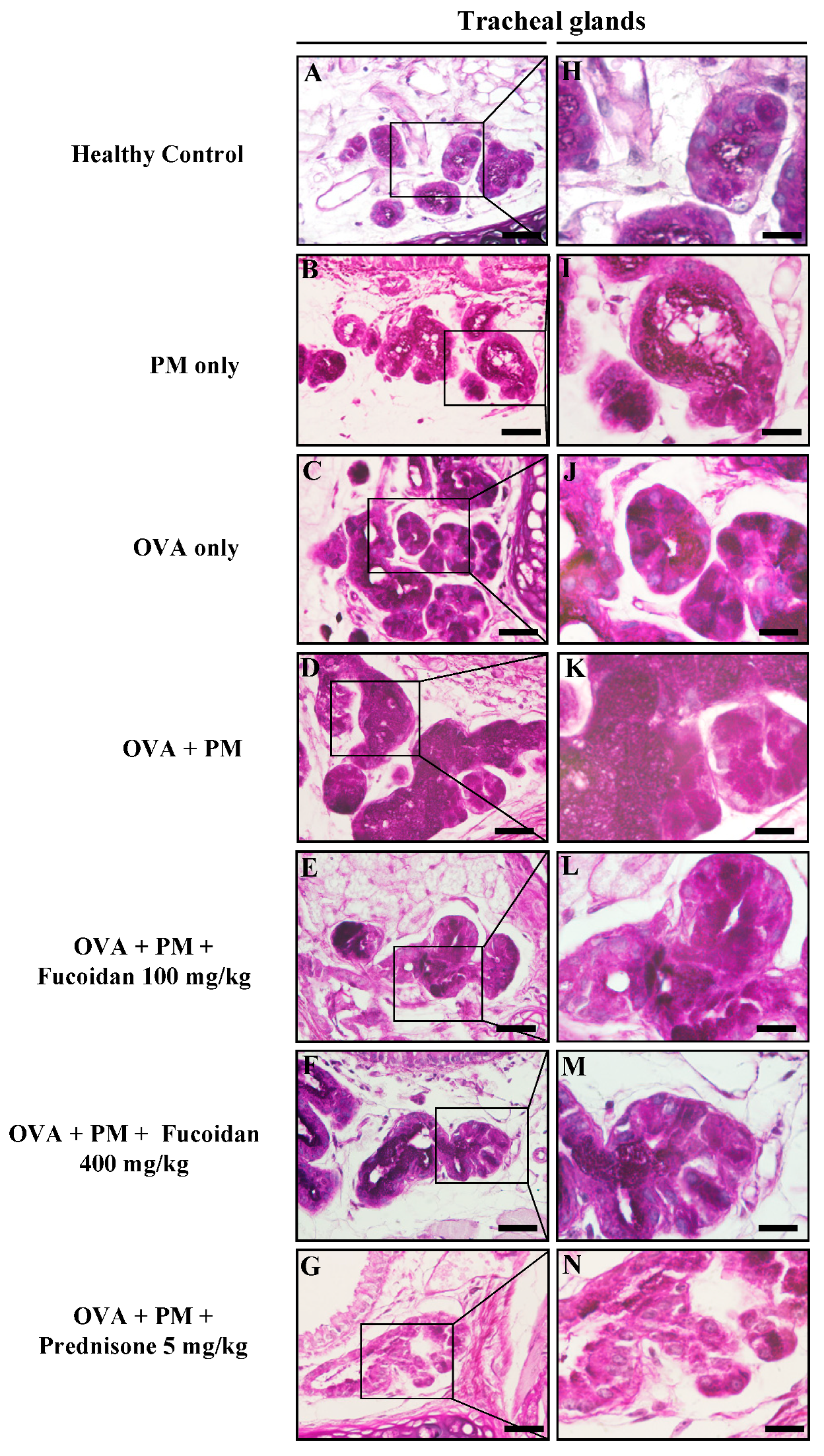
2.10. Fucoidans Attenuated Goblet Cell Hyperplasia and Mucus Secretion
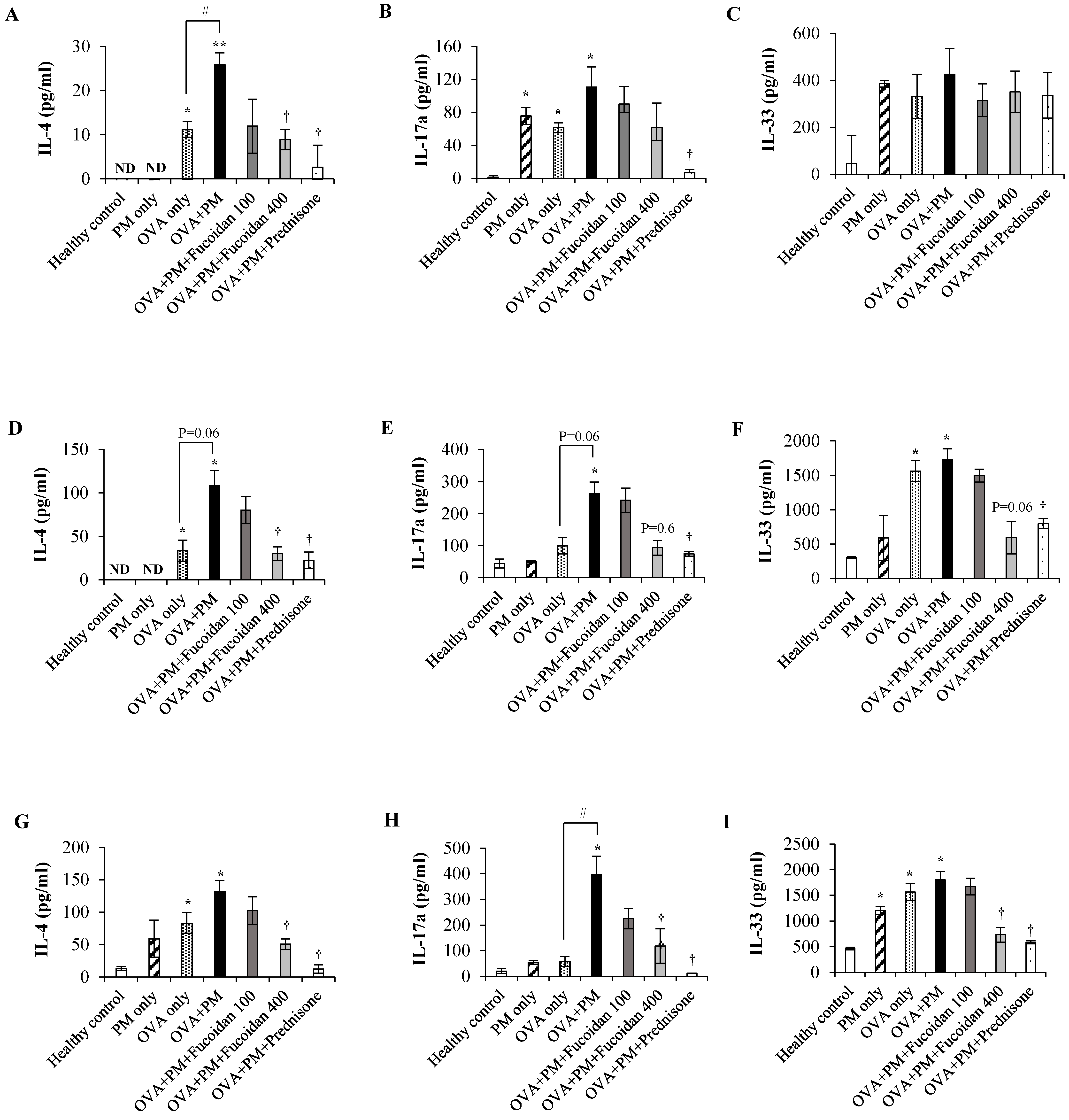
2.11. Fucoidans Attenuated the Levels of the Th2-Derived Cytokine IL-4 and the Epithelial-Cell-Derived Cytokine IL-33
3. Discussion
4. Materials and Methods
4.1. Reagents and Kits
4.2. Particulate Matter
4.3. Animals
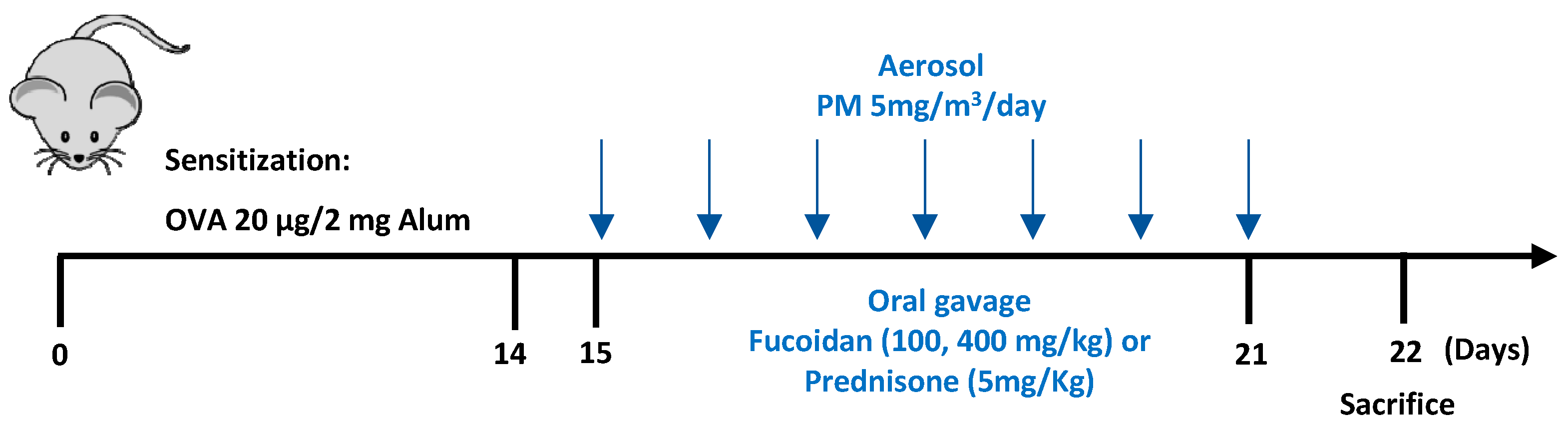
4.4. Experimental Design
4.5. Measurement of Malondialdehyde (MDA) Level in Serum
4.6. Thiobarbituric Acid Reactive Substance (TBARS) Assay
4.7. Methacholine Test for Airway Hyper-Responsiveness
4.8. Bronchoalveolar Lavage Fluid (BALF) Collection and Differential Counting
4.9. Histological Examination of Trachea and Lung
4.10. Immunohistochemical Analysis of Trachea and Lung
4.11. Enzyme-Linked Immunosorbent Assay (ELISA) Analysis
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xing, Y.-F.; Xu, Y.-H.; Shi, M.-H.; Lian, Y.-X. The impact of PM2.5 on the human respiratory system. J. Thorac. Dis. 2016, 8, 69–74. [Google Scholar] [CrossRef]
- Kurai, J.; Watanabe, M.; Sano, H.; Hantan, D.; Shimizu, E. The effect of seasonal variations in airborne particulate matter on asthma-related airway inflammation in mice. Int. J. Environ. Res. Public Health 2016, 13, 579. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.K.; Birrell, M.A.; Adcock, J.J.; Wortley, M.A.; Dubuis, E.D.; Chen, S.; McGilvery, C.M.; Hu, S.; Shaffer, M.S.P.; Bonvini, S.J.; et al. Mechanistic link between diesel exhaust particles and respiratory reflexes. J. Allergy Clin. Immunol. 2018, 141, 1074–1084. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hui, Y.; Zhao, L.; Hao, Y.; Guo, H.; Ren, F. Oral administration of Lactobacillus paracasei L9 attenuates PM2.5-induced enhancement of airway hyperresponsiveness and allergic airway response in murine model of asthma. PLoS ONE 2017, 12, e0171721. [Google Scholar] [CrossRef] [PubMed]
