Bioanalytical Method Development and Validation of Veratraldehyde and Its Metabolite Veratric Acid in Rat Plasma: An Application for a Pharmacokinetic Study
Abstract
1. Introduction
2. Results and Discussion
2.1. Bioanalytical Method Development
2.1.1. Determination of Mass Spectrometry Conditions
2.1.2. Determination of Liquid Chromatography Conditions
2.1.3. Determination of Biological Matrix and Extraction Procedure
2.2. Bioanalytic Method Validation
2.2.1. Linearity and Lower Limit of Quantification (LLOQ)
2.2.2. Specificity
2.2.3. Precision and Accuracy, Matrix Effect, and Carry Over
2.2.4. Stability
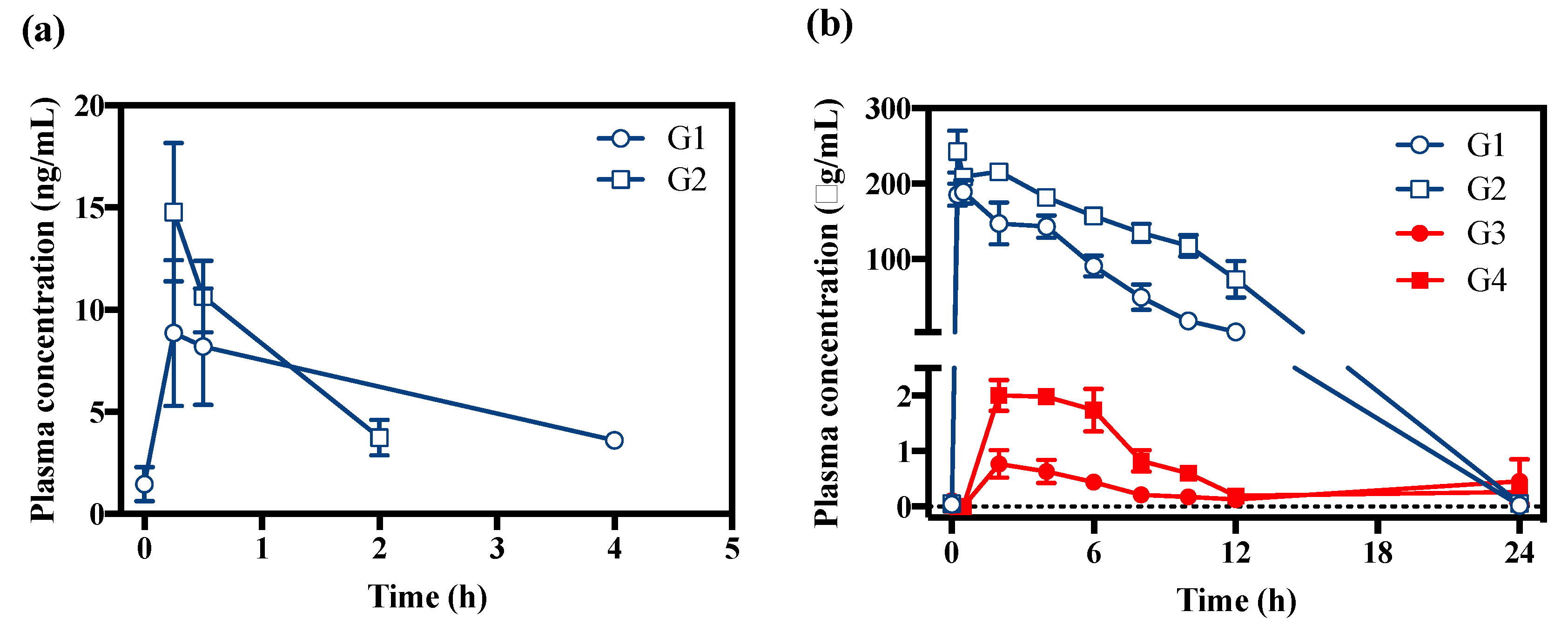
2.3. Pharmacokinetic Application
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Bioanalytical Method
3.2.1. Instrumentation and Chromatographic Conditions
3.2.2. Preparation of Stock Standards and Quality Controls (QCs)
3.2.3. Plasma Sample Extraction
3.2.4. Validation of Bioanalytical Method
3.3. Pharmacokinetic Application
3.3.1. Animals
3.3.2. Pharmacokinetics of VD
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Janssens, L.; De Pooter, H.L.; Schamp, N.M.; Vandamme, E.J. Production of flavours by microorganisms. Process. Biochem. 1992, 27, 195–215. [Google Scholar] [CrossRef]
- Melián-Rodríguez, M.; Saravanamurugan, S.; Kegnæs, S.; Riisager, A. Aerobic oxidation of veratryl alcohol to veratraldehyde with heterogeneous ruthenium catalysts. Top. Catal. 2015, 58, 1036–1042. [Google Scholar] [CrossRef]
- Fleisher, V.L.; Andryukhova, M.V. Preparative synthesis of fragrance substances based on vanillin and veratraldehyde. BSTU 2012, 4, 25–28. [Google Scholar]
- Molleti, J.; Yadav, G.D. Green synthesis of veratraldehyde using potassium promoted lanthanum–magnesium mixed oxide catalyst. Org. Process. Res. Dev. 2017, 21, 1012–1020. [Google Scholar] [CrossRef]
- Ambrose, K.; Hurisso, B.B.; Singer, R.D. Recyclable ionic liquid tagged Co(salen) catalysts for the oxidation of lignin model compounds. Can. J. Chem. 2013, 91, 1258–1261. [Google Scholar] [CrossRef]
- Prakoso, N.I.; Pangestu, P.H.; Wahyuningsih, T.D. O-methylation of natural phenolic compounds based on green chemistry using dimethyl carbonate. Mater. Sci. Eng. 2016, 107, 012065. [Google Scholar] [CrossRef]
