Antiviral Natural Products for Arbovirus Infections
Abstract
1. Introduction
2. Flaviviruses
2.1. Dengue Virus (DENV)
2.2. Japanese Encephalitis Virus (JEV)
2.3. West Nile Virus (WNV)
2.4. Zika Virus (ZIKV)
3. Alphaviruses
3.1. Chikungunya Virus (CHIKV)
3.2. Mayaro Virus
4. Natural Products as a Source of Antiviral Compounds
4.1. Antiviral Natural Compounds
4.1.1. Curcumin
4.1.2. Epigallocatechin Gallate (EGCG)
4.1.3. Pinocembrin
4.1.4. Quinine/Quinine Sulfate
4.1.5. Other Potential Antiviral Compounds against Arboviruses
4.2. Antiviral Plant Extracts
4.2.1. Psiloxylon mauritianum Extract
4.2.2. Silymarin Complex
4.2.3. Other Potential Antiviral Plant Extracts against Arboviruses
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Young, P.R.; Ng, L.F.P.; Hall, R.A.; Smith, D.W.; Johansen, C.A. Chapter 14-Arbovirus Infections. In Manson’s Tropical Infectious Diseases, 23rd ed.; Farrar, J., Hotez, P.J., Junghanss, T., Kang, G., Lalloo, D., White, N.J., Eds.; W.B. Saunders: London, UK, 2014; pp. 129–161. [Google Scholar]
- Wilder-Smith, A.; Ooi, E.-E.; Horstick, O.; Wills, B. Dengue. Lancet 2019, 393, 350–363. [Google Scholar] [CrossRef]
- Young, P.R. Arboviruses: A Family on the Move. In Dengue and Zika: Control and Antiviral Treatment Strategies; Hilgenfeld, R., Vasudevan, S.G., Eds.; Springer: Singapore, 2018; pp. 1–10. [Google Scholar]
- Wilder-Smith, A.; Gubler, D.J.; Weaver, S.C.; Monath, T.P.; Heymann, D.L.; Scott, T.W. Epidemic arboviral diseases: Priorities for research and public health. Lancet Infect. Dis. 2017, 17, e101–e106. [Google Scholar] [CrossRef]
- Marchi, S.; Trombetta, C.M.; Montomoli, E. Emerging and Re-emerging Arboviral Diseases as a Global Health Problem. In Public Health-Emerging and Re-emerging Issues; Majumder, M.A.A., Russel, K., Sayeeda, R., Eds.; InTech: London, UK, 2018. [Google Scholar] [CrossRef]
- World Health Organization. Dengue Guidelines for Diagnosis, Treatment, Prevention and Control; WHO: Geneva, Switzerland, 2009. [Google Scholar]
- Hanley, K.A.; Weaver, S.C. Chapter 16-Arbovirus Evolution. In Origin and Evolution of Viruses, 2nd ed.; Domingo, E., Parrish, C.R., Holland, J.J., Eds.; Academic Press: London, UK, 2008; pp. 351–391. [Google Scholar] [CrossRef]
- Weaver, S.C.; Reisen, W.K. Present and future arboviral threats. Antivir. Res. 2010, 85, 328–345. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Fact Sheet: Dengue and Severe Dengue. Available online: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (accessed on 1 April 2020).
- Gould, E.; Pettersson, J.; Higgs, S.; Charrel, R.; De Lamballerie, X. Emerging arboviruses: Why today? One Health 2017, 4, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Dash, A.P.; Bhatia, R.; Sunyoto, T.; Mourya, D.T. Emerging and re-emerging arboviral diseases in Southeast Asia. J. Vector Borne Dis. 2013, 50, 77–84. [Google Scholar]
- Huang, Y.-J.S.; Higgs, S.; Vanlandingham, D.L. Emergence and re-emergence of mosquito-borne arboviruses. Curr. Opin. Virol. 2019, 34, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Halstead, S.B. Chapter 3-Chikungunya and Zika Disease. In Chikungunya and Zika Viruses; Higgs, S., Vanlandingham, D.L., Powers, A.M., Eds.; Academic Press: London, UK, 2018; pp. 69–85. [Google Scholar] [CrossRef]
- Cao-Lormeau, V.-M.; Blake, A.; Mons, S.; Lastère, S.; Roche, C.; Vanhomwegen, J.; Dub, T.; Baudouin, L.; Teissier, A.; Larre, P.; et al. Guillain-Barré Syndrome outbreak associated with Zika virus infection in French Polynesia: A case-control study. Lancet 2016, 387, 1531–1539. [Google Scholar] [CrossRef]
- Sanofi Pasteur. Press Release: Dengvaxia®, World’s First Dengue Vaccine, Approved in Mexico. Available online: https://www.sanofi.com/en/media-room/press-releases/2015/2015-12-09-16-30-00 (accessed on 9 April 2020).
- World Health Organization. Japanese Encephalitis Vaccines: WHO position paper–February 2015. Wkly Epidemiol. Rec. 2015, 90, 69–88. [Google Scholar]
- Ng, T.; Hathaway, D.; Jennings, N.; Champ, D.; Chiang, Y.W.; Chu, H.J. Equine vaccine for West Nile virus. Dev. Biol. 2003, 114, 221–227. [Google Scholar]
- Long, M.T.; Gibbs, E.P.J.; Mellencamp, M.W.; Bowen, R.A.; Seino, K.K.; Zhang, S.; Beachboard, S.E.; Humphrey, P.P. Efficacy, duration, and onset of immunogenicity of a West Nile virus vaccine, live Flavivirus chimera, in horses with a clinical disease challenge model. Equine Vet. J. 2007, 39, 491–497. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Vaccines Licensed for Use in the United States. Available online: https://www.fda.gov/vaccines-blood-biologics/vaccines/vaccines-licensed-use-united-states (accessed on 1 April 2020).