- Rychlik, K.A.; Secrest, J.R.; Lau, C.; Pulczinski, J.; Zamora, M.L.; Leal, J.; Langley, R.; Myatt, L.G.; Raju, M.; Chang, R.C.-A.; et al. In utero ultrafine particulate matter exposure causes offspring pulmonary immunosuppression. Proc. Natl. Acad. Sci. USA 2019, 116, 3443–3448. [Google Scholar] [CrossRef]
- Ohta, K.; Yamaguchi, M.; Akiyama, K.; Adachi, M.; Ichinose, M.; Takahashi, K.; Nishimuta, T.; Morikawa, A.; Nishima, S. Japanese guideline for adult asthma. Allergol. Int. 2011, 60, 115–145. [Google Scholar] [CrossRef]
- Kemp, M.W.; Schmidt, A.F.; Jobe, A.H. Optimizing antenatal corticosteroid therapy. Semin. Fetal Neonatal Med. 2019, 24, 176–181. [Google Scholar] [CrossRef]
- Wijesinghe, W.; Athukorala, Y.; Jeon, Y.-J. Effect of anticoagulative sulfated polysaccharide purified from enzyme-assistant extract of a brown seaweed Ecklonia cava on Wistar rats. Carbohydr. Polym. 2011, 86, 917–921. [Google Scholar] [CrossRef]
- Sanjeewa, A.; Jayawardena, T.U.; Kim, H.-S.; Kim, S.-Y.; Fernando, S.; Wang, L.; Abetunga, D.T.U.; Kim, W.-S.; Lee, D.-S.; Jeon, Y.-J. Fucoidan isolated from Padina commersonii inhibit LPS-induced inflammation in macrophages blocking TLR/NF-κB signal pathway. Carbohydr. Polym. 2019, 244, 115195. [Google Scholar] [CrossRef]
- Tanino, Y.; Hashimoto, T.; Ojima, T.; Mizuno, M. F-fucoidan from Saccharina japonica is a novel inducer of galectin-9 and exhibits anti-allergic activity. J. Clin. Biochem. Nutr. 2016, 59, 25–30. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, Y.; Wang, J.; Ma, S.; Yu, Y.; White, W.L.; Yang, S.; Yang, F.; Lu, J. Fucoidan extracted from undaria pinnatifida: Source for nutraceuticals/functional foods. Mar. Drugs 2018, 16, 321. [Google Scholar] [CrossRef] [PubMed]
- Koh, H.S.A.; Lu, J.; Zhou, W. Structure characterization and antioxidant activity of fucoidan isolated from undaria pinnatifida grown in New Zealand. Carbohydr. Polym. 2019, 212, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Synytsya, A.; Kim, W.-J.; Kim, S.-M.; Pohl, R.; Synytsya, A.; Kvasnička, F.; Čopíková, J.; Il Park, Y. Structure and antitumour activity of fucoidan isolated from sporophyll of Korean brown seaweed undaria pinnatifida. Carbohydr. Polym. 2010, 81, 41–48. [Google Scholar] [CrossRef]
