Development of ‘Lignin-First’ Approaches for the Valorization of Lignocellulosic Biomass
Abstract
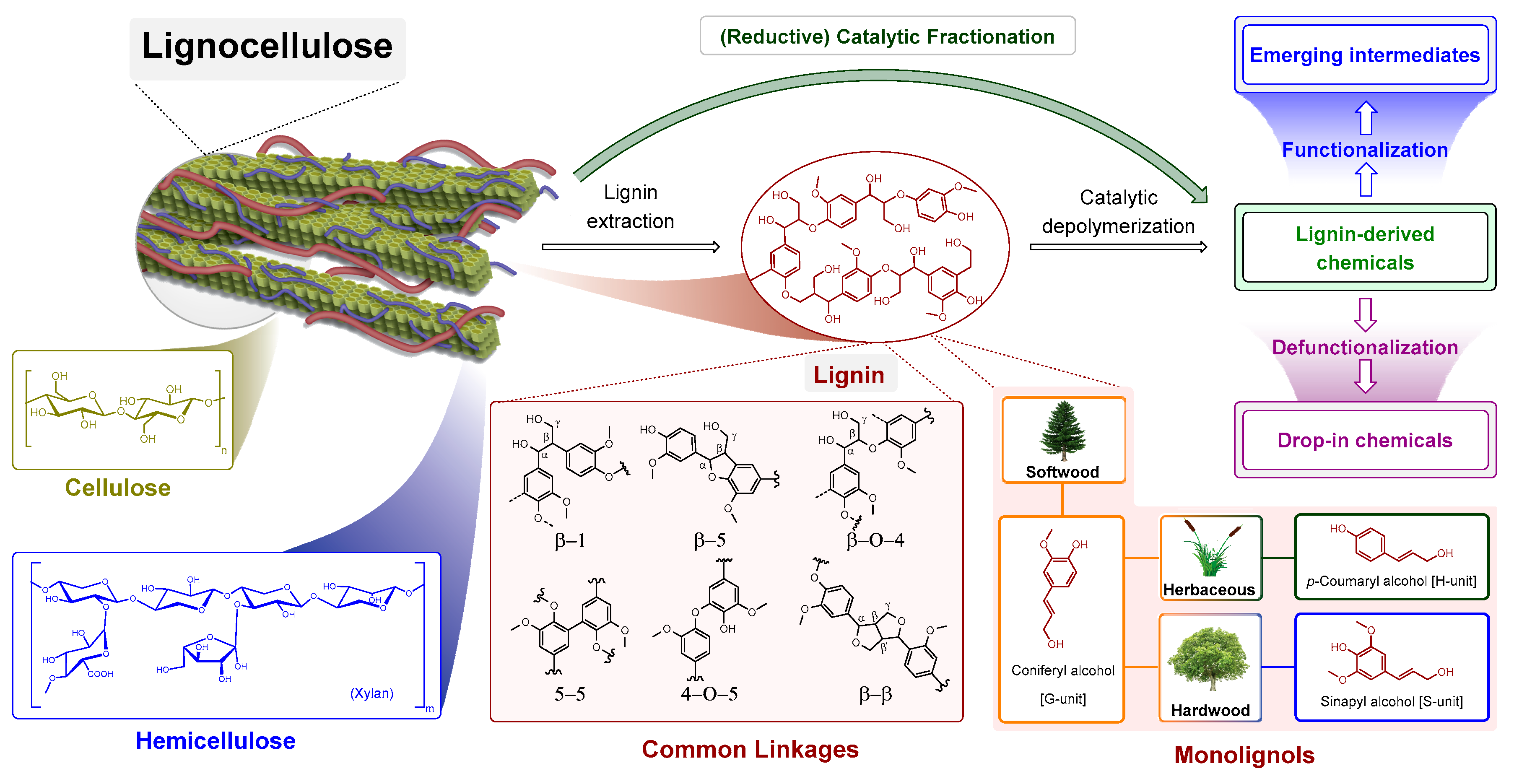
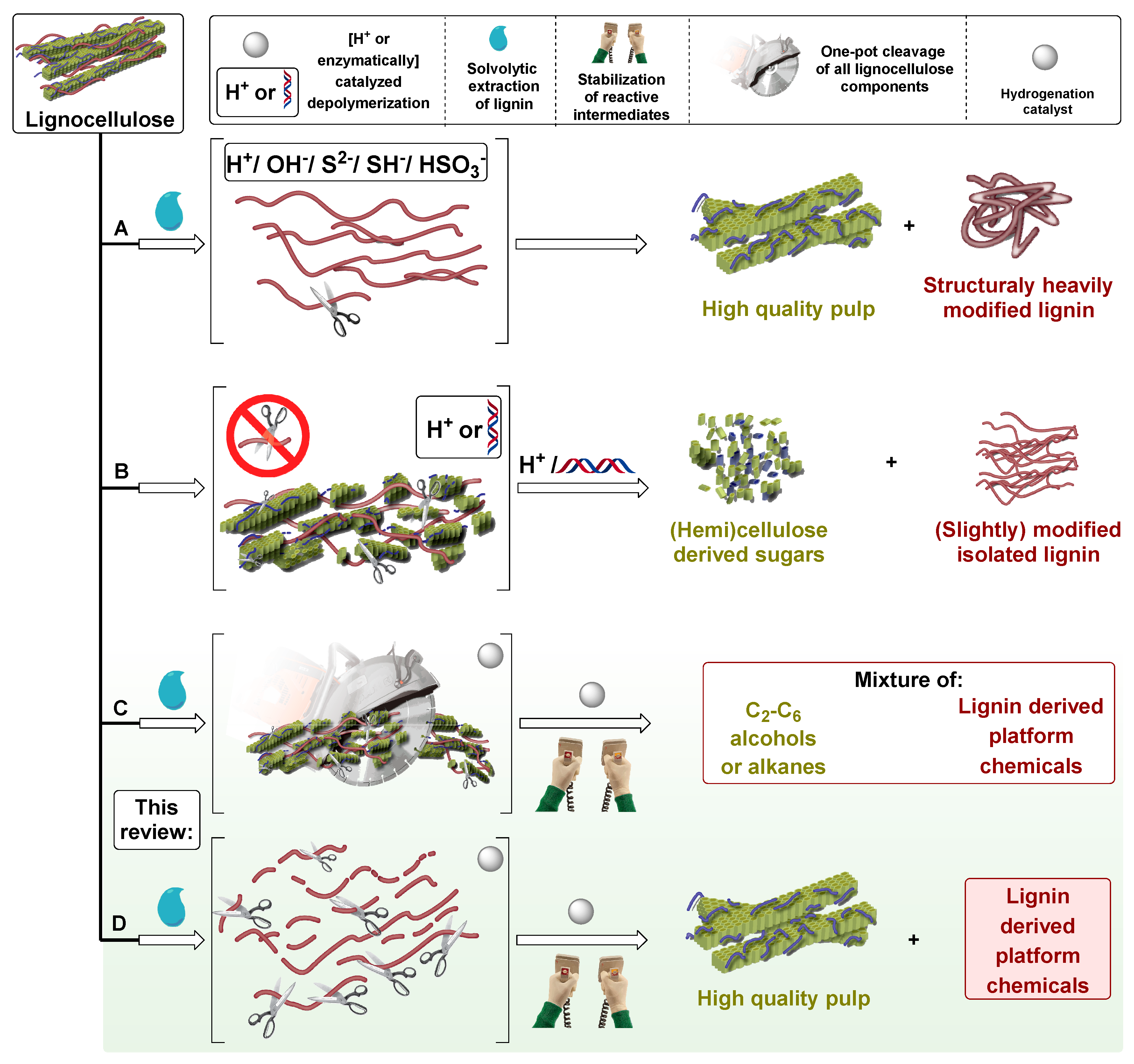
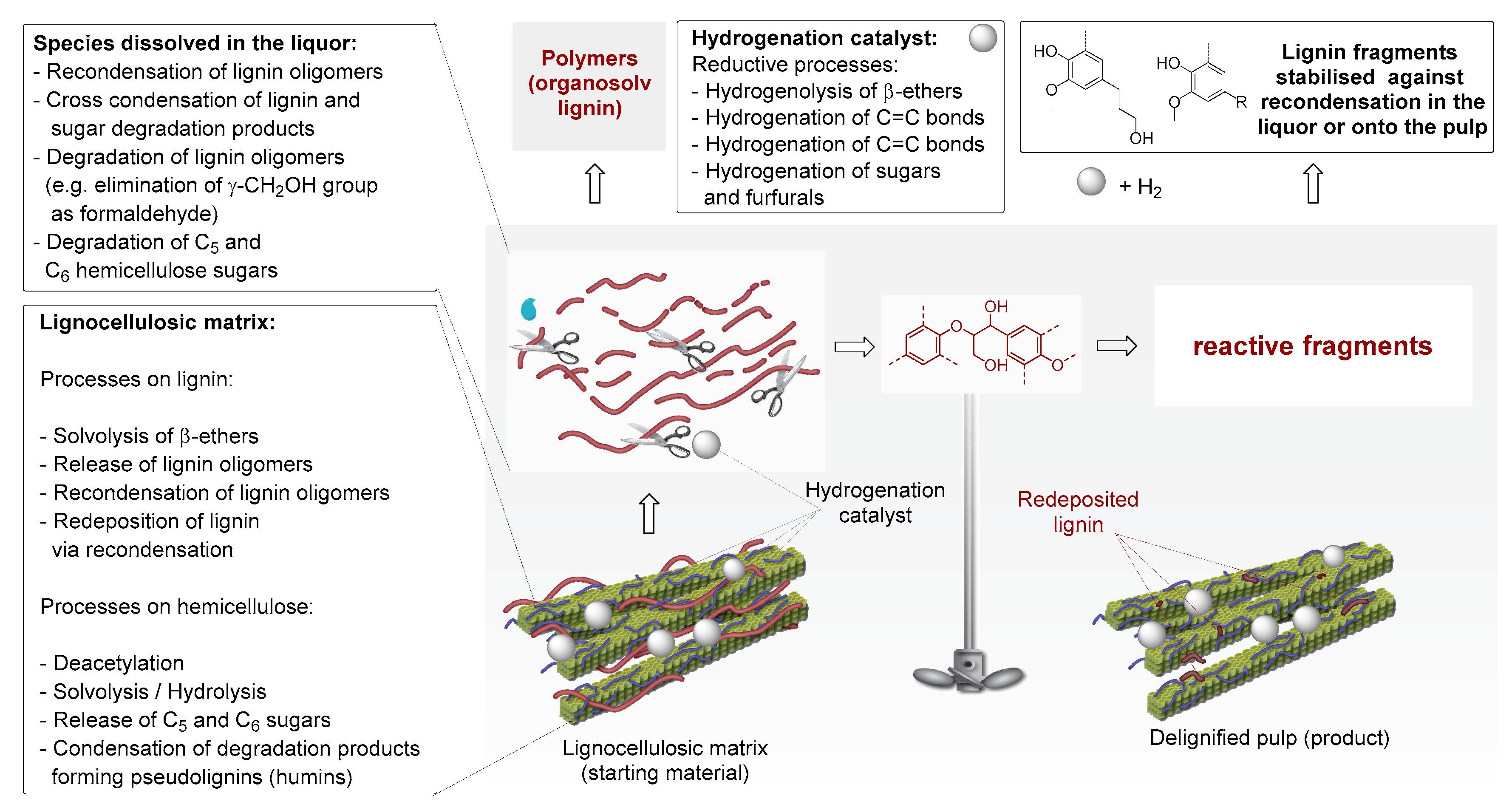
1. Introduction
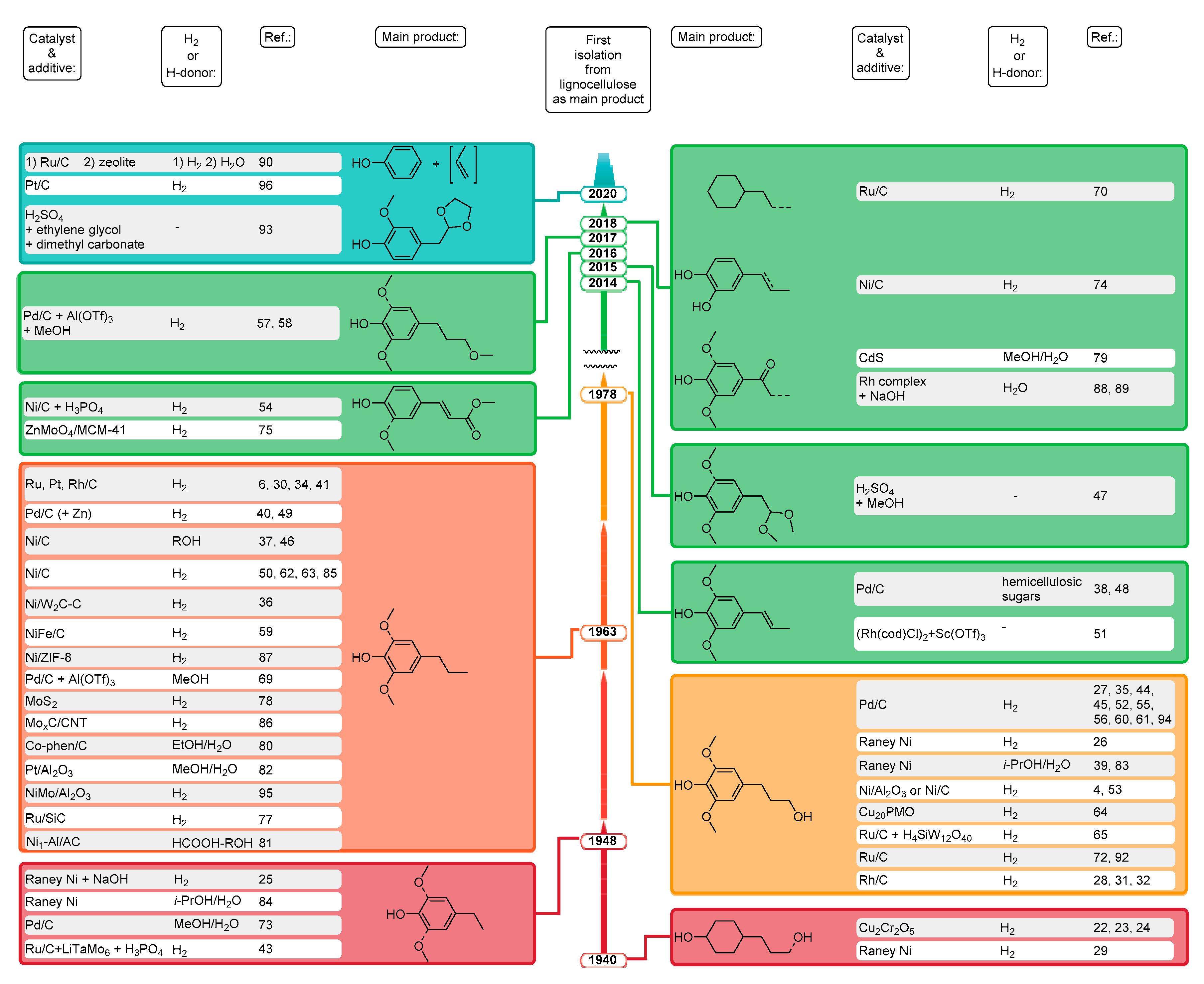
2. Chronological Overview
2.1. From 1940 to 2014
2.2. 2015
2.3. 2016
2.4. 2017
2.5. 2018
2.6. 2019
2.7. 2020
3. Summary
4. Future Perspectives and Challenges
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.F.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin valorization: Improving lignin processing in the biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef] [PubMed]
- Zakzeski, J.; Bruijnincx, P.C.A.; Jongerius, A.L.; Weckhuysen, B.M. The catalytic valorization of lignin for the production of renewable chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Fridrich, B.; de Santi, A.; Elangovan, S.; Barta, K. Bright side of lignin depolymerization: Toward new platform chemicals. Chem. Rev. 2018, 118, 614–678. [Google Scholar] [CrossRef] [PubMed]
- Van den Bosch, S.; Renders, T.; Kennis, S.; Koelewijn, S.-F.; Van den Bossche, G.; Vangeel, T.; Deneyer, A.; Depuydt, D.; Courtin, C.M.; Thevelein, J.M.; et al. Integrating lignin valorization and bio-ethanol production: On the role of Ni-Al2O3 catalyst pellets during lignin-first fractionation. Green Chem. 2017, 19, 3313–3326. [Google Scholar] [CrossRef]
- Galkin, M.V.; Samec, J.S.M. Lignin valorization through catalytic lignocellulose fractionation: A fundamental platform for the future biorefinery. ChemSusChem 2016, 9, 1544–1558. [Google Scholar] [CrossRef] [PubMed]
- Shuai, L.; Amiri, M.T.; Questell-Santiago, Y.M.; Héroguel, F.; Li, Y.; Kim, H.; Meilan, R.; Chapple, C.; Ralph, J.; Luterbacher, J.S. Formaldehyde stabilization facilitates lignin monomer production during biomass depolymerization. Science 2016, 354, 329–333. [Google Scholar] [CrossRef]
- Xu, C.; Arancon, R.A.D.; Labidi, J.; Luque, R. Lignin depolymerisation strategies: Towards valuable chemicals and fuels. Chem. Soc. Rev. 2014, 43, 7485–7500. [Google Scholar] [CrossRef]
- Kumar, C.R.; Anand, N.; Kloekhorst, A.; Cannilla, C.; Bonura, G.; Frusteri, F.; Barta, K.; Heeres, H.J. Solvent free depolymerization of kraft lignin to alkyl-phenolics using supported NiMo and CoMo catalysts. Green Chem. 2015, 17, 4921–4930. [Google Scholar] [CrossRef]
- Narani, A.; Chowdari, R.K.; Cannilla, C.; Bonura, G.; Frusteri, F.; Heeres, H.J.; Barta, K. Efficient catalytic hydrotreatment of Kraft lignin to alkylphenolics using supported NiW and NiMo catalysts in supercritical methanol. Green Chem. 2015, 17, 5046–5057. [Google Scholar] [CrossRef]
- Rahimi, A.; Ulbrich, A.; Coon, J.J.; Stahl, S.S. Formic-acid-induced depolymerization of oxidized lignin to aromatics. Nature 2014, 515, 249–252. [Google Scholar] [CrossRef]
- Zhao, C.; Xie, S.; Pu, Y.; Zhang, R.; Huang, F.; Ragauskas, A.J.; Yuan, J.S. Synergistic enzymatic and microbial lignin conversion. Green Chem. 2016, 18, 1306–1312. [Google Scholar] [CrossRef]
- Luo, H.; Abu-Omar, M.M. Chemicals from lignin. In Encyclopedia of Sustainable Technologies; Abraham, M.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 573–585. ISBN 9780128046777. [Google Scholar]
- Van den Bosch, S.; Koelewijn, S.-F.; Renders, T.; Van den Bossche, G.; Vangeel, T.; Schutyser, W.; Sels, B.F. Catalytic strategies towards lignin-derived chemicals. Top. Curr. Chem. 2018, 376, 36. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, R.; Jastrzebski, R.; Clough, M.T.; Ralph, J.; Kennema, M.; Bruijnincx, P.C.A.; Weckhuysen, B.M. Paving the way for lignin valorisation: Recent advances in bioengineering, biorefining and catalysis. Angew. Chem. Int. Ed. 2016, 55, 8164–8215. [Google Scholar] [CrossRef] [PubMed]
- Renders, T.; Van den Bosch, S.; Koelewijn, S.-F.; Schutyser, W.; Sels, B.F. Lignin-first biomass fractionation: The advent of active stabilisation strategies. Energy Environ. Sci. 2017, 10, 1551–1557. [Google Scholar] [CrossRef]
- Schutyser, W.; Renders, T.; Van den Bosch, S.; Koelewijn, S.-F.; Beckham, G.T.; Sels, B.F. Chemicals from lignin: An interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef]
- Renders, T.; Van den Bossche, G.; Vangeel, T.; Van Aelst, K.; Sels, B. Reductive catalytic fractionation: State of the art of the lignin-first biorefinery. Curr. Opin. Biotechnol. 2019, 56, 193–201. [Google Scholar] [CrossRef]