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Suroowan, S.; Mahomoodally, F.; Ragoo, L. Management and Treatment of Dengue and Chikungunya-Natural Products to the Rescue. Comb. Chem. High Throughput Screen. 2016, 19, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.K.; Munoz, A.L.; Segura, N.A.; Rangel, H.R.; Bello, F. Molecular characteristics and replication mechanism of dengue, zika and chikungunya arboviruses, and their treatments with natural extracts from plants: An updated review. EXCLI J. 2019, 18, 988–1006. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.F.; Teixeira, R.R.; Oliveira, A.S.; Souza, A.P.; Silva, M.L.; Paula, S.O. Potential Antivirals: Natural Products Targeting Replication Enzymes of Dengue and Chikungunya Viruses. Molecules 2017, 22, 505. [Google Scholar] [CrossRef]
- Brady, O.J.; Gething, P.W.; Bhatt, S.; Messina, J.P.; Brownstein, J.S.; Hoen, A.G.; Moyes, C.L.; Farlow, A.W.; Scott, T.W.; Hay, S.I. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl. Trop. Dis. 2012, 6, e1760. [Google Scholar] [CrossRef]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef]
- Waggoner, J.J.; Gresh, L.; Vargas, M.J.; Ballesteros, G.; Tellez, Y.; Soda, K.J.; Sahoo, M.K.; Nuñez, A.; Balmaseda, A.; Harris, E.; et al. Viremia and Clinical Presentation in Nicaraguan Patients Infected With Zika Virus, Chikungunya Virus, and Dengue Virus. Clin. Infect. Dis. 2016, 63, 1584–1590. [Google Scholar] [CrossRef]
- Sridhar, S.; Luedtke, A.; Langevin, E.; Zhu, M.; Bonaparte, M.; Machabert, T.; Savarino, S.; Zambrano, B.; Moureau, A.; Khromava, A.; et al. Effect of Dengue Serostatus on Dengue Vaccine Safety and Efficacy. N. Engl. J. Med. 2018, 379, 327–340. [Google Scholar] [CrossRef]
- Sanofi Pasteur. Press Release: Sanofi Updates Information on Dengue Vaccine. Available online: https://www.sanofi.com/en/media-room/press-releases/2017/2017-11-29-17-36-30 (accessed on 9 April 2020).
- Hadinegoro, S.R.; Arredondo-García, J.L.; Capeding, M.R.; Deseda, C.; Chotpitayasunondh, T.; Dietze, R.; Hj Muhammad Ismail, H.I.; Reynales, H.; Limkittikul, K.; Rivera-Medina, D.M.; et al. Efficacy and Long-Term Safety of a Dengue Vaccine in Regions of Endemic Disease. N. Engl. J. Med. 2015, 373, 1195–1206. [Google Scholar] [CrossRef]
- Campbell, G.; Hills, S.; Fischer, M.; Jacobson, J.; Hoke, C.; Hombach, J.; Marfin, A.; Solomon, T.; Tsai, T.; Tsui, V.; et al. Estimated global incidence of Japanese encephalitis. Bull. World Health Organ. 2011, 89, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Erlanger, T.E.; Weiss, S.; Keiser, J.; Utzinger, J.; Wiedenmayer, K. Past, Present, and Future of Japanese Encephalitis. Emerg. Infect. Dis. 2009, 15, 1–7. [Google Scholar] [CrossRef] [PubMed]
- King, N.J.C.; Getts, D.R.; Getts, M.T.; Rana, S.; Shrestha, B.; Kesson, A.M. Immunopathology of flavivirus infections. Immunol. Cell Biol. 2007, 85, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Solomon, T. Japanese encephalitis. J. Neurol. Neurosurg. Psychiatry 2000, 68, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Van Gessel, Y.; Klade, C.S.; Putnak, R.; Formica, A.; Krasaesub, S.; Spruth, M.; Cena, B.; Tungtaeng, A.; Gettayacamin, M.; Dewasthaly, S. Correlation of protection against Japanese encephalitis virus and JE vaccine (IXIARO®) induced neutralizing antibody titers. Vaccine 2011, 29, 5925–5931. [Google Scholar] [CrossRef]
- World Health Organization. Fact Sheet: Japanese Encephalitis. Available online: https://www.who.int/en/news-room/fact-sheets/detail/japanese-encephalitis (accessed on 7 April 2020).
- Yun, S.-I.; Lee, Y.-M. Japanese encephalitis. Hum. Vaccines Immunother. 2014, 10, 263–279. [Google Scholar] [CrossRef]
- Nash, D.; Mostashari, F.; Fine, A.; Miller, J.; O’Leary, D.; Murray, K.; Huang, A.; Rosenberg, A.; Greenberg, A.; Sherman, M.; et al. The Outbreak of West Nile Virus Infection in the New York City Area in 1999. N. Engl. J. Med. 2001, 344, 1807–1814. [Google Scholar] [CrossRef]
- Kramer, L.D.; Li, J.; Shi, P.-Y. West Nile virus. Lancet Neurol. 2007, 6, 171–181. [Google Scholar] [CrossRef]
- Nasci, R.S.; Savage, H.M.; White, D.J.; Miller, J.R.; Cropp, B.C.; Godsey, M.S.; Kerst, A.J.; Bennett, P.; Gottfried, K.; Lanciotti, R.S. West Nile Virus in Overwintering Culex Mosquitoes, New York City, 2000. Emerg. Infect. Dis. 2001, 7, 742–744. [Google Scholar] [CrossRef]
- Hayes, E.B.; Sejvar, J.J.; Zaki, S.R.; Lanciotti, R.S.; Bode, A.V.; Campbell, G.L. Virology, pathology, and clinical manifestations of West Nile virus disease. Emerg. Infect. Dis. 2005, 11, 1174–1179. [Google Scholar] [CrossRef]
- Mostashari, F.; Bunning, M.L.; Kitsutani, P.T.; Singer, D.A.; Nash, D.; Cooper, M.J.; Katz, N.; Liljebjelke, K.A.; Biggerstaff, B.J.; Fine, A.D.; et al. Epidemic West Nile encephalitis, New York, 1999: Results of a household-based seroepidemiological survey. Lancet 2001, 358, 261–264. [Google Scholar] [CrossRef]
- World Health Organization. Fact Sheet: West Nile Virus. Available online: https://www.who.int/news-room/fact-sheets/detail/west-nile-virus (accessed on 6 April 2020).