- Kim, K.-J.; Lee, O.-H.; Lee, H.-H.; Lee, B.-Y. A 4-week repeated oral dose toxicity study of fucoidan from the sporophyll of undaria pinnatifida in sprague–dawley rats. Toxicology 2010, 267, 154–158. [Google Scholar] [CrossRef]
- Kim, K.-J.; Lee, B.-Y. Fucoidan form the sporophyll of undaria pinnatifida suppresses adipocyte differentiation by inhibiton of inflammation-related cytokines in 3T3-L1 cells. Nutr. Res. 2012, 32, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-J.; Yoon, K.-Y.; Lee, B.-Y. Low molecular weight fucoidan from the sporophyll of undaria pinnatifida suppresses inflammation by promoting the inhibition of mitogen-activated protein kinases and oxidative stress in RAW264.7 cells. Fitoterapia 2012, 83, 1628–1635. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, H.; Tamauchi, H.; Hashimoto, M.; Nakano, T. Suppression of Th2 immune responses by mekabu fucoidan from undaria pinnatifida sporophylls. Int. Arch. Allergy Immunol. 2005, 137, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.F.S.; Nihei, J.; Mengel, J.; Henriques, M.G.; Penido, C. Requirement of L-selectin for γδ T lymphocyte activation and migration during allergic pleurisy: Co-relation with eosinophil accumulation. Int. Immunopharmacol. 2009, 9, 303–312. [Google Scholar] [CrossRef]
- Harb, H.; Alashkar Alhamwe, B.; Garn, H.; Renz, H.; Potaczek, D.P. Recent developments in epigenetics of pediatric asthma. Curr. Opin. Pediatr. 2016, 28, 754–763. [Google Scholar] [CrossRef]
- Potaczek, D.P.; Harb, H.; Michel, S.; Alhamwe, B.A.; Renz, H.; Tost, J. Epigenetics and allergy: From basic mechanisms to clinical applications. Epigenomics 2017, 9, 539–571. [Google Scholar] [CrossRef]