- Ha, J.-M.; Hwang, K.-R.; Kim, Y.-M.; Jae, J.; Kim, K.H.; Lee, H.W.; Kim, J.-Y.; Park, Y.-K. Recent progress in the thermal and catalytic conversion of lignin. Renew. Sustain. Energy Rev. 2019, 111, 422–441. [Google Scholar] [CrossRef]
- Song, Y. Lignin valorization via reductive depolymerization. In Chemical Catalysts For Biomass Upgrading; Crocker, M., Santillan-Jimenez, E., Eds.; Wiley: Weinheim, Germany, 2020; pp. 395–438. [Google Scholar] [CrossRef]
- Paone, E.; Tabanelli, T.; Mauriello, F. The rise of lignin biorefinery. Curr. Opin. Green Sustain. Chem. 2020, 24, 1–6. [Google Scholar] [CrossRef]
- Ferrini, P.; Rezende, C.A.; Rinaldi, R. Catalytic upstream biorefining through hydrogen transfer reactions: Understanding the process from the pulp perspective. ChemSusChem 2016, 9, 3171–3180. [Google Scholar] [CrossRef]
- Godard, H.P.; McCarthy, J.L.; Hibbert, H. Hydrogenation of wood. J. Am. Chem. Soc. 1940, 62, 988. [Google Scholar] [CrossRef]
- Godard, H.P.; McCarthy, J.L.; Hibbert, H. Studies on lignin and related compounds. LXII. high pressure hydrogenation of wood using copper chromite catalyst (part 1). J. Am. Chem. Soc. 1941, 63, 3061–3066. [Google Scholar] [CrossRef]
- Bower, J.R.; Cooke, L.M.; Hibbert, H. Studies on lignin and related compounds. LXX. hydrogenolysis and hydrogenation of maple wood. J. Am. Chem. Soc. 1943, 65, 1192–1195. [Google Scholar] [CrossRef]
- Pepper, J.M.; Hibbert, H. Studies on lignin and related compounds. LXXXVII. high pressure hydrogenation of maple wood. J. Am. Chem. Soc. 1948, 70, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Pepper, J.M.; Steck, W. The effect of time and temperature on the hydrogenation of aspen lignin. Can. J. Chem. 1963, 41, 2867–2875. [Google Scholar] [CrossRef]
- Pepper, J.M.; Steck, W.F.; Swoboda, R.; Karapally, J.C. Hydrogenation of lignin using nickel and palladium catalysts. Lignin Struct. React. 1966, 238–248. [Google Scholar] [CrossRef]
- Pepper, J.M.; Lee, Y.W. Lignin and related compounds. I. A comparative study of catalysts for lignin hydrogenolysis. Can. J. Chem. 1969, 47, 723–727. [Google Scholar] [CrossRef]
- Pepper, J.M.; Lee, Y.W. Lignin and related compounds. II. studies using ruthenium and raney nickel as catalysts for lignin hydrogenolysis. Can. J. Chem. 1970, 48, 477–479. [Google Scholar] [CrossRef]
- Pepper, J.M.; Fleming, R.W. Lignin and related compounds. V. The hydrogenolysis of aspen wood lignin using rhodium-on-charcoal as catalyst. Can. J. Chem. 1978, 56, 896–898. [Google Scholar] [CrossRef]
- Pepper, J.M.; Supathna, P. Lignin and related compounds. VI. A study of variables affecting the hydrogenolysis of spruce wood lignin using a rhodium-on-charcoal catalyst. Can. J. Chem. 1978, 56, 899–902. [Google Scholar] [CrossRef]
- Pepper, J.M.; Rahman, M.D. Lignin and related compounds. XI: Selective degradation of aspen poplar lignin by catalytic hydrogenolysis. Cellul. Chem. Technol. 1987, 21, 233–239. [Google Scholar]
- Kaplunova, T.S.; Abduazimov, K.A.; Pulatov, B.K.; Ikramutdinova, M.T.; Abidova, M.F. Hydrogenolysis of Rice Husk Lignin. Chem. Nat. Compd. 1993, 29, 530–532. [Google Scholar] [CrossRef]
- Yan, N.; Zhao, C.; Dyson, P.J.; Wang, C.; Liu, L.T.; Kou, Y. Selective degradation of wood lignin over noble-metal catalysts in a two-step process. ChemSusChem 2008, 1, 626–629. [Google Scholar] [CrossRef] [PubMed]
- Torr, K.M.; van de Pas, D.J.; Cazeils, E.; Suckling, I.D. Mild hydrogenolysis of in-situ and isolated pinus radiata lignins. Bioresour. Technol. 2011, 102, 7608–7611. [Google Scholar] [CrossRef]
- Li, C.; Zheng, M.; Wang, A.; Zhang, T. One-pot catalytic hydrocracking of raw woody biomass into chemicals over supported carbide catalysts: Simultaneous conversion of cellulose, hemicellulose and lignin. Energy Environ. Sci. 2012, 5, 6383–6390. [Google Scholar] [CrossRef]
- Song, Q.; Wang, F.; Cai, J.; Wang, Y.; Zhang, J.; Yu, W.; Xu, J. Lignin depolymerization (LDP) in alcohol over nickel-based catalysts via a fragmentation–hydrogenolysis process. Energy Environ. Sci. 2013, 6, 994–1007. [Google Scholar] [CrossRef]
- Galkin, M.V.; Samec, J.S.M. Selective route to 2-propenyl aryls directly from wood by a tandem organosolv and palladium-catalysed transfer hydrogenolysis. ChemSusChem 2014, 7, 2154–2158. [Google Scholar] [CrossRef] [PubMed]