- Murray, K.O.; Walker, C.; Gould, E. The virology, epidemiology, and clinical impact of West Nile virus: A decade of advancements in research since its introduction into the Western Hemisphere. Epidemiol. Infect. 2011, 139, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Sejvar, J.J. The Long-Term Outcomes of Human West Nile Virus Infection. Clin. Infect. Dis. 2007, 44, 1617–1624. [Google Scholar] [CrossRef] [PubMed]
- Pacenti, M.; Sinigaglia, A.; Franchin, E.; Pagni, S.; Lavezzo, E.; Montarsi, F.; Capelli, G.; Barzon, L. Human West Nile Virus Lineage 2 Infection: Epidemiological, Clinical, and Virological Findings. Viruses 2020, 12, 458. [Google Scholar] [CrossRef] [PubMed]
- Dick, G.W.A.; Kitchen, S.F.; Haddow, A.J. Zika Virus (I). Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 509–520. [Google Scholar] [CrossRef]
- Duffy, M.R.; Chen, T.-H.; Hancock, W.T.; Powers, A.M.; Kool, J.L.; Lanciotti, R.S.; Pretrick, M.; Marfel, M.; Holzbauer, S.; Dubray, C.; et al. Zika Virus Outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med. 2009, 360, 2536–2543. [Google Scholar] [CrossRef] [PubMed]
- Cao-Lormeau, V.-M.; Roche, C.; Teissier, A.; Robin, E.; Berry, A.-L.; Mallet, H.-P.; Sall, A.A.; Musso, D. Zika Virus, French Polynesia, South Pacific, 2013. Emerg. Infect. Dis. 2014, 20, 1084–1086. [Google Scholar] [CrossRef] [PubMed]
- Dupont-Rouzeyrol, M.; O’Connor, O.; Calvez, E.; Daurès, M.; John, M.; Grangeon, J.-P.; Gourinat, A.-C. Co-infection with Zika and Dengue Viruses in 2 Patients, New Caledonia, 2014. Emerg. Infect. Dis. 2015, 21, 381–382. [Google Scholar] [CrossRef]
- Musso, D. Zika Virus Transmission from French Polynesia to Brazil. Emerg. Infect. Dis. 2015, 21, 1887. [Google Scholar] [CrossRef]
- Campos, G.S.; Bandeira, A.C.; Sardi, S.I. Zika Virus Outbreak, Bahia, Brazil. Emerg. Infect. Dis. 2015, 21, 1885–1886. [Google Scholar] [CrossRef]
- Mlakar, J.; Korva, M.; Tul, N.; Popović, M.; Poljšak-Prijatelj, M.; Mraz, J.; Kolenc, M.; Resman Rus, K.; Vesnaver Vipotnik, T.; Fabjan Vodušek, V.; et al. Zika Virus Associated with Microcephaly. N. Engl. J. Med. 2016, 374, 951–958. [Google Scholar] [CrossRef]
- Schuler-Faccini, L.; Ribeiro, E.M.; Feitosa, I.M.L.; Horovitz, D.D.G.; Cavalcanti, D.P.; Pessoa, A.; DoriquI, M.J.R.; Neri, J.I.; de Pina Neto, J.M.; Wanderley, H.Y.C.; et al. Possible Association Between Zika Virus Infection and Microcephaly—Brazil, 2015. Morb. Mortal. Wkly Rep. 2016, 65, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Mécharles, S.M.D.; Herrmann, C.M.D.; Poullain, P.M.D.; Tran, T.-H.M.B.; Deschamps, N.M.B.; Mathon, G.M.D.; Landais, A.M.D.; Breurec, S.M.D.; Lannuzel, A.P. Acute myelitis due to Zika virus infection. Lancet 2016, 387, 1481. [Google Scholar] [CrossRef]
- Carteaux, G.; Maquart, M.; Bedet, A.; Contou, D.; Brugières, P.; Fourati, S.; de Langavant, L.C.; de Broucker, T.; Brun-Buisson, C.; Leparc-Goffart, I.; et al. Zika Virus Associated with Meningoencephalitis. N. Engl. J. Med. 2016, 374, 1595–1596. [Google Scholar] [CrossRef] [PubMed]
- Powers, A.M. Chapter 1-The Origins of Chikungunya and Zika Viruses-History of the Discoveries. In Chikungunya and Zika Viruses; Higgs, S., Vanlandingham, D.L., Powers, A.M., Eds.; Academic Press: London, UK, 2018; pp. 1–13. [Google Scholar] [CrossRef]
- Robinson, M.C. An epidemic of virus disease in Southern Province, Tanganyika territory, in 1952–1953. Trans. R. Soc. Trop. Med. Hyg. 1955, 49, 28–32. [Google Scholar] [CrossRef]
- Caglioti, C.; Lalle, E.; Castilletti, C.; Carletti, F.; Capobianchi, M.R.; Bordi, L. Chikungunya virus infection: An overview. New Microbiol. 2013, 36, 211. [Google Scholar]
- Hammon, W.M.; Rudnick, A.; Sather, G.E. Viruses Associated with Epidemic Hemorrhagic Fevers of the Philippines and Thailand. Science 1960, 131, 1102–1103. [Google Scholar] [CrossRef]
- Weaver, S.C. Arrival of Chikungunya Virus in the New World: Prospects for Spread and Impact on Public Health. PLoS Negl. Trop. Dis. 2014, 8, e2921. [Google Scholar] [CrossRef]
- Sourisseau, M.; Schilte, C.; Casartelli, N.; Trouillet, C.; Guivel-Benhassine, F.; Rudnicka, D.; Sol-Foulon, N.; Roux, K.L.; Prevost, M.-C.; Fsihi, H.; et al. Characterization of Reemerging Chikungunya Virus. PLoS Pathog. 2007, 3, e89. [Google Scholar] [CrossRef]
- Schuffenecker, I.; Iteman, I.; Michault, A.; Murri, S.; Frangeul, L.; Vaney, M.-C.; Lavenir, R.; Pardigon, N.; Reynes, J.-M.; Pettinelli, F.; et al. Genome Microevolution of Chikungunya Viruses Causing the Indian Ocean Outbreak. PLoS Med. 2006, 3, e263. [Google Scholar] [CrossRef]
- Enserink, M. Massive Outbreak Draws Fresh Attention to Little-Known Virus. Science 2006, 311, 1085a. [Google Scholar] [CrossRef]
