Hormesis and Ginseng: Ginseng Mixtures and Individual Constituents Commonly Display Hormesis Dose Responses, Especially for Neuroprotective Effects
Abstract
:1. Introduction
2. Search Strategy
3. Ginseng Constituent RG1
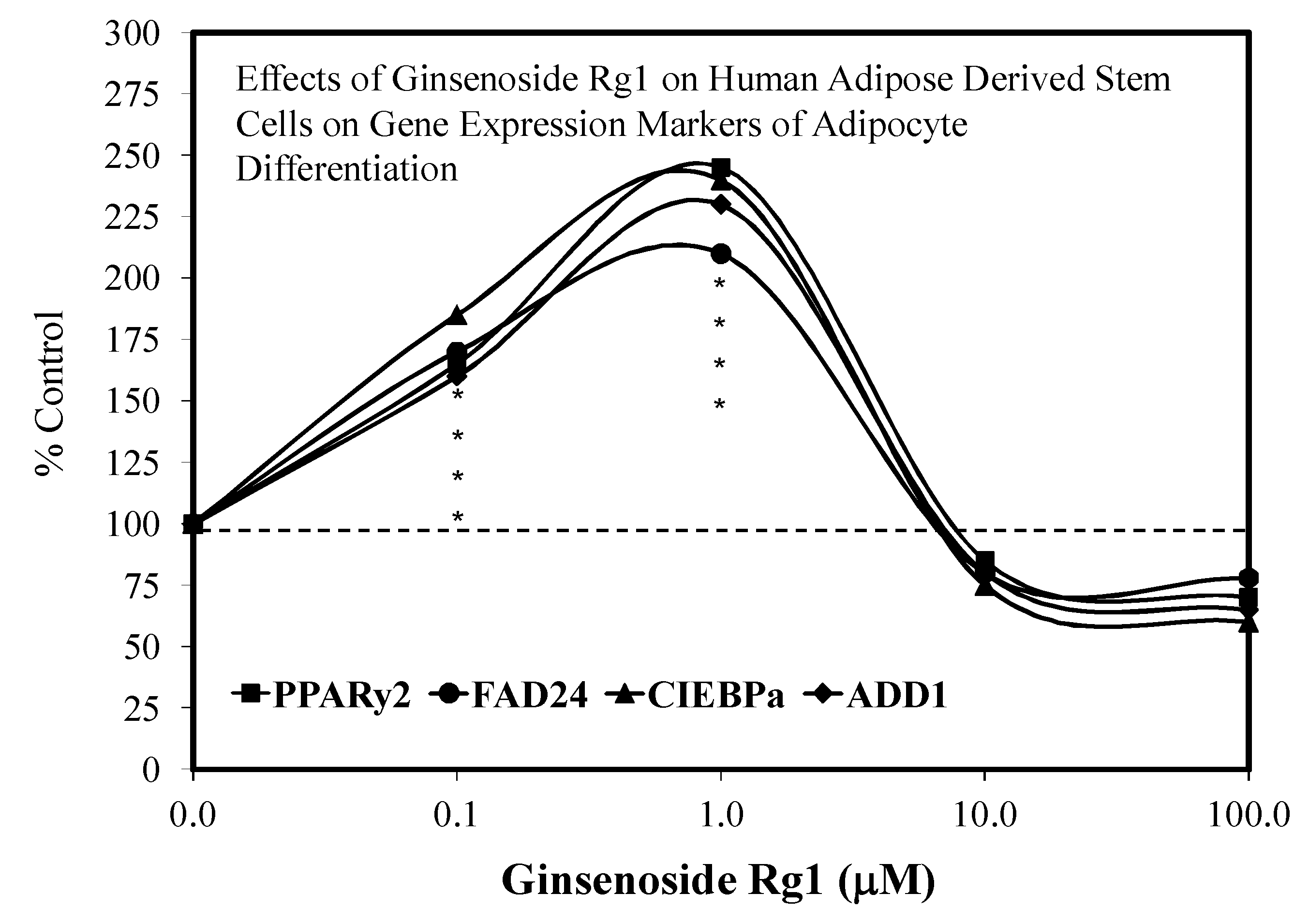
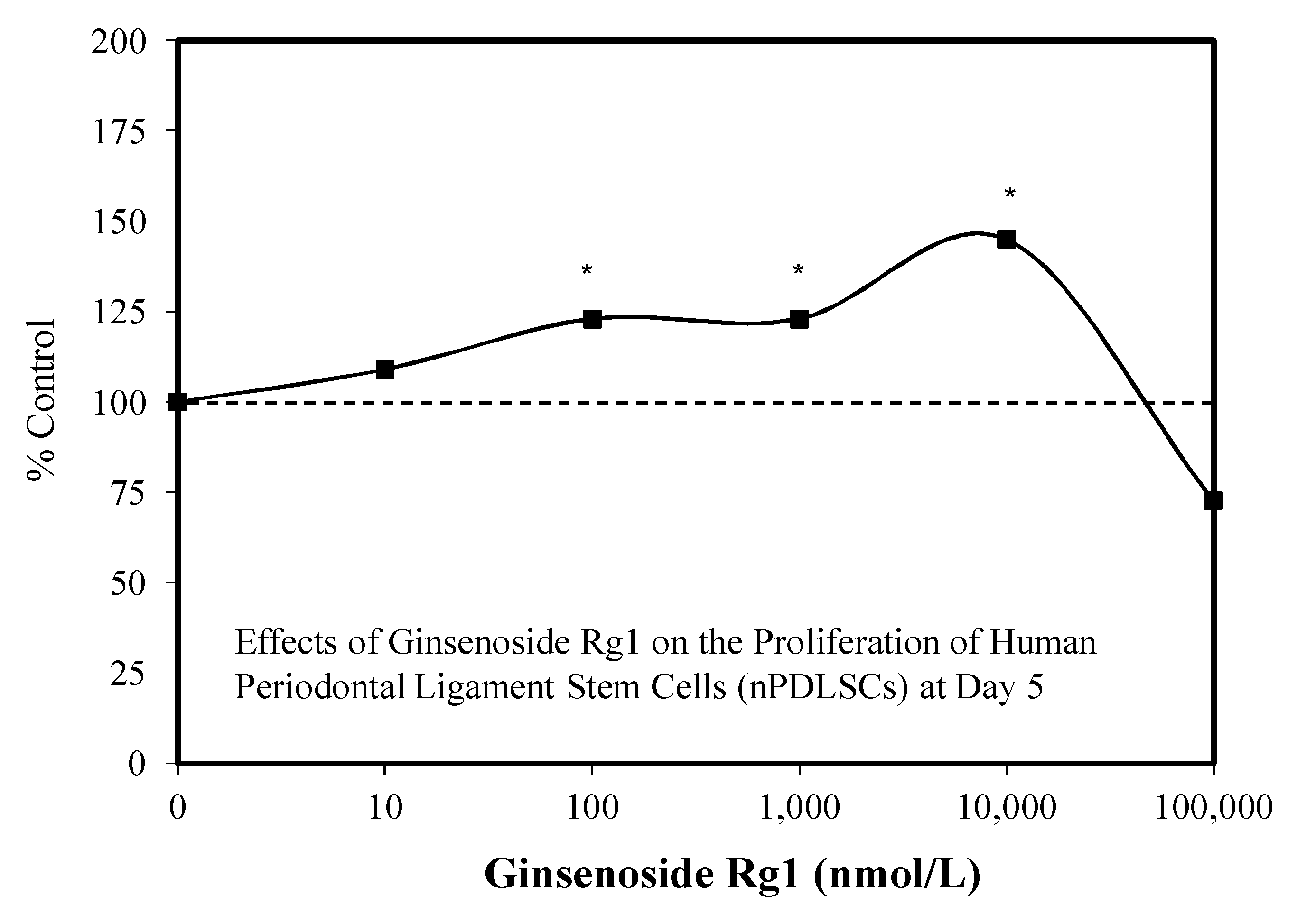
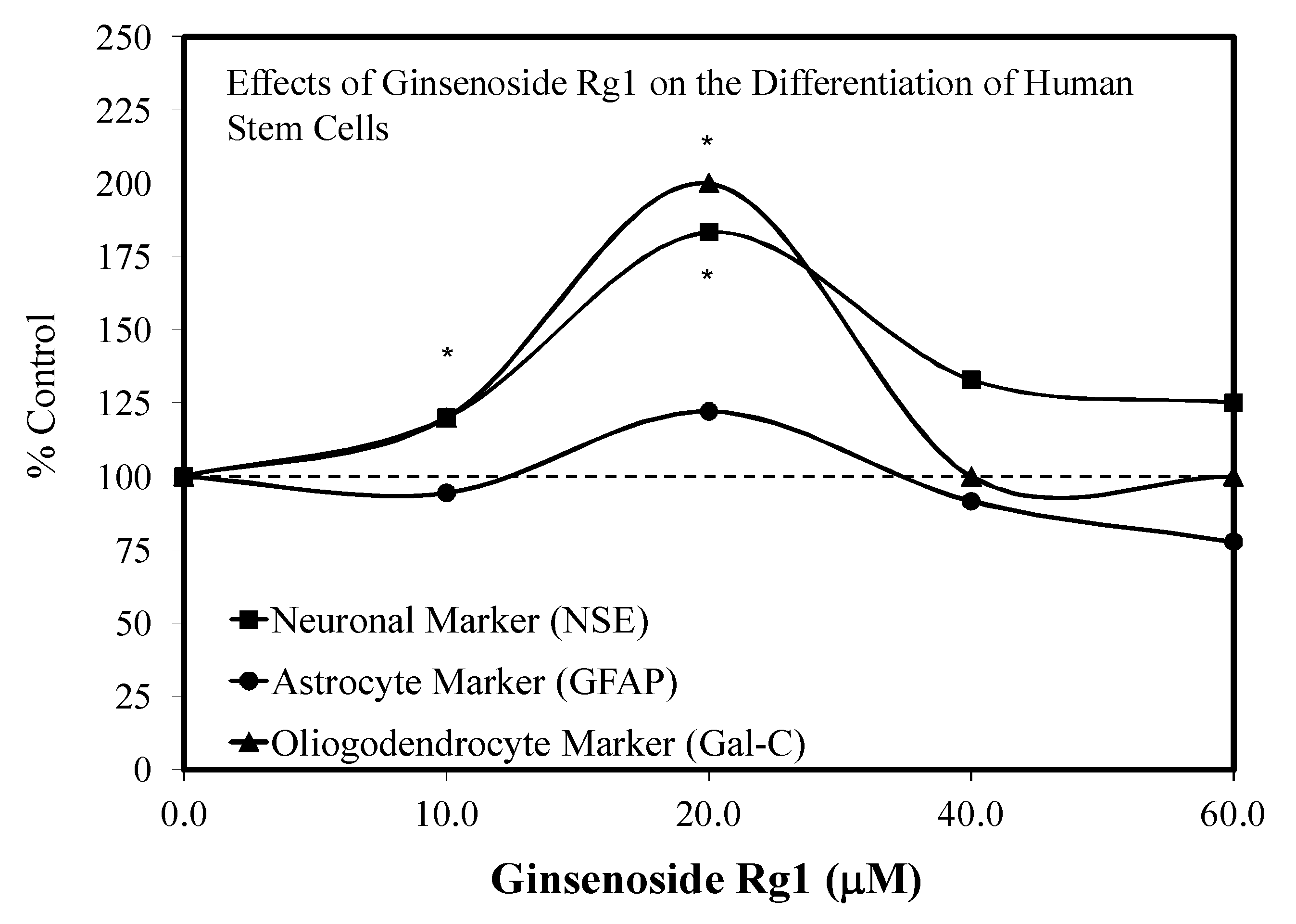
3.1. Rg1-Stem Cell Proliferation and Differentiation
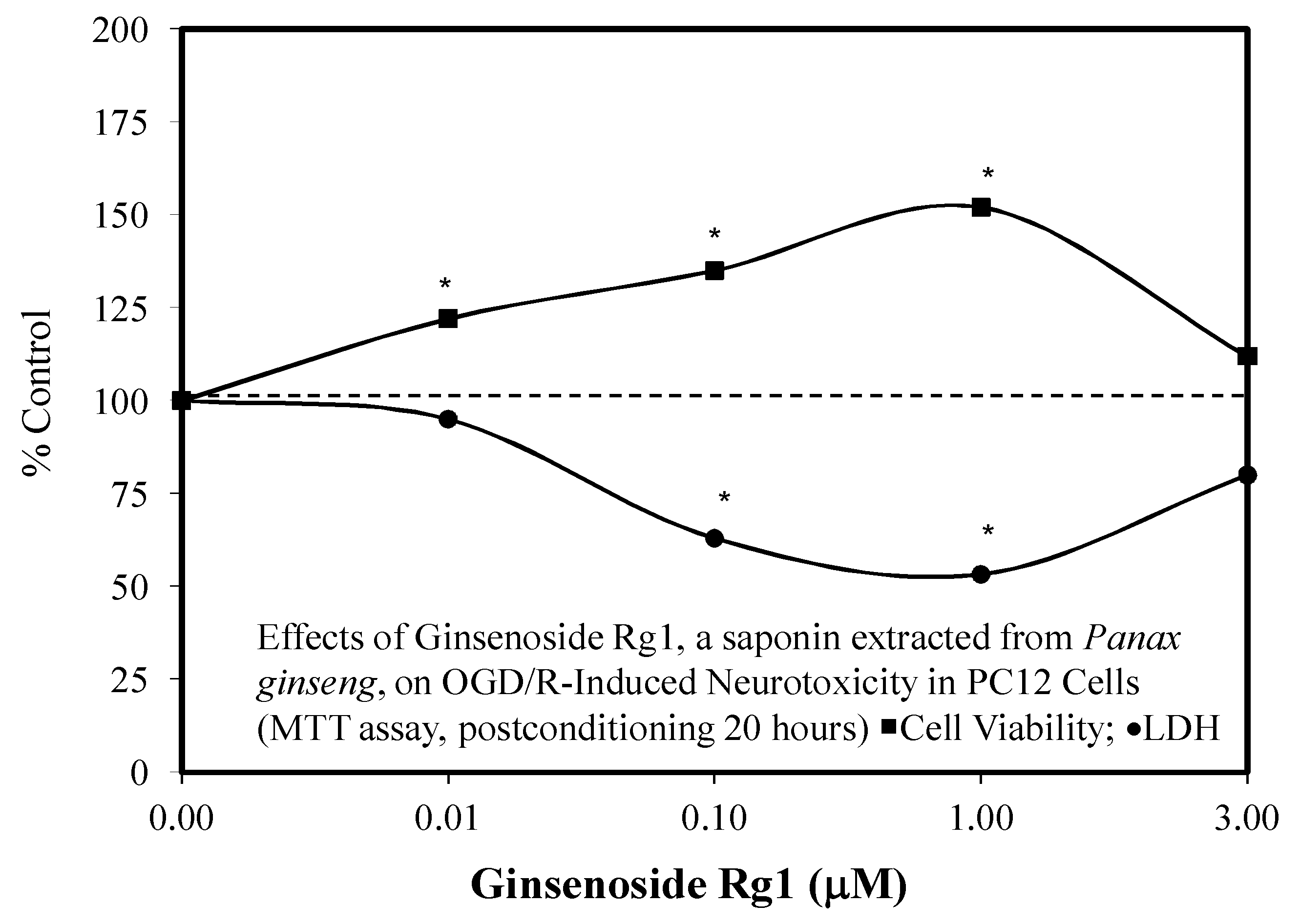
3.2. Rg1 Neuroprotection
3.3. Parkinson’s Disease
3.3.1. Introduction
3.3.2. In Vitro Studies
3.3.3. In Vivo Studies
3.3.4. Summary
3.4. Alzheimer’s Disease
3.5. Stroke
3.6. Neonatal Brain Hypoxia
4. Neuronal Wound Healing
4.1. Role of Schwann Cells
4.2. Role of Angiogenesis
4.3. Nerve Regeneration-Role of Schwann Cells
4.4. Heart
4.5. Immune Responses
4.6. Diabetes
5. Ginseng Constituent RB1
5.1. Rb1: Neuroprotection
Alzheimer’s and Parkinson’s Diseases and Stroke Models
5.2. Neuronal Repair/Regeneration Role of Schwann Cells
5.3. Heart: Cardiomyocytes
5.4. Diabetes
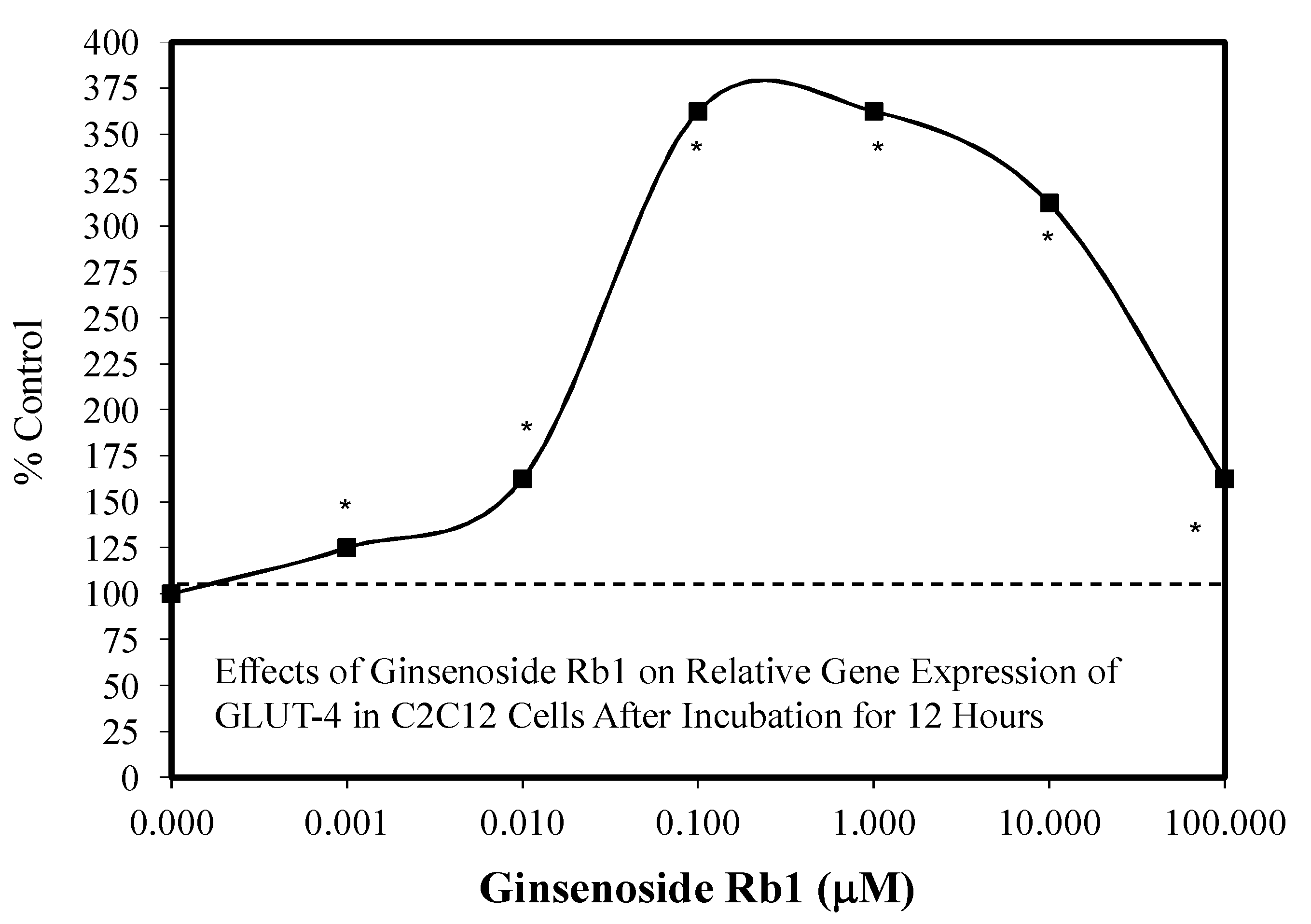
5.5. Adipocyte Differentiation
5.6. White to Brown Adipocyte Transformation
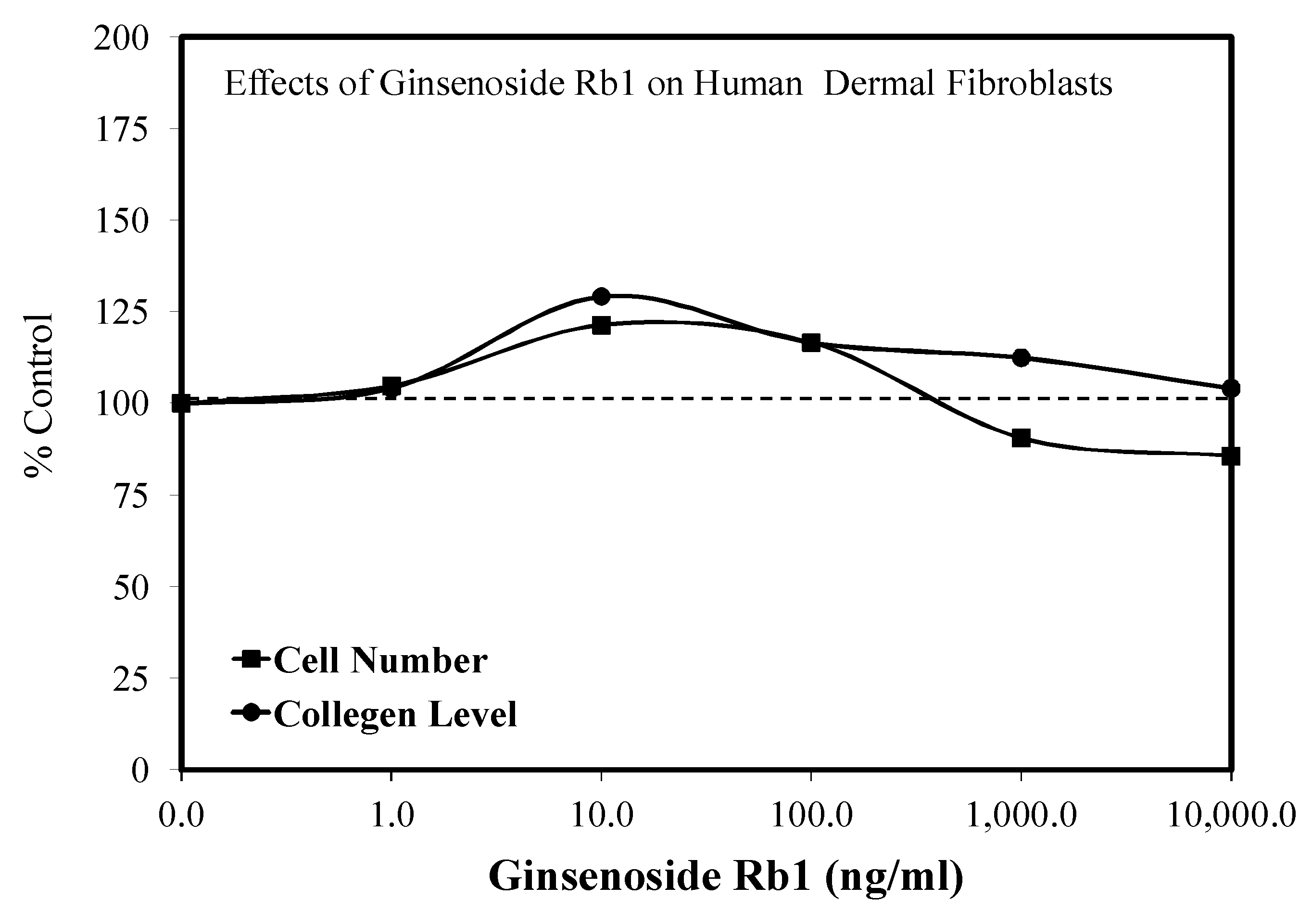
5.7. Wound Healing
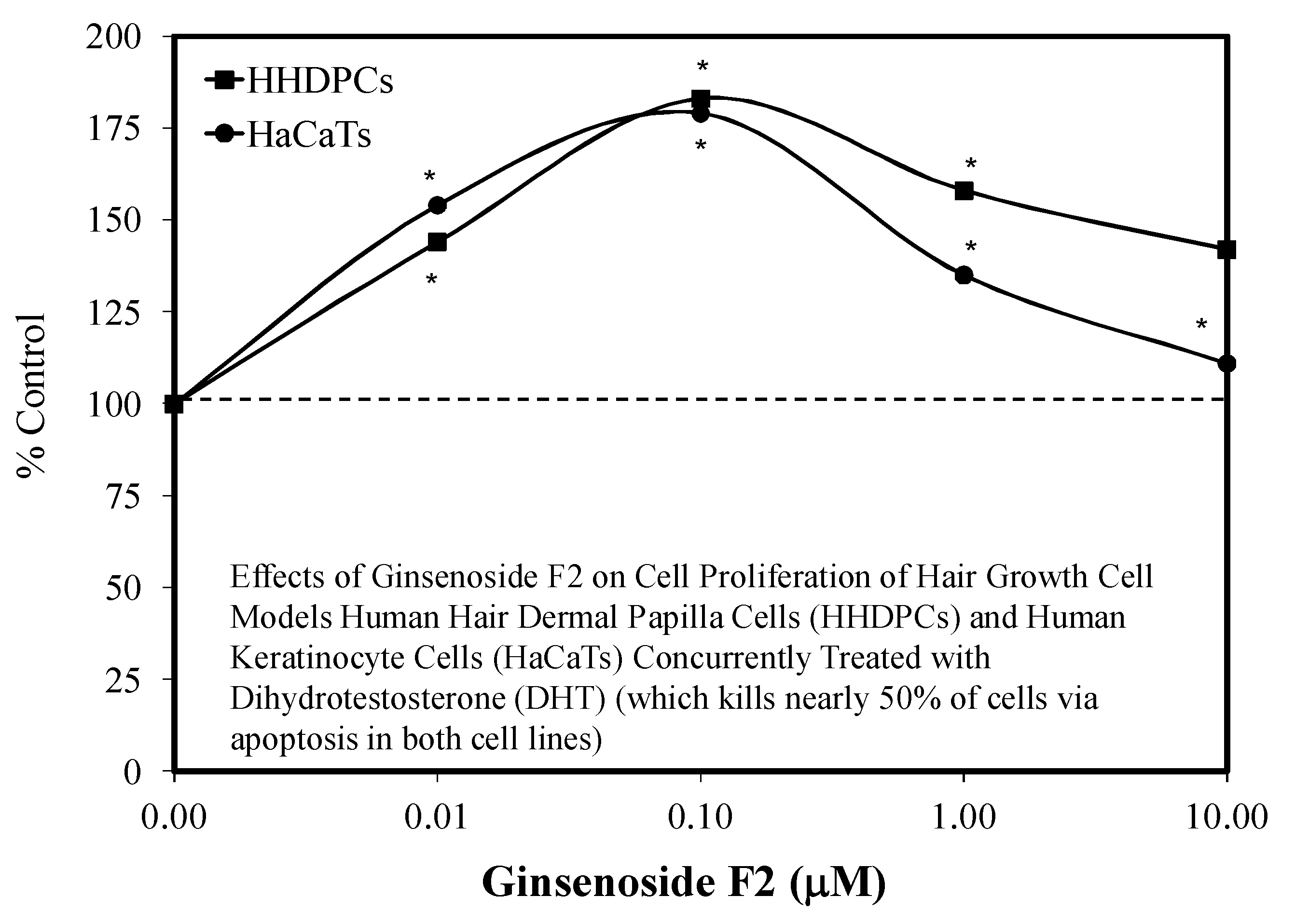
5.8. Hair Growth
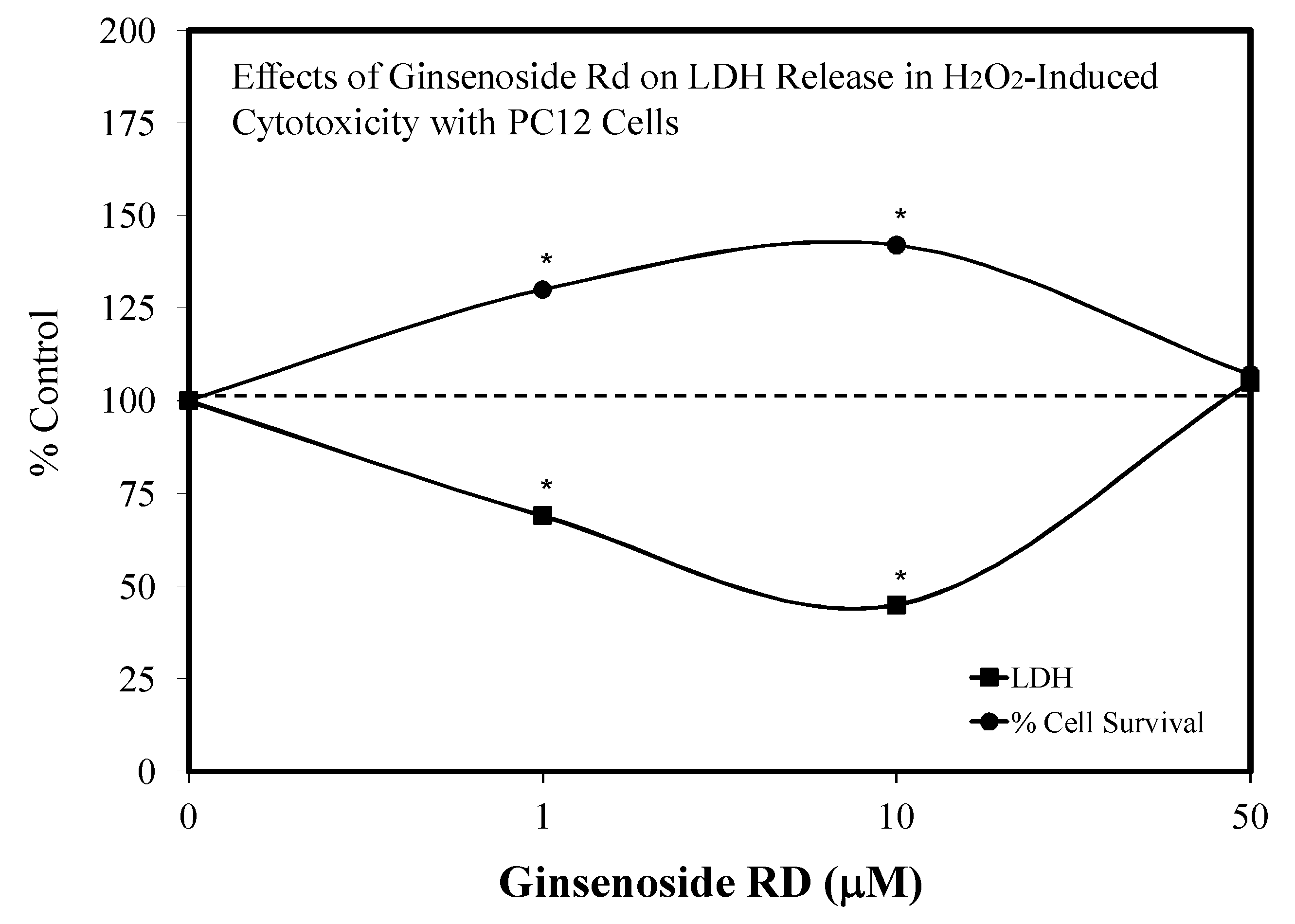
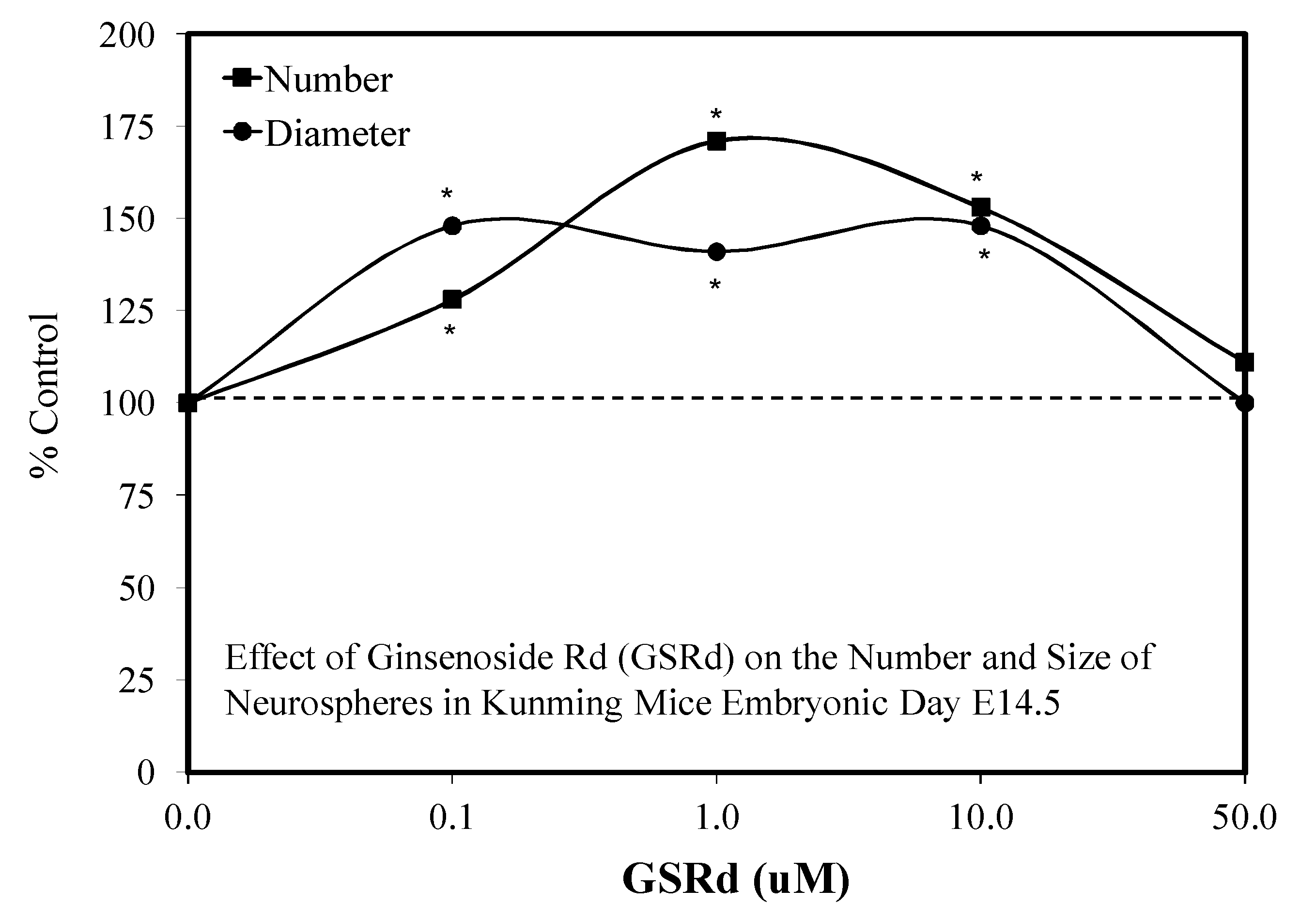
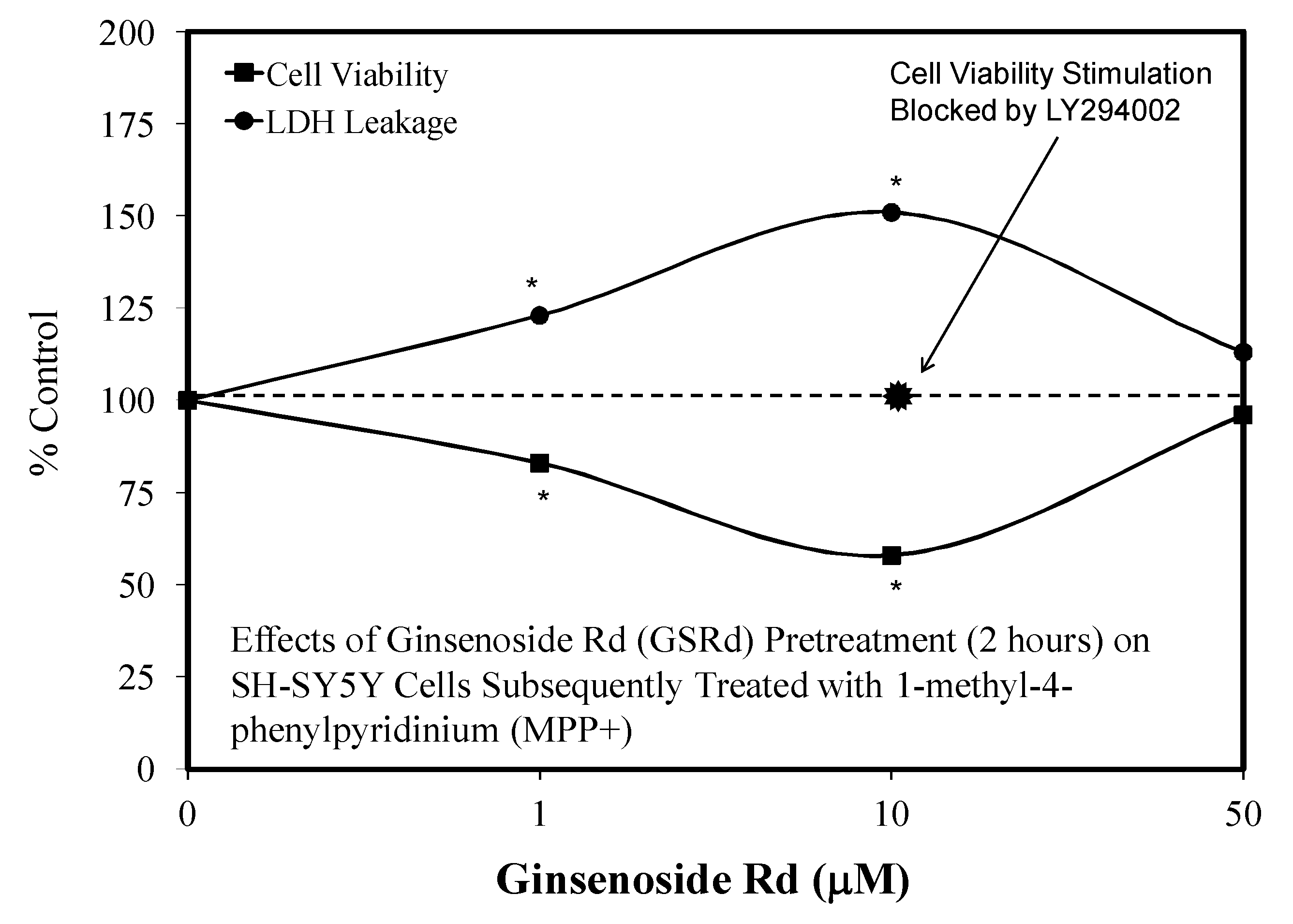
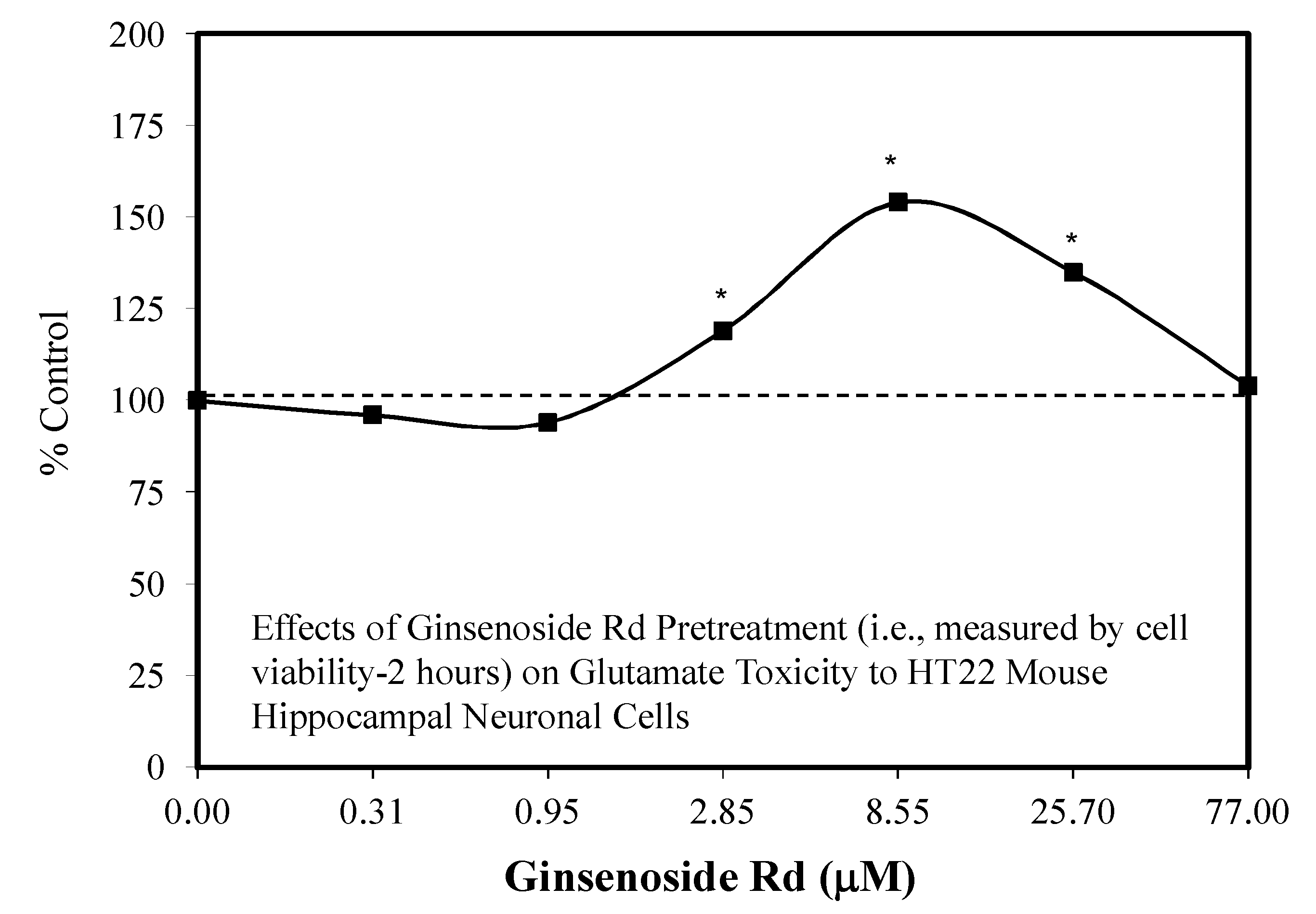
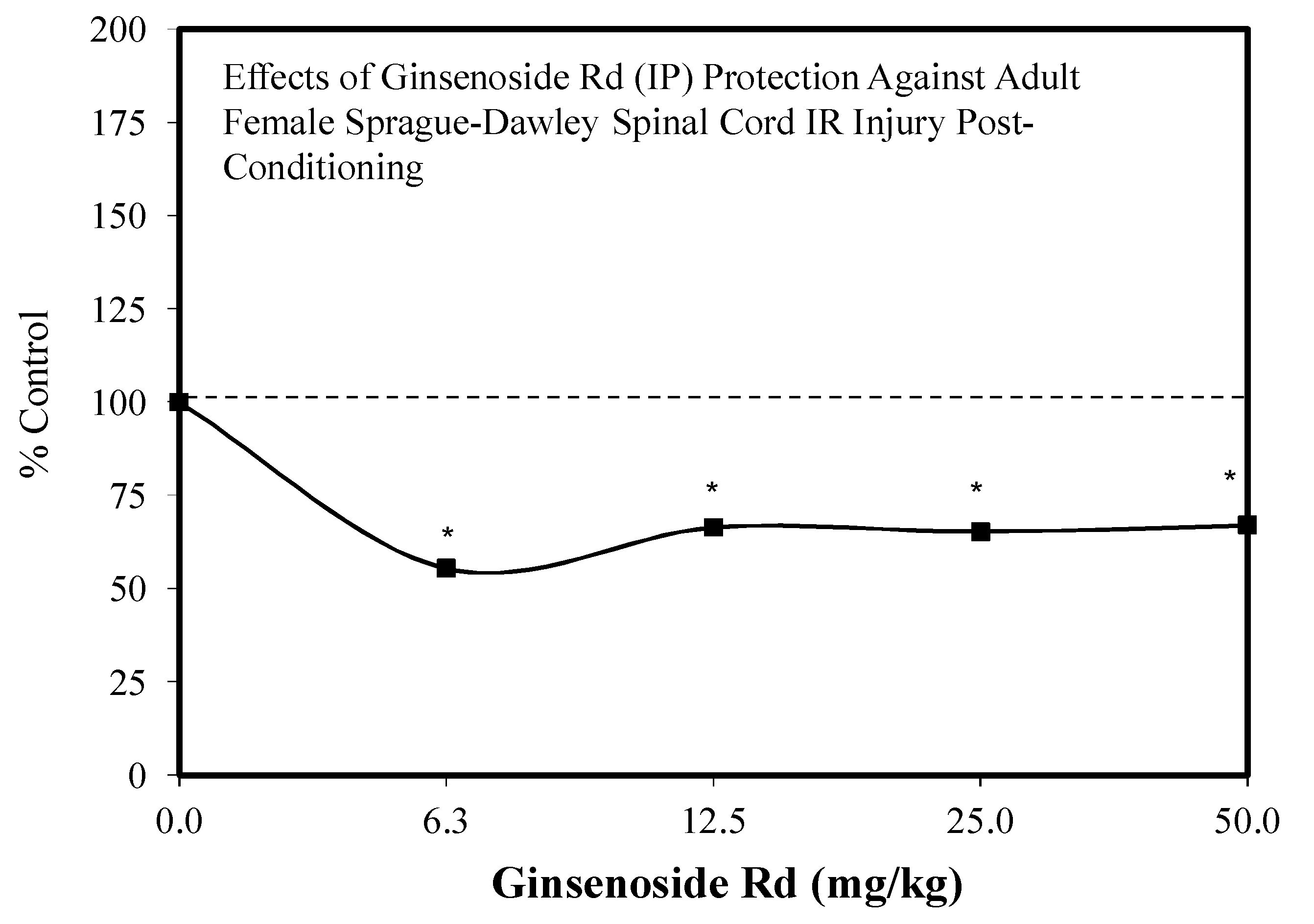
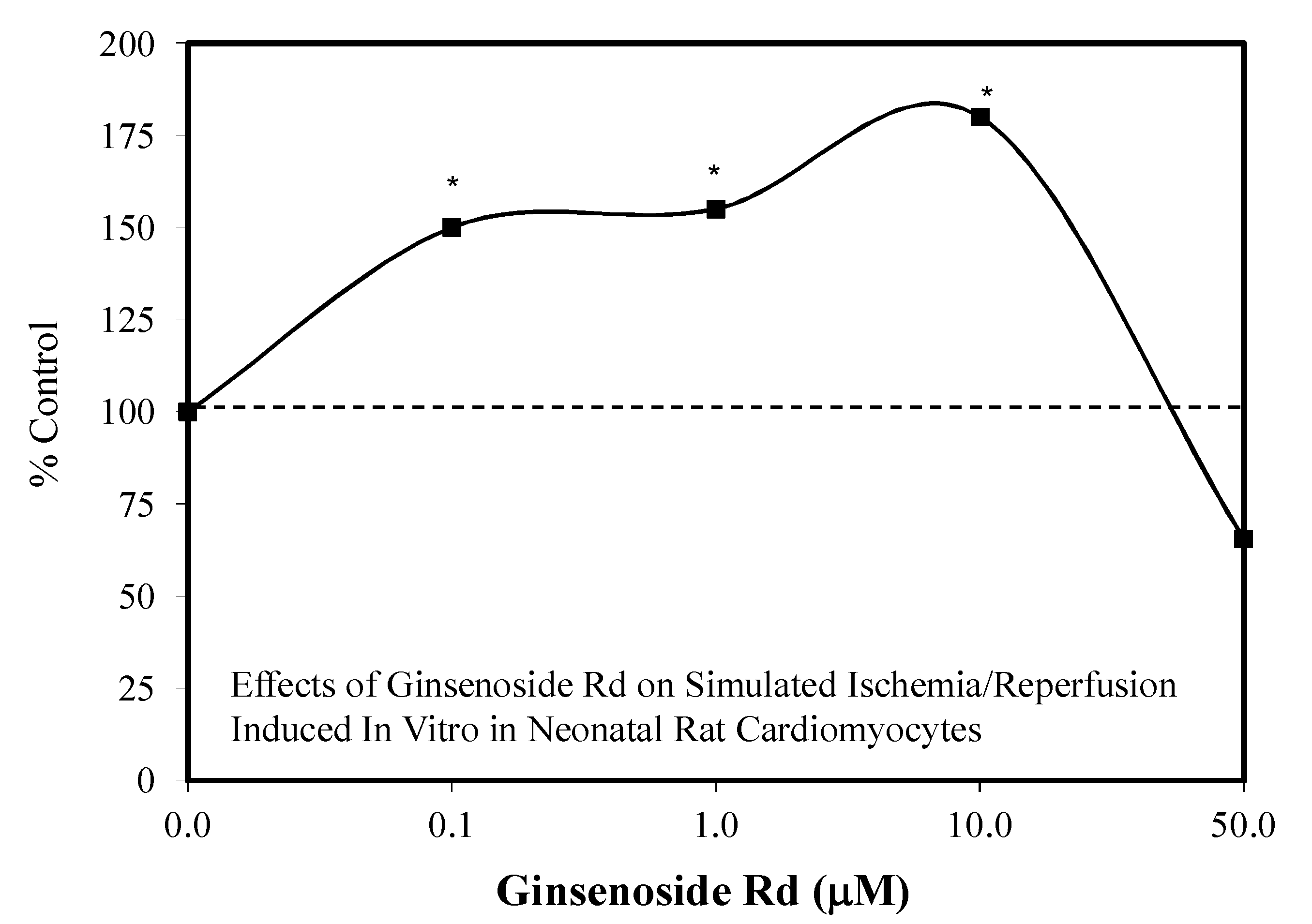
5.9. Ginseng-Rd
5.10. Stroke
5.11. Other Neuroprotective Studies
5.12. Heart
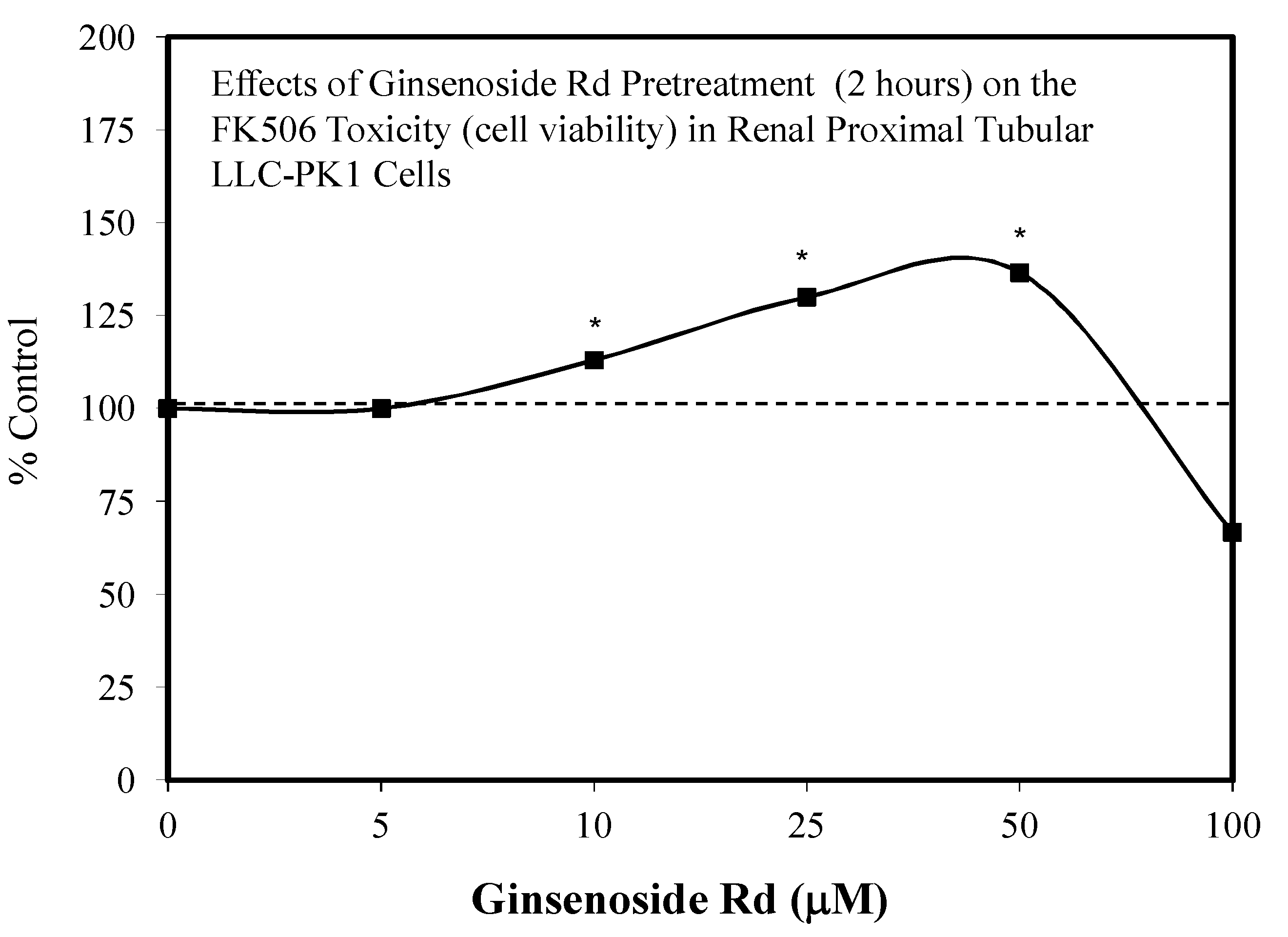
5.13. Kidney
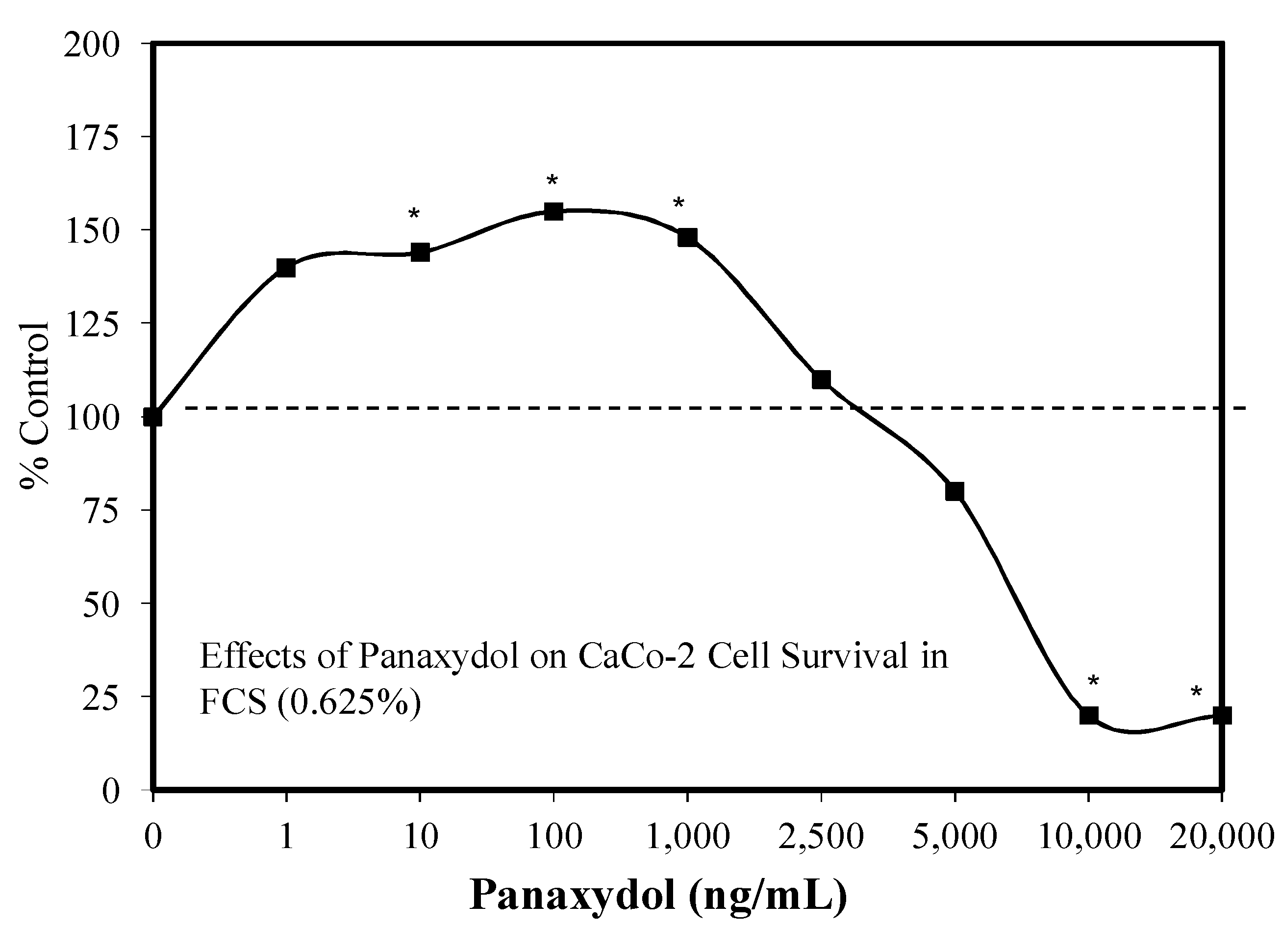
5.14. Polyacetylenes
Introduction
5.15. Alzheimer’s Disease
5.16. Nerve Regeneration
5.17. Rg3
Pain/Tobacco Toxicity/Diabetes/Vero Cells
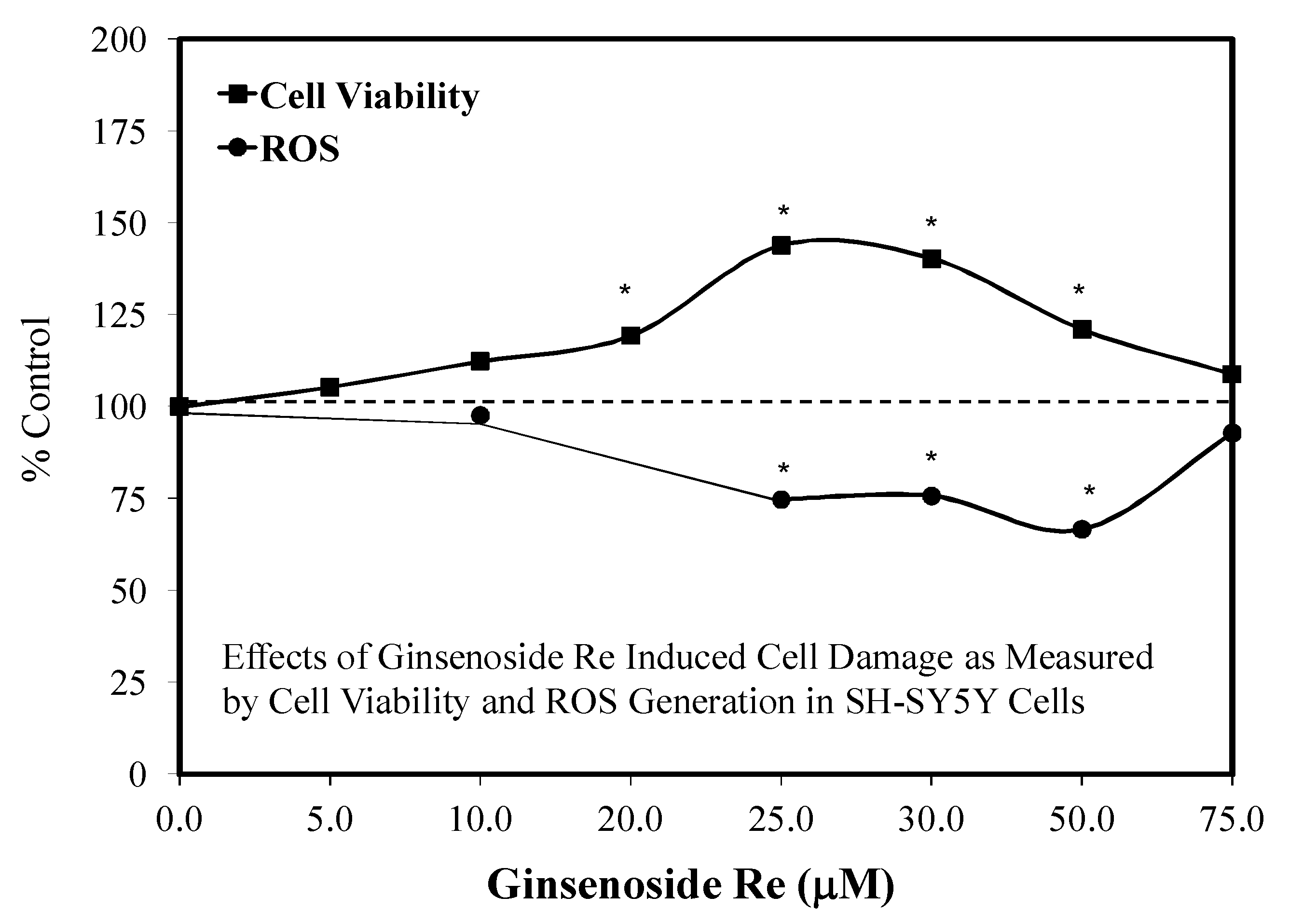
6. Ginseng Constituent Re
6.1. Introduction
6.2. Nerve Injury and Regeneration
6.3. Alzheimer’s Disease
6.4. Atherosclerosis
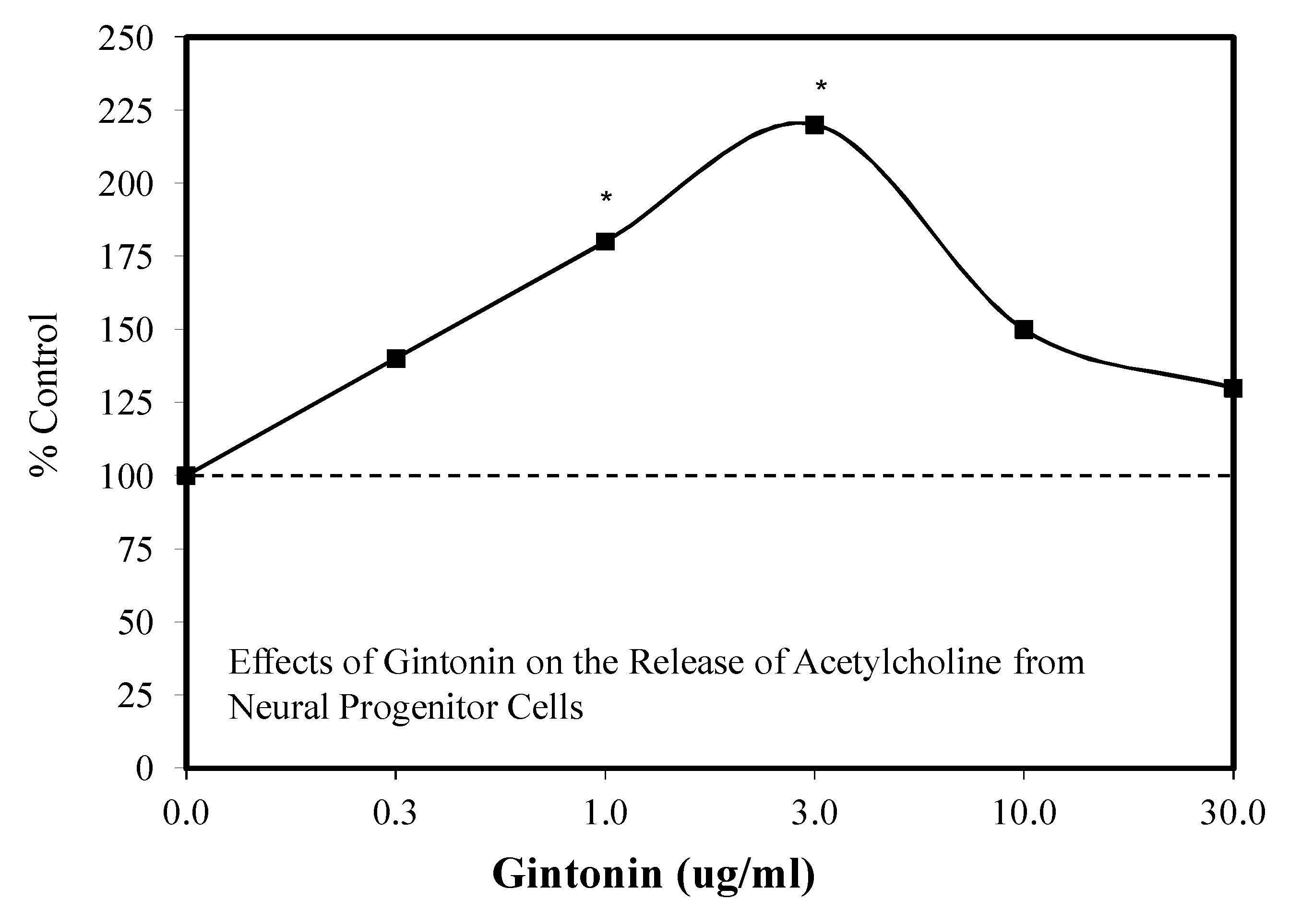
6.5. Gintonin
Introduction
6.6. Corneal Injury
6.7. Alzheimer’s Disease
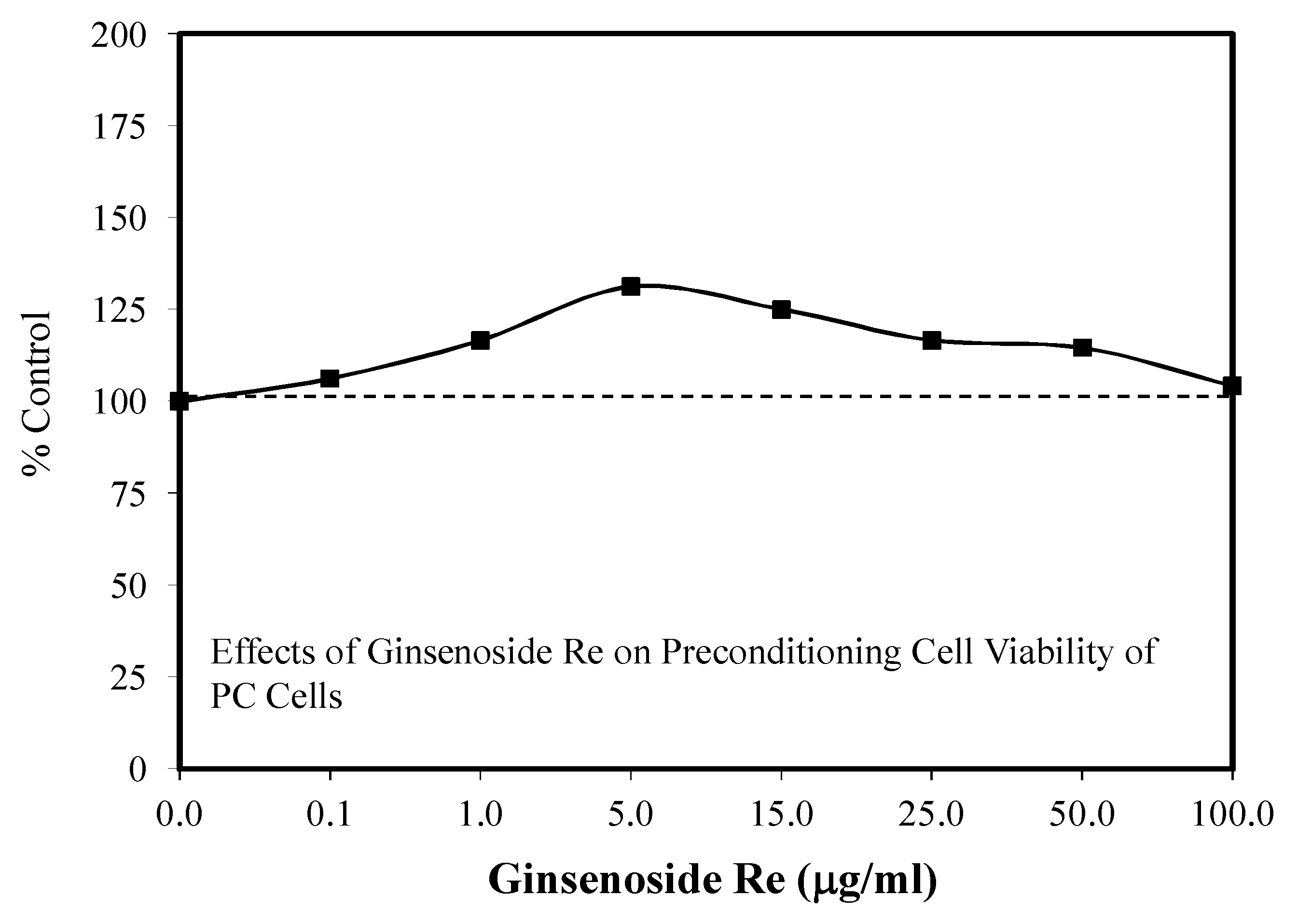
6.8. Astrocyte Glycogenolysis and Preconditioning
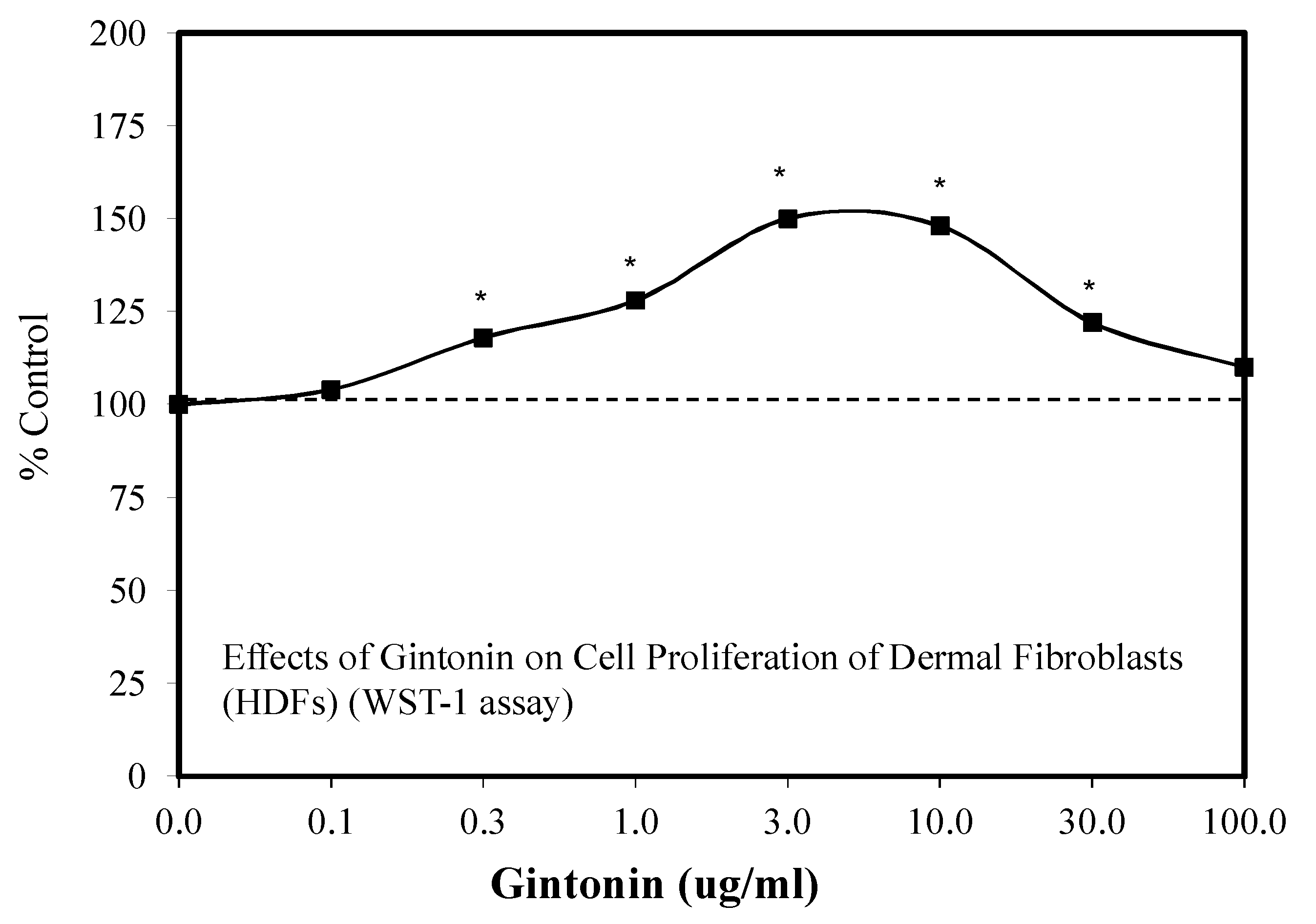
6.9. Wound Healing
7. Ginseng Mixtures
7.1. Introduction
7.2. Kidney Toxicity
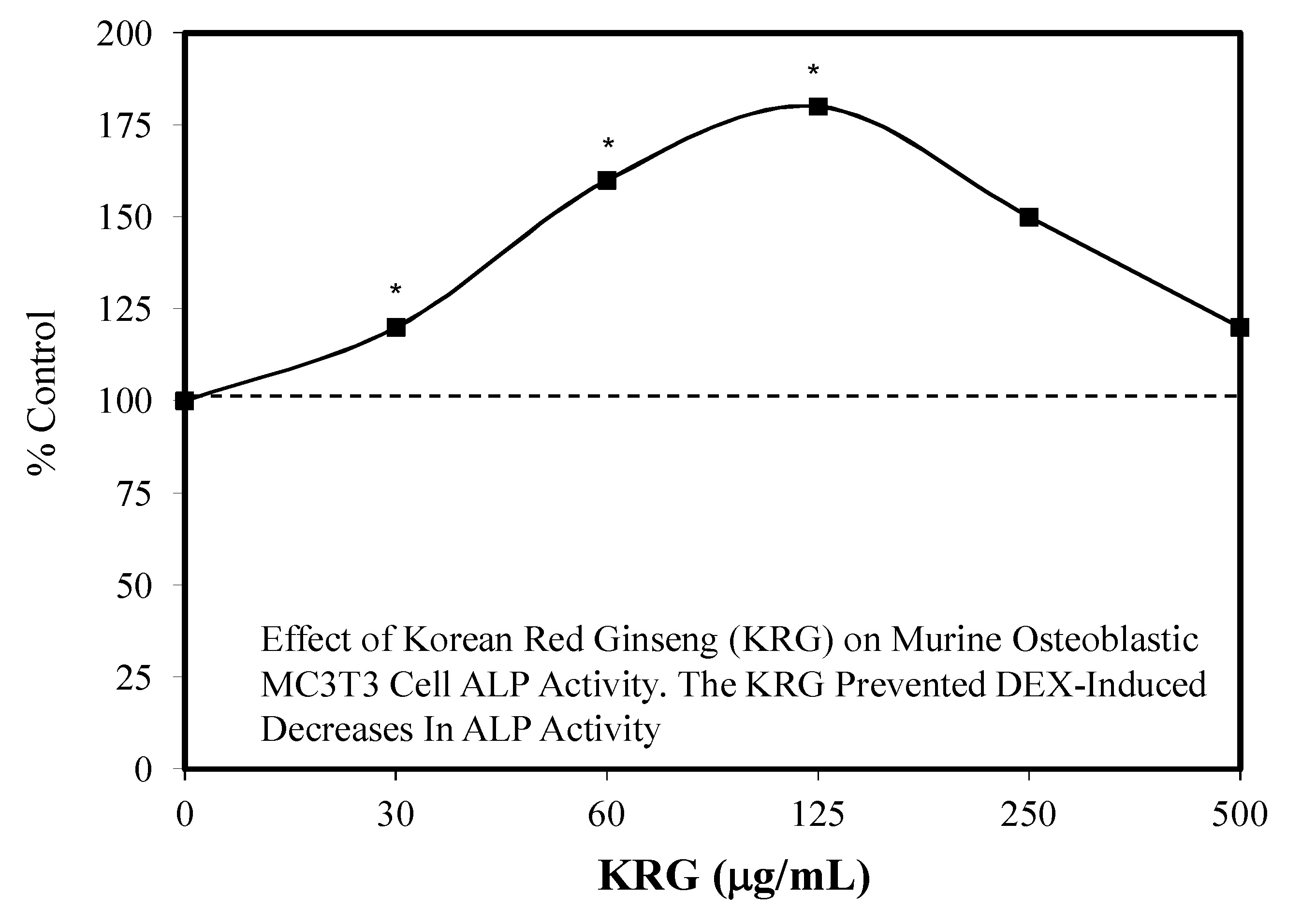
7.3. Bone Toxicity
7.4. Learning and Memory
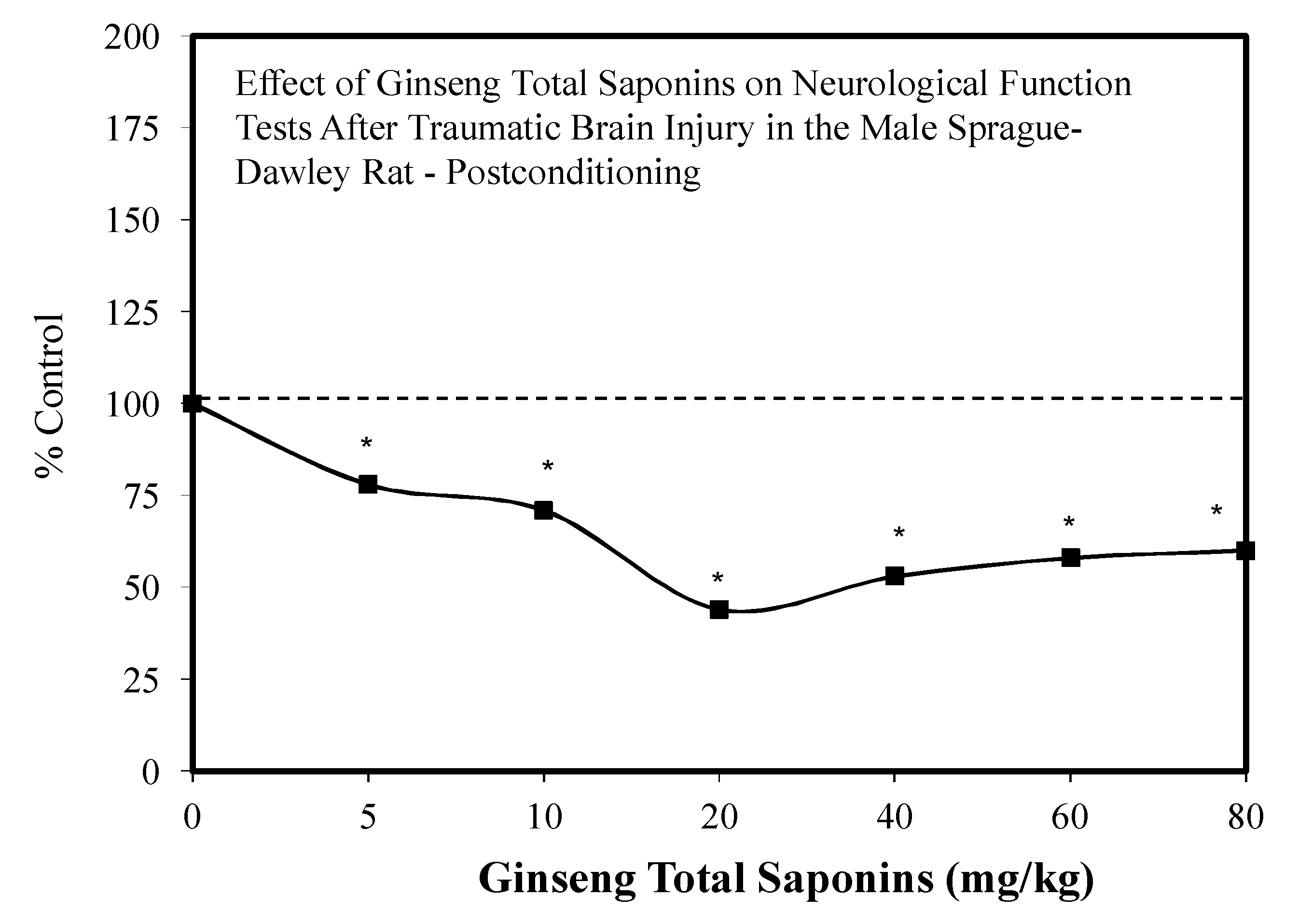
7.5. Brain Traumatic Injury
7.6. Muscle Injury
7.7. Liver Injury and Repair
8. Discussion
Funding
Conflicts of Interest
References
- Fujitani, I. Essay on the chemistry and pharmacology of ginseng root. Arch. Int. Pharmacodyn. Ther. 1905, 14, 355. [Google Scholar]
- Calabrese, E.J.; Baldwin, L.A. Defining hormesis. Hum. Exp. Toxicol. 2002, 21, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Mattson, M.P. How does hormesis impact biology, toxicology, and medicine? Aging Mech. Dis. 2017, 3, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calabrese, E.J.; Blain, R.B. The hormesis database: The occurrence of hormesis dose responses in the toxicological literature. Regul. Toxicol. Pharmacol. 2011, 61, 73–81. [Google Scholar] [CrossRef]
- Calabrese, E.J. Biphasic dose responses in biology, toxicology and medicine: Accounting for their generality and quantitative feature. Environ. Pollut. 2013, 182, 452–460. [Google Scholar] [CrossRef]
- Calabrese, E.J. Preconditioning is hormesis. Part 1: Documentation, dose-response features and mechanistic foundations. Pharmacol. Res. 2016, 110, 242–264. [Google Scholar] [CrossRef]
- Calabrese, E.J. Hormesis: Why it is important to toxicology and toxicologists. Environ. Chem. Toxicol. 2008, 27, 1451–1474. [Google Scholar] [CrossRef]
- Calabrese, E.J. Hormetic mechanisms. Crit. Rev. Toxicol. 2013, 43, 580–606. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Dhawan, G.; Kapoor, R.; Mattson, M.P.; Rattan, S.I. Curcumin and hormesis with particular emphasis on neural cells. Food Chem. Toxicol. 2019, 129, 399–404. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Calabrese, V.; Tsatsakis, A.; Giordano, J.J. Hormesis and Ginkgo biloba (GB): Numerous biological effects of GB are mediated via hormesis. Aging Res. Rev. 2020, in press. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Tsatsakis, A.M.; Agathokleous, E.; Giordano, J.; Calabrese, V. Hormesis and Green Tea. Dose-Response 2020, in press. [Google Scholar]