- Ferrini, P.; Rinaldi, R. Catalytic biorefining of plant biomass to non-pyrolytic lignin bio-oil and carbohydrates through hydrogen transfer reactions. Angew. Chem. Int. Ed. 2014, 53, 8634–8639. [Google Scholar] [CrossRef]
- Parsell, T.; Yohe, S.; Degenstein, J.; Jarrell, T.; Klein, I.; Gencer, E.; Hewetson, B.; Hurt, M.; Kim, J.I.; Choudhari, H.; et al. A synergistic biorefinery based on catalytic conversion of lignin prior to cellulose starting from lignocellulosic biomass. Green Chem. 2015, 17, 1492–1499. [Google Scholar] [CrossRef]
- Van den Bosch, S.; Schutyser, W.; Vanholme, R.; Driessen, T.; Koelewijn, S.-F.; Renders, T.; De Meester, B.; Huijgen, W.J.J.; Dehaen, W.; Courtin, C.M.; et al. Reductive lignocellulose fractionation into soluble lignin-derived phenolic monomers and dimers and processable carbohydrate pulps. Energy Environ. Sci. 2015, 8, 1748–1763. [Google Scholar] [CrossRef]
- Dusselier, M.J.; Op De Beeck, B.; Sels, B.F. Biphasic Solvent Catalytic Process for the Direct Production of Light Naphta from Carbohydrate-Containing Feedstock. Patent 10,407,623, 10 September 2019. [Google Scholar]
- Liu, Y.; Chen, L.; Wang, T.; Zhang, Q.; Wang, C.; Yan, J.; Ma, L. One-pot catalytic conversion of raw lignocellulosic biomass into gasoline alkanes and chemicals over LiTaMoO6 and Ru/C in aqueous phosphoric acid. ACS Sustain. Chem. Eng. 2015, 3, 1745–1755. [Google Scholar] [CrossRef]
- Van den Bosch, S.; Schutyser, W.; Koelewijn, S.-F.; Renders, T.; Courtin, C.M.; Sels, B.F. Tuning the lignin oil OH-content with Ru and Pd catalysts during lignin hydrogenolysis on birch wood. Chem. Commun. 2015, 51, 13158–13161. [Google Scholar] [CrossRef] [PubMed]
- Schutyser, W.; Van den Bosch, S.; Renders, T.; De Boe, T.; Koelewijn, S.-F.; Dewaele, A.; Ennaert, T.; Verkinderen, O.; Goderis, B.; Courtin, C.M.; et al. Influence of bio-based solvents on the catalytic reductive fractionation of birch wood. Green Chem. 2015, 17, 5035–5045. [Google Scholar] [CrossRef]
- Klein, I.; Saha, B.; Abu-Omar, M.M. Lignin depolymerization over Ni/C catalyst in methanol, a continuation: Effect of substrate and catalyst loading. Catal. Sci. Technol. 2015, 5, 3242–3245. [Google Scholar] [CrossRef]
- Kaiho, A.; Kogo, M.; Sakai, R.; Saito, K.; Watanabe, T. In situ trapping of enol intermediates with alcohol during acid-catalysed de-polymerisation of lignin in a nonpolar solvent. Green Chem. 2015, 17, 2780–2783. [Google Scholar] [CrossRef]
- Galkin, M.V.; Smit, A.T.; Subbotina, E.; Artemenko, K.A.; Bergquist, J.; Huijgen, W.J.J.; Samec, J.S.M. Hydrogen-free catalytic fractionation of woody biomass. ChemSusChem 2016, 9, 3280–3287. [Google Scholar] [CrossRef]
- Klein, I.; Marcum, C.; Kenttämaa, H.; Abu-Omar, M.M. Mechanistic investigation of the Zn/Pd/C catalyzed cleavage and hydrodeoxygenation of lignin. Green Chem. 2016, 18, 2399–2405. [Google Scholar] [CrossRef]
- Luo, H.; Klein, I.M.; Jiang, Y.; Zhu, H.; Liu, B.; Kenttämaa, H.I.; Abu-Omar, M.M. Total utilization of miscanthus biomass, lignin and carbohydrates, using earth abundant nickel catalyst. ACS Sustain. Chem. Eng. 2016, 4, 2316–2322. [Google Scholar] [CrossRef]
- Jastrzebski, R.; Constant, S.; Lancefield, C.S.; Westwood, N.J.; Weckhuysen, B.M.; Bruijnincx, P.C.A. Tandem catalytic depolymerization of lignin by water-tolerant lewis acids and rhodium complexes. ChemSusChem 2016, 9, 2074–2079. [Google Scholar] [CrossRef]
- Huang, X.; Zhu, J.; Korányi, T.I.; Boot, M.D.; Hensen, E.J.M. Effective release of lignin fragments from lignocellulose by lewis acid metal triflates in the lignin-first approach. ChemSusChem 2016, 9, 3262–3267. [Google Scholar] [CrossRef]
- Chen, J.; Lu, F.; Si, X.; Nie, X.; Chen, J.; Lu, R.; Xu, J. High yield production of natural phenolic alcohols from woody biomass using a nickel-based catalyst. ChemSusChem 2016, 9, 3353–3360. [Google Scholar] [CrossRef]
- Anderson, E.M.; Katahira, R.; Reed, M.; Resch, M.G.; Karp, E.M.; Beckham, G.T.; Román-Leshkov, Y. Reductive catalytic fractionation of corn stover lignin. ACS Sustain. Chem. Eng. 2016, 4, 6940–6950. [Google Scholar] [CrossRef]
- Renders, T.; Schutyser, W.; Van Den Bosch, S.; Koelewijn, S.F.; Vangeel, T.; Courtin, C.M.; Sels, B.F. Influence of acidic (H3PO4) and alkaline (NaOH) additives on the catalytic reductive fractionation of lignocellulose. ACS Catal. 2016, 6, 2055–2066. [Google Scholar] [CrossRef]
