Zingerone in the Flower of Passiflora maliformis Attracts an Australian Fruit Fly, Bactrocera jarvisi (Tryon)
Abstract
1. Introduction
2. Results
2.1. Chemical Profiles of P. maliformis Flowers
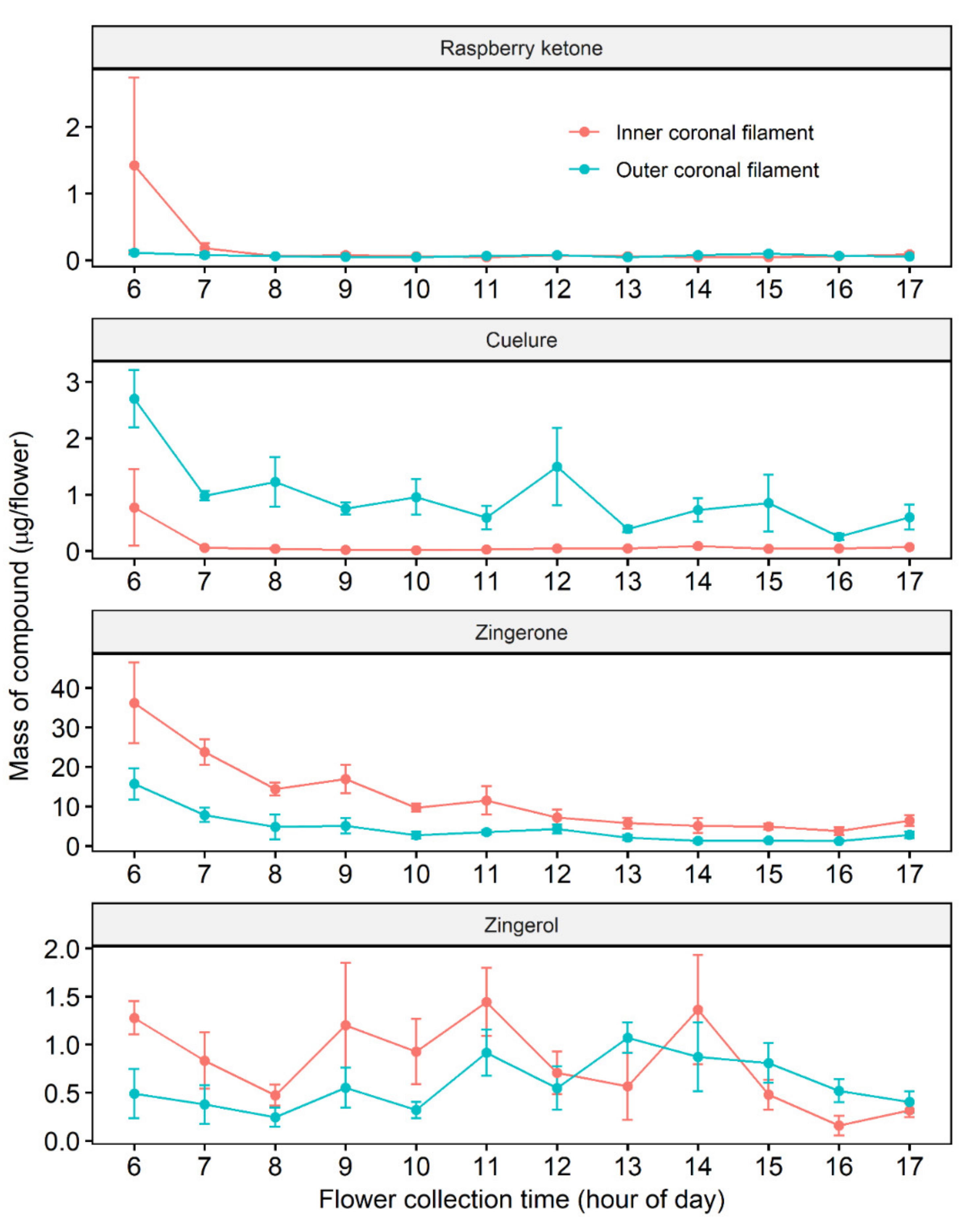
2.2. Diurnal Changes in Raspberry Ketone, Cuelure, Zingerone and Zingerol in P. maliformis Coronal Filaments
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Flower Extraction
4.3. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
4.4. Quantification of Known Bactrocera Attractants in P. maliformis
4.5. Chromatographic Purification of Unknown Compounds
4.6. Data Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Metcalf, R.L.; Metcalf, E.R. Plant Kairomones in Insect Ecology and Control; Champmon and Hall: New York, NY, USA, 1992. [Google Scholar]
- Tan, K.H.; Nishida, R. Zingerone in the floral synomone of Bulbophyllum baileyi (Orchidaceae) attracts Bactrocera fruit flies during pollination. Biochem. Syst. Ecol. 2007, 35, 334–341. [Google Scholar] [CrossRef]
- Tan, K.H.; Tan, L.T.; Nishida, R. Floral phenylpropanoid cocktail and architecture of Bulbophyllum vinaceum orchid in attracting fruit flies for pollination. J. Chem. Ecol. 2006, 32, 2429–2441. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.H.; Nishida, R. Synomone or kairomone?—Bulbophyllum apertum flower releases raspberry ketone to attract Bactrocera fruit flies. J. Chem. Ecol. 2005, 31, 497–507. [Google Scholar]
- Nishida, R.; Tan, K.-H.; Wee, S.-L.; Hee, A.K.-W.; Toong, Y.-C. Phenylpropanoids in the fragrance of the fruit fly orchid, Bulbophyllum cheiri, and their relationship to the pollinator, Bactrocera papayae. Biochem. Syst. Ecol. 2004, 32, 245–252. [Google Scholar] [CrossRef]
- Tan, K.H.; Nishida, R.; Toong, Y.C. Floral synomone of a wild orchid, Bulbophyllum cheiri, lures Bactrocera fruit flies for pollination. J. Chem. Ecol. 2002, 28, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.H. Fruit fly pests as pollinators of wild orchids. In Proceedings of the 7th International Symposium on Fruit Flies of Economic Importance, Salvador, Brazil, 10‒15 September 2006. [Google Scholar]
- Tan, K.H. Fruit flies (Bactrocera spp.) as pests and pollinators. Sab. Soc. J. 2000, 17, 37–56. [Google Scholar]
- Tan, K.H.; Nishida, R. Pollination of bactrocerophilous Bulbophyllum orchids. In Proceedings of the 20th World Orchid Conference, Singapore, 13–20 November 2011. [Google Scholar]
- Park, S.J.; Morelli, R.; Hanssen, B.L.; Jamie, J.F.; Jamie, I.M.; Siderhurst, M.S.; Taylor, P.W. Raspberry ketone analogs: Vapour pressure measurements and attractiveness to Queensland fruit fly, Bactrocera tryoni (Froggatt) (Diptera: Tephritidae). PLoS ONE 2016, 11. [Google Scholar] [CrossRef]
- Park, S.J.; Siderhurst, M.S.; Jamie, I.; Taylor, P.W. Hydrolysis of Queensland fruit fly, Bactrocera tryoni (Froggatt), attractants: Kinetics and implications for biological activity. Aust. J. Chem. 2016, 69, 1162–1166. [Google Scholar] [CrossRef]