- Patterson, J.; Sammon, M.; Garg, M. Dengue, Zika and Chikungunya: Emerging Arboviruses in the New World. West. J. Emerg. Med. 2016, 17, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.Z.; Chu, J.J.H. The Interplay of Viral and Host Factors in Chikungunya Virus Infection: Targets for Antiviral Strategies. Viruses 2018, 10, 294. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.L.; Oh, H.M.L.; Ooi, E.E. Chikungunya in Singapore: Imported Cases Among Travelers Visiting Friends and Relatives. J. Travel Med. 2009, 16, 289–291. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hapuarachchi, H.C.; Bandara, K.B.A.T.; Sumanadasa, S.D.M.; Hapugoda, M.D.; Lai, Y.L.; Lee, K.S.; Tan, L.K.; Lin, R.T.P.; Ng, L.F.P.; Bucht, G.; et al. Re-emergence of Chikungunya virus in South-east Asia: Virological evidence from Sri Lanka and Singapore. J. Gen. Virol. 2010, 91, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Hochedez, P.; Hausfater, P.; Jaureguiberry, S.; Gay, F.; Datry, A.; Danis, M.; Bricaire, F.; Bossi, P. Cases of chikungunya fever imported from the islands of the South West Indian Ocean to Paris, France. Eurosurveillance 2007, 12, 13–14. [Google Scholar] [CrossRef]
- Abubakar, S.; Sam, I.C.; Wong, P.-F.; Matrahim, N.; Hooi, P.-S.; Roslan, N. Reemergence of Endemic Chikungunya, Malaysia. Emerg. Infect. Dis. 2007, 13, 147–149. [Google Scholar] [CrossRef]
- Lanciotti, R.S.; Kosoy, O.L.; Laven, J.J.; Panella, A.J.; Velez, J.O.; Lambert, A.J.; Campbell, G.L. Chikungunya Virus in US Travelers Returning from India, 2006. Emerg. Infect. Dis. 2007, 13, 764–767. [Google Scholar] [CrossRef]
- Azevedo, R.S.S.; Silva, E.V.P.; Carvalho, V.L.; Rodrigues, S.G.; Nunes Neto, J.P.; Monteiro, H.A.O.; Peixoto, V.S.; Chiang, J.O.; Nunes, M.R.T.; Vasconcelos, P.F.C. Mayaro Fever Virus, Brazilian Amazon. Emerg. Infect. Dis. 2009, 15, 1830. [Google Scholar] [CrossRef]
- Auguste, A.J.; Liria, J.; Forrester, N.L.; Giambalvo, D.; Moncada, M.; Long, K.C.; Morón, D.; de Manzione, N.; Tesh, R.B.; Halsey, E.S.; et al. Evolutionary and Ecological Characterization of Mayaro Virus Strains Isolated during an Outbreak, Venezuela, 2010. Emerg. Infect. Dis. 2015, 21, 1742–1750. [Google Scholar] [CrossRef]
- Zuchi, N.; Heinen, L.B.D.S.; Santos, M.A.M.D.; Pereira, F.C.; Slhessarenko, R.D. Molecular detection of Mayaro virus during a dengue outbreak in the state of Mato Grosso, Central-West Brazil. Mem. Inst. Oswaldo Cruz 2014, 109, 820–823. [Google Scholar] [CrossRef]
- Long, K.C.; Ziegler, S.A.; Thangamani, S.; Hausser, N.L.; Kocher, T.J.; Higgs, S.; Tesh, R.B. Experimental Transmission of Mayaro Virus by Aedes aegypti. Am. J. Trop. Med. Hyg. 2011, 85, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Halsey, E.S.; Siles, C.; Guevara, C.; Vilcarromero, S.; Jhonston, E.J.; Ramal, C.; Aguilar, P.V.; Ampuero, J.S. Mayaro Virus Infection, Amazon Basin region, Peru, 2010–2013. Emerg. Infect. Dis. 2013, 19, 1839. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Mota, M.T.; Ribeiro, M.R.; Vedovello, D.; Nogueira, M.L. Mayaro virus: A neglected arbovirus of the Americas. Future Virol. 2015, 10, 1109–1122. [Google Scholar] [CrossRef]
- Vieira, C.J.d.S.P.; Silva, D.J.F.d.; Barreto, E.S.; Siqueira, C.E.H.; Colombo, T.E.; Ozanic, K.; Schmidt, D.J.; Drumond, B.P.; Mondini, A.; Nogueira, M.L.; et al. Detection of Mayaro virus infections during a dengue outbreak in Mato Grosso, Brazil. Acta Trop. 2015, 147, 12–16. [Google Scholar] [CrossRef]
- Mackay, I.M.; Arden, K.E. Mayaro virus: A forest virus primed for a trip to the city? Microbes Infect. 2016, 18, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Hotez, P.J.; Murray, K.O. Dengue, West Nile virus, chikungunya, Zika-and now Mayaro? PLoS Negl. Trop. Dis. 2017, 11, e0005462. [Google Scholar] [CrossRef]
- World Health Organization. WHO Traditional Medicine Strategy 2002–2005; WHO: Geneva, Switzerland, 2002. [Google Scholar]
- Clain, E.; Sinigaglia, L.; Koishi, A.C.; Gorgette, O.; Gadea, G.; Viranaicken, W.; Krejbich-Trotot, P.; Mavingui, P.; Desprès, P.; dos Santos, C.N.D.; et al. Extract from Aphloia theiformis, an edible indigenous plant from Reunion Island, impairs Zika virus attachment to the host cell surface. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Dos Santos, A.E.; Kuster, R.M.; Yamamoto, K.A.; Salles, T.S.; Campos, R.; De Meneses, M.D.; Soares, M.R.; Ferreira, D. Quercetin and quercetin 3-O-glycosides from Bauhinia longifolia (Bong.) Steud. show anti-Mayaro virus activity. Parasites Vectors 2014, 7, 130. [Google Scholar] [CrossRef]