- Renders, T.; Van den Bosch, S.; Vangeel, T.; Ennaert, T.; Koelewijn, S.-F.; Van den Bossche, G.; Courtin, C.M.; Schutyser, W.; Sels, B.F. Synergetic effects of alcohol/water mixing on the catalytic reductive fractionation of poplar wood. ACS Sustain. Chem. Eng. 2016, 4, 6894–6904. [Google Scholar] [CrossRef]
- Huang, X.; Morales Gonzalez, O.M.; Zhu, J.; Korányi, T.I.; Boot, M.D.; Hensen, E.J.M. Reductive fractionation of woody biomass into lignin monomers and cellulose by tandem metal triflate and Pd/C catalysis. Green Chem. 2017, 19, 175–187. [Google Scholar] [CrossRef]
- Huang, X.; Ouyang, X.; Hendriks, B.; Gonzalez, O.M.M.; Zhu, J.; Korányi, T.I.; Boot, M.; Hensen, E.J.M. Selective production of mono-aromatics from lignocellulose over Pd/C catalyst: The influence of acid co-catalysts. Faraday Discuss. 2017, 202, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Li, C.; Xu, G.; Ma, Y.; Liu, X.; Zhang, Y. Depolymerization of lignin via a non-precious Ni–Fe alloy catalyst supported on activated carbon. Green Chem. 2017, 19, 1895–1903. [Google Scholar] [CrossRef]
- van de Pas, D.J.; Torr, K.M. Biobased epoxy resins from deconstructed native softwood lignin. Biomacromolecules 2017, 18, 2640–2648. [Google Scholar] [CrossRef]
- Kumaniaev, I.; Subbotina, E.; Sävmarker, J.; Larhed, M.; Galkin, M.V.; Samec, J. Lignin depolymerization to monophenolic compounds in a flow-through system. Green Chem. 2017, 19, 5767–5771. [Google Scholar] [CrossRef]
- Anderson, E.M.; Stone, M.L.; Katahira, R.; Reed, M.; Beckham, G.T.; Román-Leshkov, Y. Flowthrough reductive catalytic fractionation of biomass. Joule. 2017, 1, 613–622. [Google Scholar] [CrossRef]
- Anderson, E.M.; Stone, M.L.; Hülsey, M.J.; Beckham, G.T.; Román-Leshkov, Y. Kinetic studies of lignin solvolysis and reduction by reductive catalytic fractionation decoupled in flow-through reactors. ACS Sustain. Chem. Eng. 2018, 6, 7951–7959. [Google Scholar] [CrossRef]
- Sun, Z.; Bottari, G.; Afanasenko, A.; Stuart, M.C.A.; Deuss, P.J.; Fridrich, B.; Barta, K. Complete lignocellulose conversion with integrated catalyst recycling yielding valuable aromatics and fuels. Nat. Catal. 2018, 1, 82–92. [Google Scholar] [CrossRef]
- Deneyer, A.; Peeters, E.; Renders, T.; Van den Bosch, S.; Van Oeckel, N.; Ennaert, T.; Szarvas, T.; Korányi, T.I.; Dusselier, M.; Sels, B.F. Direct upstream integration of biogasoline production into current light straight run naphtha petrorefinery processes. Nat. Energy 2018, 3, 969–977. [Google Scholar] [CrossRef]
- Cao, Z.; Dierks, M.; Clough, M.T.; de Castro, I.B.D.; Rinaldi, R. A convergent approach for a deep converting lignin-first biorefinery rendering high-energy-density drop-in fuels. Joule. 2018, 2, 1118–1133. [Google Scholar] [CrossRef] [PubMed]
- Graça, I.; Woodward, R.T.; Kennema, M.; Rinaldi, R. Formation and fate of carboxylic acids in the lignin-first biorefining of lignocellulose via h-transfer catalyzed by raney Ni. ACS Sustain. Chem. Eng. 2018, 6, 13408–13419. [Google Scholar] [CrossRef]
- Sagues, W.J.; Bao, H.; Nemenyi, J.L.; Tong, Z. A lignin-first approach to biorefining: Utilizing fenton’s reagent and supercritical ethanol for the production of phenolics and sugars. ACS Sustain. Chem. Eng. 2018, 6, 4958–4965. [Google Scholar] [CrossRef]
- Ouyang, X.; Huang, X.; Hendriks, B.M.S.; Boot, M.D.; Hensen, E.J.M. Coupling organosolv fractionation and reductive depolymerization of woody biomass in a two-step catalytic process. Green Chem. 2018, 20, 2308–2319. [Google Scholar] [CrossRef]
- Li, X.; Guo, T.; Xia, Q.; Liu, X.; Wang, Y. One-pot catalytic transformation of lignocellulosic biomass into alkylcyclohexanes and polyols. ACS Sustain. Chem. Eng. 2018, 6, 4390–4399. [Google Scholar] [CrossRef]
- Guo, T.; Li, X.; Liu, X.; Guo, Y.; Wang, Y. Catalytic transformation of lignocellulosic biomass into arenes, 5-hydroxymethylfurfural, and furfural. ChemSusChem 2018, 11, 2758–2765. [Google Scholar] [CrossRef]
- Renders, T.; Cooreman, E.; Van den Bosch, S.; Schutyser, W.; Koelewijn, S.-F.; Vangeel, T.; Deneyer, A.; Van den Bossche, G.; Courtin, C.M.; Sels, B.F. Catalytic lignocellulose biorefining in n-butanol/water: A one-pot approach toward phenolics, polyols, and cellulose. Green Chem. 2018, 20, 4607–4619. [Google Scholar] [CrossRef]
- Kumaniaev, I.; Samec, J.S.M. Valorization of quercus suber bark toward hydrocarbon bio-oil and 4-ethylguaiacol. ACS Sustain. Chem. Eng. 2018, 6, 5737–5742. [Google Scholar] [CrossRef]