- Suma, A.A.T.; Wahyuningsih, T.D.; Pranowo, D. Synthesis and antibacterial activities of n-phenylpyrazolines from veratraldehyde. Mater. Sci. Forum 2017, 901, 124–132. [Google Scholar] [CrossRef]
- Wahyuningsih, T.D.; Suma, A.A.T.; Astuti, E. Synthesis, anticancer activity, and docking study of N-acetyl pyrazolines from veratraldehyde. J. Appl. Pharm. 2019, 9, 14–20. [Google Scholar]
- Basappa; Doreswamy, B.H.; Mahendra, M.; Mantelingu, K.; Sridhar, M.A.; Prasad, J.S.; Rangappa, K.S. Reduction of aldehydes and oximes to their corresponding alcohols and amines by catalytic hydrogenation method. Indian J. Chem. B 2005, 44, 148–151. [Google Scholar]
- Tingjun, D.; Fengyin, M.; Chongping, Y. Reversed phase extraction purification process characteristics improvement of veratraldehyde. Guangdong Chem. Ind. 2012, 11, 29. [Google Scholar]
- Chen, X.Q.; Zhang, B.; Zhang, Z. Application of veratraldehyde as a temporary consolidant for relics at underwater cultural heritage sites. Archaeometry 2019, 61, 1417–1429. [Google Scholar] [CrossRef]
- Hang, C.; Fang-fang, Z.; Jian-xin, Z.; Xi, Z.; Zhu, T.; Sai-feng, X.; Qian-jiang, Z. Rapid transformation of benzylic alcohols to aldehyde in the presence of cucurbit[8]uril. Catal. Commun. 2009, 11, 167–170. [Google Scholar] [CrossRef]
- Kim, S.-I.; Chun, B.N.; Suh, J.K.; Park, S.Y.; Inc, W.B. Hematophagous Arthropod Repellent Composition. U.S. Patent 16/485,935, 16 January 2020. [Google Scholar]
- Nerio, L.S.; Olivero-Verbel, J.; Stashenko, E. Repellent activity of essential oils: A review. Bioresour. Technol. 2010, 101, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Alankar, S.A. Review on peppermint oil. Asian J. Pharm. Clin. Res. 2009, 2, 27–33. [Google Scholar]
- Ansari, M.A.; Vasudevan, P.; Tandon, M.; Razdan, R.K. Larvicidal and mosquito repellent action of peppermint (Mentha piperita) oil. Bioresour. Technol. 2000, 71, 267–271. [Google Scholar] [CrossRef]
- Guillén, F.; Christine, S.E. Anisaldehyde and veratraldehyde acting as redox cycling agents for H2O2 production by Pleurotus eryngii. Appl. Environ. Microbiol. 1994, 60, 2811–2817. [Google Scholar] [CrossRef]
