Fruit and Vegetable Peels: Utilization of High Value Horticultural Waste in Novel Industrial Applications
Abstract
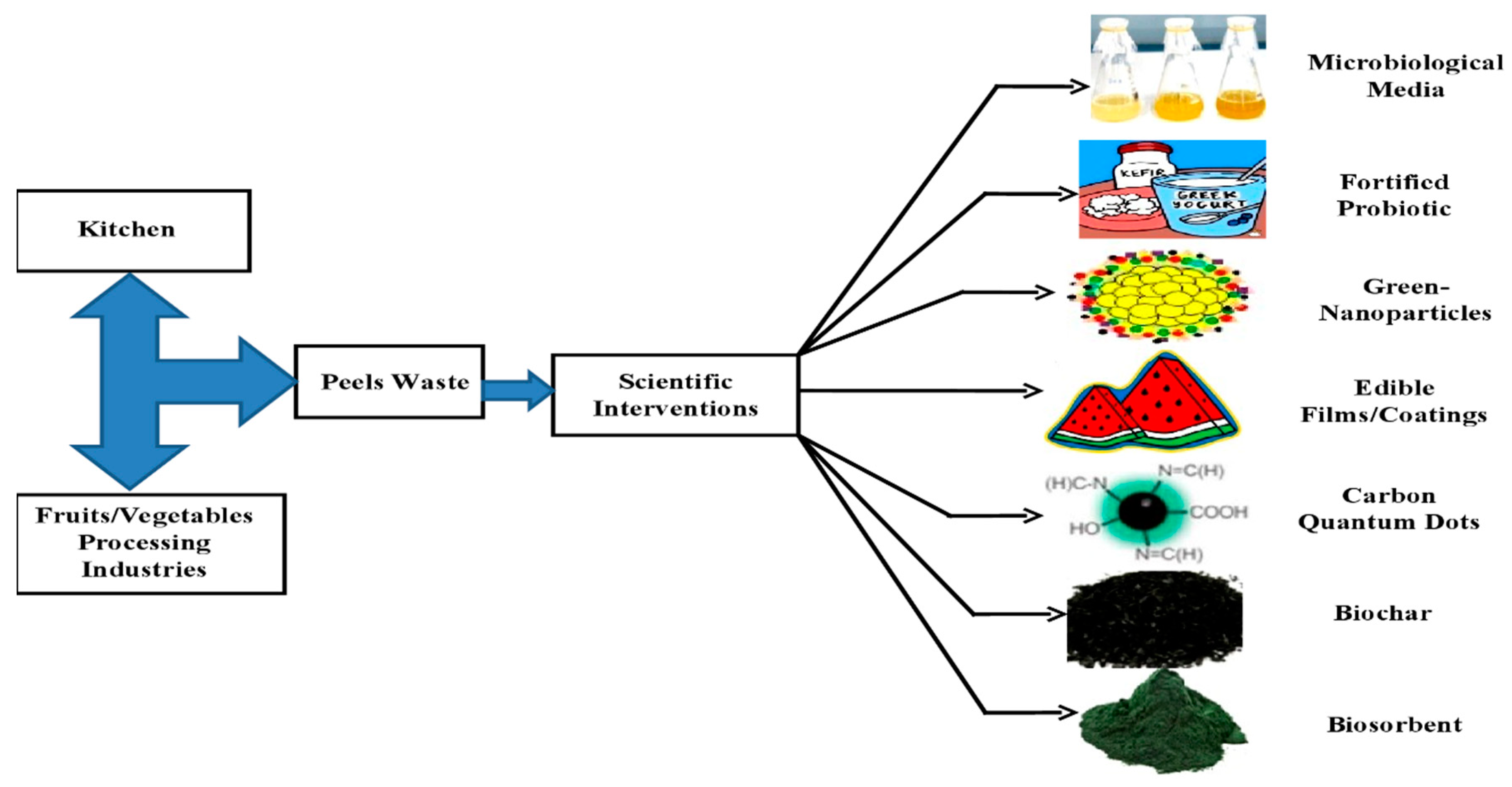
1. Introduction
2. Fruit and Vegetable Peel Based Edible Coatings/Films
3. Fruit and Vegetable Peel Fortified Probiotics
4. Fruit and Vegetable Peel-Derived Metallic Nanoparticles
5. Fruit and Vegetable Peel Derived Carbon Dots
6. Fruit and Vegetable Peel Based Microbiological Media
7. Fruit and Vegetable Peel Derived Biochar
8. Fruit and Vegetable Peel Derived Biosorbents
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Plazzotta, S.; Manzocco, L.; Nicoli, M.C. Fruit and vegetable waste management and the challenge of fresh-cut salad. Trends Food Sci. Technol. 2017, 63, 51–59. [Google Scholar] [CrossRef]
- National Academy of Agricultural Sciences. Saving the Harvest: Reducing the Food Loss and Waste; Policy Brief No. 5.; National Academy of Agricultural Sciences: New Delhi, India, 2019. Available online: http://naasindia.org/documents/Saving%20the%20Harvest.pdf (accessed on 8 October 2019).
- Chang, J.I.; Tsai, J.J.; Wu, K.H. Composting of vegetable waste. Waste Manag. Res. 2006, 24, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.K.; Mishra, S.S.; Kayitesi, E.; Ray, R.C. Microbial-processing of fruit and vegetable wastes for production of vital enzymes and organic acids: Biotechnology and scopes. Environ. Res. 2016, 146, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, C.M. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Rudra, S.G.; Nishad, J.; Jakhar, N.; Kaur, C. Food industry waste: Mine of nutraceuticals. Int. J. Sci. Environ. 2015, 4, 205–229. [Google Scholar]
- Gorinstein, S.; Martín-Belloso, O.; Park, Y.-S.; Haruenkit, R.; Lojek, A.; Ĉíž, M.; Caspi, A.; Libman, I.; Trakhtenberg, S. Comparison of some biochemical characteristics of different citrus fruits. Food Chem. 2001, 74, 309–315. [Google Scholar] [CrossRef]
- Soong, Y.-Y.; Barlow, P.J. Antioxidant activity and phenolic content of selected fruit seeds. Food Chem. 2004, 88, 411–417. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and Vegetable Waste: Bioactive Compounds, Their Extraction, and Possible Utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef]
- Siddiqui, A.; Salahuddin, T.; Riaz, A.; Zohra, R.R.; Naheed, S. Production of amylase from Bacillus sp. AY3 using fruit peels as substrate. FUUAST J. Biol. 2014, 4, 213–215. [Google Scholar]
- Santo, A.P.D.E.; Cartolano, N.S.; Silva, T.F.; Soares, F.A.S.D.M.; Gioielli, L.; Perego, P.; Converti, A.; De Oliveira, M.N. Fibers from fruit by-products enhance probiotic viability and fatty acid profile and increase CLA content in yoghurts. Int. J. Food Microbiol. 2012, 154, 135–144. [Google Scholar] [CrossRef]
- Shanmugavadivu, M.; Kuppusamy, S.; Ranjithkumar, R. Synthesis of pomegranate peel extract mediated silver nanoparticles and its antibacterial activity. Am. J. Adv. Drug. Deliv. 2014, 2, 174–182. [Google Scholar]
- Nasirifar, S.Z.; Maghsoudlou, A.; Oliyaei, N. Effect of active lipid-based coating incorporated with nanoclay and orange peel essential oil on physicochemical properties of Citrus sinensis. Food Sci. Nutr. 2018, 6, 1508–1518. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.-Y.; Li, L.-S.; Qin, S.; Zhang, Y.; Huang, K.; Xu, L. The synthesis of fluorescent carbon dots from mango peel and their multiple applications. Colloids Surfaces A: Physicochem. Eng. Asp. 2019, 577, 306–314. [Google Scholar] [CrossRef]
- Zhou, N.; Chen, H.; Xi, J.; Yao, D.; Zhou, Z.; Tian, Y.; Lu, X. Biochars with excellent Pb(II) adsorption property produced from fresh and dehydrated banana peels via hydrothermal carbonization. Bioresour. Technol. 2017, 232, 204–210. [Google Scholar] [CrossRef]
- Enniya, I.; Jourani, A. Study of Methylene Blue Removal by a biosorbent prepared with Apple peels. J. Mater. Environ. Sci. 2017, 8, 4573–4581. [Google Scholar] [CrossRef][Green Version]
- Zaragoza, M.D.L.L.Z.; González-Reza, R.M.; Mendoza-Munoz, N.; Miranda-Linares, V.; Bernal-Couoh, T.F.; Mendoza-Elvira, S.; Quintanar-Guerrero, D. Nanosystems in Edible Coatings: A Novel Strategy for Food Preservation. Int. J. Mol. Sci. 2018, 19, 705. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, Y.; Wang, Q. Antioxidant and antimicrobial properties of essential oils encapsulated in zein nanoparticles prepared by liquid–liquid dispersion method. LWT 2012, 48, 283–290. [Google Scholar] [CrossRef]
- Prakash, A.; Baskaran, R.; Vadivel, V. Citral nanoemulsion incorporated edible coating to extend the shelf life of fresh cut pineapples. LWT 2020, 118, 108851. [Google Scholar] [CrossRef]
- Raghav, P.K.; Agarwal, N.; Saini, M. Edible coating of fruits and vegetables: A review. Int. J. Sci. Res. Mod. Edu. 2016, 1, 188–204. [Google Scholar]
- Ullah, A.; Abbasi, N.; Shafique, M.; Qureshi, A.A. Influence of Edible Coatings on Biochemical Fruit Quality and Storage Life of Bell Pepper cv. “Yolo Wonder.”. J. Food Qual. 2017, 2017, 1–11. [Google Scholar] [CrossRef]
- Adukwu, E.; Allen, S.C.; Phillips, C.A. The anti-biofilm activity of lemongrass (Cymbopogon flexuosus) and grapefruit (Citrus paradisi) essential oils against five strains of Staphylococcus aureus. J. Appl. Microbiol. 2012, 113, 1217–1227. [Google Scholar] [CrossRef] [PubMed]
- Alparslan, Y.; Metin, C.; Yapıcı, H.H.; Baygar, T.; Günlü, A.; Baygar, T. Combined effect of orange peel essential oil and gelatin coating on the quality and shelf life of shrimps. J. Food Saf. Food Qual. 2017, 68, 69–78. [Google Scholar]
- Etxabide, A.; Urdanpilleta, M.; Gómez-Arriaran, I.; De La Caba, K.; Guerrero, P. Effect of pH and lactose on cross-linking extension and structure of fish gelatin films. React. Funct. Polym. 2017, 117, 140–146. [Google Scholar] [CrossRef]
- Hanani, Z.A.N.; Yee, F.C.; Nor-Khaizura, M. Effect of pomegranate (Punica granatum L.) peel powder on the antioxidant and antimicrobial properties of fish gelatin films as active packaging. Food Hydrocoll. 2019, 89, 253–259. [Google Scholar] [CrossRef]