- Stone, M.L.; Anderson, E.M.; Meek, K.M.; Reed, M.; Katahira, R.; Chen, F.; Dixon, R.A.; Beckham, G.T.; Román-Leshkov, Y. Reductive catalytic fractionation of c-lignin. ACS Sustain. Chem. Eng. 2018, 6, 11211–11218. [Google Scholar] [CrossRef]
- Wang, S.; Gao, W.; Li, H.; Xiao, L.-P.; Sun, R.-C.; Song, G. Selective fragmentation of biorefinery corncob lignin into p-hydroxycinnamic esters with a supported ZnMoO4 catalyst. ChemSusChem 2018, 11, 2114–2123. [Google Scholar] [CrossRef] [PubMed]
- McCallum, C.S.; Strachan, N.; Bennett, S.C.; Forsythe, W.G.; Garrett, M.D.; Hardacre, C.; Morgan, K.; Sheldrake, G.N. Catalytic depolymerisation of suberin rich biomass with precious metal catalysts. Green Chem. 2018, 20, 2702–2705. [Google Scholar] [CrossRef]
- Huang, Y.; Duan, Y.; Qiu, S.; Wang, M.; Ju, C.; Cao, H.; Fang, Y.; Tan, T. Lignin-first biorefinery: A reusable catalyst for lignin depolymerization and application of lignin oil to jet fuel aromatics and polyurethane feedstock. Sustain. Energy Fuels 2018, 2, 637–647. [Google Scholar] [CrossRef]
- Li, S.; Li, W.; Zhang, Q.; Shu, R.; Wang, H.; Xin, H.; Ma, L. Lignin-first depolymerization of native corn stover with an unsupported MoS2 catalyst. Rsc Adv. 2018, 8, 1361–1370. [Google Scholar] [CrossRef]
- Wu, X.; Fan, X.; Xie, S.; Lin, J.; Cheng, J.; Zhang, Q.; Chen, L.; Wang, Y. Solar energy-driven lignin-first approach to full utilization of lignocellulosic biomass under mild conditions. Nat. Catal. 2018, 1, 772–780. [Google Scholar] [CrossRef]
- Rautiainen, S.; Di Francesco, D.; Katea, S.; Westin, G.; Tungasita, D.; Samec, J. Lignin valorization by cobalt-catalyzed fractionation of lignocellulose to yield monophenolic compounds. ChemSusChem 2019, 12, 404–408. [Google Scholar] [CrossRef]
- Park, J.; Riaz, A.; Verma, D.; Lee, H.J.; Woo, H.M.; Kim, J. Fractionation of lignocellulosic biomass over core-shell Ni-alumina catalysts with formic acid as a co-catalyst and hydrogen source. ChemSusChem 2019, 12, 1743–1762. [Google Scholar] [CrossRef]
- Ouyang, X.; Huang, X.; Zhu, J.; Boot, M.D.; Hensen, E.J.M. Catalytic conversion of lignin in woody biomass into phenolic monomers in methanol/water mixtures without external hydrogen. ACS Sustain. Chem. Eng. 2019, 7, 13764–13773. [Google Scholar] [CrossRef]
- Sultan, Z.; Graça, I.; Li, Y.; Lima, S.; Peeva, L.G.; Kim, D.; Ebrahim, M.A.A.; Rinaldi, R.; Livingston, A. Membrane fractionation of liquors from lignin-first biorefining. ChemSusChem 2019, 12, 1203–1212. [Google Scholar] [CrossRef]
- Rinaldi, R.; Woodward, R.T.; Ferrini, P.; Riverac, H.J.E. Lignin-first biorefining of lignocellulose: The impact of process severity on the uniformity of lignin oil composition. J. Braz. Chem. Soc. 2019, 30, 479–491. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, X.; Luo, H.; Liu, B.; Shiga, T.M.; Li, X.; Kim, J.I.; Rubinelli, P.; Overton, J.C.; Subramanyam, V.; et al. Overcoming cellulose recalcitrance in woody biomass for the lignin-first biorefinery. Biotechnol. Biofuels 2019, 12, 171. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Guo, X.; Huang, Y.; Fang, Y.; Tan, T. Task-specific catalyst development for lignin-first biorefinery toward hemicellulose retention or feedstock extension. ChemSusChem 2019, 12, 944–954. [Google Scholar] [CrossRef]
- Liu, X.; Li, H.; Xiao, L.-P.; Sun, R.-C.; Song, G. Chemodivergent hydrogenolysis of eucalyptus lignin with Ni@ZIF-8 catalyst. Green Chem. 2019, 21, 1498–1504. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Miao, W.; Tang, W.; Xue, D.; Li, C.; Zhang, B.; Xiao, J.; Wang, A.; Zhang, T.; et al. Mild redox-neutral depolymerization of lignin with a binuclear rh complex in water. ACS Catal. 2019, 9, 4441–4447. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Miao, W.; Tang, W.; Xue, D.; Xiao, J.; Zhang, T.; Wang, C. Rhodium-terpyridine catalyzed redox-neutral depolymerization of lignin in water. Green Chem. 2020, 22, 33–38. [Google Scholar] [CrossRef]
- Liao, Y.; Koelewijn, S.-F.; Van den Bossche, G.; Van Aelst, J.; Van den Bosch, S.; Renders, T.; Navare, K.; Nicolaï, T.; Van Aelst, K.; Maesen, M.; et al. A sustainable wood biorefinery for low–carbon footprint chemicals production. Science 2020, 367, 1385–1390. [Google Scholar] [CrossRef]
- Sels, B.; Renders, T.; Cooreman, E.; Van den Bosch, S. Fractionation and depolymerisation of lignocellulosic material. International Application No. PCT/EP2019/066887, 2 January 2020. [Google Scholar]