- Scheline, R.R. The metabolism of some aromatic aldehydes and alcohols by the rat intestinal microflora. Xenobiotica 1972, 2, 227–236. [Google Scholar] [CrossRef]
- Sammons, H.G.; Williams, R.T. Studies in detoxication; the metabolism of veratraldehyde and veratric acid in the rabbit. Biochem. J. 1946, 40, 223–227. [Google Scholar] [CrossRef]
- Peng, Y.-S.; Liu, L.-J.; Zhao, C.; Yang, X.; Liu, C.; Wang, R.-F. Pharmacokinetics and tissue distributions of veratric acid after intravenous administration in rats. Chin. J. Nat. Med. 2015, 13, 535–539. [Google Scholar] [CrossRef]
- Khatri, C.A.; Ch, V.P.; Pharm, K.J.J. Development and validation of bioanalytical method for simultaneous quantification of veratric acid, mebeverine acid and desmethyl mebeverine acid in human edta plasma by using LC-MS/MS. J. Pharm. Chem. 2012, 6, 11–18. [Google Scholar]
- Jeong, S.H.; Jang, J.H.; Cho, H.Y.; Oh, I.J.; Lee, Y.B. A sensitive UPLC–ESI–MS/MS method for the quantification of cinnamic acid in vivo and in vitro: Application to pharmacokinetic and protein binding study in human plasma. J. Pharm. Investig. 2020, 50, 159–172. [Google Scholar] [CrossRef]
- Meirinho, S.; Rodrigues, M.; Fortuna, A.; Falcão, A.; Alves, G. Novel bioanalytical method for the quantification of rufinamide in mouse plasma and tissues using HPLC-UV: A tool to support pharmacokinetic studies. J. Chromatogr. B 2019, 1124, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Dalvi, A.V.; Uppuluri, C.T.; Bommireddy, E.P.; Ravi, P.R. Design of experiments-based RP–HPLC bioanalytical method development for estimation of Rufinamide in rat plasma and brain and its application in pharmacokinetic study. J. Chromatogr. B 2018, 1102, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Bessegato, T.C.; Niehues, M.; Buqui, G.A.; Lopes, N.P.; Pitta, I.R.; Galdino, S.L.; Dalla Costa, T. Development and validation of a UHPLC-MS/MS bioanalytical method to quantify in plasma the analgesic candidate PT-31 following a preliminary pharmacokinetic study in rats. Biomed. Chromatogr. 2016, 30, 852–856. [Google Scholar] [CrossRef]