- Shamshuddin, N.S.S.; Mohd Zohdi, R. Gelam honey attenuates ovalbumin-induced airway inflammation in a mice model of allergic asthma. J. Tradit. Complement. Med. 2018, 8, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Herath, K.H.I.N.M.; Kim, H.J.; Mihindukulasooriya, S.; Kim, A.; Kim, H.; Jeon, Y.-J.; Jee, Y. Sargassum horneri extract containing mojabanchromanol attenuates the particulate matter exacerbated allergic asthma through reduction of Th2 and Th17 response in mice. Environ. Pollut. 2020. [Google Scholar] [CrossRef]
- Herath, K.H.I.N.M.; Mihindukulasooriya, S.P.; Kim, H.J.; Kim, A.; Kim, H.J.; Jeon, Y.-J.; Jee, Y. Oral administration of polyphenol-rich Sargassum horneri suppresses particulate matter exacerbated airway inflammation in murine allergic asthma: Relevance to the TLR mediated NF-κB pathway inhibition. J. Funct. Foods 2020, 71, 103991. [Google Scholar] [CrossRef]
- Ling, M.F.; Luster, A.D. Allergen-specific CD4+ T cells in human asthma. Ann. Am. Thorac. Soc. 2016, 13, 25–30. [Google Scholar]
- Gavett, S.H.; Chen, X.; Finkelman, F.; Wills-Karp, M. Depletion of murine CD4+ T lymphocytes prevents antigen-induced airway hyperreactivity and pulmonary eosinophilia. Am. J. Respir. Cell. Mol. Biol. 1994, 10, 587–593. [Google Scholar] [CrossRef]
- Tian, T.; Chang, H.; He, K.; Ni, Y.; Li, C.; Hou, M.; Chen, L.; Xu, Z.; Chen, B.; Ji, M. Fucoidan from seaweed fucus vesiculosus inhibits 2,4-dinitrochlorobenzene-induced atopic dermatitis. Int. Immunopharmacol. 2019, 75, 105823. [Google Scholar] [CrossRef]
- Tanaka, K.; Ito, M.; Kodama, M.; Tomita, M.; Kimura, S.; Hoyano, M.; Mitsuma, W.; Hirono, S.; Hanawa, H.; Aizawa, Y. Sulfated polysaccharide fucoidan ameliorates experimental autoimmune myocarditis in rats. J. Cardiovasc. Pharmacol. Ther. 2010, 16, 79–86. [Google Scholar] [CrossRef]
- Ivetic, A.; Hoskins Green, H.L.; Hart, S.J. L-selectin: A major regulator of leukocyte adhesion, migration and signaling. Front. Immunol. 2019, 10, 1068. [Google Scholar] [CrossRef]