- Tan, K.H.; Nishida, R. Mutual reproductive benefits between a wild orchid, Bulbophyllum patens, and Bactrocera fruit flies via a floral synomone. J. Chem. Ecol. 2000, 26, 533–546. [Google Scholar] [CrossRef]
- Shelly, T.E.; Dewire, A.-L.M. Chemically mediated mating success in male oriental fruit flies (Diptera: Tephritidae). Ann. Entomol. Soc. Am. 1994, 87, 375–382. [Google Scholar] [CrossRef]
- Wee, S.-L.; Tan, K.H.; Nishida, R. Pharmacophagy of methyl eugenol by males enhances sexual selection of Bactrocera carambolae. J. Chem. Ecol. 2007, 33, 1272–1282. [Google Scholar] [CrossRef] [PubMed]
- Kumaran, N.; Balagawi, S.; Schutze, M.K.; Clarke, A.R. Evolution of lure response in tephritid fruit flies: Phytochemicals as drivers of sexual selection. Anim. Behav. 2013, 85, 781–789. [Google Scholar] [CrossRef]
- Kumaran, N.; Clarke, A.R. Indirect effects of phytochemicals on offspring performance of Queensland fruit fly, Bactrocera tryoni (Diptera: Tephritidae). J. Appl. Entomol. 2014, 138, 361–367. [Google Scholar] [CrossRef]
- Kumaran, N.; Hayes, R.A.; Clarke, A.R. Cuelure but not zingerone make the sex pheromone of male Bactrocera tryoni (Tephritidae: Diptera) more attractive to females. J. Insect Physiol. 2014, 68, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Hancock, D.L.; Hamacek, E.L.; Lloyd, A.C.; Elson-Harris, M.M. The Distribution and Host Plants of Fruit Flies (Diptera: Tephritidae) in Australia; Information Series (Queensland Department of Primary Industries); DPI Publications: Brisbane, Australia, 2000. [Google Scholar]
- Fitt, G.P. The influence of a shortage of hosts on the specificity of oviposition behaviour in species of Dacus (Diptera, Tephritidae). Physiol. Entomol. 1986, 11, 133–143. [Google Scholar] [CrossRef]
- Drew, R.A.I. The tropical fruit flies (Diptera: Tephritidae: Dacinae) of the Australian and Oceanian regions. Mem. Queensl. Mus. 1989, 26, 521. [Google Scholar]
- Wee, S.-L.; Peek, T.; Clarke, A.R. The responsiveness of Bactrocera jarvisi (Diptera: Tephritidae) to two naturally occurring phenylbutaonids, zingerone and raspberry ketone. J. Insect Physiol. 2018, 109, 41–46. [Google Scholar] [CrossRef]
- Fay, H.A.C. A highly effective and selective male lure for Bactrocera jarvisi (Tryon) (Diptera: Tephritidae). Aust. J. Entomol. 2012, 51, 189–197. [Google Scholar] [CrossRef]
- May, A.W.S. Queensland host records for the Dacinae (fam. Try-petidae). Qld. J. Agric. Sci. 1953, 17, 195–200. [Google Scholar]
- Halevy, A.H. Handbook of Flowering; Taylor & Francis: Boca Raton, FL, USA, 1989. [Google Scholar]
- Nyanzi, S.A.; Carstensen, B.; Schwack, W. A comparative study of fatty acid profiles of Passiflora seed oils from Uganda. J. Am. Oil Chem. Soc. 2005, 82, 41–44. [Google Scholar] [CrossRef]