- Voces, J.; Alvarez, A.I.; Vila, L.; Ferrando, A.; Cabral de Oliverira, C.; Prieto, H.G. Effects of administration of the standardized Panax ginseng extract G115 on hepatic antioxidant function after exhaustive exercise. Comp. Biochem. Physiol. Part C 1999, 123, 175–184. [Google Scholar] [CrossRef]
- Voces, J.; Cabral de Oliveira, A.C.; Prieto, J.G.; Vila, L.; Perez, A.C.; Duarte, E.D.G.; Alvarez, A.I. Ginseng administration protects skeletal muscle from oxidative stress induced acute exercise in rats. Braz. J. Med. Biol. Res. 2004, 37, 1863–1871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Xiao, Z.; Li, B.; Wang, H.; Qi, J.; Wang, Y. Ginsenoside exhibits concentration-dependent dual effects on HepG2 cell proliferation via regulation of c-Myc and HNF-4a. Eur. J. Pharm. 2016, 792, 26–32. [Google Scholar] [CrossRef]
- Sung, W.N.; Kwok, H.H.; Rhee, M.H.; Yue, R.Y.K.; Wong, R.N.S. Korean Red Ginseng extract induces angiogenesis through activation of glucocorticoid receptor. J. Ginseng Res. 2017, 41, 477–486. [Google Scholar] [CrossRef]
- Song, H.; Lee, Y.J. Inhibition of hypoxia-induced cyclooxygenase-2 by Korean Red Ginseng is dependent on peroxisome proliferator-activated receptor gamma. J. Ginseng Res. 2017, 41, 240–246. [Google Scholar] [CrossRef] [Green Version]
- Seghinsara, A.M.; Shoorei, H.; Taheri, A.A.H.; Khaki, A.; Shokoohi, M.; Tahmasebi, M.; Khaki, A.A.; Eyni, H.; Ghorbani, S.; Rad, H.R.; et al. Panax ginseng extract improves follicular development after mouse preantral follicle 3D culture. Cell J. 2019, 21, 210–219. [Google Scholar]
- Lim, B.V.; Shin, M.C.; Jang, M.J.; Lee, T.H.; Kim, Y.P.; Kim, H.B.; Lee, K.S.; Kim, H.; Kim, E.H.; Kim, C.J. Ginseng radix increases cell proliferation in dentate gyrus of rats with streptozotocin-induced diabetes. Biol. Pharm. Bull. 2002, 25, 1550–1554. [Google Scholar] [CrossRef] [Green Version]
- Kanzaki, T.; Morisaki, N.; Shiina, R.; Saito, Y. Role of transforming growth factor-beta pathway in the mechanism of wound healing by saponin from Ginseng Radix rubra. Br. J. Pharmacol. 1998, 125, 255–262. [Google Scholar] [CrossRef] [Green Version]
- Morisaki, N.; Watanebe, S.; Tezuka, M.; Zenibayashi, M.; Shiina, R.; Koyama, N.; Kanzaki, T.; Saito, Y. Mechanism of angiogenic effects of saponin from ginseng Radix rubra in human umbilical vein endothelial cells. Br. J. Pharmacol. 1995, 115, 1188–1193. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.; Yu, W.; Yang, T.; Liu, W.; Zao, Y.; Sun, Y.; Cahi, L.; Gao, Y.; Dong, B.; Zhu, L. Panax notoginseng saponins provide neuroprotection by regulating NgR1/RhoA/ROCK2 pathway expression, in vitro and in vivo. J. Ethnopharmacol. 2016, 190, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.Y.; Liu, X.J.; Qiang, R.; Jiang, Z.L.; Xu, L.H.; Wang, G.H.; Li, X.; Peng, B. Treatment with ginseng total saponins improves the neurorestoration of rat after traumatic brain injury. J. Ethnopharmacol. 2014, 155, 1243–1255. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Jiang, Z.-J.; Wang, G.-H.; Hu, B.-Y.; Ke, K.-F. Treatment with ginseng total saponins reduces the secondary brain injury in rat after cortical impact. J. Neurosci. Res. 2012, 90, 1424–1436. [Google Scholar] [CrossRef] [PubMed]
- Liao, B.; Newmark, H.; Zhou, R. Neuroprotective effects of ginseng total saponin and ginsenosides Rb1 and Rg1 on spinal cord neurons in vitro. Exp. Neurol. 2002, 173, 224–234. [Google Scholar] [CrossRef]
- Zhang, C.; Li, C.; Chen, S.; Li, Z.; Ma, L.; Ji, X.; Wang, K.; Bao, J.; Liang, Y.; Chem, M.; et al. Hormetic effect of panaxatriol saponins confers neuroprotection in PC12 cells and zebrafish through P13K/AKT/mTOR and AMPK/SIRT1FOX03 pathways. Sci. Rep. 2017, 7, 41082. [Google Scholar] [CrossRef]
- Naval, M.V.; Gomez-Serranillo, A.P.; Carretero, M.E.; Villar, A.M. Neuroprotective effect of a ginseng (Panax ginseng) root extract on astrocytes primary culture. J. Ethnopharmacol. 2007, 112, 262–270. [Google Scholar] [CrossRef]