- Vo, T.S.; Ngo, D.H.; Kang, K.H.; Jung, W.K.; Kim, S.K. The beneficial properties of marine polysaccharides in alleviation of allergic responses. Mol. Nutr. Food Res. 2015, 59, 129–138. [Google Scholar] [CrossRef]
- Oomizu, S.; Yanase, Y.; Suzuki, H.; Kameyoshi, Y.; Hide, M. Fucoidan prevents Cε germline transcription and NFκB p52 translocation for IgE production in B cells. Biochem. Biophys. Res. Commun. 2006, 350, 501–507. [Google Scholar] [CrossRef]
- Jin, Y.; Zhu, M.; Guo, Y.; Foreman, D.; Feng, F.; Duan, G.; Wu, W.; Zhang, W. Fine particulate matter (PM2.5) enhances FcεRI-mediated signaling and mast cell function. Cell. Signal. 2019, 57, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Parulekar, A.D.; Diamant, Z.; Hanania, N.A. Role of T2 inflammation biomarkers in severe asthma. Curr. Opin. Pulm. Med. 2016, 22, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.M.; Hellewell, P.G. The effect of the selectin binding polysaccharide fucoidin on eosinophil recruitment in vivo. Br. J. Pharmacol. 1997, 120, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Li, Y.; Han, C.; Lin, R.; Qian, W.; Hou, X. L-Fucose ameliorates DSS-induced acute colitis via inhibiting macrophage M1 polarization and inhibiting NLRP3 inflammasome and NF-kB activation. Int. Immunopharmacol. 2019, 73, 379–388. [Google Scholar] [CrossRef]
- Tan, H.T.; Hagner, S.; Ruchti, F.; Radzikowska, U.; Tan, G.; Altunbulakli, C.; Eljaszewicz, A.; Moniuszko, M.; Akdis, M.; Akdis, C.A.; et al. Tight junction, mucin, and inflammasome-related molecules are differentially expressed in eosinophilic, mixed, and neutrophilic experimental asthma in mice. Allergy 2019, 74, 294–307. [Google Scholar] [CrossRef]
- Liu, W.; Liu, S.; Verma, M.; Zafar, I.; Good, J.T.; Rollins, D.; Groshong, S.; Gorska, M.M.; Martin, R.J.; Alam, R. Mechanism of T(H)2/T(H)17-predominant and neutrophilic T(H)2/T(H)17-low subtypes of asthma. J. Allergy Clin. Immunol. 2017, 139, 1548–1558. [Google Scholar] [CrossRef]