- Shin, S.-H.; Chang, Y.; Lacroix, M.; Han, J. Control of microbial growth and lipid oxidation on beef product using an apple peel-based edible coating treatment. LWT 2017, 84, 183–188. [Google Scholar] [CrossRef]
- Al-Anbari, I.H.; Dakhel, A.M.; Adnan, A. The effect of adding local orange peel powder to microbial inhibition and oxidative reaction within edible film component. Plant Arc. 2019, 19, 1006–1012. [Google Scholar]
- Moghadam, M.; Salami, M.; Mohammadian, M.; Khodadadi, M.; Emam-Djomeh, Z. Development of antioxidant edible films based on mung bean protein enriched with pomegranate peel. Food Hydrocoll. 2020. [Google Scholar] [CrossRef]
- Tammineni, N.; Ünlü, G.; Min, S.C. Development of antimicrobial potato peel waste-based edible films with oregano essential oil to inhibit Listeria monocytogenes on cold-smoked salmon. Int. J. Food Sci. Tech. 2013, 48, 211–214. [Google Scholar] [CrossRef]
- Alparslan, Y.; Baygar, T. Effect of Chitosan Film Coating Combined with Orange Peel Essential Oil on the Shelf Life of Deepwater Pink Shrimp. Food Bioprocess Technol. 2017, 10, 842–853. [Google Scholar] [CrossRef]
- Rahmawati, D.; Chandra, M.; Santoso, S.; Puteri, M.G. Application of lemon peel essential oil with edible coating agent to prolong shelf life of tofu and strawberry. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2017. [Google Scholar] [CrossRef]
- Radi, M.; Akhavan, H.-R.; Amiri, S.; Akhavan-Darabi, S. The use of orange peel essential oil microemulsion and nanoemulsion in pectin-based coating to extend the shelf life of fresh-cut orange. J. Food Process. Preserv. 2017. [Google Scholar] [CrossRef]
- Borah, P.P.; Das, P.; Badwaik, L.S. Ultrasound treated potato peel and sweet lime pomace based biopolymer film development. Ultrason. Sonochem. 2017, 36, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Hanani, Z.A.N.; Husna, A.A.; Syahida, S.N.; Khaizura, M.N.; Jamilah, B. Effect of different fruit peels on the functional properties of gelatin/polyethylene bilayer films for active packaging. Food Packag. Shelf Life 2018, 18, 201–211. [Google Scholar] [CrossRef]
- Abdel-Hamid, M.; Romeih, E.; Huang, Z.; Enomoto, T.; Huang, L.; Li, L. Bioactive properties of probiotic set-yogurt supplemented with Siraitia grosvenorii fruit extract. Food Chem. 2019. [Google Scholar] [CrossRef]
- Cerezal, P.; Duarte, G. Use of skin in the elaboration of concentrated products of cactus pear (Opuntia ficus-indica (L.) Miller). J. Prof. Assoc. Cactus. 2005, 7, 61–83. [Google Scholar]
- Crizel, T.D.M.; Jablonski, A.; Rios, A.D.O.; Rech, R.; Flôres, S.H. Dietary fiber from orange byproducts as a potential fat replacer. LWT 2013, 53, 9–14. [Google Scholar] [CrossRef]
- Chan, C.-L.; Gan, R.-Y.; Shah, N.P.; Corke, H. Enhancing antioxidant capacity of Lactobacillus acidophilus-fermented milk fortified with pomegranate peel extracts. Food Biosci. 2018, 26, 185–192. [Google Scholar] [CrossRef]
- Coelho, E.M.; Souza, M.E.; Corrêa, L.C.; Viana, A.C.; De Azevêdo, L.C.; Lima, M.D.S. Bioactive Compounds and Antioxidant Activity of Mango Peel Liqueurs (Mangifera indica L.) Produced by Different Methods of Maceration. Antioxidants 2019, 8, 102. [Google Scholar] [CrossRef]
- Drago, L. Probiotics and Colon Cancer. Microorganisms 2019, 7, 66. [Google Scholar] [CrossRef]
- Sah, B.N.P.; Vasiljevic, T.; McKechnie, S.; Donkor, O. Effect of pineapple waste powder on probiotic growth, antioxidant and antimutagenic activities of yogurt. J. Food Sci. Technol. 2015, 53, 1698–1708. [Google Scholar] [CrossRef]
- Vicenssuto, G.M.; De Castro, R.J.S. Development of a novel probiotic milk product with enhanced antioxidant properties using mango peel as a fermentation substrate. Biocatal. Agric. Biotechnol. 2020, 24, 101564. [Google Scholar] [CrossRef]
- Pgi, D.; Jwa, S.; Rmusk, R. Formulation and development of composite fruit peel powder incorporated fat and sugar-free probiotic set yogurt. GSC Boil. Pharm. Sci. 2020, 11, 93–99. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Panwar, J.; Yun, Y.-S. Biogenic Synthesis of Metallic Nanoparticles by Plant Extracts. ACS Sustain. Chem. Eng. 2013, 1, 591–602. [Google Scholar] [CrossRef]
- Ghosh, P.R.; Fawcett, D.; Sharma, S.B.; Poinern, G.E.J. Production of High-Value Nanoparticles via Biogenic Processes Using Aquacultural and Horticultural Food Waste. Materials 2017, 10, 852. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Fawcett, D.; Sharma, S.; Tripathy, S.K.; Poinern, G.E.J. Green Synthesis of Metallic Nanoparticles via Biological Entities. Materials 2015, 8, 7278–7308. [Google Scholar] [CrossRef]
- Fawcett, D.; Verduin, J.; Shah, M.; Sharma, S.B.; Poinern, G.E.J. A Review of Current Research into the Biogenic Synthesis of Metal and Metal Oxide Nanoparticles via Marine Algae and Seagrasses. J. Nanosci. 2017, 2017, 1–15. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Thakkar, K.; Mhatre, S.S.; Parikh, R.Y. Biological synthesis of metallic nanoparticles. Nanomed. Nanotech. Boil. Med. 2010, 6, 257–262. [Google Scholar] [CrossRef]
- Kumar, H.; Bhardwaj, K.; Kuca, K.; Kalia, A.; Nepovimova, E.; Verma, R.; Kumar, D. Flower-Based Green Synthesis of Metallic Nanoparticles: Applications beyond Fragrance. Nanomaterials 2020, 10, 766. [Google Scholar] [CrossRef]
- Naganathan, K.; Thirunavukkarasu, S. Green way genesis of silver nanoparticles using multiple fruit peels waste and its antimicrobial, anti-oxidant and anti-tumor cell line studies. In Proceedings of the IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017; Volume 191, p. 12009. [Google Scholar]
- Shet, A.R.; Tantri, S.; Bennal, A. Economical biosynthesis of silver nanoparticles using fruit waste. J. Chem. Pharm. Sci. 2016, 9, 2306–2311. [Google Scholar]
- Reenaa, M.; Menon, A.S. Synthesis of Silver Nanoparticles from Different Citrus Fruit Peel Extracts and a Comparative Analysis on its Antibacterial Activity. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 2358–2365. [Google Scholar] [CrossRef]
- Skiba, M.I.; Vorobyova, V.I. Synthesis of Silver Nanoparticles Using Orange Peel Extract Prepared by Plasmochemical Extraction Method and Degradation of Methylene Blue under Solar Irradiation. Adv. Mater. Sci. Eng. 2019, 2019, 1–8. [Google Scholar] [CrossRef]