- Gao, Y.; Li, J.; Chu, S.; Zhang, Z.; Chen, N.; Li, L.; Zhang, L. Ginsenoside Rg1 protects mice against streptozotocin–induced type 1 diabetic by modulating the NLRP3 and Keap1/Nrf2/HO-1 pathways. Eur. J. Pharm. 2020, 866, 172801. [Google Scholar] [CrossRef]
- Boonlert, W.; Benya-Aphikul, H.; Welbat, J.M.; Rodsiri, R. Ginseng extract G115 attenuates ethanol-induced depression in mice b increasing brain BDNF levels. Nutrients 2017, 9, 931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, L.; Zhang, J. NMDA receptor and iNOs are involved in the effects of ginsenoside Rg1 on hippocampal neurogenesis in ischemic gerbils. Neurol. Res. 2007, 29, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Shi, A.W.; Wang, X.B.; Lu, F.X.; Zhu, M.M.; Kong, X.Q.; Cao, K.J. Ginsenoside Rg1 promotes endothelial progenitor cell migration and proliferation. Acta Pharmacol. Sin. 2009, 30, 299–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, F.T.; Li, H.M.; Yin, Q.S.; Cui, S.E.; Liu, D.L.; Nan, H.; Han, Z.A.; Xu, K.M. Effect of ginsenoside Rg1 on proliferation and neural phenotype differentiation of human adipose-derived stem cells in vitro. Can. J. Physiol. Pharmacol. 2014, 92, 467–475. [Google Scholar] [CrossRef]
- Dong, J.; Zhu, G.; Wang, T.C.; Shi, F.S. Ginsenoside Rg1 promotes neural differentiation of mouse adipose-derived stem cells via the miRNA-124 signaling pathway. J. Zhejiang Univ. Sci. B 2017, 18, 445–448. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.W.; Tian, S.J.; Barry, D.; Luu, B. Panaxadiol glycosides that induce neuronal differentiation in neurosphere stem cells. J. Nat. Prod. 2007, 70, 1329–1334. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.J.; Lu, Z.; Zhu, D.D.; Yi, X.L.; Wu, F.X.; He, N.; Tang, C.; Wei, C.Y.; Li, I.; Li, H.M. Ginsenoside Rg1 accelerates paracrine activity and adipogenic differentiation of human breast adipose-derived stem cells in a dose-dependent manner in vitro. Cell Transplant. 2019, 28, 286–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, J.; Wan, F.; Tian, M.; Li, Y.; Li, Y.; Zhang, J.; Wang, Y.; Huang, X.; Zhang, L.; Si, Y.C. Effects of ginsenoside-Rg1 on the proliferation and glial-like directed differentiation of embryonic rat cortical neural stem cells in vitro. Mol. Med. Rep. 2017, 16, 8875–8881. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wei, X.; Zhou, Y.; Wang, Y.P.; Yang, K.; Zhang, F.J.; Jiang, R. Effect of ginsenoside Rg1 on proliferation and differentiation of human dental pulp cells in vitro. Aust. Dent. J. 2012, 57, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Yin, L.-H.; Cheng, W.-X.; Qin, A.-S.; Sun, K.M.; Zhong, M.; Wang, J.K.; Gao, W.Y.; Yu, Z.H. Effects of ginsenoside (Rg1) on the proliferation and osteogenic differentiation of human periodontal ligament stem cells. Chin. J. Integr. Med. 2015, 21, 676–681. [Google Scholar] [CrossRef]
- Kim, S.H.; Choi, K.H.; Lee, D.K.; Oh, J.M.; Hwang, J.Y.; Park, C.H.; Lee, C.K. Ginsenoside Rg1 improves in vitro-produced embryo quality by increasing glucose uptake in procine blastocytes. Asian-Australas. J. Anim. Sci. 2016, 29, 1095–1101. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.B.; Wang, Y.; Tang, J.P.; Chen, D.; Wang, S.L. Neuroprotective effects of ginsenoside Rg1-induced neural stem cell transplantation on hypoxic-ischemic encephalopathy. Neural Regen. Res. 2015, 5, 753–759. [Google Scholar]
- Chen, C.; Ahou, Y.C.; Chen, Y.; Zhu, Y.G.; Fang, F.; Chen, L.M. Ginsenoside Rg1 reduces MPTP-induced substantia nigra neuron loss by suppressing oxidative stress. Acta Pharmacol. Sin. 2005, 26, 56–62. [Google Scholar] [CrossRef] [Green Version]
- Gao, Q.G.; Chen, W.F.; Xie, J.X.; Wong, M.S. Ginsenoside Rg1 protect against 6-OHDA–induced neurotoxicity in neuroblastoma SK-N-SH cells via IGF-1 receptor and estrogen receptor pathways. J. Neurochem. 2009, 109, 1338–1347. [Google Scholar] [CrossRef] [PubMed]
- Ge, K.L.; Chen, W.F.; Xie, J.X.; Wong, M.S. Ginsenoside Rg1 protects against 6-OHDA-induced toxicity in MES23.5 cells via Akt and ERK signaling pathways. J. Ethnopharmacol. 2010, 127, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Moriano, C.; Gonzalez-Burgos, E.; Iglesian, I.; Lozano, R.; Gomez-Serranillos, M.P. Evaluation of the adaptogenic potential exerted by ginsenosides Rb1 and Rg1 against oxidative stress-mediated neurotoxicity in an in vitro neuronal model. PLoS ONE 2017, 12, e0182933. [Google Scholar] [CrossRef]