- Shiamala, D.R.; Sidik, B.J.; Harah, Z.M.; Sing, K.W.; Arif, S.S.M. Sugars, ascorbic acid, total phenolic content and total antioxidant activity in passion fruit (Passiflora) cultivars. J. Sci. Food Agric. 2013, 93, 1198–1205. [Google Scholar]
- Willis, J.M.G. Passion fruits and granadillas. Qld. Agric. J. 1954, 79, 205–217. [Google Scholar]
- Restrepo, P.; Duque, C. Componentes volatiles de la gulupa Passiflora maliformis. Rev. Colomb. Quim. 1988, 17, 57–63. [Google Scholar]
- Brieze-Stegeman, R.; Rice, M.J.; Hooper, G.H.S. Daily periodicity in attraction of male tephritid fruit flies to synthetic chemical lures. Aust. J. Entomol. 1978, 17, 341–346. [Google Scholar] [CrossRef]
- Weldon, C.W.; Perez-Staples, D.; Taylor, P.W. Feeding on yeast hydrolysate enhances attraction to cue-lure in Queensland fruit flies, Bactrocera tryoni. Entomol. Exp. Appl. 2008, 129, 200–209. [Google Scholar] [CrossRef]
- Manoukis, N.C.; Jang, E.B. The diurnal rhythmicity of Bactrocera cucurbitae (Diptera: Tephritidae) attraction to cuelure: Insights from an interruptable lure and computer vision. Ann. Entomol. Soc. Am. 2013, 106, 136–142. [Google Scholar] [CrossRef]
- Hazzit, M.; Baaliouamer, A.; Faleiro, M.L.; Miguel, M.G. Composition of the essential oils of Thymus and Origanum species from Algeria and their antioxidant and antimicrobial activities. J. Agric. Food. Chem. 2006, 54, 6314–6321. [Google Scholar] [CrossRef]
- Angioni, A.; Barra, A.; Cereti, E.; Barile, D.; Coïsson, J.D.; Arlorio, M.; Dessi, S.; Coroneo, V.; Cabras, P. Chemical composition, plant genetic differences, antimicrobial and antifungal activity investigation of the essential oil of Rosmarinus officinalis L. J. Agric. Food. Chem. 2004, 52, 3530–3535. [Google Scholar] [CrossRef]
- Mebazaa, R.; Mahmoudi, A.; Fouchet, M.; Santos, M.D.; Kamissoko, F.; Nafti, A.; Cheikh, R.B.; Rega, B.; Camel, V. Characterisation of volatile compounds in Tunisian fenugreek seeds. Food Chem. 2009, 115, 1326–1336. [Google Scholar] [CrossRef]
- Kotowska, U.; Żalikowski, M.; Isidorov, V.A. HS-SPME/GC–MS analysis of volatile and semi-volatile organic compounds emitted from municipal sewage sludge. Environ. Monit. Assess. 2012, 184, 2893–2907. [Google Scholar] [CrossRef]
- Zhou, Q.; Wintersteen, C.L.; Cadwallader, K.R. Identification and quantification of aroma-active components that contribute to the distinct malty flavor of buckwheat honey. J. Agric. Food. Chem. 2002, 50, 2016–2021. [Google Scholar] [CrossRef] [PubMed]
- Pino, J.A.; Mesa, J.; Muñoz, Y.; Martí, M.P.; Marbot, R. Volatile components from mango (Mangifera indica L.) cultivars. J. Agric. Food. Chem. 2005, 53, 2213–2223. [Google Scholar] [CrossRef] [PubMed]
- Dob, T.; Dahmane, D.; Chelghoum, C. Essential oil composition of Juniperus oxycedrus growing in Algeria. Pharm. Biol. 2006, 44, 1–6. [Google Scholar] [CrossRef]