- Potaczek, D.P.; Miethe, S.; Schindler, V.; Alhamdan, F.; Garn, H. Role of airway epithelial cells in the development of different asthma phenotypes. Cell. Signal. 2020, 69, 109523. [Google Scholar] [CrossRef]
- Lloyd, C.M.; Saglani, S. Epithelial cytokines and pulmonary allergic inflammation. Curr. Opin. Immunol. 2015, 34, 52–58. [Google Scholar] [CrossRef]
- Desai, M.; Oppenheimer, J. Elucidating asthma phenotypes and endotypes: Progress towards personalized medicine. Ann. Allergy Asthma Immunol. 2016, 116, 394–401. [Google Scholar] [CrossRef]
- Falcon-Rodriguez, C.I.; Garcia, L.; Segura-Medina, P. Particulate matter inside of the alveolar macrophage. Acta Toxicol. Argent. 2017, 25, 23–25. [Google Scholar]
- Kim, T.-M.; Paudel, K.R.; Kim, D.-W. Eriobotrya japonica leaf extract attenuates airway inflammation in ovalbumin-induced mice model of asthma. J. Ethnopharmacol. 2019, 253, 112082. [Google Scholar] [CrossRef] [PubMed]
- Umehara, K.; Hayakawa, H.; Myrvik, Q.N. L-Fucose blocks MIF/MAF priming of rabbit alveolar macrophages for a PMA-induced oxidative response. Cell. Immunol. 1989, 119, 67–72. [Google Scholar] [CrossRef]
- Zhu, T.; Chen, Z.; Chen, G.; Wang, D.; Tang, S.; Deng, H.; Wang, J.; Li, S.; Lan, J.; Tong, J.; et al. Curcumin attenuates asthmatic airway inflammation and mucus hypersecretion involving a pparγ-dependent nf-κb signaling pathway in vivo and in vitro. Mediat. Inflamm. 2019, 2019, 4927430. [Google Scholar] [CrossRef] [PubMed]
- Sanjeewa, K.K.A.; Jayawardena, T.U.; Lee, H.G.; Herath, K.; Jee, Y.; Jeon, Y.J. The protective effect of Sargassum horneri against particulate matter-induced inflammation in lung tissues of an in vivo mouse asthma model. Food Funct. 2019, 10, 7995–8004. [Google Scholar] [CrossRef]
- Madushani Herath, K.H.I.N.; Bing, S.J.; Cho, J.; Kim, A.; Kim, G.; Kim, J.-S.; Kim, J.-B.; Doh, Y.H.; Jee, Y. Sasa quelpaertensis leaves ameliorate alcohol-induced liver injury by attenuating oxidative stress in HepG2 cells and mice. Acta Histochem. 2018, 120, 477–489. [Google Scholar] [CrossRef]