- Johari, J.; Kianmehr, A.; Mustafa, M.; Abubakar, S.; Zandi, K. Antiviral Activity of Baicalein and Quercetin against the Japanese Encephalitis Virus. Int. J. Mol. Sci. 2012, 13, 16785–16795. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C. Flavonoid Antioxidants. Curr. Med. Chem. 2001, 8, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Orhan, D.D.; Özçelik, B.; Özgen, S.; Ergun, F. Antibacterial, antifungal, and antiviral activities of some flavonoids. Microbiol. Res. 2010, 165, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Kaul, T.N.; Middleton, E.; Ogra, P.L. Antiviral Effect of Flavonoids on Human Viruses. J. Med. Virol. 1985, 15, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Chahar, M.K.; Sharma, N.; Dobhal, M.P.; Joshi, Y.C. Flavonoids: A versatile source of anticancer drugs. Pharmacogn. Rev. 2011, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Calvo, Á.; Jiménez de Oya, N.; Martín-Acebes, M.A.; Garcia-Moruno, E.; Saiz, J.-C. Antiviral Properties of the Natural Polyphenols Delphinidin and Epigallocatechin Gallate against the Flaviviruses West Nile Virus, Zika Virus, and Dengue Virus. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef]
- Colpitts, C.C.; Schang, L.M. A Small Molecule Inhibits Virion Attachment to Heparan Sulfate- or Sialic Acid-Containing Glycans. J. Virol. 2014, 88, 7806–7817. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Thomas, S.G.; Kunnumakkara, A.B.; Sundaram, C.; Harikumar, K.B.; Sung, B.; Tharakan, S.T.; Misra, K.; Priyadarsini, I.K.; Rajasekharan, K.N.; et al. Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature. Biochem. Pharmacol. 2008, 76, 1590–1611. [Google Scholar] [CrossRef] [PubMed]
- Lani, R.; Hassandarvish, P.; Shu, M.-H.; Phoon, W.H.; Chu, J.J.H.; Higgs, S.; Vanlandingham, D.; Abu Bakar, S.; Zandi, K. Antiviral activity of selected flavonoids against Chikungunya virus. Antivir. Res. 2016, 133, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, E.; Teoh, B.-T.; Sam, S.-S.; Lani, R.; Hassandarvish, P.; Chik, Z.; Yueh, A.; Abubakar, S.; Zandi, K. Baicalin, a metabolite of baicalein with antiviral activity against dengue virus. Sci. Rep. 2014, 4, 5452. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, A.; Pilankatta, R.; Teramoto, T.; Sajith, A.M.; Nwulia, E.; Kulkarni, A.; Padmanabhan, R. Inhibition of dengue virus by curcuminoids. Antivir. Res. 2019, 162, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-Y.; Chen, D.-Y.; Wen, H.-W.; Ou, J.-L.; Chiou, S.-S.; Chen, J.-M.; Wong, M.-L.; Hsu, W.-L. Inhibition of enveloped viruses infectivity by curcumin. PLoS ONE 2013, 8, e62482. [Google Scholar] [CrossRef] [PubMed]
- Mounce, B.C.; Cesaro, T.; Carrau, L.; Vallet, T.; Vignuzzi, M. Curcumin inhibits Zika and chikungunya virus infection by inhibiting cell binding. Antivir. Res. 2017, 142, 148–157. [Google Scholar] [CrossRef]
- Weber, C.; Sliva, K.; Von Rhein, C.; Kümmerer, B.M.; Schnierle, B.S. The green tea catechin, epigallocatechin gallate inhibits chikungunya virus infection. Antivir. Res. 2015, 113, 1–3. [Google Scholar] [CrossRef]
- Carneiro, B.M.; Batista, M.N.; Braga, A.C.S.; Nogueira, M.L.; Rahal, P. The green tea molecule EGCG inhibits Zika virus entry. Virology 2016, 496, 215–218. [Google Scholar] [CrossRef]
- Kaur, P.; Thiruchelvan, M.; Lee, R.C.H.; Chen, H.; Chen, K.C.; Ng, M.L.; Chu, J.J.H. Inhibition of Chikungunya Virus Replication by Harringtonine, a Novel Antiviral That Suppresses Viral Protein Expression. Antimicrob. Agents Chemother. 2013, 57, 155–167. [Google Scholar] [CrossRef]
- Fang, C.-Y.; Chen, S.-J.; Wu, H.-N.; Ping, Y.-H.; Lin, C.-Y.; Shiuan, D.; Chen, C.-L.; Lee, Y.-R.; Huang, K.-J. Honokiol, a Lignan Biphenol Derived from the Magnolia Tree, Inhibits Dengue Virus Type 2 Infection. Viruses 2015, 7, 4894–4910. [Google Scholar] [CrossRef]
- Gaudry, A.; Bos, S.; Viranaicken, W.; Roche, M.; Krejbich-Trotot, P.; Gadea, G.; Desprès, P.; El-Kalamouni, C. The Flavonoid Isoquercitrin Precludes Initiation of Zika Virus Infection in Human Cells. Int. J. Mol. Sci. 2018, 19, 1093. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, Z.; Du, J.; Hu, Y.; Liu, L.; Yang, F.; Jin, Q. Anti-Japanese-Encephalitis-Viral Effects of Kaempferol and Daidzin and Their RNA-Binding Characteristics. PLoS ONE 2012, 7, e30259. [Google Scholar] [CrossRef] [PubMed]
- Frabasile, S.; Koishi, A.C.; Kuczera, D.; Silveira, G.F.; Verri, W.A., Jr.; Duarte Dos Santos, C.N.; Bordignon, J. The citrus flavanone naringenin impairs dengue virus replication in human cells. Sci. Rep. 2017, 7, 41864. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.L.; Loe, M.W.C.; Lee, R.C.H.; Chu, J.J.H. Antiviral activity of pinocembrin against Zika virus replication. Antivir. Res. 2019, 167, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Zandi, K.; Teoh, B.-T.; Sam, S.-S.; Wong, P.-F.; Mustafa, M.R.; Abubakar, S. Antiviral activity of four types of bioflavonoid against dengue virus type-2. Virol. J. 2011, 8, 560. [Google Scholar] [CrossRef] [PubMed]
- Malakar, S.; Sreelatha, L.; Dechtawewat, T.; Noisakran, S.; Yenchitsomanus, P.-T.; Chu, J.J.H.; Limjindaporn, T. Drug repurposing of quinine as antiviral against dengue virus infection. Virus Res. 2018, 255, 171–178. [Google Scholar] [CrossRef]