- Boussaada, O.; Ammar, S.; Saidana, D.; Chriaa, J.; Chraif, I.; Daami, M.; Helal, A.N.; Mighri, Z. Chemical composition and antimicrobial activity of volatile components from capitula and aerial parts of Rhaponticum acaule DC growing wild in Tunisia. Microbiol. Res. 2008, 163, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Roussis, V.; Tsoukatou, M.; Petrakis, P.V.; Ioanna, C.; Skoula, M.; Harborne, J.B. Volatile constituents of four Helichrysum species growing in Greece. Biochem. Syst. Ecol. 2000, 28, 163–175. [Google Scholar] [CrossRef]
- Radulović, N.; Blagojević, P.; Palić, R. Comparative study of the leaf volatiles of Arctostaphylos uva-ursi (L.) Spreng.; Vaccinium vitis-idaea L. (Ericaceae). Molecules 2010, 15, 6168–6185. [Google Scholar]
- Ferhat, M.A.; Tigrine-Kordjani, N.; Chemat, S.; Meklati, B.Y.; Chemat, F. Rapid extraction of volatile compounds using a new simultaneous microwave distillation: Solvent extraction device. Chromatographia 2007, 65, 217–222. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, J.; Xu, Y.; Duan, H.; Fan, W.; Zhao, G. Extraction, preparation and identification of volatile compounds in Changyu XO brandy. Chin. J. Chrom. 2008, 26, 212–222. [Google Scholar] [CrossRef]
- Zhao, C.-X.; Li, X.-N.; Liang, Y.-Z.; Fang, H.-Z.; Huang, L.-F.; Guo, F.-Q. Comparative analysis of chemical components of essential oils from different samples of Rhododendron with the help of chemometrics methods. Chemometrics Intellig. Lab. Syst. 2006, 82, 218–228. [Google Scholar] [CrossRef]
- Benkaci–Ali, F.; Baaliouamer, A.; Meklati, B.Y.; Chemat, F. Chemical composition of seed essential oils from Algerian Nigella sativa extracted by microwave and hydrodistillation. Flavour. Fragr. J. 2007, 22, 148–153. [Google Scholar] [CrossRef]
- Povolo, M.; Pelizzola, V.; Ravera, D.; Contarini, G. Significance of the nonvolatile minor compounds of the neutral lipid fraction as markers of the origin of dairy products. J. Agric. Food. Chem. 2009, 57, 7387–7394. [Google Scholar] [CrossRef]
- Grünler, J.; Ericsson, J.; Dallner, G. Branch-point reactions in the biosynthesis of cholesterol, dolichol, ubiquinone and prenylated proteins. Biochim. Biophys. Acta 1994, 1212, 259–277. [Google Scholar] [CrossRef]
- Begley, T.P.; Keeling, C.I.; Bohlmann, J. Plant Terpenoids. In Wiley Encyclopedia of Chemical Biology; Begley, T.P., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; pp. 1–10. [Google Scholar]
- Lincoln, D.E.; Lawrence, B.M. The volatile constituents of camphorweed, Heterotheca subaxillaris. Phytochemistry 1984, 23, 933–934. [Google Scholar] [CrossRef]
- Wong, K.C.; Ong, K.S.; Lim, C.L. Compositon of the essential oil of rhizomes of Kaempferia galanga L. Flavour. Fragr. J. 1992, 7, 263–266. [Google Scholar] [CrossRef]
- Jafari, E.; Ghanbarian, G.; Bahmanzadegan, A. Essential oil composition of aerial parts of Micromeria persica Boiss from Western of Shiraz, Iran. Nat. Prod. Res. 2018, 32, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.I.; Cheng, M.C.; Peng, S.M. Structure of terpin. Aeta Cryst. 1986, 42, 1787–1789. [Google Scholar] [CrossRef]