- Samreen, F.G.; Muzaffar, R.; Nawaz, M.; Gul, S.; Basra, M.A.R. Synthesis, Characterization and Anti-Microbial Activity of Citrus limon Mediated Nanoparticles. Preprints 2018, 2018110417. [Google Scholar] [CrossRef]
- Ibrahim, H.M.M. Green synthesis and characterization of silvernanoparticles using banana peel extract and their antimicrobial activity against representative microorganisms. J. Radiat. Res. Appl. Sci. 2015, 8, 265–275. [Google Scholar] [CrossRef]
- Devanesan, S.; AlSalhi, M.; Balaji, R.V.; A Ranjitsingh, A.J.; Ahamed, A.; Alfuraydi, A.; Alqahtani, F.Y.; Aleanizy, F.S.; Othman, A.H. Antimicrobial and Cytotoxicity Effects of Synthesized Silver Nanoparticles from Punica granatum Peel Extract. Nanoscale Res. Lett. 2018, 13, 315. [Google Scholar] [CrossRef] [PubMed]
- Ajmal, N.; Saraswat, K.; Sharma, V.; Zafar, M.E. Synthesis and antibacterial activity of silver nanoparticles from Prunus armeniaca (Apricot) fruit peel extract. Bull. Environ. Pharmacol. Life Sci. 2016, 5, 91–94. [Google Scholar]
- Kokila, T.; Ramesh, P.S.; Geetha, D. Biosynthesis of silver nanoparticles from Cavendish banana peel extract and its antibacterial and free radical scavenging assay: A novel biological approach. Appl. Nanosci. 2015, 5, 911–920. [Google Scholar] [CrossRef]
- Barros, C.H.N.; Cruz, G.C.F.; Mayrink, W.; Tasic, L. Bio-based synthesis of silver nanoparticles from orange waste: Effects of distinct biomolecule coatings on size, morphology, and antimicrobial activity. Nanotechnol. Sci. Appl. 2018, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Nava, O.; Soto-Robles, C.; Gómez-Gutiérrez, C.; Vilchis-Nestor, A.; Castro-Beltrán, A.; Olivas, A.; Morales, P.L. Fruit peel extract mediated green synthesis of zinc oxide nanoparticles. J. Mol. Struct. 2017, 1147, 1–6. [Google Scholar] [CrossRef]
- Das, G.; Patra, J.K.; Basavegowda, N.; Vishnuprasad, C.N.; Shin, H.-S. Comparative study on antidiabetic, cytotoxicity, antioxidant and antibacterial properties of biosynthesized silver nanoparticles using outer peels of two varieties of Ipomoea batatas (L.) Lam. Int. J. Nanomed. 2019, 14, 4741–4754. [Google Scholar] [CrossRef]
- Bhuvaneswari, S.; Subashini, G.; Subramaniyam, S. Green synthesis of zinc oxide nanoparticles using potato peel and degradation of textile mill effluent by photocatalytic activity. World J. Pharm. Res. 2017, 6, 774–785. [Google Scholar] [CrossRef]
- Patra, J.K.; Kwon, Y.; Baek, K.-H. Green biosynthesis of gold nanoparticles by onion peel extract: Synthesis, characterization and biological activities. Adv. Powder Technol. 2016, 27, 2204–2213. [Google Scholar] [CrossRef]
- Kumar, V.; Verma, S.; Choudhury, S.; Tyagi, M.; Chatterjee, S.; Variyar, P.S. Biocompatible silver nanoparticles from vegetable waste: Its characterization and bio-efficacy. Int. J. Nanomater. Sci. 2015, 4, 70–86. [Google Scholar]
- Tamileswari, R.; Nisha, M.H.; Jesurani, S.; Kanagesan, S.; Hashim, M.; Catherine, S.; Alexander, P. Synthesis of silver nanoparticles using the vegetable extract of Raphanus sativus (radish) and assessment of their antibacterial activity. Int. J. Adv. Technol. Eng. Sci. 2015, 3, 207–212. [Google Scholar]
- Fan, H.; Zhang, M.; Bhandari, B.; Yang, C.-H. Food waste as a carbon source in carbon quantum dots technology and their applications in food safety detection. Trends Food Sci. Technol. 2020, 95, 86–96. [Google Scholar] [CrossRef]
- Kumar, H.; Kuča, K.; Bhatia, S.K.; Saini, K.; Kaushal, A.; Verma, R.; Bhalla, T.C.; Kumar, D. Applications of Nanotechnology in Sensor-Based Detection of Foodborne Pathogens. Sensors 2020, 20, 1966. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, A. Carbon quantum dots: Synthesis, properties and applications. J. Mater. Chem. C 2014, 2, 6921. [Google Scholar] [CrossRef]
- Ajila, C.; Bhat, S.; Rao, U.P. Valuable components of raw and ripe peels from two Indian mango varieties. Food Chem. 2007, 102, 1006–1011. [Google Scholar] [CrossRef]
- Rattanapoltee, P.; Kaewkannetra, P. Utilization of Agricultural Residues of Pineapple Peels and Sugarcane Bagasse as Cost-Saving Raw Materials in Scenedesmus acutus for Lipid Accumulation and Biodiesel Production. Appl. Biochem. Biotechnol. 2014, 173, 1495–1510. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Saura-Calixto, F. Fruit peels as sources of non-extractable polyphenols or macromolecular antioxidants: Analysis and nutritional implications. Food Res. Int. 2018, 111, 148–152. [Google Scholar] [CrossRef]
- Cao, L.; Meziani, M.J.; Sahu, S.; Sun, Y.-P. Photoluminescence Properties of Graphene versus Other Carbon Nanomaterials. Accounts Chem. Res. 2012, 46, 171–180. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Green synthesis, biomedical and biotechnological applications of carbon and graphene quantum dots. A review. Environ. Chem. Lett. 2020, 18, 703–727. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.; Tripathi, K.M.; Choudhary, S.; Singh, N.; Gupta, R.K. Green synthesis of carbon quantum dots from lemon peel waste: Applications in sensing and photocatalysis. RSC Adv. 2016, 6, 72423–72432. [Google Scholar] [CrossRef]
- Huang, C.-C.; Hung, Y.-S.; Weng, Y.-M.; Chen, W.; Lai, Y.-S. Sustainable development of carbon nanodots technology: Natural products as a carbon source and applications to food safety. Trends Food Sci. Technol. 2019, 86, 144–152. [Google Scholar] [CrossRef]
- Vandarkuzhali, S.A.A.; Natarajan, S.; Jeyabalan, S.; Sivaraman, G.; Singaravadivel, S.; Muthusubramanian, S.; Viswanathan, B. Pineapple Peel-Derived Carbon Dots: Applications as Sensor, Molecular Keypad Lock, and Memory Device. ACS Omega 2018, 3, 12584–12592. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Ghosal, K.; Mohammad, S.A.; Sarkar, K. Dendrimer functionalized carbon quantum dot for selective detection of breast cancer and gene therapy. Chem. Eng. J. 2019, 373, 468–484. [Google Scholar] [CrossRef]
- Huang, Q.; Lin, X.; Zhu, J.J.; Tong, Q.X. Pd-Au@ carbon dots nanocomposite: Facile synthesis and application as an ultrasensitive electrochemical biosensor for determination of colitoxin DNA in human serum. Biosens. Bioelectron. 2017, 94, 507–512. [Google Scholar] [CrossRef]