- Mohd, A.; Zainal, N.; Tan, K.-K.; Abubakar, S. Resveratrol affects Zika virus replication in vitro. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- Lee, J.K.; Chui, J.L.M.; Lee, R.C.H.; Kong, H.Y.; Chin, W.-X.; Chu, J.J.H. Antiviral activity of ST081006 against the dengue virus. Antivir. Res. 2019, 171, 104589. [Google Scholar] [CrossRef]
- Ellan, K.; Thayan, R.; Raman, J.; Hidari, K.I.P.J.; Ismail, N.; Sabaratnam, V. Anti-viral activity of culinary and medicinal mushroom extracts against dengue virus serotype 2: An in-vitro study. BMC Complement. Altern. Med. 2019, 19, 260. [Google Scholar] [CrossRef]
- Clain, E.; Haddad, J.G.; Koishi, A.C.; Sinigaglia, L.; Rachidi, W.; Desprès, P.; Duarte Dos Santos, C.N.; Guiraud, P.; Jouvenet, N.; El Kalamouni, C. The Polyphenol-Rich Extract from Psiloxylon mauritianum, an Endemic Medicinal Plant from Reunion Island, Inhibits the Early Stages of Dengue and Zika Virus Infection. Int. J. Mol. Sci. 2019, 20, 1860. [Google Scholar] [CrossRef]
- Lani, R.; Hassandarvish, P.; Chiam, C.W.; Moghaddam, E.; Chu, J.J.H.; Rausalu, K.; Merits, A.; Higgs, S.; Vanlandingham, D.; Abu Bakar, S.; et al. Antiviral activity of silymarin against chikungunya virus. Sci. Rep. 2015, 5, 11421. [Google Scholar] [CrossRef]
- Camini, F.C.; da Silva, T.F.; da Silva Caetano, C.C.; Almeida, L.T.; Ferraz, A.C.; Alves Vitoreti, V.M.; de Mello Silva, B.; de Queiroz Silva, S.; de Magalhaes, J.C.; de Brito Magalhaes, C.L. Antiviral activity of silymarin against Mayaro virus and protective effect in virus-induced oxidative stress. Antivir. Res. 2018, 158, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.; Sanderson, I.R.; MacDonald, T.T. Curcumin as a therapeutic agent: The evidence from in vitro, animal and human studies. Br. J. Nutr. 2010, 103, 1545–1557. [Google Scholar] [CrossRef] [PubMed]
- Lao, C.D.; Ruffin, M.T.; Normolle, D.; Heath, D.D.; Murray, S.I.; Bailey, J.M.; Boggs, M.E.; Crowell, J.; Rock, C.L.; Brenner, D.E. Dose escalation of a curcuminoid formulation. BMC Complement. Altern. Med. 2006, 6. [Google Scholar] [CrossRef] [PubMed]
- Strimpakos, A.S.; Sharma, R.A. Curcumin: Preventive and therapeutic properties in laboratory studies and clinical trials. Antioxid. Redox Signal. 2008, 10, 511–546. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.A. Phase I Clinical Trial of Oral Curcumin: Biomarkers of Systemic Activity and Compliance. Clin. Cancer Res. 2004, 10, 6847–6854. [Google Scholar] [CrossRef]
- Colpitts, C.C.; Schang, L.M.; Rachmawati, H.; Frentzen, A.; Pfaender, S.; Behrendt, P.; Brown, R.J.P.; Bankwitz, D.; Steinmann, J.; Ott, M.; et al. Turmeric curcumin inhibits entry of all hepatitis C virus genotypes into human liver cells. Gut 2014, 63, 1137–1149. [Google Scholar] [CrossRef]
- Mazumder, A.; Raghavan, K.; Weinstein, J.; Kohn, K.W.; Pommier, Y. Inhibition of human immunodeficiency virus type-1 integrase by curcumin. Biochem. Pharmacol. 1995, 49, 1165–1170. [Google Scholar] [CrossRef]
- Calland, N.; Albecka, A.; Belouzard, S.; Wychowski, C.; Duverlie, G.; Descamps, V.; Hober, D.; Dubuisson, J.; Rouillé, Y.; Séron, K. (−)-Epigallocatechin-3-gallate is a new inhibitor of hepatitis C virus entry. Hepatology 2012, 55, 720–729. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Honda, M.; Ikigai, H.; Hara, Y.; Shimamura, T. Inhibitory effects of (−)-epigallocatechin gallate on the life cycle of human immunodeficiency virus type 1 (HIV-1). Antivir. Res. 2002, 53, 19–34. [Google Scholar] [CrossRef]
- Weisburg, J.H.; Weissman, D.B.; Sedaghat, T.; Babich, H. In Vitro Cytotoxicity of Epigallocatechin Gallate and Tea Extracts to Cancerous and Normal Cells from the Human Oral Cavity. Basic Clin. Pharmacol. Toxicol. 2004, 95, 191–200. [Google Scholar] [CrossRef]
- Chu, K.O.; Wang, C.C.; Chu, C.Y.; Choy, K.W.; Pang, C.P.; Rogers, M.S. Uptake and distribution of catechins in fetal organs following in utero exposure in rats. Hum. Reprod. 2007, 22, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Wang, W.; Li, Q.; Wang, J. The natural flavonoid pinocembrin: Molecular targets and potential therapeutic applications. Mol. Neurobiol. 2016, 53, 1794–1801. [Google Scholar] [CrossRef]
- Shen, X.; Liu, Y.; Luo, X.; Yang, Z. Advances in Biosynthesis, Pharmacology, and Pharmacokinetics of Pinocembrin, a Promising Natural Small-Molecule Drug. Molecules 2019, 24, 2323. [Google Scholar] [CrossRef]
- Yang, Z.-H.; Sun, X.; Qi, Y.; Mei, C.; Sun, X.-B.; Du, G.-H. Uptake characteristics of pinocembrin and its effect on p-glycoprotein at the blood–brain barrier inin vitrocell experiments. J. Asian Nat. Prod. Res. 2012, 14, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Ying, P.; Yan, B.; Xue, W.; Li, K.; Shi, A.; Sun, T.; Yan, J.; Hu, X. Pharmacokinetics, safety, and tolerability of single and multiple-doses of pinocembrin injection administered intravenously in healthy subjects. J. Ethnopharmacol. 2015, 168, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Achan, J.; Talisuna, A.O.; Erhart, A.; Yeka, A.; Tibenderana, J.K.; Baliraine, F.N.; Rosenthal, P.J.; D’Alessandro, U. Quinine, an old anti-malarial drug in a modern world: Role in the treatment of malaria. Malar. J. 2011, 10, 144. [Google Scholar] [CrossRef] [PubMed]