- Loreto, F.; Dicke, M.; Schnitzler, J.-P.; Turlings, T.C.J. Plant volatiles and the environment. Plant. Cell Environ. 2014, 37, 1905–1908. [Google Scholar] [CrossRef]
- Tholl, D. Biosynthesis and biological functions of terpenoids in plants. In Biotechnology of Isoprenoids; Schrader, J., Bohlmann, J., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 63–106. [Google Scholar]
- Dicke, M.; Baldwin, I.T. The evolutionary context for herbivore-induced plant volatiles: Beyond the ‘cry for help’. Trends Plant. Sci. 2010, 15, 167–175. [Google Scholar] [CrossRef]
- Gershenzon, J. Metabolic costs of terpenoid accumulation in higher plants. J. Chem. Ecol. 1994, 20, 1281–1328. [Google Scholar] [CrossRef]
- Pichersky, E.; Raguso, R.A.; Lewinsohn, E.; Croteau, R. Floral scent production in Clarkia (Onagraceae) (I. Localization and developmental modulation of monoterpene emission and linalool synthase activity). Plant. Physiol. 1994, 106, 1533–1540. [Google Scholar] [CrossRef]
- Heidi, E.M.D.; Groth, I.; Bergstrom, G. Pollen advertisement: Chemical contrasts between whole-flower and pollen odors. Am. J. Bot. 1996, 83, 877–885. [Google Scholar]
- Evans, K.A.; Allen-Williams, L.J. Electroantennogram responses of the cabbage seed weevil, Ceutorhynchus assimilis, to oilseed rape, Brassica napus ssp. oleifera, volatiles. J. Chem. Ecol. 1992, 18, 1641–1659. [Google Scholar] [CrossRef] [PubMed]
- Robertson, G.W.; Griffiths, D.W.; Smith, W.M.; Butcher, R.D. The application of thermal desorption-gas chromatography-mass spectrometry to the analyses of flower volatiles from five varieties of oilseed rape (Brassica napus spp. oleifera). Phytochem. Anal. 1993, 4, 152–157. [Google Scholar] [CrossRef]
- Baldwin, I.T. Plant volatiles. Curr. Biol. 2010, 20, 392–397. [Google Scholar] [CrossRef]
- Raffaele, S.; Leger, A.; Roby, D. Very long chain fatty acid and lipid signaling in the response of plants to pathogens. Plant. Signal. Behav. 2009, 4, 94–99. [Google Scholar] [CrossRef]
- Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant. 2010, 3, 2–20. [Google Scholar] [CrossRef]
- Dudareva, N.; Pichersky, E.; Gershenzon, J. Biochemistry of plant volatiles. Plant. Physiol. 2004, 135, 1893–1902. [Google Scholar] [CrossRef]
- Horiuchi, J.; Badri, D.V.; Kimball, B.A.; Negre, F.; Dudareva, N.; Paschke, M.W.; Vivanco, J.M. The floral volatile, methyl benzoate, from snapdragon (Antirrhinum majus) triggers phytotoxic effects in Arabidopsis thaliana. Planta 2007, 226, 1–10. [Google Scholar] [CrossRef]
- Lu, S.; Xu, R.; Jia, J.-W.; Pang, J.; Matsuda, S.P.T.; Chen, X.-Y. Cloning and functional characterization of a β-pinene synthase from Artemisia annua that shows a circadian pattern of expression. Plant. Physiol. 2002, 130, 477–486. [Google Scholar] [CrossRef]
- Helsper, J.P.F.G.; Davies, J.A.; Bouwmeester, H.J.; Krol, A.F.; van Kampen, M.H. Circadian rhythmicity in emission of volatile compounds by flowers of Rosa hybrida L. cv. Honesty. Planta 1998, 207, 88–95. [Google Scholar] [CrossRef]