- Lu, W.; Qin, X.; Liu, S.; Chang, G.; Zhang, Y.; Luo, Y.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. Economical, Green Synthesis of Fluorescent Carbon Nanoparticles and Their Use as Probes for Sensitive and Selective Detection of Mercury(II) Ions. Anal. Chem. 2012, 84, 5351–5357. [Google Scholar] [CrossRef]
- Xiao, P.; Ke, Y.; Lu, J.; Huang, Z.; Zhu, X.; Wei, B.; Huang, L. Photoluminescence immunoassay based on grapefruit peel-extracted carbon quantum dots encapsulated into silica nanospheres for p53 protein. Biochem. Eng. J. 2018, 139, 109–116. [Google Scholar] [CrossRef]
- Bankoti, K.; Rameshbabu, A.P.; Datta, S.; Das, B.; Mitra, A.; Dhara, S. Onion derived carbon nanodots for live cell imaging and accelerated skin wound healing. J. Mater. Chem. B 2017, 5, 6579–6592. [Google Scholar] [CrossRef]
- Zhou, J.; Sheng, Z.; Han, H.-Y.; Zhou, M.; Li, C. Facile synthesis of fluorescent carbon dots using watermelon peel as a carbon source. Mater. Lett. 2012, 66, 222–224. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Kasouni, A.; Sygellou, L.; Avgeropoulos, A.; Troganis, A.; Stalikas, C. Two of a kind but different: Luminescent carbon quantum dots from Citrus peels for iron and tartrazine sensing and cell imaging. Talanta 2017, 175, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Prasannan, A.; Imae, T. One-Pot Synthesis of Fluorescent Carbon Dots from Orange Waste Peels. Ind. Eng. Chem. Res. 2013, 52, 15673–15678. [Google Scholar] [CrossRef]
- Aji, M.P.; Susanto; Wiguna, P.A. Sulhadi Facile synthesis of luminescent carbon dots from mangosteen peel by pyrolysis method. J. Theor. Appl. Phys. 2017, 22, 119–126. [Google Scholar] [CrossRef]
- Yadav, H.A.; Eraiah, B.; Basavaraj, R.; Nagabhushana, H.; Darshan, G.; Sharma, S.; Prasad, B.D.; Nithya, R.; Shanthi, S. Rapid synthesis of C-dot@TiO2 core-shell composite labeling agent: Probing of complex fingerprints recovery in fresh water. J. Alloy Compd. 2018, 742, 1006–1018. [Google Scholar] [CrossRef]
- Vikneswaran, R.; Ramesh, S.; Yahya, R. Green synthesized carbon nanodots as a fluorescent probe for selective and sensitive detection of iron(III) ions. Mater. Lett. 2014, 136, 179–182. [Google Scholar] [CrossRef]
- Jadhav, P.; Sonne, M.; Kadam, A.; Patil, S.; Dahigaonkar, K.; Oberoi, J.K. Formulation of cost effective alternative bacterial culture media using fruit and vegetables waste. Int. J. Curr. Res. Rev. 2018, 10, 6–15. [Google Scholar]
- Basu, S.; Bose, C.; Ojha, N.; Das, N.; Das, J.; Pal, M.; Khurana, S. Evolution of bacterial and fungal growth media. Bioinformation 2015, 11, 182–184. [Google Scholar] [CrossRef]
- Deivanayaki, M.; Iruthayaraj, A.P. Alternative vegetable nutrient source for microbial growth. Inter. J. Biosci. 2012, 2, 47–51. [Google Scholar]
- Wasas, A.D.; Huebner, R.E.; Klugman, K.P. Use of dorset egg medium for maintenance and transport of Neisseria meningitides and Haemophilus influenzae Type b. J. Clin. Microbiol. 1999, 37, 2045–2046. [Google Scholar] [CrossRef]
- Tijani, I.D.R.; Jamal, P.; Alam, M.; Mirghani, M. Optimization of cassava peel medium to an enriched animal feed by the white rot fungi Panus tigrinus M609RQY. Int. Food Res. J. 2012, 19, 427–432. [Google Scholar]
- Jamal, P.; Saheed, O.K.; Kari, M.I.A.; Alam, Z.; Muyibi, S.A. Cellulolytic Fruits Wastes: A Potential Support for Enzyme Assisted Protein Production. J. Boil. Sci. 2013, 13, 379–385. [Google Scholar] [CrossRef]
- Kahraman, S.; Gurdal, I.H. Effect of synthetic and natural culture media on laccase production by white rot fungi. Bioresour. Technol. 2002, 82, 215–217. [Google Scholar] [CrossRef]
- Milala, M.; Shugaba, A.; Gidado, A.; Ene, A.; Wafar, J. Studies on the use of agricultural wastes for cellulase enzyme production by Aspergillus niger. Res. J. Agric. Biol. Sci. 2005, 1, 325–328. [Google Scholar]
- Putri, C.H.; Janica, L.; Jannah, M.; Ariana, P.P. Utilization of dragon fruit peel waste as microbial growth media. In Proceedings of the 10th CISAK, Daejeon, Korea, July 2017; pp. 91–95. [Google Scholar]
- Kindo, A.; Tupaki-Sreepurna, A.; Yuvaraj, M. Banana peel culture as an indigenous medium for easy identification of late-sporulation human fungal pathogens. Indian J. Med. Microbiol. 2016, 34, 457. [Google Scholar] [CrossRef] [PubMed]
- Hasanin, M.; Hashem, A.H. Eco-friendly, economic fungal universal medium from watermelon peel waste. J. Microbiol. Methods 2020, 168, 105802. [Google Scholar] [CrossRef]
- Carota, E.; Petruccioli, M.; D’Annibale, A.; Gallo, A.M.; Crognale, S. Orange peel waste–based liquid medium for biodiesel production by oleaginous yeasts. Appl. Microbiol. Biotechnol. 2020, 104, 4617–4628. [Google Scholar] [CrossRef]
- Verma, N.; Kumar, V.; Bansal, M. Utility of Luffa cylindrica and Litchi chinensis peel, an agricultural waste biomass in cellulase production by Trichoderma reesei under solid state cultivation. Biocatal. Agric. Biotechnol. 2018, 16, 483–492. [Google Scholar] [CrossRef]
- Verma, N.; Bansal, M.C.; Kumar, V. Pea peel waste: A lignocellulosic waste and its utility in cellulase production by Trichoderma reesei under solid state cultivation. Bioresources 2011, 6, 1505–1519. [Google Scholar]
- Kadam, A.; Patil, S.; Sonne, M.; Dahigaonkar, K.; Oberoi, J.K.; Jadhav, P. Cost effective alternative fungal culture media formulation using fruit and vegetables waste. Int. J. Curr. Res. 2017, 9, 56887–56893. [Google Scholar]
- Bruno, G.; Mike, P.; Christelle, B.; Goodspeed, K. Biochar is carbon negative. Nature Geosci. 2009, 2, 2. [Google Scholar] [CrossRef]
- Oh, J.-I.; Lee, J.; Lee, T.; Ok, Y.S.; Lee, S.-R.; Kwon, E.E. Strategic CO 2 utilization for shifting carbon distribution from pyrolytic oil to syngas in pyrolysis of food waste. J. CO2 Util. 2017, 20, 150–155. [Google Scholar] [CrossRef]