- De Santi, A.; Galkin, M.V.; Lahive, C.W.; Deuss, P.J.; Barta, K. Lignin-first fractionation of softwood lignocellulose using a mild dimethyl carbonate and ethylene glycol organosolv process. ChemSusChem 2020, in press. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, K.; Xiao, L.-P.; Sun, R.-C.; Song, G. Total utilization of lignin and carbohydrates in eucalyptus grandis: An integrated biorefinery strategy towards phenolics, levulinic acid, and furfural. Biotechnol. Biofuels 2020, 13, 1–10. [Google Scholar] [CrossRef]
- Parto, S.G.; Jørgensen, E.K.; Christensen, J.M.; Pedersen, L.S.; Larsen, D.B.; Duus, J.Ø.; Jensen, A.D. Solvent assisted catalytic conversion of beech wood and organosolv lignin over NiMo/γ-Al2O3. Sustain. Energy Fuels 2020, 4, 1844–1854. [Google Scholar] [CrossRef]
- Ouyang, X.; Huang, X.; Boot, M.D.; Hensen, E.J.M. Efficient conversion of pine wood lignin to phenol. Chemsuschem 2020, 13, 1705–1709. [Google Scholar] [CrossRef] [PubMed]
- Subbotina, E.; Velty, A.; Samec, J.S.M.; Corma, A. Zeolite-assisted lignin-first fractionation of lignocellulose: Overcoming lignin recondensation via shape-selective catalysis. ChemSusChem 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Thornburg, N.E.; Pecha, M.B.; Brandner, D.G.; Reed, M.L.; Vermaas, J.V.; Michener, W.E.; Katahira, R.; Vinzant, T.B.; Foust, T.D.; Donohoe, B.S.; et al. Mesoscale reaction-diffusion phenomena governing lignin-first biomass fractionation. ChemSusChem 2020, in press. [Google Scholar] [CrossRef]
- Lourencon, T.V.; Greca, L.G.; Tarasov, D.; Borrega, M.; Tamminen, T.; Rojas, O.J.; Balakshin, M.Y. Lignin-first integrated hydrothermal treatment (HTT) and synthesis of low-cost biorefinery particles. ACS Sustain. Chem. Eng. 2020, 8, 1230–1239. [Google Scholar] [CrossRef]
- Xu, J.; Dai, L.; Gui, Y.; Yuan, L.; Zhang, C.; Lei, Y. Synergistic benefits from a lignin-first biorefinery of poplar via coupling acesulfamate ionic liquid followed by mild alkaline extraction. Bioresour. Technol. 2020, 303, 122888. [Google Scholar] [CrossRef] [PubMed]
- Nandiwale, K.Y.; Danby, A.M.; Ramanathan, A.; Chaudhari, R.V.; Motagamwala, A.H.; Dumesic, J.A.; Subramaniam, B. Enhanced acid-catalyzed lignin depolymerization in a continuous reactor with stable activity. ACS Sustain. Chem. Eng. 2020, 8, 4096–4106. [Google Scholar] [CrossRef]
- Tschulkow, M.; Compernolle, T.; Van den Bosch, S.; Van Aelst, J.; Storms, I.; Van Dael, M.; Van den Bossche, G.; Sels, B.; Van Passel, S. Integrated techno-economic assessment of a biorefinery process: The high-end valorization of the lignocellulosic fraction in wood streams. J. Clean. Prod. 2020, 266, 122022. [Google Scholar] [CrossRef]
| Entry | Year | Feedstock | Catalyst | Solvent | Reaction Conditions | Yield of Main Products | Lignin Monomer | Reference | ||
|---|---|---|---|---|---|---|---|---|---|---|
| T (°C) | p (bar) | t(h) | wt % | Yield (wt %) | ||||||
| 1 | 1940 | maple, spruce | Cu2Cr2O5 | dioxane | 250 | 333 (H2) | 12 | 4-propylcyclohexanol, 4-propylcyclohexanediol | [22] | |
| 2 | 1941 | maple, spruce | Cu2Cr2O5 | dioxane | 280 | H2 | 10 | 4-propylcyclohexanol | 36 | [23] |
| 3 | 1943 | maple | Cu2Cr2O5 | dioxane | 280 | H2 | 20 | 4-propylcyclohexanol, 3-cyclohexyl-1-propanol | 40 | [24] |
| 4 | 1948 | maple | Raney-Ni + NaOH | dioxane/H2O (1:1) | 173 | 210 (H2) | 6 | 15% 4-ethylsyringol, 6% 4-ethanolsyringol | 27 | [25] |
| 5 | 1963 | aspen | Raney-Ni | dioxane/H2O (1:1) | 220 | 35 (H2) | 5 | 29% 4-propylsyringol, 13% 4-propanolsyringol | 59 | [26] |
| 6 | 1966 | spruce | Pd/C | dioxane/H2O (1:1) | 195 | 35 (H2) | 10 | 17% 4-propanolguaiacol | 17 | [27] |
| 7 | 1969 | spruce | Rh/C | dioxane/H2O (1:1) | 195 | 35 (H2) | 5 | 11% 4-propanolguaiacol | 34 | [28] |
| 8 | 1970 | spruce | Raney-Ni | dioxane/H2O (1:1) | 195 | 35 (H2) | 5 | 17% 4-propanol-cyclohexanol | 17 | [29] |
| 9 | 1978 | aspen | Rh/C | dioxane/H2O (1:1) | 195 | 34(H2) | 5 | 26% 4-propanolsyringol, 13% 4-propylsyringol | 50 | [30] |
| 10 | 1978 | spruce | Rh/C | dioxane/H2O (1:1) | 195 | 35(H2) | 5 | 21% 4-propanolguaiacol | 21 | [31] |
| 11 | 1986 | aspen poplar | Rh/C | dioxane/H2O (1:1) | 195 | 35(H2) | 5 | 21% 4-propanolguaiacol | 21 | [32] |
| 12 | 1993 | rice husks | polyvalent metals | dioxane | 250 | 50(H2) | 2 | 33% 4-propylsyringol | 33 | [33] |