- Jakobsen, H.B.; Olsen, C.E. Influence of climatic factors on emission of flower volatiles in situ. Planta 1994, 192, 365–371. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H. Analysis of volatile components in mulberry fruit sparkling wine by gas chromatography/mass spectrometry. Canye Kexue 2010, 36, 152–156. [Google Scholar]
- Nishida, R.; Tan, K. Search for new fruit fly attractants from plants: A review. In Proceedings of the 9th International Symposium on Fruit Flies of Economic Importance, Bangkok, Thailand, 12‒16 May 2014. [Google Scholar]
- Katte, T.; Tan, K.H.; Su, Z.-H.; Ono, H.; Nishida, R. Floral fragrances in two closely related fruit fly orchids, Bulbophyllum hortorum and B. macranthoides (Orchidaceae): Assortments of phenylbutanoids to attract tephritid fruit fly males. Appl. Entomol. Zool. 2020, 55, 55–64. [Google Scholar] [CrossRef]
- Christenson, L.D.; Foote, R.H. Biology of fruit flies. Annu. Rev. Entomol. 1960, 5, 171–192. [Google Scholar] [CrossRef]
- Tan, K.H.; Nishida, R.; Jang, E.B.; Shelly, T.E. Pheromones, male lures, and trapping of tephritid fruit flies. In Trapping and the Detection, Control, and Regulation of Tephritid Fruit Flies: Lures, Area-Wide Programs, and Trade Implications; Tan, K.H., Nishida, R., Jang, E.B., Shelly, T.E., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 15–74. [Google Scholar]
- Shivanna, K.R. Reproductive assurance through unusual autogamy in the absence of pollinators in Passiflora edulis (passion fruit). Curr. Sci. 2012, 103, 1091–1096. [Google Scholar]
- Yamamoto, M.; da Silva, C.I.; Augusto, S.C.; Barbosa, A.A.A.; Oliveira, P.E. The role of bee diversity in pollination and fruit set of yellow passion fruit (Passiflora edulis forma flavicarpa, Passifloraceae) crop in central Brazil. Apidologie 2012, 43, 515–526. [Google Scholar] [CrossRef]
- Janzen, D.H. Reproductive behavior in the Passifloraceae and some of its pollinators in Central America. Behaviour 1968, 32, 33–48. [Google Scholar] [CrossRef]
- Sazima, M.; Sazima, I. Bat pollination of the passion flower, Passiflora mucronata, in Southeastern Brazil. Biotropica 1978, 10, 100–109. [Google Scholar] [CrossRef]
- Varela, G.; Cocucci, A.; Sersic, A.N. Role of the corona in Passiflora caerulea (Passifloraceae) as attractant to their pollinators. Boletin de la Sociedad Argentina de Botanica 2016, 51, 99–110. [Google Scholar] [CrossRef]
| No | Identity | RI | Lit. RI [ref] | MM | Diagnostic Ions | ICF/% (SE) | OCF/% (SE) | Anther/% (SE) | Stigma/% (SE) | Ovary/% (SE) | Petal/% (SE) | Sepal/% (SE) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | β-Pinene | 980 | 978 [32] | 136.2 | 136, 121, 93, 79, 69, 41 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 26.27 (8.86) | 25.39 (9.21) |