- Carmona-Cabello, M.; Garcia, I.L.; Leiva-Candia, D.; Dorado, M.P. Valorization of food waste based on its composition through the concept of biorefinery. Curr. Opin. Green Sustain. Chem. 2018, 14, 67–79. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, F.-S. Removal of lead from water using biochars prepared from hydrothermal liquefaction of biomass. J. Hazard Mater. 2009, 167, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Inyang, M.; Gao, B.; Yao, Y.; Xue, Y.; Zimmerman, A.R.; Pullammanappallil, P.; Cao, X. Removal of heavy metals from aqueous solution by biochars derived from anaerobically digested biomass. Bioresour. Technol. 2012, 110, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Cao, X.; Zhao, L. Comparison of rice husk- and dairy manure-derived biochars for simultaneously removing heavy metals from aqueous solutions: Role of mineral components in biochars. Chemosphere 2013, 92, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Ma, L.; Gao, B.; Harris, W. Dairy-manure derived biochar effectively sorbs lead and atrazine. Environ. Sci. Technol. 2009, 43, 3285–3291. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Gao, B.; Inyang, M.; Zimmerman, A.R.; Cao, X.; Pullammanappallil, P.; Yang, L. Biochar derived from anaerobically digested sugar beet tailings: Characterization and phosphate removal potential. Bioresour. Technol. 2011, 102, 6273–6278. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.S.; Liew, R.K.; Cheng, C.-K.; Rasit, N.; Ooi, C.K.; Ma, N.L.; Ng, J.-H.; Lam, W.H.; Chong, C.T.; Chase, H.A. Pyrolysis production of fruit peel biochar for potential use in treatment of palm oil mill effluent. J. Environ. Manag. 2018, 213, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wu, Y.; Cha, L. Removal of methyl orange dye using activated biochar derived from pomelo peel wastes: Performance, isotherm, and kinetic studies. J. Dispers. Sci. Technol. 2019, 41, 125–136. [Google Scholar] [CrossRef]
- Wang, C.; Gu, L.; Liu, X.; Zhang, X.; Cao, L.; Hu, X. Sorption behavior of Cr(VI) on pineapple-peel-derived biochar and the influence of coexisting pyrene. Int. Biodeterior. Biodegradation 2016, 111, 78–84. [Google Scholar] [CrossRef]
- Fu, B.; Ge, C.; Yue, L.; Luo, J.; Feng, D.; Deng, H.; Yu, H. Characterization of Biochar Derived from Pineapple Peel Waste and Its Application for Sorption of Oxytetracycline from Aqueous Solution. Bioresouces 2016, 11, 9017–9035. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, X.; Ngo, H.H.; Guo, W.; Wen, H.; Li, C.; Zhang, Y.; Ma, C. Comparison study on the ammonium adsorption of the biochars derived from different kinds of fruit peel. Sci. Total Environ. 2020, 707, 135544. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Cha, L.; Fane, Y.; Fang, P.; Ming, Z.; Sha, H. Activated Biochar Prepared by Pomelo Peel Using H3PO4 for the Adsorption of Hexavalent Chromium: Performance and Mechanism. Water Air Soil Pollut. 2017, 228, 405. [Google Scholar] [CrossRef]
- Wu, J.; Yang, J.; Feng, P.; Huang, G.; Xu, C.; Lin, B. High-efficiency removal of dyes from wastewater by fully recycling litchi peel biochar. Chemosphere 2020, 246, 125734. [Google Scholar] [CrossRef] [PubMed]
- Selvanathan, M.; Yann, K.T.; Chung, C.H.; Selvarajoo, A.; Arumugasamy, S.K.; Sethu, V. Adsorption of copper(II) ion from aqueous solution using biochar derived from rambutan (Nephelium lappaceum) peel: Feedforward neural network modelling study. Water Air Soil Pollut. 2017. [Google Scholar] [CrossRef]
- Cao, Q.; Huang, Z.; Liu, S.; Wu, Y. Potential of Punica granatum biochar to adsorb Cu(II) in soil. Sci. Rep. 2019. [Google Scholar] [CrossRef]
- Shakya, A.; Núñez-Delgado, A.; Agarwal, T. Biochar synthesis from sweet lime peel for hexavalent chromium remediation from aqueous solution. J. Environ. Manag. 2019. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, G.; Zhang, L.; Sun, Z. Preparation of high performance H 2 S removal biochar by direct fluidized bed carbonization using potato peel waste. Process. Saf. Environ. Prot. 2017, 107, 281–288. [Google Scholar] [CrossRef]
- Niazi, N.K.; Murtaza, B.; Bibi, I.; Shahid, M.; White, J.; Nawaz, M.; Bashir, S.; Shakoor, M.; Choppala, G.; Murtaza, G.; et al. Removal and Recovery of Metals by Biosorbents and Biochars Derived From Biowastes. In Environmental Materials and Waste; Elsevier BV: Amsterdam, The Netherlands, 2016; pp. 149–177. [Google Scholar]
- Vijayaraghavan, K.; Yun, Y.-S. Bacterial biosorbents and biosorption. Biotechnol. Adv. 2008, 26, 266–291. [Google Scholar] [CrossRef]
- Wang, J.; Chen, C. Biosorbents for heavy metals removal and their future. Biotechnol. Adv. 2009, 27, 195–226. [Google Scholar] [CrossRef]
- Park, N.; Yun, Y.-S.; Park, J.M. The past, present, and future trends of biosorption. Biotechnol. Bioprocess Eng. 2010, 15, 86–102. [Google Scholar] [CrossRef]
- Abdi, O.; Kazemi, M. A review study of biosorption of heavy metals and comparison between different biosorbents. J. Mater. Environ. Sci. 2015, 6, 1386–1399. [Google Scholar]
- Hameed, B.; Ahmad, A. Batch adsorption of methylene blue from aqueous solution by garlic peel, an agricultural waste biomass. J. Hazard. Mater. 2009, 164, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Krishni, R.; Foo, K.Y.; Hameed, B. Food cannery effluent, pineapple peel as an effective low-cost biosorbent for removing cationic dye from aqueous solutions. Desalination Water Treat. 2013, 52, 6096–6103. [Google Scholar] [CrossRef]
- Shakoor, S.; Nasar, A. Adsorptive treatment of hazardous methylene blue dye from artificially contaminated water using cucumis sativus peel waste as a low-cost adsorbent. Groundw. Sustain. Dev. 2017, 5, 152–159. [Google Scholar] [CrossRef]
- Jawad, A.H.; Kadhum, A.M.; Ngoh, Y.S. Applicability of dragon fruit (Hylocereus polyrhizus) peels as low-cost biosorbent for adsorption of methylene blue from aqueous solution: Kinetics, equilibrium and thermodynamics studies. DESALINATION Water Treat. 2018, 109, 231–240. [Google Scholar] [CrossRef]
- Romero-Cano, L.A.; González-Gutiérrez, L.V.; A Baldenegro-Pérez, L.; Carrasco-Marin, F. Grapefruit peels as biosorbent: Characterization and use in batch and fixed bed column for Cu(II) uptake from wastewater. J. Chem. Technol. Biotechnol. 2017, 92, 1650–1658. [Google Scholar] [CrossRef]
- Ibisi, N.E.; Asoluka, C.A. Use of agro-waste (Musa paradisiaca peels) as a sustainable biosorbent for toxic metal ions removal from contaminated water. Chem. Int. 2018, 4, 52–59. [Google Scholar]