- Baroni, A.; Paoletti, I.; Ruocco, E.; Ayala, F.; Corrado, F.; Wolf, R.; Tufano, M.A.; Donnarumma, G. Antiviral effects of quinine sulfate on HSV-1 HaCat cells infected: Analysis of the molecular mechanisms involved. J. Dermatol. Sci. 2007, 47, 253–255. [Google Scholar] [CrossRef]
- Marois, I.; Cloutier, A.; Meunier, I.; Weingartl, H.M.; Cantin, A.M.; Richter, M.V. Inhibition of Influenza Virus Replication by Targeting Broad Host Cell Pathways. PLoS ONE 2014, 9, e110631. [Google Scholar] [CrossRef]
- Ashburn, T.T.; Thor, K.B. Drug repositioning: Identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 2004, 3, 673–683. [Google Scholar] [CrossRef]
- Federico, A.; Dallio, M.; Loguercio, C. Silymarin/Silybin and Chronic Liver Disease: A Marriage of Many Years. Molecules 2017, 22, 191. [Google Scholar] [CrossRef]
- Dunnick, J.K.; Singh, B.; Nyska, A.; Peckham, J.; Kissling, G.E.; Sanders, J.M. Investigating the Potential for Toxicity from Long-Term Use of the Herbal Products, Goldenseal and Milk Thistle. Toxicol. Pathol. 2011, 39, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Saller, R.; Brignoli, R.; Melzer, J.; Meier, R. An Updated Systematic Review with Meta-Analysis for the Clinical Evidence of Silymarin. Complement. Med. Res. 2008, 15, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Wagoner, J.; Negash, A.; Kane, O.J.; Martinez, L.E.; Nahmias, Y.; Bourne, N.; Owen, D.M.; Grove, J.; Brimacombe, C.; McKeating, J.A.; et al. Multiple effects of silymarin on the hepatitis C virus lifecycle. Hepatology 2010, 51, 1912–1921. [Google Scholar] [CrossRef]
- Wilder-Smith, A.; Vannice, K.; Durbin, A.; Hombach, J.; Thomas, S.J.; Irani, T.; Simmons, C.P. Zika vaccines and therapeutics: Landscape analysis and challenges ahead. BMC Med. 2018, 16, 84. [Google Scholar] [CrossRef] [PubMed]
- Saiz, J.-C.; Oya, N.; Blázquez, A.-B.; Escribano-Romero, E.; Martín-Acebes, M. Host-Directed Antivirals: A Realistic Alternative to Fight Zika Virus. Viruses 2018, 10, 453. [Google Scholar] [CrossRef] [PubMed]
- Schiborr, C.; Kocher, A.; Behnam, D.; Jandasek, J.; Toelstede, S.; Frank, J. The oral bioavailability of curcumin from micronized powder and liquid micelles is significantly increased in healthy humans and differs between sexes. Mol. Nutr. Food Res. 2014, 58, 516–527. [Google Scholar] [CrossRef]
- Sasaki, H.; Sunagawa, Y.; Takahashi, K.; Imaizumi, A.; Fukuda, H.; Hashimoto, T.; Wada, H.; Katanasaka, Y.; Kakeya, H.; Fujita, M.; et al. Innovative Preparation of Curcumin for Improved Oral Bioavailability. Biol. Pharm. Bull. 2011, 34, 660–665. [Google Scholar] [CrossRef]
- Cheng, C.; Peng, S.; Li, Z.; Zou, L.; Liu, W.; Liu, C. Improved bioavailability of curcumin in liposomes prepared using a pH-driven, organic solvent-free, easily scalable process. RSC Adv. 2017, 7, 25978–25986. [Google Scholar] [CrossRef]
- Minnelli, C.; Laudadio, E.; Galeazzi, R.; Barucca, G.; Notarstefano, V.; Cantarini, M.; Armeni, T.; Mobbili, G. Encapsulation of a Neutral Molecule into a Cationic Clay Material: Structural Insight and Cytotoxicity of Resveratrol/Layered Double Hydroxide/BSA Nanocomposites. Nanomaterials 2019, 10, 33. [Google Scholar] [CrossRef]
- Minnelli, C.; Galeazzi, R.; Laudadio, E.; Amici, A.; Rusciano, D.; Armeni, T.; Cantarini, M.; Stipa, P.; Mobbili, G. Monoalkylated Epigallocatechin-3-gallate (C18-EGCG) as Novel Lipophilic EGCG Derivative: Characterization and Antioxidant Evaluation. Antioxidants 2020, 9, 208. [Google Scholar] [CrossRef]
- Nekhai, S.; Kumari, N.; Kulkarni, A.; Lin, X.; McLean, C.; Ammosova, T.; Ivanov, A.; Hipolito, M.; Nwulia, E. Inhibition of HIV-1 by curcumin A, a novel curcumin analog. Drug Des. Dev. Ther. 2015, 5051–5060. [Google Scholar] [CrossRef] [PubMed]
- Li, A.-N.; Li, S.; Zhang, Y.-J.; Xu, X.-R.; Chen, Y.-M.; Li, H.-B. Resources and Biological Activities of Natural Polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef] [PubMed]
| Virus | Family/Genus | Transmission Vectors | Symptoms | Treatment Available? | Vaccine Available? |
|---|---|---|---|---|---|
| DENV | Flaviviridae (genus Flavivirus) | Aedes aegypti and Aedes albopticus | Fever, hemorrhagic fever. | No | Yes [15] |
| JEV | Flaviviridae (genus Flavivirus) | Culex spp. | Fever, headache, seizures, encephalitis. | No | Yes [16] |
| WNV | Flaviviridae (genus Flavivirus) | Culex spp. | Fever, muscle weakness, encephalitis, meningitis. | No | Not for humans. Vaccines for horses are available [17,18]. |
| ZIKV | Flaviviridae (genus Flavivirus) | Aedes spp. | Fever, arthralgia, and myalgia. Neurological manifestations. | No | No |