- Chan, R.Y.; Chen, W.F.; Dong, A.; Guo, D.A.; Wong, M.S. Estrogen-like activity of ginsenoside Rg1 derived from Panax notoginseng. J. Clin. Endocrinol. Metab. 2002, 87, 3691–3695. [Google Scholar] [CrossRef] [PubMed]
- Green, P.S.; Gridley, K.E.; Simpkins, J.W. Estradiol protects against beta-amyloid (25-3)5-induced toxicity in SK-N-SH human neuroblastoma cells. Neurosci. Lett. 1996, 218, 165–168. [Google Scholar] [CrossRef]
- Xu, H.; Gouras, G.K.; Greefield, J.P.; Vincent, B.; Naslund, J.; Mazzarelli, L.; Fried, G.; Jovanoic, J.N.; Seeger, M.; Relkin, N.R.; et al. Estrogen reduces neuronal generation of Alzheimer beta-amyloid peptides. Nat. Med. 1998, 4, 447–451. [Google Scholar] [CrossRef]
- Goodenough, S.; Schafer, M.; Behl, C. Estrogen-induced cell signaling in a cellular model of Alzheimer’s disease. J. Steroid Biochem. Mol. Biol. 2003, 84, 301–305. [Google Scholar] [CrossRef]
- Gong, L.; Li, S.L.; Li, H.; Zhang, L. Ginsenoside Rg1 protects primary cultured rat hippocampal neurons from cell apoptosis induced by B-amyloid protein. Pharm. Biol. 2011, 49, 501–507. [Google Scholar] [CrossRef]
- Nie, L.; Xia, J.; Li, H.; Zhang, Z.; Yang, Y.; Huang, X.; He, Z.; Liu, J.; Yang, X. Ginsenoside Rg1 ameliorates behavioral abnormalities and modulates the hippocampal proteomic change in triple transgenic mice of Alzheimer’s Disease. Oxid. Med. Cell. Longev. 2017, 2017, 6473506. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Wang, J.; Xing, Y.; Gong, L.; Li, H.; Wu, Z.; Li, Y.; Wang, J.; Wang, Y.; Dong, L.; et al. Effects of ginsenoside Rg1 or 17b-estradiol on a cognitively impaired, ovariectomized rat model of Alzheimer’s Disease. Neuroscience 2012, 220, 191–200. [Google Scholar] [CrossRef]
- Chu, S.F.; Zhang, Z.; Zhou, X.; He, W.B.; Chen, C.; Luo, P.; Liu, D.D.; Gong, H.F.; Wang, Z.Z.; Sun, H.S.; et al. Ginsenoside Rg1 protects against ischemic/reperfusion-induced neuronal injury through miR-144/Nrf2/ARE pathway. Acta Pharmacol. Sin. 2019, 40, 13–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Z.; Zhang, M.; Ling, C.; Zhu, Y.; Ren, H.; Hong, C.; Qin, J.; Lu, T.; Wang, J. Neuroprotective effects of ginsenosides against cerebral ischemia. Molecules 2019, 24, 1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Guam, Y.; Liu, N.; Shao, C.; Liu, Z.; Chen, J.; Wang, Q.; Pan, X.; Sun, H.; Ahang, Y. Brain concentration of ginsenosides and pharmacokinetics after oral administration of mountain-cultivated ginseng. J. Chin. Chem. Soc. 2017, 64, 395–403. [Google Scholar] [CrossRef]
- Li, Y.; Suo, L.; Li, H.; Xue, W. Protective effects of ginsenoside Rg1 against oxygen-glucose-deprivation-induced apoptosis in neural stem cells. J. Neurol. Sci. 2017, 373, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.C.; Lai, T.Y.; Hwang, J.M.; Chen, H.T.; Chang, S.H.; Tsai, F.J.; Wang, H.L.; Lin, C.C.; Kuo, W.W.; Huang, C.Y. Proliferation- and migration-enhancing effects of ginseng and ginsenoside Rg1 though IGF-I- and FGF-2-signaling pathways on RSC96 Schwann cells. Cell Biochem. Funct. 2009, 27, 186–192. [Google Scholar] [CrossRef]
- Chan, L.S.; Yue, P.Y.K.; Wong, Y.Y.; Wong, R.N.S. MicroRNA-15b contributes to ginsenoside-Rg1-induced angiogenesis through increased expression of VEGFR-2. Biochem. Pharmacol. 2013, 86, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.X.; Wang, W.; Chen, H.J. Study progression in neuroprotective effects of ginsenoside Rg1. J. Bethune Med. Coll. 2005, 10, 291–293. [Google Scholar]
- Wei, C.B.; Jia, J.P.; Wang, F. Effects of ginsenosides Rg1 and Rb1 on metabolism pathway of amyloid protein. Chin. J. Inf. Tradit. Chin. Med. 2008, 9, 28–30. [Google Scholar]
- Zhang, Z.J.; Jiang, W. Effects and its mechanism of ginsenosides on dilating rabbit basal artery. J. Heart 2003, 15, 313–315. [Google Scholar]
- Lu, Z.F.; Shen, Y.X.; Zhang, P. Ginsenoside Rg1 promote proliferation and neurotrophin expression of olfactory ensheating cells. J. Asian Nat. Res. 2010, 12, 265–272. [Google Scholar] [CrossRef]