| 2 | 3-Ethyl-2,3-dihydro-4H-pyran-4-one * | 1042 | 126.1 | 126, 98, 81, 70, 53, | 5.93 (1.96) | 3.43 (1.08) | 0.0 | 1.19 (0.74) | 1.21 (0.77) | 1.21 (0.69) | 1.78 (1.00) | |
| 3 | Borneol | 1175 | 1171 [33] | 154.2 | 154, 139, 123, 110, 95, 55, 41 | 0.0 | 0.0 | 0.0 | 0.52 (0.12) | 0.38 (0.06) | 0.49 (0.11) | 0.50 (0.20) |
| 4 | 1-(4-Methyl-1,3-dioxolan-2-yl)pentan-3-one * | 1227 | 172.2 | 172, 143, 128, 99, 85, 82, 72, 57 | 8.91 (2.71) | 5.77 (2.21) | 2.57 (1.66) | 8.38 (3.33) | 8.28 (3.43) | 4.78 (2.00) | 3.22 (1.74) | |
| 6 | Nonanoic acid | 1278 | 1275 [34] | 158.2 | 129, 115, 73, 60, 57, 41 | 0.20 (0.04) | 0.12 (0.05) | 1.03 (0.82) | 0.52 (0.14) | 0.53 (0.14)) | 0.42 (0.07) | 0.10 (0.04) |
| 7 | Indole | 1295 | 1294 [35] | 117.1 | 117, 90 | 0.16 (0.01) | 0.13 (0.04) | 0.09 (0.05) | 0.0 | 0.09 (0.06) | 0.19 (0.05) | 0.18 (0.06) |
| 8 | 4-(2-Hydroxyisopropyl) -1-methylcyclohexanol (Terpin) | 1315 | 172.3 | 139, 96, 81, 59, 43 | 0.0 | 0.0 | 2.02 (1.04) | 1.22 (0.63) | 0.0 | 0.0 | 1.04 (0.71) | |
| 9 | 4-(4-Hydroxyphenyl)-2-butanone (Raspberry ketone) | 1560 | 1556 [36] | 164.2 | 164, 107 | 0.22 (0.04) | 0.11 (0.09) | 0.0 | 0.0 | 0.0 | 0.05 (0.04) | 0.06 (0.03) |
| 10 | 4-(4-Acetoxyphenyl)-2-butanone (Cuelure) | 1652 | 206.2 | 206, 164, 107 | 0.16 (0.06) | 1.11 (0.30) | 0.0 | 0.0 | 0.0 | 0.03 (0.02) | 0.04 (0.02) | |
| 11 | 4-(4-Hydroxy-3-methoxyphenyl)-2-butanone (Zingerone) | 1663 | 194.2 | 194, 137 | 1.10 (0.24) | 0.12 (0.07) | 0.0 | 0.0 | 0.0 | 0.06 (0.02) | 0.06 (0.02) | |
| 12 | 4-(4-Hydroxy-3-methoxyphenyl)-2-butanol (Zingerol) | 1688 | 196.2 | 196, 137 | 0.22 (0.04) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| 13 | Propyl laurate | 1690 | 1685 [37] | 242.4 | 242, 201, 183, 115, 102, 73, 61, 43 | 0.0 | 0.0 | 0.0 | 1.88 (1.00) | 0.0 | 0.0 | 0.0 |
| 14 | (2E,6E)-3,7,11-Trimethyldodeca-2,6,10-trien-1-ol (Farnesol) | 1732 | 1728 [38] | 222.4 | 222, 136, 121, 107, 93, 81, 69, 41 | 5.87 (3.22) | 3.92 (1.45) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 15 | 3,7,11-Trimethyl-2,6,10-dodecatrien-1-ol acetate (Farnesyl acetate) | 1849 | 1846 [39] | 264.4 | 264, 204, 161, 136, 121, 107, 93, 81, 69, 43 | 7.78 (2.34) | 8.31 (2.45) | 0.42 (0.18) | 1.76 (1.10) | 1.02 (0.47) | 0.58 (0.20) | 0.52 (0.22) |
| 16 | Palmitic acid | 1978 | 1977 [40] | 256.4 | 256, 312, 185, 171, 157, 129, 73, 57 | 8.76 (1.07) | 17.82 (5.78) | 12.27 (4.84) | 12.52 (5.02) | 13.47 (5.62) | 7.78 (3.12) | 8.42 (3.88) |
| 17 | Ethyl palmitate | 1997 | 1996 [41] | 284.5 | 284, 241, 157, 101, 88, 70, 55 | 6.87 (1.17) | 2.33 (0.43) | 19.08 (6.66) | 14.89 (5.98) | 15.08 (6.13) | 4.85 (1.96) | 5.32 (2.24) |
| 18 | Phytol | 2121 | 2117 [41] | 296.5 | 123, 111, 97, 81, 71m, 69, 57 | 0.23 (0.06) | 0.24 (0.09) | 0.08 (0.03) | 3.86 (2.02) | 5.03 (2.38) | 1.82 (0.98) | 11.45 (4.44) |
| 19 | Linoleic acid | 2157 | 2156 [42] | 280.4 | 280, 150, 138, 124, 109, 95, 81, 67, 55 | 2.38 (0.62) | 2.10 (0.64) | 8.28 (3.35) | 4.42 (2.56) | 5.86 (2.18) | 4.33 (1.94) | 5.78 (2.21) |