- European Medicines Agency. Guideline Bioanalytical Method Validation. 2015. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-bioanalytical-method-validation_en.pdf (accessed on 3 June 2015).
Sample Availability: Samples of the compounds are not available from the authors. |

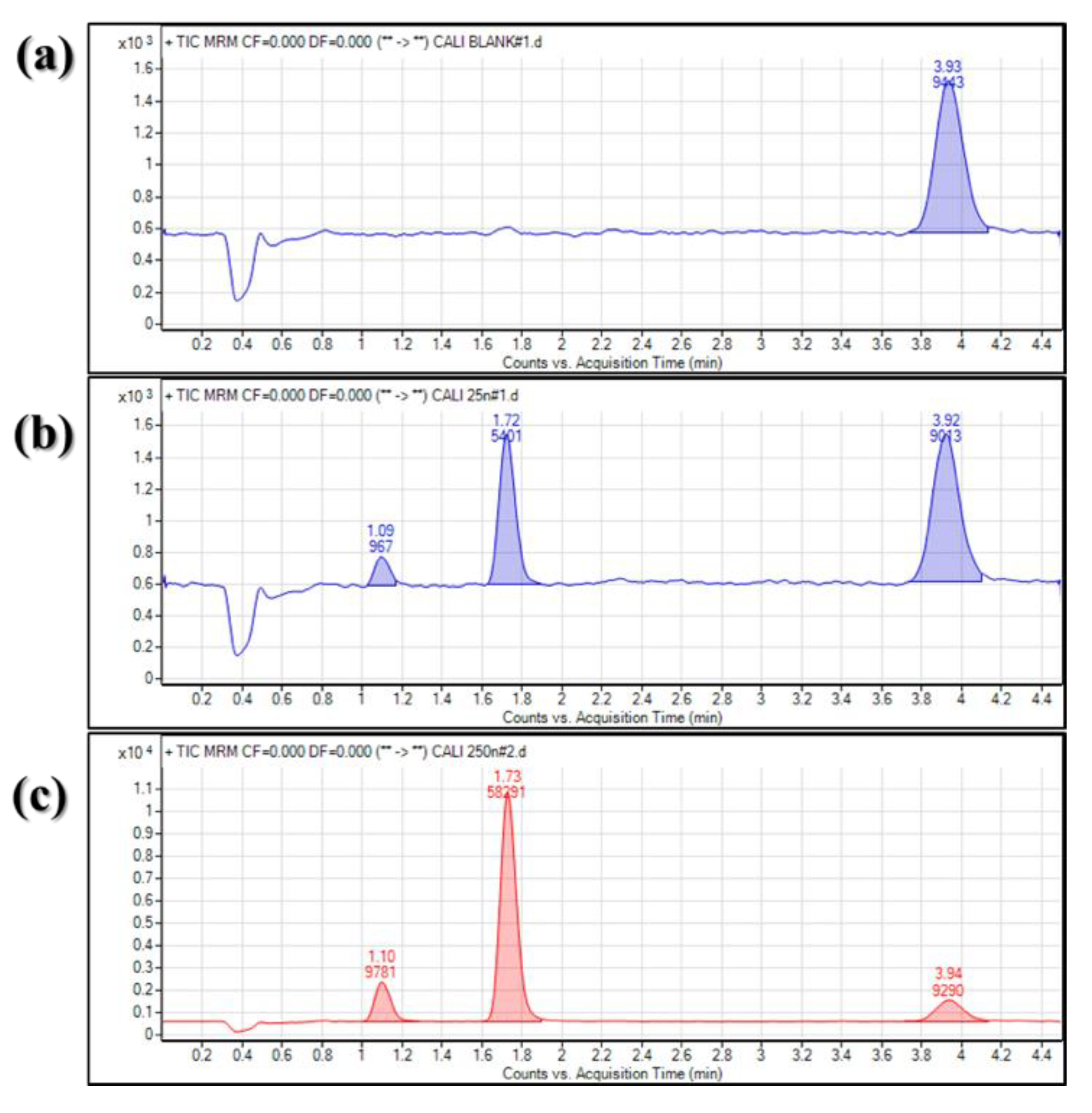
| Compound | Parent Ion (m/z, [M + H]+) | Product Ion (m/z) | Retention Time (RT) (min) |
|---|---|---|---|
| (CE) | |||
| Veratraldehyde | 167.07 | 139.00 (11) | 1.72 |
| Veratric acid | 183.07 | 139.00 (11) | 1.10 |
| Cinnamaldehyde (IS) | 133.00 | 55.00 (11) | 3.92 |
| Compound | Linearity | LLOQ | ||||||||||
| Equation | Range (ng/mL) | Correlation Coefficient (R2) | Nominal Concentration (ng/mL) | Mean | RSD | S/N | ||||||
| VD | Y = 0.031263x + 0.018961 | 3–1000 | 0.9986 | 3 | 2.64 | 9.87 | 15.5 | |||||
| VA | Y = 0.003814x + 0.008264 | 10–10,000 | 0.9977 | 10 | 10.09 | 12.37 | 8.04 | |||||
| Linearity of Veratraldehyde and Veratric Acid | ||||||||||||
| Veratraldehyde | Veratric Acid | |||||||||||
| Nominal Concentration (ng/mL) | Concentration (ng/mL) | Bias (%) | Nominal Concentration (ng/mL) | Concentration (ng/mL) | Bias (%) | |||||||
| 3 | 2.64 ± 0.26 | 88.01 | 10 | 10.09 ± 1.24 | 100.95 | |||||||
| 5 | 5.54 ± 0.22 | 110.83 | 25 | 28.07 ± 1.54 | 112.30 | |||||||
| 10 | 10.16 ± 0.14 | 101.69 | 50 | 47.75 ± 2.11 | 95.51 | |||||||
| 25 | 26.37 ± 0.71 | 105.49 | 100 | 103.36 ± 3.40 | 103.36 | |||||||
| 50 | 47.75 ± 2.53 | 95.51 | 250 | 233.29 ± 0.92 | 93.32 | |||||||
| 100 | 103.62 ± 3.06 | 103.62 | 500 | 476.86 ± 6.32 | 95.37 | |||||||
| 250 | 233.87 ± 3.80 | 93.55 | 1000 | 952.92 ± 21.84 | 95.29 | |||||||
| 500 | 499.98 ± 7.38 | 100.00 | 2500 | 2638.20 ± 248.29 | 105.53 | |||||||
| 1000 | 1013.04 ± 23.77 | 101.30 | 5000 | 4891.51 ± 70.52 | 97.83 | |||||||
| 10,000 | 10,052.90 ± 145.56 | 100.53 | ||||||||||
| Analytes | Nominal Concentration (ng/mL) | Intra-Day | Inter-Day | Matrix Effect | |||||
|---|---|---|---|---|---|---|---|---|---|
| Calculated Concentration (ng/mL) | RSD (%) | RE (%) | Calculated Concentration (ng/mL) | RSD (%) | RE (%) | Mean | RSD | ||