- Lam, Y.F.; Lee, L.Y.; Chua, S.J.; Lim, S.S.; Gan, S. Insights into the equilibrium, kinetic and thermodynamics of nickel removal by environmental friendly Lansium domesticum peel biosorbent. Ecotoxicol. Environ. Saf. 2016, 127, 61–70. [Google Scholar] [CrossRef]
- Pavan, F.A.; Mazzocato, A.C.; Jacques, R.A.; Dias, S.L.P. Ponkan peel: A potential biosorbent for removal of Pb(II) ions from aqueous solution. Biochem. Eng. J. 2008, 40, 357–362. [Google Scholar] [CrossRef]
- Mohammed, R.; Chong, M.F. Treatment and decolorization of biologically treated Palm Oil Mill Effluent (POME) using banana peel as novel biosorbent. J. Environ. Manag. 2014, 132, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Pavan, F.A.; Lima, I.S.; Lima, E.C.; Airoldi, C.; Gushikem, Y. Use of Ponkan mandarin peels as biosorbent for toxic metals uptake from aqueous solutions. J. Hazard. Mater. 2006, 137, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Parveen, N.; Gupta, H. Adsorptive decontamination of rhodamine-B from water using banana peel powder: A biosorbent. Environ. Technol. Innov. 2018, 12, 189–195. [Google Scholar] [CrossRef]
- Ahmed, D.; Abid, H.; Riaz, A. Lagenaria siceraria peel biomass as a potential biosorbent for the removal of toxic metals from industrial wastewaters. Int. J. Environ. Stud. 2018, 75, 1–11. [Google Scholar] [CrossRef]
- Ng, H.W.; Lee, L.Y.; Chan, W.L.; Gan, S.; Chemmangattuvalappil, N.G. Luffa acutangula peel as an effective natural biosorbent for malachite green removal in aqueous media: Equilibrium, kinetic and thermodynamic investigations. DESALINATION Water Treat. 2015, 57, 1–10. [Google Scholar] [CrossRef]
- Gill, R.; Mahmood, A.; Nazir, R. Biosorption potential and kinetic studies of vegetable waste mixture for the removal of Nickel(II). J. Mater. Cycles Waste Manag. 2013, 15, 115–121. [Google Scholar] [CrossRef]
| Fruit/Vegetable Common Name | Scientific Name | Matrix | Applied on Food Items | Technique Used | Beneficial Effects | Ref |
|---|---|---|---|---|---|---|
| Apple | Malus domestica | Carboxy methylcellulose | Fresh beef patties | Microfluidization | A complete inhibition of lipid oxidation, and efficient suppression of the growth of microbes on raw beef patties. No effect on the sensory characteristics of raw and cooked beef patties | [26] |
| Orange | Citrus sinensis | Gelatin | Cupcake | ND | Increase in peroxide value by 3.60–4.80 (mL.eq./kg fat) in refrigerated storage for 1 week and decrease in microbial growth | [27] |
| Pomegranate | Punica granatum | Mung bean protein | NS | ND | The films enriched with pomegranate peel also showed higher total phenolic content; antioxidant activity, antibacterial capacity compared to the control mung bean protein film. These films found their use in food industry to develop bio-functional edible films intended for packaging of food products | [28] |
| Potato | Solanum tuberosum | Oregano essential oil (OEO) | Cold-smoked salmon | ND | When samples were coated with Potato processing waste-based-oregano oil-incorporating film (PPW-OO), the Listeria population decreased from 6.7 to 4.7 log CFU/g by the end of storage. Incorporation of oil into the films reduced the film strength and increased their water vapor permeability. The PPW-OO film reduced the growth of Listeria monocytogenes on cold-smoked salmon during storage under vacuum conditions at 4 °C for 28 days | [29] |
| Orange | Citrus sinensis (L.) Osbeck | Chitosan film | Deepwater pink shrimp | Casting | The combination of chitosan film with 2% orange peel essential oil concentration was effective in prolonging the shelf life of fresh shrimps to 15 days | [30] |
| Orange | Citrus sinensis (L.) Osbeck | Gelatin | Shrimps | ND | Gelatin coating combined with orange peel essential oil preserved shrimp quality during cold storage with a shelf-life extension of about 6 days | [23] |
| Lemon | Citrus limon | Cassava starch and sodium alginate | Tofu, Strawberry | ND | The addition of 0.6% lemon peel essential oil (LPEO) to tofu and 1% LPEO to strawberry with each of edible coating agents was significantly able to reduce their degradation | [31] |
| Orange | Citrus sinensis | Carnauba wax, montmorillonite nanoclay | Blood orange | ND | Blood orange coated by carnauba wax with montmorillonite nanoclay (MMT) had the least deformation and dissolved solid and the highest acidity compared to other treatments. Fruits coating with MMT showed better brightness | [13] |
| Orange | Citrus sinensis | Pectin-coating | Fresh-cut orange | ND | The results showed that the nanoemulsion-based edible coatings containing orange peel essential oil can extend the shelf life of orange slices without any undesirable impacts on sensory attributes | [32] |
| Fruit/Vegetable Common Name | Scientific Name | Types of Nanoparticles Synthesized | Reaction Time | Morphology | Size | Applications | Ref |
|---|---|---|---|---|---|---|---|
| Pomegranate; Orange; Banana and Apple | Punica granatum; Citrus sinensis; Musa; Malus domestica | Silver | 2 min | Sphere | 25 nm | Antibacterial activity against Salmonella sp., Escherichia coli, Pseudomonas sp., Aeromonas hydrophila; Antifungal activity against Fuarium sp.; Antioxidant activity using 2,2-diphenyl-1-picrylhydrazyl (DPPH); Cytotoxicity against human breast cancer cells MCF-7 | [51] |
| Orange; Banana | Citrus sinensis; Musa | Silver | 1 h | Sphere | ND | Antibacterial activity against Staphylococcus aureus, Proteus vulgaris | [52] |
| Orange; Lemon; Sweet lemon | Citrus sinensis; Citrus limon; Citrus limetta | Silver | 24 h | ND | ND | Antibacterial activity against Pseudomonas aeruginosa, E. coli and Salmonella typhimurium | [53] |
| Orange | Citrus sinensis | Silver | 10 min | Sphere | 47–53 nm | Photocatalytic against methylene blue | [54] |
| Lemon | Citrus limon | Silver | 30 min | Sphere | 2–5 nm | Antibacterial activity against P. aeruginosa, E. coli, Acinetobacter baumannii, Streptococcus mutans, Proteus mirabilis; Antifungal activity against Candida albicans | [55] |