| 20 | Linolenic acid | 2162 | 2162 [34] | 278.4 | 278, 222, 149, 135, 121, 108, 93, 95, 79, 67, 55 | 1.83 (0.76) | 4.32 (1.76) | 2.22 (1.08) | 5.11 (2.88) | 4.63 (2.23) | 2.82 (1.21) | 3.61 (1.22) |
| 21 | Ethyl linoleate | 2171 | 2171 [43] | 308.5 | 308, 262, 109, 95, 81, 67, 55 | 9.54 (2.24) | 15.23 (4.63) | 18.41 (6.33) | 19.45 (7.21) | 17.94 (6.12) | 16.36 (5.05) | 16.50 (5.55) |
| 22 | Ethyl linolenate | 2174 | 2173 [44] | 306.5 | 306, 264, 149, 135, 121, 108, 95, 79, 67, 55 | 2.03 (1.34) | 2.21 (1.46) | 23.77 (7.45) | 9.52 (3.32) | 9.44 (3.33) | 3.70 (1.83) | 3.82 (2.00) |
| 23 | Stearic acid | 2188 | 2188 [45] | 284.5 | 284, 241, 185, 129, 73, 60, 43 | 1.84 (0.78) | 2.10 (1.05) | 1.12 (0.32) | 3.83 (1.23) | 6.52 (2.28) | 1.84 (0.92) | 1.79 (1.04) |
| 24 | Ethyl sterate | 2198 | 2197 [46] | 312.5 | 312, 269, 213, 157, 101, 88, 670, 55 | 2.13 (0.83) | 1.43 (0.93) | 4.03 (2.72) | 6.02 (3.44) | 4.77 (1.75) | 0.72 (0.32) | 1.02 (0.41) |
| 25 | Squalene | 2834 | 2833 [41] | 410.7 | 367, 341, 203, 175, 161, 136, 121, 85, 81, 69 | 33.83 (10.01) | 32.58 (10.21) | 4.49 (2.15) | 4.74 (2.83) | 5.65 (2.71) | 19.85 (7.02) | 9.35 (4.44) |
| Compound | Flower Part | A (SE) | ε (SE) | k (SE) | T1/2, Hour | RSS (RSE) |
|---|---|---|---|---|---|---|
| Raspberry ketone | Inner coronal filament | 3429000 (6557000) | 0.060 (0.116) | 2.456 (3.662) | 0.282 | 34.62 (0.793) |
| Cuelure | Outer coronal filament | 4200 (245700) | 0.768 (0.112) | 1.667 (1.789) | 0.964 | 31.51 (0.756) |
| Zingerone | Inner coronal filament | 365 (259) | 4.807 (2.127) | 0.412 (1.331) | 0.416 | 3454 (7.925) |
| Outer coronal filament | 968 (1166) | 2.247 (0.575) | 0.719 (1.307) | 1.682 | 563.4 (3.200) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.J.; De Faveri, S.G.; Cheesman, J.; Hanssen, B.L.; Cameron, D.N.S.; Jamie, I.M.; Taylor, P.W. Zingerone in the Flower of Passiflora maliformis Attracts an Australian Fruit Fly, Bactrocera jarvisi (Tryon). Molecules 2020, 25, 2877. https://doi.org/10.3390/molecules25122877
Park SJ, De Faveri SG, Cheesman J, Hanssen BL, Cameron DNS, Jamie IM, Taylor PW. Zingerone in the Flower of Passiflora maliformis Attracts an Australian Fruit Fly, Bactrocera jarvisi (Tryon). Molecules. 2020; 25(12):2877. https://doi.org/10.3390/molecules25122877
Chicago/Turabian StylePark, Soo Jean, Stefano G. De Faveri, Jodie Cheesman, Benjamin L. Hanssen, Donald N. S. Cameron, Ian M. Jamie, and Phillip W. Taylor. 2020. "Zingerone in the Flower of Passiflora maliformis Attracts an Australian Fruit Fly, Bactrocera jarvisi (Tryon)" Molecules 25, no. 12: 2877. https://doi.org/10.3390/molecules25122877
APA StylePark, S. J., De Faveri, S. G., Cheesman, J., Hanssen, B. L., Cameron, D. N. S., Jamie, I. M., & Taylor, P. W. (2020). Zingerone in the Flower of Passiflora maliformis Attracts an Australian Fruit Fly, Bactrocera jarvisi (Tryon). Molecules, 25(12), 2877. https://doi.org/10.3390/molecules25122877