| CHIKV | Togaviridae (genus Alphavirus) | Aedes spp. | Fever, arthralgia, and myalgia. | No | No |
| S/N | Compound Name/Chemical Structure | Source | Virus(es) Affected | Proposed Mode of Inhibition | Assay Used | Ref. |
|---|---|---|---|---|---|---|
| 1 | Baicalein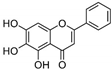 | Roots of Scutellaria baicalensis and Scutellaria lateriflora | CHIKV |
|
| [95] |
| JEV |
|
| [83] | |||
| 2 | Baicalin (main metabolite of baicalein)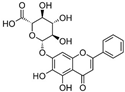 | Roots of Scutellaria baicalensis and Scutellaria lateriflora | DEN-2 |
|
| [96] |
| 3 | Curcumin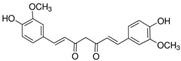 | Curcuma longa (turmeric) | DEN-2 |
|
| [97] |
|
| [98] | ||||
| CHIKV |
|
| [99] | |||
| ZIKV JEV |
|
| [99] | |||
|
| [98] | ||||
| 4 | Delphinidin | Pigment found in various flowers and fruits | DEN-1 to -4 |
|
| [92] |
| WNV |
|
| [92] | |||
| ZIKV |
|
| [92] | |||
| 5 | Epigallocatechin gallate (EGCG)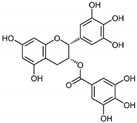 | Leaves of Camellia sinensis (green tea) | DEN-1 to -4 |
|
| [92] |
| CHIKV |
|
| [100] | |||
| WNV |
|
| [92] | |||
| ZIKV |
|
| [101] | |||
|
| [92] | ||||
| 6 | Fisetin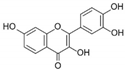 | Pigment found in various flowers and fruits | CHIKV |
|
| [95] |
| 7 | Harringtonine | Cephalotaxus harringtonia (Japanese plum yew) | CHIKV |
|
| [102] |
| 8 | Honokiol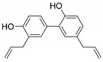 | Bark or seed cones of Magnolia tree | DEN-2 |
|
| [103] |
| 9 | Isoquercitrin | Various plants, including leaves of Annona squamosa (sugar apple) and Camellia sinensis (green tea) | ZIKV |
|
| [104] |
| 10 | Kaempferol | Various plant sources including tea, broccoli, grapefruit and apples | JEV |
|
| [105] |
| 11 | Naringenin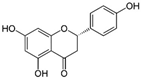 | Citrus fruits such as grapefruit, bergamot, and tomatoes | DEN-1 to -4 |
|
| [106] |
| 12 | Pinocembrin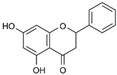 | Honey, tea and red wine | ZIKV |
|
| [107] |
| 13 | Quercetin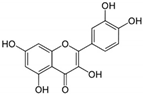 | Bauhinia longifolia leaves, tea, apple, onion and tomato | DEN-2 |
|
| [108] |
| MAYV * |
|
| [82] | |||
| 14 | Quercetagetin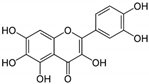 | Leaves of Eriocaulon species. | CHIKV |
|
| [95] |
| 15 | Quinine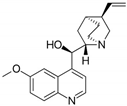 | Cinchona tree | DEN-1 to -4 |
|
| [109] |
| 16 | Resveratrol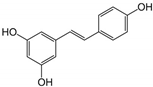 | Grapes and peanuts | ZIKV |
|
| [110] |
| 17 | ST081006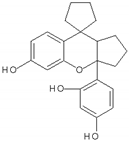 | Synthetic flavonoid from flavonoid-derivative library | DEN-1 to -4 |
|
| [111] |
| S/N | Plant Extract (active compound) | Source | Virus(es) Affected | Mode of Inhibition | Assay Used | Ref. |
|---|---|---|---|---|---|---|
| 1 | Aphloia theiformis extract^ | Aphloia theiformis | DEN-1 to -4 |
|
| [81] |
| ZIKV |
| |||||
| 2 | Mushroom extracts^ | L. rhinocerotis, P. giganteus, H. erinaceus and S. commune | DEN-2 |
|
| [112] |
| 3 | Psiloxylon mauritianum extract^ | Aerial parts of Psiloxylon mauritianum | DEN-1 to -4 |
|
| [113] |
| ZIKV |
| |||||
| 4 | Silymarin complex (Silybin) | Seeds of Silybum marianum (Milk thistle) | CHIKV |
|
| [114] |
| MAYV * |
|
| [115] |
| Type of Inhibitors | Antiviral Natural Compounds 1 |
|---|---|
| DENV inhibitors | Baicalein, curcumin, delphinidin, EGCG, honokiol, naringenin, quercetin, quinine, and ST081006 |
| JEV inhibitors | Baicalein, curcumin, and kaempferol |
| WNV inhibitors | Delphinidin and EGCG |
| ZIKV inhibitors | Curcumin, delphinidin, EGCG, isoquercitrin, pinocembrin, and resveratrol |
| CHIKV inhibitors | Baicalein, curcumin, EGCG, fisetin, harringtonine, and quercetagetin |
| MAYV inhibitor | Quercetin |
| Type of Inhibitors | Antiviral Plant Extracts 1 |
|---|---|
| DENV inhibitors | Aphloia theiformis extract, mushroom extracts and Psiloxylon mauritianum extract |
| ZIKV inhibitors | Aphloia theiformis extract and Psiloxylon mauritianum extract |
| CHIKV inhibitors | Silymarin complex |
| MAYV inhibitor | Silymarin complex |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goh, V.S.L.; Mok, C.-K.; Chu, J.J.H. Antiviral Natural Products for Arbovirus Infections. Molecules 2020, 25, 2796. https://doi.org/10.3390/molecules25122796
Goh VSL, Mok C-K, Chu JJH. Antiviral Natural Products for Arbovirus Infections. Molecules. 2020; 25(12):2796. https://doi.org/10.3390/molecules25122796
Chicago/Turabian StyleGoh, Vanessa Shi Li, Chee-Keng Mok, and Justin Jang Hann Chu. 2020. "Antiviral Natural Products for Arbovirus Infections" Molecules 25, no. 12: 2796. https://doi.org/10.3390/molecules25122796
APA StyleGoh, V. S. L., Mok, C.-K., & Chu, J. J. H. (2020). Antiviral Natural Products for Arbovirus Infections. Molecules, 25(12), 2796. https://doi.org/10.3390/molecules25122796