| Pomegranate | Punica granatum | Silver | 24 h | ND | 5–50 nm | Antibacterial activity against S. aureus, P. aeruginosa, E. coli | [12] |
| Banana | Musa paradisiaca | Silver | 1 h | Sphere | 23.7 nm | Antibacterial activity against P. aeruginosa, E. coli, S. aureus, Bacillus subtilis; Antifungal activity against C. albicans | [56] |
| Pomegranate | Punica granatum | Silver | 24 h | Sphere | 20–40 nm | Antibacterial activity against E. coli, P. vulgaris, P. aeruginosa, S. typhimurium, S. aureus, Staphylococcus epidermidis, Klebsiella pneumonia; Cytotoxicity against human colon cancer cell line RKO: ATCC® CRL-2577™ | [57] |
| Apricot | Prunusa rmeniaca | Silver | NS | Rod | 50 nm | Antibacterial activity against E. coli, S. aureus, P. aeruginosa, B. subtilis | [58] |
| Cavendish banana | Musa acuminata | Silver | 30 min | Sphere | 55 nm | Antibacterial activity against S. aureus, B. subtilis, E. coli, K. pneumonia; Antioxidant activity (DPPH), 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) | [59] |
| Orange | Citrus sinensis | Silver | 5 h | ND | 48.1 nm | Antibacterial activity against Xanthomonas axonopodis pv. citri (Xac) | [60] |
| Tomato; Orange; Grapefruit; Lemon | Lycopersicon esculentum; Citrus sinensis; Citrus Paradise; Citrus aurantifolia | Zinc Oxide | 1 h | Hexagonal | 9.01 nm; 12.55 nm; 19.66 nm; 11.39 nm | Photocatalytic against methylene blue | [61] |
| Sweet Potato | Ipomoea batatas (L.) Lam.(Ib) | Silver | 1–12 h | Agglomerated | ND | Antibacterial activity against Enterococcus feacium, Salmonella enteritica, Listeria monocytogenes, B. cereus, S. aureus; Antidiabetic; Antioxidant activity (DPPH, ABTS, nitrite/nitrate oxide (NOx)); Cytotoxicity against HepG2 cancer cells | [62] |
| Potato | Solanum tuberosum | Zinc Oxide | 24 h | Hexagonal | 30–150 nm | Photocatalytic against methylene blue and azo dyes | [63] |
| Onion | Allium cepa | Gold | 24 h | Sphere and Triangle | 45.42 nm | Synergistic antimicrobial potential against B. cereus, E. coli, L. monocytogenes, S. aureus, S. typhimurium; Antifungal activity against C. albicans, C. glabrata, C. glochares; Antioxidant activity (DPPH, ABTS, NOx) | [64] |
| Bottle gourd | Lagenaria siceraria | Silver | 20 h | Sphere | 5–40 nm | Cytotoxicity against A431, (skin carcinoma, p53 mutant) and A549, (lung carcinoma, p53 wild type); Antibacterial activity against S. typhi | [65] |
| Radish | Raphanus sativus | Silver | 15 min | Polygonal | 30–60 nm | Antibacterial activity against S. aureus, B. subtilis, E. coli, K. pneumonia | [66] |
| Fruits/Vegetable Common Name | Scientific Name | Production Conditions | Detection Limit of Heavy Metals | Applications | Ref |
|---|---|---|---|---|---|
| Mango | Mangifera indica | Hydrothermal/300 °C/2 h | 1.2 µM | Cellular labeling ferrous ion (Fe2+) detection | [14] |
| Pineapple | Ananas comosus | Hydrothermal/200 °C/3 h | 4.5 nM | Electronic security devices mercury ion (Hg2+) quantification | [77] |
| Lemon | Citrus limon (L.) | Hydrothermal/200 °C/8 h | 73 nM | Cr6+ sensing; Photocatalysis effect | [75] |
| Sweet lemon | Citrus limetta | Hydrothermal/180 °C/3 h | NA | Breast cancer detection gene therapy | [78] |
| Banana | Musa acuminata | Microwave-assisted/500 W/20 min | NA | Determination of colitoxin DNA | [79] |
| Pomelo | Citrus maxima | Hydrothermal/200 °C/3 h | 0.23 nM | Hg2+ sensing | [80] |
| Grapefruit | Citrus paradisi | Hydrothermal/190 °C/12 h | NA | Photoluminescence immunoassay | [81] |
| Onion | Allium cepa | Microwave-assisted/1000 W/a specific time intervals | NA | Skin wound healing; Living cells imaging | [82] |
| Watermelon | Citrullus lanatus | Hydrothermal/220 °C/2 h | NA | Imaging probe | [83] |
| Citrus | Citrus sinensis, Citrus limon | Hydrothermal/180 °C/2 h | 0.01 µM | Ferric ion (Fe3+) and tartrazine sensing; Cell imaging | [84] |
| Orange | Citrus sinensis | Hydrothermal/150 °C/10 h | NA | Photocatalytic activity | [85] |
| Mangosteen | Garcinia mangostana | Hydrothermal/200 °C/30 min | NA | Cells imaging | [86] |
| Pomegranate | Punica granatum | Hydrothermal/180 °C/36 h | NA | Recovery of latent prints | [87] |
| Banana | Musa acuminata | Hydrothermal/200 °C/2 h | 211 nM | Selective and sensitive detection of Fe3+ ions | [88] |
| Fruit/Vegetable Common Name | Scientific Name | Medium Composition | Purpose/Utilization | Ref |
|---|---|---|---|---|
| Dragon fruit | Hylocereus undatus | Dragon fruit peel powder (33.3 g/L), peptone (20 mg/mL) and agar (1.5%) | Viability analysis of Escherichia coli | [97] |
| Orange; Potato; Drum stick | Citrus sinensis; Solanum tuberosum; Moringa oleifera | Peel powder of orange (0.20 g/100 mL), potato (0.25 g/100 mL), drum stick (1 g/100mL) and agar (2%) | Growth and pigment production analysis of E. coli, Serratia sp., Pseudomonas sp. | [89] |
| Banana; Melon; Grapefruit | Musa; Cucumis melo; Citrus paradise | Luria-Bertani medium contained 1% (w/v) starch, banana, grape fruit and melon peel powder | Amylase production from Bacillus sp. AY3 | [10] |
| Banana | Musa | Autoclave banana peel directly inoculated with fungi | Growth of human fungal pathogens viz. Lasiodiplodia theobromae, Macrophomina phaseolina, Nigrospora sphaerica, Chaetomium murorum, Nattrassia mangiferae and Schizophyllum commune | [98] |
| Watermelon | Citrullus lanatus | Watermelon peel waste extract (500 g/L) and dextrose (20 g/L) | Evaluation of fungal growth such as Rhizopus oryzae, Lichtheimia corymbifera, Aspergillus niger, Penicillium Expansium and Fusarium oxysporum | [99] |
| Orange | Citrus sinensis | Orange peel extract (19.8 g/L), (NH4)2SO4 (0.6 g/L) | Biodiesel production using oleaginous yeasts | [100] |
| Sponge gourd; Lychee | Luffa cylindrica; Litchi chinensis | Sponge gourd peel bed soaked with urea (0.3 g/L), (NH4)2SO4 (1.4 g/L), KH2PO4 (2.0 g/L), MgSO4 7H2O (0.3 g/L), peptone (1 g/L), tween 80 (0.2 g/L), FeSO4 7H2O (0.005 g/L), MnSO4.7H2O (0.0016 g/L), ZnSO4. 7H2O (0.0014 g/L) CaCl2 2H2O (0.4 g/L), CoCl2 6H2O (0.02 g/L); same composition with lychee peel | Cellulase production using Trichoderma reesei | [101] |