| 13 | 2008 | birch | H3PO4 + Pt/C | dioxane/H2O (1:1) | 200 | 40(H2) | 4 | 21% 4-propylsyringol, 15% 4-propanolsyringol | 46 | [34] |
| 14 | 2011 | pine | Pd/C | dioxane/H2O (1:1) | 195 | 35(H2) | 24 | 21% 4-propanolguaiacol | 22 | [35] |
| 15 | 2012 | birch | Ni-W2C/C | ethylene glycol | 235 | 60(H2) | 4 | 18% 4-propylsyringol, 10% 4-propanolsyringol | 47 | [36] |
| 16 | 2013 | birch | Ni/C | MeOH | 200 | 1(Ar) | 6 | 36% 4-propylsyringol, 12% 4-propylguaiacol | 54 | [37] |
| 17 | 2014 | birch | Pd/C | EtOH/H2O (1:1) | 195 | 4(Ar) | 1 | 49% 4-propenylsyringol | 49 | [38] |
| 18 | 2014 | poplar | Raney-Ni | 2-PrOH/H2O (7:3) | 180 | autogenous | 3 | 4-propanolsyringol | 25 | [39] |
| 19 | 2015 | poplar | Zn-Pd/C | MeOH | 225 | 35(H2) | 12 | 30% 4-propylsyringol, 24% 4-propylguaiacol | 54 | [40] |
| 20 | 2015 | birch | Ru/C | MeOH | 250 | 30(H2) | 6 | 31% 4-propylsyringol, 10% 4-propylguaiacol | 52 | [41] |
| 21 | 2015 | lignocellulose | Ru/C + H4SiW12O40 | org.phase/H2O | 140–300 | 50(H2) | < 24 | n-hexane, cyclohexane, methylcyclopentane | 82 * | [42] |
| 22 | 2015 | corn stalk | Ru/C + LiTaMoO6 | H3PO4 | 230 | 60(H2) | 24 | 6% 4-ethylphenol | 24 | [43] |
| 23 | 2015 | birch | Pd/C | MeOH | 250 | 30(H2) | 3 | 35% propanolsyringol, 10% propanolguaiacol | 49 | [44] |
| 24 | 2015 | birch | Pd/C | ethylene glycol | 250 | 30(H2) | 3 | 35% propanolsyringol, 12% propanolguaiacol | 50 | [45] |
| 25 | 2015 | birch | Ni/C | MeOH | 200 | 2(N2) | 6 | 18% 4-propylsyringol, 10% 4-propylguaiacol | 32 | [46] |
| 26 | 2015 | cedar | H2SO4 | toluene/MeOH | 170 | 1(air) | 0.1 | 5% homovanillyl aldehyde dimethyl acetal | 10 | [47] |
| 27 | 2016 | poplar | Ru/C | MeOH | 250 | 40(H2) | 15 | 49% propylsyringol, 22% propanolsyringol | 78 * | [6] |
| 28 | 2016 | birch | Pd/C | EtOH/H2O (1:1) | 210 | 1(Ar) | 15 | 20% 4-propylsyringol, 11% 4-propenylsyringol | 36 | [48] |
| 29 | 2016 | poplar | Zn-Pd/C | MeOH | 225 | 35(H2) | 12 | 28% 4-propylsyringol, 14% 4-propylguaiacol | 43 | [49] |
| 30 | 2016 | miscanthus | Ni/C | MeOH | 225 | 60(H2) | 12 | 19% 4-propylsyringol, 21% 4-propylguaiacol | 68 | [50] |
| 31 | 2016 | poplar | (Rh(cod)Cl)2 + Sc(OTf)3 | Dioxane/H2O | 175 | 2 | 3% 4-propenylsyringol, 2% 4-methylguaiacol | 10 | [51] | |
| 32 | 2016 | birch | Pd/C + Yb(OTf)3 | MeOH | 200 | 30(H2) | 2 | 4-propanolsyringol, 4-propanolguaiacol | 48 | [52] |
| 33 | 2016 | beech | Ni/C | MeOH/H2O (3:2) | 200 | 60(H2) | 5 | 29% 4-propanolsyringol, 10% 4-propanolguaiacol | 51 | [53] |
| 34 | 2016 | corn stover | H3PO4 + Ni/C | MeOH | 200 | 30(H2) | 6 | 15% methyl coumarate, 15% methyl ferulate | 38 | [54] |
| 35 | 2016 | poplar | H3PO4 + Pd/C | MeOH | 200 | 20(H2) | 3 | 21% 4-propanolsyringol, 14% 4-propanolguaiacol | 42 | [55] |
| 36 | 2016 | poplar | Pd/C | MeOH/H2O (7:3) | 200 | 20(H2) | 3 | 23% 4-propanolsyringol, 13% 4-propanolguaiacol | 44 | [56] |
| 37 | 2017 | birch | Pd/C + Al(OTf)3 | MeOH | 180 | 30(H2) | 2 | 34% methoxypropylsyringol, 8% methoxypropylguaiacol | 55 | [57] |
| 38 | 2017 | oak | Pd/C + Al(OTf)3 | MeOH | 180 | 30(H2) | 2 | 12% methoxypropylsyringol, 10% propanolsyringol | 46 | [58] |
| 39 | 2017 | birch | NiFe/C | MeOH | 200 | 20(H2) | 6 | 24% 4-propylsyringol, 11% 4-propylguaiacol | 40 | [59] |
| 40 | 2017 | pinus radiata | Pd/C | Dioxane/H2O (1:1) | 195 | 34(H2) | 24 | 22% propanolguaiacol, 3% propylguaiacol | 78 + | [60] |
| 41 | 2017 | birch | Ni/Al2O3 | MeOH | 250 | 30(H2) | 3 | 21% propanolsyringol, 5% propylsyringol | 36 | [4] |
| 42 | 2017 | birch | H3PO4 + Pd/C | MeOH/H2O (7:3) | 180 | 30(H2) | 3 | 18% propanolsyringol, 11% propylsyringol | 37 | [61] |
| 43 | 2017 | poplar | Ni/C | MeOH | 190 | 60(H2) | 3 | 12% propylguaiacol and propylsyringol | 17 | [62] |
| 44 | 2018 | poplar | Ni/C | MeOH | 200 | 30(H2) | 1 | 8% propylsyringol, 5% propylguaiacol | 48 | [63] |
| 45 | 2018 | pine | Cu20PMO | MeOH | 220 | 40(H2) | 18 | 8% propanolguaiacol, 4% propylguaiacol | 13 | [64] |
| 46 | 2018 | birch | Ru/C + H4SiW12O40 | petrol/H2O | 220 | 50(H2) | 5 | 21% propanolsyringol, 5% propylsyringol | 39 | [65] |
| 47 | 2018 | poplar, spruce | Raney-Ni, Ni2P/SiO2 | 2-PrOH/H2O (7:3) | 180 | autogenous | 3 | 50–60% phenolic species | 20–25 | [66] |
| 48 | 2018 | poplar, spruce | Raney-Ni | 2-PrOH/H2O (7:3) | 200 | autogenous | 6 | [67] | ||
| 49 | 2018 | sorghum | Fenton | EtOH | 250 | autogenous | 12 | phenolic oil | 76 | [68] |