- Zhou, P.; Dong, L.L.; Yang, Z.J.; Fang, Z. A study of effects of ginsenoside Rg1 on regeneration of rat sciatic nerves. Inn. Mong. Med. J. 2011, 43, 413–415. [Google Scholar]
- Huo, D.-S.; Zhang, M.; Cai, Z.-P.; Dong, C.-X.; Wang, H.; Yang, Z.-J. The role of nerve growth factor in ginsenoside Rg1-induced regeneration of injured rat sciatic nerve. J. Toxicol. Environ. Health Part A 2015, 78, 1328. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.M.; Li, C.B.; Liu, Q.L.; Yang, H.; Li, P. Ginsenoside Rg1 protects H9c2 cells against nutritional stress-induced injury via aldolase/AMPK/PINK1. J. Cell. Biochem. 2019, 120, 18388–18397. [Google Scholar] [CrossRef]
- Tong, L.S.; Chao, C.Y. Effects of ginsenoside Rg1 of Panax ginseng on mitosis in human blood lymphocytes in vitro. Am. J. Chin. Med. 1980, 8, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, S.; Liu, H.; Yang, L.; Nan, G. Stimulatory effect of saponin from Panax ginseng on immune function of lymphocytes in the elderly. Mech. Ageing Dev. 1995, 83, 43–53. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Yan, L.; Kang, X.P.; Dou, C.Y.; Zhou, R.G.; Huang, S.Q.; Peng, L.; Wen, L. Ginsenoside Rb1 confers neuroprotection via promotion of glutamate transporters in a mouse model of Parkinson’s disease. Neuropharmacology 2013, 131, 223–237. [Google Scholar] [CrossRef]
- Tohda, C.; Matsumoto, N.; Zou, K.; Meselhy, M.R.; Komatsu, K. AB (25-35)-induced memory impairment, axonal atrophy, and synaptic loss are ameliorated by MI, a metabolite of protopanaxadiol-type saponins. Neuropsycopharmacology 2004, 29, 860–868. [Google Scholar] [CrossRef]
- Kim, Y.C.; Kim, S.R.; Markelonis, G.J.; Oh, T.H. GInsenosides RB1 and Rg3 protect cultured rat cortical cells from glutamate-induced neurodegeneration. J. Neurosci. Res. 1998, 53, 426–432. [Google Scholar] [CrossRef]
- Ni, N.; Liu, Q.; Ren, H.; Wu, D.; Luo, C.; Li, P.; Wan, J.B.; Su, H. Ginsenoside Rb1 protects rat neural progenitor cells against oxidative injury. Molecules 2014, 19, 3012–3024. [Google Scholar] [CrossRef]
- Hadlock, T.; Sunback, C.; Hunter, D.; Cheney, M.; Vacanti, J.P. A polymer foam conduit seeded with Schwann cells promotes guided peripheral nerve regeneration. Tissue Eng. 2000, 6, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.J. The effect of pulse-released nerve growth factor from genipin-crosslinked gelatin in Schwann cell-seeded polycaprolactone conduits on large-gap peripheral nerve regeneration. Tissue Eng. Part A 2009, 15, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Ye, Z.; Hu, Z.; Lu, L.; Luo, Z. Electrical stimulation induces calcium-dependent release of NGF from cultured Schwann cells. Glia 2010, 58, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Hu, X.; Lu, L.; Ye, Z.; Zhang, Q.; Luo, Z. Electrical regulation of Schwann cells using conductive polypyrrole/chitosan polymers. J. Biomed. Mater. Res. 2010, 93A, 164–174. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Ding, W.L.; Li, F.; Li, F.; Wang, W.J.; Zhu, H. Panaxydol treatment enhances the biological properties of Schwann cells in vitro. Chem.-Biol. Interact. 2009, 177, 34–39. [Google Scholar] [CrossRef]
- Liang, W.; Ge, S.; Yang, L.; Yang, M.; Ye, Z.M.; Yan, M.; Du, J.; Luo, Z. Ginsenosides RB1 and Rg1 promote proliferation and expression of neurotrophic factors in primary Schwann cell cultures. Brain Res. 2010, 1357, 18–25. [Google Scholar] [CrossRef]
- Xia, R.; Zhao, B.; Wu, Y.; Hou, J.B.; Zhang, L.; Xu, J.J.; Xia, Z.Y. Ginsenoside Rb1 preconditioning enhances eNOS expression and attenuates myocardial ischemia/reperfusion injury in diabetic rats. J. Biomed. Biotechnol. 2011, 2011, 767930. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Shao, Z.H.; Xie, J.Z.; Wang, C.Z.; Ramachandran, S.; Yin, J.J.; Aung, H.; Li, C.Q.; Qin, G.; Vanden Hoek, T.; et al. The effects of ginsenoside Rb1 on JNK in oxidative injury in cardiomyocytes. Arch. Pharmacal Res. 2012, 35, 1259–1267. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Xia, Z.Y.; Dou, J.J.; Zhang, L.; Xu, J.J.; Zhao, B.; Lei, S.Q.; Liu, H.M. Rb1 against myocardial ischemia/reperfusion injury in streptozotocin-induced diabetic rats. Mol. Biol. Rep. 2011, 38, 4327–4335. [Google Scholar] [CrossRef]