| Veratraldehyde (VD) | 3 | 3.3 ± 0.3 | 8.5 | 12.06 | 3.4 ± 0.3 | 12 | 11.2 | ||
| 10 | 10.2 ± 0.2 | 1.6 | 1.7 | 10.1 ± 0.8 | 7.50 | 1.44 | 9.3 | 3.07 | |
| 500 | 450 ± 6 | 1.4 | −9.9 | 485 ± 13 | 2.73 | −2.98 | - | - | |
| 1000 | 885 ± 15 | 1.7 | −11.4 | 989 ± 31 | 3.10 | −1.12 | 1084 | 4.6 | |
| Veratric acid (VA) | 10 | 11.3 ± 0.4 | 12 | 6.7 | 11.7 ± 0.4 | 17 | 15.4 | ||
| 30 | 27.3 ± 1.1 | 4.1 | −8.9 | 28.0 ± 0.9 | 3.18 | −6.53 | 29.9 | 7.8 | |
| 5000 | 4422 ± 111 | 2.9 | −11.6 | 4515 ± 84 | 1.85 | −9.70 | - | - | |
| 10,000 | 9930 ± 372 | 3.7 | −0.7 | 9921 ± 281 | 2.83 | −0.79 | 9224 | 1.4 | |
| (a) | ||||
| Veratraldehyde | Concentration (ng/mL) | RSD (%) | RE (%) | |
| Nominal | Calculated | |||
| Short-term: exposure at RT for 24 h | 10 | 8.6 ± 0.1 | 1.3 | 13.5 |
| 1000 | 1017 ± 8 | 0.8 | 1.7 | |
| Long-term: storage at −70 °C for 30 days | 10 | 8.9 ± 0.2 | 2.6 | 10.2 |
| 1000 | 990 ± 24 | 2.5 | 1.0 | |
| Freeze and thaw for three cycles: freezing at −70 °C and thawing at RT | 10 | 8.0 ± 0.2 | 2.4 | 19.6 |
| 1000 | 934 ± 22 | 2.3 | 6.7 | |
| Post-preparation: auto-sampler (4 °C) for 24 h | 10 | 9.7 ± 0.2 | 2.1 | 3.1 |
| 500 | 445 ± 6 | 4.7 | 11.1 | |
| 1000 | 888 ± 12 | 4.8 | 11.2 | |
| (b) | ||||
| Veratric Acid | Concentration (ng/mL) | RSD (%) | RE (%) | |
| Nominal | Calculated | |||
| Short-term: exposure at RT for 24 h | 30 | 29.12 ± 3.7 | 12.7 | 2.93 |
| 10,000 | 9663 ± 315 | 3.3 | 3.37 | |
| Long-term: storage at −70 °C for 30 days | 30 | 28.9 ± 2.0 | 6.8 | 3.75 |
| 10,000 | 9487 ± 271 | 2.9 | 5.13 | |
| Freeze and thaw for three cycles: freezing at −70 °C and thawing at RT | 30 | 25.9 ± 1.0 | 4.0 | 13.77 |
| 10,000 | 8946 ± 39 | 0.4 | 10.54 | |
| Post-preparation: auto-sampler (4 °C) for 24 h | 30 | 25.1 ± 0.9 | 3.5 | 16.24 |
| 5000 | 4362 ± 130 | 3.0 | 12.76 | |
| 10,000 | 9830 ± 409 | 4.2 | 1.70 | |
| Parameters (unit) | Veratraldehyde | Veratric Acid | ||||
|---|---|---|---|---|---|---|
| G1 | G2 | G1 | G2 | G3 | G4 | |
| T1/2 (h) | NA | 1.39 ± 0.50 | 1.15 ± 0.25 | 14.56 ± 3.06 | 73.62 ± 96.06 | |
| Tmax (h) | 0.34 ± 0.15 | 0.25 ± 0.00 | 0.42 ± 0.15 | 0.84 ± 1.02 | 10.67 ± 11.55 | 3.34 ± 2.31 |