| Pea | Pisum sativum | Pea peel powder soaked with urea (0.3 g/L), (NH4)2SO4 (1.4 g/L), KH2PO4 (2.0 g/L), MgSO4.7H2O (0.3g/L), peptone (1g/L), tween 80 (0.2 g/L), FeSO4 7H2O (0.005 g/L), MnSO4.7H2O (0.0016g/L), ZnSO4. 7H2O (0.0014 g/L) CaCl2.2H2O (0.2 g/L), CoCl2. 6H2O (0.2 g/L) | Cellulase production using Trichoderma reesei | [102] |
| Orange; Potato; Drum stick | Citrus sinensis; Solanum tuberosum; Moringa oleifera | Peel powder of orange (0.20 g/100 mL), potato (0.25 g/100 mL), drum stick (1 g/100 mL) and agar (2.5%) | Growth analysis of Trichoderma sp., Aspergillus sp. | [103] |
| Fruit/Vegetable Common Name | Scientific Name | Process conditions Required for Biochar Formation | Applications | Ref |
|---|---|---|---|---|
| Orange; Banana | Citrus sinensis; Musa | Pyrolysis at 500 °C for 10 min | Showed good performance in reducing the concentration of biochemical oxygen demand (BOD), chemical oxygen demand (COD), total suspended solid (TSS) and oil and grease of Palm oil Mil effluent (POME) to an acceptable level below the discharge | [112] |
| Banana | Musa | Hydrothermal carbonization at 230 °C for 2 h | Showed excellent lead clarification capability of 359 mg/g and 193 mg/g, respectively | [15] |
| Pomelo | Citrus maxima | Pyrolysis at 450 °C for 1 h | One gram of biochar adsorb 150 mg/L methyl orange dye | [113] |
| Pineapple | Ananas comosus | Pyrolysis at 750 °C for 2 h | Sorption capacity for hexavalent chromium: Cr (VI) was 7.44 mg/g | [114] |
| Pineapple | Ananas comosus | Pyrolysis at 200 °C for 2 h and then heated at 650 °C for 3 h | Sorption of oxytetracycline | [115] |
| Orange; Pineapple; Dragon fruit | Citrus sinensis; Ananas comosus; Hylocereus undatus | Pyrolysis at 300 °C for 2 h | Maximum ammonium cation (NH4+) adsorption capacities were associated with biochars of orange peel (4.71 mg/g) and pineapple peel (5.60 mg/g) produced at 300 °C for 2 h. The maximum NH4+ adsorption capacity of the dragon fruit (pitaya) peel biochar produced at 400 °C for 2 h was 2.65 mg/g | [116] |
| Pomelo | Citrus maxima | Pyrolysis at 450 °C for 1 h | A 0.05 g of biochar adsorbed 57.637 mg/g of Cr (VI) | [117] |
| Litchi | Litchi chinensis | Hydrothermal carbonization at 180 °C for 12 h | Adsorption capacity for congored and malachite green was 404.4 and 2468 mg/g | [118] |
| Rambutan | Nephelium lappaceum | Pyrolysis at 600 °C for 3 h | Adsorption for removal of copper ion: Cu(II) from aqueous solutions of 50 and 100 mg/L at 0.2 and 0.4 g/L adsorbent dosages, respectively | [119] |
| Pomegranate | Punica granatum | Pyrolysis at 300 °C for 2 h | Adsorption of Cu(II) was 52 mg/g | [120] |
| Sweet lime | Citrus limetta | Pyrolysis at 450 °C for 1 h | Maximum removal efficiency was found to be 95% with 120 mg/L of initial Cr(VI) concentration with 3 g/L of biochar dose | [121] |
| Potato | Solanum tuberosum | Pyrolysis at 500 °C for 5 min | Hydrogen sulfide (H2S) was achieved 53 mg/g at 500 °C, under space velocity (8000 L min–1kg–1) | [122] |
| Fruit/Vegetable Common Name | Scientific Name | Drying Temperature/Time | Applications | Ref |
|---|---|---|---|---|
| Apple | Malus domestica | 60 °C/24 h | Adsorbed 107.52 mg/g of methylene blue | [16] |
| Dragon fruit | Hylocereus undatus | 105 °C/24 h | A dosage of 0.06 g adsorbed 192.31 mg/g of methylene blue | [131] |
| Pineapple | Ananas comosus | 70 °C/48 h | Adsorbed 97.09 mg/g of methylene blue | [129] |
| Grapefruit | Citrus paradisi | 105 °C/24 h | Adsorbed 52.48 mg/g copper ion: Cu(II) | [132] |
| Banana | Musa paradisiaca | 60 °C/5 h | Removed 90% lead (II) and cadmium (II) ions | [133] |
| Langast | Lansium domesticum | 60 °C/24 h | Adsorbed 10.1 mg/g of nickel | [134] |
| Ponkan fruits/Mandarin orange | Citrus reticulata | RT/days | Adsorbed 112.1 mg/g of lead (II) ions | [135] |
| Banana | Musa | 80 °C/48 h | Adsorbed 97 mg/g color, 25 mg/g TSS, and 90.5 mg/g COD removed from Palm oil mill effluent (Natural banana peel); Adsorbed 137.5 mg/g, 28.5 mg/g and 93 mg/g for color, TSS and COD removed (Methylated banana peel) | [136] |
| Ponkan fruits/Mandarin orange | Citrus reticulata | 60 °C/24 h | Adsorbed 1.92, 1.37 and 1.31 mmol/g of nickel (II), cobalt (II) and copper (II) ions | [137] |
| Banana | Musa | RT/4 days | A dosage of 0.3 g adsorbed 81.07% of rhodamine-B | [138] |
| Bottle gourd | Lagenaria siceraria | 80 °C/24 h | Adsorbed 99% copper, 95% silver and iron | [139] |
| Sponge gourd | Luffa acutangula | 60 °C/24 h | A dosage of 8 g/L adsorbed 69.64 mg/g of malachite green | [140] |
| Potato; Carrot | Solanum tuberosum/ Daucus carota subsp. sativus | 60 °C/48 h | A dosage of 3.0 g adsorbed 79.32% of nickel | [141] |
| Cucumber | Cucumis sativus | 95 °C/24 h | A dosage of 4 g/L adsorbed 81.4% methylene blue | [130] |
| Garlic | Allium sativum | 60 °C/24 h | Adsorbed 142.86 mg/g of methylene blue | [128] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, H.; Bhardwaj, K.; Sharma, R.; Nepovimova, E.; Kuča, K.; Dhanjal, D.S.; Verma, R.; Bhardwaj, P.; Sharma, S.; Kumar, D. Fruit and Vegetable Peels: Utilization of High Value Horticultural Waste in Novel Industrial Applications. Molecules 2020, 25, 2812. https://doi.org/10.3390/molecules25122812
Kumar H, Bhardwaj K, Sharma R, Nepovimova E, Kuča K, Dhanjal DS, Verma R, Bhardwaj P, Sharma S, Kumar D. Fruit and Vegetable Peels: Utilization of High Value Horticultural Waste in Novel Industrial Applications. Molecules. 2020; 25(12):2812. https://doi.org/10.3390/molecules25122812
Chicago/Turabian StyleKumar, Harsh, Kanchan Bhardwaj, Ruchi Sharma, Eugenie Nepovimova, Kamil Kuča, Daljeet Singh Dhanjal, Rachna Verma, Prerna Bhardwaj, Somesh Sharma, and Dinesh Kumar. 2020. "Fruit and Vegetable Peels: Utilization of High Value Horticultural Waste in Novel Industrial Applications" Molecules 25, no. 12: 2812. https://doi.org/10.3390/molecules25122812
APA StyleKumar, H., Bhardwaj, K., Sharma, R., Nepovimova, E., Kuča, K., Dhanjal, D. S., Verma, R., Bhardwaj, P., Sharma, S., & Kumar, D. (2020). Fruit and Vegetable Peels: Utilization of High Value Horticultural Waste in Novel Industrial Applications. Molecules, 25(12), 2812. https://doi.org/10.3390/molecules25122812