- Yan, X.; Tian, J.; Wu, H.; Liu, Y.; Ren, J.; Zheng, S.; Zhang, C.; Yang, C.; Li, Y.; Wang, S. Ginsenoside Rb1 protects neonatal rat cardiomyocytes from hypoxia/ischemia induced apoptosis and inhibits activation of the mitochondrial apoptotic pathway. Evid.-Based Complement. Altern. Med. 2014, 2014, 149195. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Xue, J.; Wang, S.; Liu, Y.; Zheng, S.; Zhang, C.; Yang, C. Ginsenodie-Rb1 protests hypoxic-and ischemic-damaged cardiomyocyte by regulating expression of miRNAs. Evid.-Based Complement. Altern. Med. 2015, 2015, 171306. [Google Scholar] [CrossRef]
- Yang, C.; Li, B.; Liu, Y.; Xing, Y. Ginsenoside Rb1 protects cardiomyocyres from oxygen-glucose deprivation injuries by targeting microRNA-21. Exp. Ther. Med. 2019, 17, 3709–3716. [Google Scholar] [PubMed] [Green Version]
- Shang, W.; Yang, Y.; Jiang, B.; Jin, H.; Zhou, L.; Liu, S.; Chen, M. Ginsenoside Rb1 promotes adipogenesis in 3T3-L1 cells by enhancing PPARy2 and C/EBPa gene expression. Life Sci. 2007, 80, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Shang, W.; Yang, Y.; Zhou, L.; Jiang, B.; Jin, H.; Cjen, M. Ginsenoside Rb1 stimulates glucose uptake through insulin-like signaling pathway in 3T3-L1 adipocytes. J. Endocrinol. 2008, 198, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Fang, X.; Li, X.; Zhao, D.; Mo, F.; Jiang, G.; Yu, N.; Zhang, Y.; Guo, Y.; Fu, M.; et al. Ginsenoside Rb1 promotes browning through regulation of PPARy in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2015, 466, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.S.; Tabandeh, M.R.; Namin, A.S.M. Promoting effect of ginsenoside Rb1 for GLUT-4 gene expression and cellular synthesis in C1C12 cells. Int. J. Pharm. Res. Allied Sci. 2016, 5, 151–158. [Google Scholar]
- Lee, G.Y.; Park, K.G.; Namgoong, S.; Han, S.K.; Jeong, S.H.; Dhong, E.S.; Kim, W.K. Effects of Panax ginseng extract on human dermal fibroblast proliferation and collagen synthesis. Int. Wound J. 2015, 13 (Suppl. S1), 42–46. [Google Scholar]
- Kim, Y.G.; Sumiyoshi, M.; Kawahira, K.; Sakanaka, M.; Kimura, Y. Effects of Red Ginseng extract on ultraviolet B-irradiated skin change in C57Bl mice. Phytother. Res. 2008, 22, 1423–1427. [Google Scholar] [CrossRef]
- Kim, W.K.; Song, S.Y.; Oh, W.K.; Kaewsuwan, S.; Tran, T.L.; Kim, W.S.; Sung, J.H. Wound-healing effect of ginsenosdide Rd from leaves of Panax ginseng via cyclic AMP-dependent protein kinase patway. Eur. J. Pharm. 2013, 702, 285–293. [Google Scholar] [CrossRef]
- Kimura, Y.; Sumiyoshi, M.; Kawahira, K.; Sakanaka, M. Effects of ginseng saponins isolated from Red Ginseng roots on burn wound healing in mice. Br. J. Pharmacol. 2006, 148, 860–870. [Google Scholar] [CrossRef] [Green Version]
- Shedoeva, A.; Leavesley, D.; Upton, Z.; Fan, C. Wound healing and the use of medicinal plants. Evid.-Based Complement. Altern. Med. 2019, 2019, 2684108. [Google Scholar] [CrossRef]
- Calabrese, E.J. Stimulating hair growth via hormesis: Experimental foundations and clinical implications. Pharmacol. Res. 2020, 152, 104599. [Google Scholar] [CrossRef]
- Kubo, M.; Matsuda, H.; Fukui, M.; Nakai, Y. Development studies of cuticle drugs from natural resources. 1. Effects of crude drug extracts on hair growth in mice. Yakugaku Zasshi J. Pharm. Soc. Jpn. 1988, 108, 971–978. [Google Scholar] [CrossRef] [Green Version]
- Shin, H.S.; Park, S.Y.; Hwang, E.S.; Lee, D.G.; Song, G.T.; Mavlonov, T.H. The inductive effect of ginsenosdie F2 on hair growth by altering the ENT signal pathway in telogen mouse cells. Eur. J. Pharm. 2014, 730, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.S.; Park, S.Y.; Hwang, E.S.; Lee, D.G.; Mavlonov, T.H. Ginsenoside F2 reduces hair loss by controlling apoptosis through the sterol regulatory element-binding protein cleavage activating protein and transforming growth factor-b pathways in a dihydrotestosterone-induced mouse model. Biol. Pharm. Bull. 2014, 37, 755–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, G.H.; Park, K.Y.; Cho, H.I.; Lee, S.M.; Han, J.S.; Won, C.H.; Chang, S.E.; Lee, M.W.; Choi, J.H.; Moon, K.C.; et al. Red ginseng extract promotes the hair growth in cultured human hair follicles. J. Med. Food 2015, 18, 354–362. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Su, Y.; Wang, J.; Gao, F.; Yang, F.; Li, G. Ginsenoside Rb1 promotes the growth of mink hair follicle via P13K/AKT/GSK-3B signaling pathway. Life Sci. 2019, 229, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Yi, S.M.; Choi, J.E.; Son, S.E. Study of the efficacy of Korean red Ginseng in the treatment of androgenic alopecia. J. Ginseng Res. 2009, 33, 223–228. [Google Scholar]