| 50 | 2018 | oak | Al(OTf)3 + Pd/C | MeOH | 160 | autogenous | 2 | 9% propylsyringol, 5% propylguaiacol | 25 | [69] |
| 51 | 2018 | cornstalk | Ru/C | H2O | 200 | 30(H2) | 8 | 67% ethylcyclohexane, 16% propylcyclohexane | 97 * | [70] |
| 52 | 2018 | birch | Pd/C+Yb(OTf)3 | MeOH | 250 | 20(H2) | 20 | lignin-oil | 83 * | [71] |
| 53 | 2018 | eucalyptus | Ru/C | BuOH/H2O (1:1) | 200 | 30(H2) | 2 | 41% propanolsyringol and propanolguaiacol | 49 | [72] |
| 54 | 2018 | bark | Pd/C | MeOH/H2O (2:1) | 200 | 2 | 3% ethylguaiacol | 42 | [73] | |
| 55 | 2018 | vanilla seeds | Ni/C | MeOH | 250 | 30(H2) | 3 | 18% propylcatechol, 3% propenylcatechol | 21 | [74] |
| 56 | 2018 | corncob | ZnMoO4/MCM-41 | MeOH | 220 | 30(H2) | 4 | 16% methyl coumarate, 13% methyl ferulate | 38 | [75] |
| 57 | 2018 | cork | Rh/C | 2-methyl tetrahydrofuran | 200 | 40(H2) | 4 | bio-oil | 43 | [76] |
| 58 | 2018 | apple wood | Ru/SiC | MeOH | 250 | 10(H2) | 3 | propylsyringol and ethylsyringol | 48 | [77] |
| 59 | 2018 | corn stover | MoS2 | MeOH | 20 | 30(H2) | 2 | 4% propylguaiacol, 3% ethylphenol | 18 | [78] |
| 60 | 2018 | birch | CdS | MeOH/H2O | r.t. | 1(N2) | 8 | 14% propanonesyringol, 7% propanoneguaiacol | 27 | [79] |
| 61 | 2019 | birch | Co-phen/C | EtOH/H2O (1:1) | 200 | autogenous | 4 | 10% 4-propylsyringol, 9% 4-propenylsyringol | 34 | [80] |
| 62 | 2019 | oak | Ni-Al/AC | HCOOH/EtOH/H2O | 190 | autogenous | 3 | 9% propylsyringol, 5% propylguaiacol | 23 x | [81] |
| 63 | 2019 | birch | Pt/Al2O3 | MeOH/H2O (1:2) | 230 | autogenous | 3 | 40% propylsyringol, 6% propylguaiacol | 49 | [82] |
| 64 | 2019 | poplar | Raney-Ni | 2-PrOH/H2O (7:3) | 200 | autogenous | 3 | 11% propanolsyringol, 10% propanolguaiacol | 34 | [83] |
| 65 | 2019 | poplar, spruce | Raney-Ni | 2-PrOH/H2O (7:3) | 220 | autogenous | 3 | 10% ethylsyringol, 7% propylsyringol | 36 | [84] |
| 66 | 2019 | poplar | Ni/C | MeOH | 225 | 35(H2) | 12 | propylsyringol, propylguaiacol | 90 | [85] |
| 67 | 2019 | apple wood | MoxC/CNT | MeOH | 250 | 10(H2) | 3 | propylsyringol, propylguaiacol | 42 | [86] |
| 68 | 2019 | eucalyptus | Ni@ZIF-8 | MeOH | 260 | 30(H2) | 8 | 24% propylsyringol + propylguaiacol | 44 | [87] |
| 69 | 2019 | basswood | binuclear Rh complex | NaOH/H2O | 110 | 1(Ar) | 24 | 2% propanonesyringol, 1.6% ethanonesyringol | 5 | [88] |
| 70 | 2020 | poplar | binuclear Rh complex | NaOH/H2O | 110 | 1(Ar) | 12 | 9% ethanonesyringol, 6% ethanoneguaiacol | 17 | [89] |
| 71 | 2020 | birch | Ru/C | MeOH | 235 | 30(H2) | 3 | 20% phenol, 9% propylene | 29 | [90] |
| 72 | 2020 | eucalyptus sawdust | Ru/C | BuOH/H2O (1:1) | 200 | 30(H2) | 2 | propanol-substituted phenolics | 49 | [91] |
| 73 | 2020 | pine | H2SO4 | dimethyl carbonate | 140 | autogenous | 0.67 | 8% G-C2-acetal | 9 | [92] |
| 74 | 2020 | eucalyptus | Pd/C | MeOH | 240 | 30(H2) | 4 | 32% propanolsyringol, 13% propanolguaiacol | 50 | [93] |
| 75 | 2020 | beech | NiMo/Al2O3 | EtOH | 260 | 26(H2) | 3 | 11% propylsyringol, 6% propylguaiacol | 20 | [94] |
| 76 | 2020 | pine | Pt/C | MeOH/H2O | 230 | 30(H2) | 10 mol% phenol | 15 | [95] | |
| 77 | 2020 | birch | H-BEA | EtOH/H2O | 220 | 2 | 20 | [96] | ||
| 78 | 2020 | poplar | 250 | autogenous | 0.33 | [97] | ||||
| 79 | 2020 | birch | H2O | 195 | [98] | |||||
| 80 | 2020 | poplar | emimAce | emimAce | 110 | 1(air) | 4 | 38% lignin | [99] | |
| 81 | 2020 | maple | Zr-KIT-5 | γ-valerolactone | 250 | 30(H2) | 18 | 3.5% 2-phenylpropan-2yl acetate, 1.6% 3,4-dimethoxyphenol | 7 | [100] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korányi, T.I.; Fridrich, B.; Pineda, A.; Barta, K. Development of ‘Lignin-First’ Approaches for the Valorization of Lignocellulosic Biomass. Molecules 2020, 25, 2815. https://doi.org/10.3390/molecules25122815
Korányi TI, Fridrich B, Pineda A, Barta K. Development of ‘Lignin-First’ Approaches for the Valorization of Lignocellulosic Biomass. Molecules. 2020; 25(12):2815. https://doi.org/10.3390/molecules25122815
Chicago/Turabian StyleKorányi, Tamás I., Bálint Fridrich, Antonio Pineda, and Katalin Barta. 2020. "Development of ‘Lignin-First’ Approaches for the Valorization of Lignocellulosic Biomass" Molecules 25, no. 12: 2815. https://doi.org/10.3390/molecules25122815
APA StyleKorányi, T. I., Fridrich, B., Pineda, A., & Barta, K. (2020). Development of ‘Lignin-First’ Approaches for the Valorization of Lignocellulosic Biomass. Molecules, 25(12), 2815. https://doi.org/10.3390/molecules25122815