| Cmax (ng/mL) | 9.18 ± 6.16 | 14.78 ± 5.87 | 192,374 ± 24,898 | 246,439 ± 43,352 | 880 ± 474 | 2344 ± 99 |
| AUClast (ng⋅h/mL) | 12.80 ± 18.40 | 15.82 ± 4.79 | 1,092,969 ± 270,449 | 2,315,618 ± 333,017 | 14,423 ± 13,381 | 25,813 ± 2606 |
| MR (VD/VA) | 4.5 × 10−5 | 3.6 × 10−5 | NA | NA | NA | NA |
| Group | Sample | Dose | Route | Dose Volume |
|---|---|---|---|---|
| G1 | Veratraldehyde (Low dose) | 300 mg/kg | Oral | 10 mL/kg |
| G2 | Veratraldehyde (Middle dose) | 600 mg/kg | Oral | 10 mL/kg |
| G3 | Veratraldehyde (Low dose) | 300 mg/kg | Percutaneous | 10 mL/kg |
| G4 | Veratraldehyde (Middle dose) | 600 mg/kg | Percutaneous | 10 mL/kg |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huh, H.W.; Song, H.-Y.; Na, Y.-G.; Kim, M.; Han, M.; Pham, T.M.A.; Lee, H.; Suh, J.; Lee, S.-J.; Lee, H.-K.; et al. Bioanalytical Method Development and Validation of Veratraldehyde and Its Metabolite Veratric Acid in Rat Plasma: An Application for a Pharmacokinetic Study. Molecules 2020, 25, 2800. https://doi.org/10.3390/molecules25122800
Huh HW, Song H-Y, Na Y-G, Kim M, Han M, Pham TMA, Lee H, Suh J, Lee S-J, Lee H-K, et al. Bioanalytical Method Development and Validation of Veratraldehyde and Its Metabolite Veratric Acid in Rat Plasma: An Application for a Pharmacokinetic Study. Molecules. 2020; 25(12):2800. https://doi.org/10.3390/molecules25122800
Chicago/Turabian StyleHuh, Hyun Wook, Hee-Yong Song, Young-Guk Na, Minki Kim, Mingu Han, Thi Mai Anh Pham, Hyeonmin Lee, Jungkyu Suh, Seok-Jong Lee, Hong-Ki Lee, and et al. 2020. "Bioanalytical Method Development and Validation of Veratraldehyde and Its Metabolite Veratric Acid in Rat Plasma: An Application for a Pharmacokinetic Study" Molecules 25, no. 12: 2800. https://doi.org/10.3390/molecules25122800
APA StyleHuh, H. W., Song, H.-Y., Na, Y.-G., Kim, M., Han, M., Pham, T. M. A., Lee, H., Suh, J., Lee, S.-J., Lee, H.-K., & Cho, C.-W. (2020). Bioanalytical Method Development and Validation of Veratraldehyde and Its Metabolite Veratric Acid in Rat Plasma: An Application for a Pharmacokinetic Study. Molecules, 25(12), 2800. https://doi.org/10.3390/molecules25122800