- Yokozawa, T.; Kiu, Z.W.; Dong, E.B. A study of ginsenoside-Rd in a renal ischemia-reperfusion model. Nephron 1998, 78, 201–206. [Google Scholar] [CrossRef]
- Yokozawa, T.; Owada, S. Effect of ginsenoside-Rd in cephaloridine-induced renal disorder. Nephron 1999, 81, 200–207. [Google Scholar] [CrossRef]
- Yokozawa, T.; Satoh, A.; Cho, E.J. Ginsenoside-rd attenuates oxidative damage related to aging in senescence-accelerated mice. J. Pharm. Pharmacol. 2004, 56, 107–113. [Google Scholar] [CrossRef]
- Lopez, M.V.N.; Gomez-Serranillo Cuadrado, M.P.G.S.; Ruiz-Poveda, O.M.P.; Del Fresno, A.M.V.; Accame, M.E.C. Neuroprotective effect of individual ginsenosides on astrocytes primary culture. Biochem. Biophys Acta 2007, 1770, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.; Han, J.; Zhao, L.; Cao, R.; Rao, Z.; Zhao, G. Protection effects of ginsenoside Rd on PC 12 cells against hydrogen peroxide. Biol. Pharm. Bull. 2008, 31, 1923–1927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, R.; Kong, X.; Yang, Q.; Zhang, Y.; Zhao, G. Ginsenoside RD attenuates redox imbalance and improves stroke outcome after focal cerebral ischemia in aged mice. Neuropharmacology 2011, 61, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Liu, Y.; Shi, M.; Li, L.; Liu, Y.; Zhao, G. Promotive effect of ginsenoside Rd on proliferation of neural stem cells in vivo and in vitro. J. Ethnopharmacol. 2012, 142, 754–761. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, R.-Y.; Zhao, J.; Dong, Z.; Feng, D.-Y.; Wu, R.; Shi, M.; Zhao, G. Ginsenoside Rd protects SH-SY5Y cells against 1-methyl-4-phenylpyridinium induced injury. Int. J. Mol. Sci. 2015, 16, 14395–14408. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.H.; Kim, D.W.; Jung, B.H.; Lee, J.H.; Lee, H.; Hwang, G.S.; Kang, K.S.; Lee, J.W. Ginsenoide Rb2 suppresses the glutamate-mediated oxidative stress and neuronal cell death in HT22 cells. J. Ginseng Res. 2019, 41, 326–334. [Google Scholar] [CrossRef]
- Wang, B.; Zhu, Q.; Man, X.; Guo, L.; Hao, L. Ginsenoside Rd inhibits apoptosis following spinal cord ischemia/reperfusion injury. Neural Regen. Res. 2014, 9, 1678–1687. [Google Scholar]
- Yang, I.; Deng, Y.; Xu, S.; Zeng, X. In vivo pharmacokinetic and metabolism studies of ginsenoside Rd. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007, 854, 77–84. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Wang, X.; Lau, W.; Wang, Y.; Xing, Y.; Zhang, X.; Ma, X.; Gao, F. Ginsenoside Rd attenuates myocardial ischemia/reperfusion injury via AKT, GSK-3B signaling and inhibition of the mitochondria-dependent apoptotic pathway. PLoS ONE 2013, 8, e70956. [Google Scholar]
- Lee, D.; Lee, D.S.; Jung, K.; Hwang, G.S.; Lee, H.L.; Yamabe, N.; Lee, H.J.; Eom, W.; Kim, K.H.; Kang, K.S. Protective effect of ginsenoside Rb1 against tacrolimus-induced apoptosis in renal proximal tubular LLC-PK1 cells. J. Ginseng Res. 2018, 42, 75–80. [Google Scholar] [CrossRef]
- Purup, S.; Larsen, E.; Christensen, L.P. Differential effects of falcarinol and related aliphatic C17-polyacetylene on intestinal cell proliferation. J. Agric. Food Chem. 2009, 57, 8290–8296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
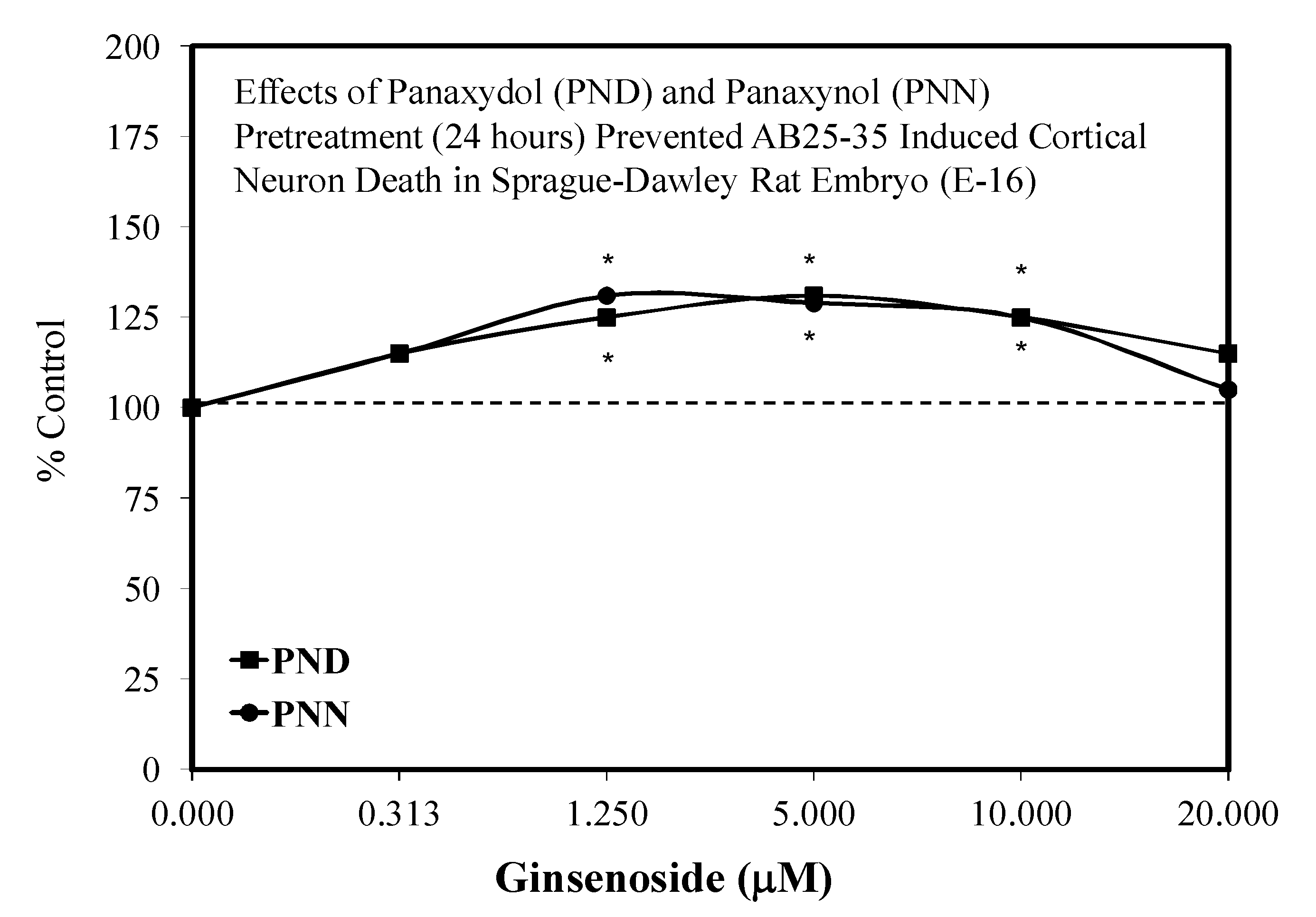
- Nie, B.M.; Jiang, X.Y.; Cai, J.X.; Fum, S.L.; Yang, L.M.; Lin, L.; Hang, Q.; Lu, P.L.; Lu, Y. Panaxydol and panaxynol protect cultured cortical neurons against AB25-35-induced toxicity. Neuropharmacology 2008, 54, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Ahn, E.J.; Choi, G.J.; Kang, H.; Baek, C.W.; Jung, Y.H.; Woo, Y.C.; Bang, S.R. Antinociceptive effects of ginsenoside Rg3 in a rat model of incisional pain. Eur. Surg. Res. 2016, 57, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, X.; Jin, W.; Xu, X.; Li, X.; Sun, L. Ginsenoside Rb3 exerts protective properties against cigarette smoke extract-induced cell injury by inhibiting the p38 MAPK/NF-κB and TGF-β1/VEGF pathways in fibroblasts and epithelial cells. Biomed. Pharmacother. 2018, 108, 1751–1758. [Google Scholar] [CrossRef] [PubMed]