- Qin, Q.; Wang, Z.; Pan, P.; Cao, Z.; Xia, Q.; Tan, H.; Hu, C. Lung dendritic cells undergo maturation and polarization towards a T helper type 2-stimulating phenotype in a mouse model of asthma: Role of nerve growth factor. Exp. Ther. Med. 2014, 8, 1402–1408. [Google Scholar] [CrossRef]
Sample Availability: Not available. |

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herath, K.H.I.N.M.; Kim, H.J.; Kim, A.; Sook, C.E.; Lee, B.-Y.; Jee, Y. The Role of Fucoidans Isolated from the Sporophylls of Undaria pinnatifida against Particulate-Matter-Induced Allergic Airway Inflammation: Evidence of the Attenuation of Oxidative Stress and Inflammatory Responses. Molecules 2020, 25, 2869. https://doi.org/10.3390/molecules25122869
Herath KHINM, Kim HJ, Kim A, Sook CE, Lee B-Y, Jee Y. The Role of Fucoidans Isolated from the Sporophylls of Undaria pinnatifida against Particulate-Matter-Induced Allergic Airway Inflammation: Evidence of the Attenuation of Oxidative Stress and Inflammatory Responses. Molecules. 2020; 25(12):2869. https://doi.org/10.3390/molecules25122869
Chicago/Turabian StyleHerath, Kalahe Hewage Iresha Nadeeka Madushani, Hyo Jin Kim, Areum Kim, Chung Eui Sook, Boo-Yong Lee, and Youngheun Jee. 2020. "The Role of Fucoidans Isolated from the Sporophylls of Undaria pinnatifida against Particulate-Matter-Induced Allergic Airway Inflammation: Evidence of the Attenuation of Oxidative Stress and Inflammatory Responses" Molecules 25, no. 12: 2869. https://doi.org/10.3390/molecules25122869
APA StyleHerath, K. H. I. N. M., Kim, H. J., Kim, A., Sook, C. E., Lee, B.-Y., & Jee, Y. (2020). The Role of Fucoidans Isolated from the Sporophylls of Undaria pinnatifida against Particulate-Matter-Induced Allergic Airway Inflammation: Evidence of the Attenuation of Oxidative Stress and Inflammatory Responses. Molecules, 25(12), 2869. https://doi.org/10.3390/molecules25122869