- Park, M.W.; Ha, J.; Chung, S.H. 20(S)-ginsenoside Rg3 enhances glucose-stimulated insulin secretion and activates AMPK. Biol. Pharm. Bull. 2008, 31, 748–751. [Google Scholar] [CrossRef] [Green Version]
- Wright, S.M.; Allman, E. Inhibition of Herbes Simplex virues, Types 1 and 2, by ginsenoside 20(s)-Rg3. J. Microbiol. Biotechnol. 2020, 30, 101–108. [Google Scholar] [CrossRef]
- Song, J.H.; Choi, H.J.; Song, H.H.; Hong, E.H.; Lee, B.R.; Oh, S.R.; Choi, K.; Yeo, S.G.; Lee, Y.P.; Cho, S.; et al. Antiviral activity of ginsenosides against coxsackievirus B, enterovirus 71, and human rhinovirus 3. J. Ginseng Res. 2014, 38, 173–179. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.D.; Zhong, X.F.; Deng, A.Y.; Zeng, R. Proteomic analysis of ginsenoside Re attenuates hydrogen peroxide-induced oxidative stress in human umbilical vein endothelial cells. Food Funct. 2016, 7, 2451–2461. [Google Scholar] [CrossRef]
- Yang, K.; Luo, Y.; Lu, S.; Hu, R.; Du, R.; Liao, P.; Sun, G.; Sun, X. Salvianolic acid B and ginsenoside Re synergistically protect against Ox-LDL-induced endothelial apoptosis through the antioxidative and anti-inflammatory mechanisms. Front. Pharmacol. 2018, 9, 662. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Yuan, D.; Zhang, D.; Zhang, W.; Liu, C.; Cheng, H.; Song, Y.; Tan, Q. Ginsenoside Re promotes nerve regeneration by facilitating the proliferation, differentiation and migration of Schwann Cells via the ERK- and JNK-dependent pathway in rat model of sciatic nerve crush injury. Cell. Mol. Neurobiol. 2015, 35, 827–840. [Google Scholar] [CrossRef]
- Liu, M.; Bai, X.; Yu, S.; Zhao, W.; Qiao, J.; Liu, Y.; Zhao, D.; Wang, J.; Wang, S. Ginenoside Re inhibits ROS/ASK-1dependent mitochondrial apoptosis pathway and activation of Nrf2-antioxidant response in beta-amyloid challenged SH-SY5Y cells. Molecules 2019, 24, 2687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Kim, M.G.; Ko, S.K.; Kim, H.K.; Leem, K.H.; Kim, Y.J. Protective effect of ginsenoside Re on acute gastric mucosal lesion induced b compound 48/80. J. Ginseng Res. 2014, 38, 8–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, R.; Lee, N.-E.; Hwang, H.; Rhim, H.; Cho, I.-H.; Nah, S.-Y. Ginseng gintonin enhances hyaluronic acid and collagen release from human dermal fibroblasts through lysophosphatidic acid receptor interaction. Molecules 2019, 24, 4438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, A.E.; Park, S.D.; Hwang, H.; Choi, S.H.; Lee, R.M.; Nam, S.M.; Choi, J.H.; Rhim, H.; Cho, I.H.; Kim, H.C.; et al. Effects of a gintonin-enriched fraction on hair growth: An in vitro and in vivo study. J. Ginseng Res. 2020, 44, 168–177. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, J.Y.; Lee, B.H.; Choi, S.H.; Rhim, H.; Kim, H.C.; Ahn, S.Y.; Jeong, S.W.; Jang, M.; Cho, I.H.; et al. Gintonin, an exogenous ginseng-derived LPA receptor ligand, promotes corneal wound healing. J. Vet. Sci. 2017, 18, 387–397. [Google Scholar] [CrossRef]
- Kim, D.G.; Jang, M.; Choi, S.H.; Kim, H.J.; Jhun, H.; Kim, H.C.; Rhim, H.; Cho, I.H.; Nah, S.Y. Gintonin, a ginseng-derived exogenous lysophosphatidic acid receptor ligand, enhances blood-brain barrier permeability and brain delivery. Int. J. Biol. Macromol. 2018, 114, 1325–1337. [Google Scholar] [CrossRef]
- Hwang, S.H.; Lee, B.H.; Choi, S.H.; Kim, H.J.; Won, K.J.; Lee, H.M.; Rhim, H.; Kim, H.C.; Nah, S.Y. Effects of gintonin on the proliferation, migration, and tube formation of human umbilical-vein endothelial cells: Involvement of lysophosphatidic acid receptors and vascular endothelial growth factor signaling. J. Ginseng Res. 2016, 40, 325–333. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.H.; Kim, H.J.; Cho, H.J.; Park, S.D.; Lee, N.E.; Hwang, S.H.; Cho, I.H.; Hwang, H.; Rhim, H.; Kim, H.C.; et al. Gintonin, a ginseng-derived exogenous lysophophatidic acid receptor ligand, protects astrocytes from hypoxic and re-oxygenation stresses through stimulation of astrocytic glycogenolysis. Mol. Neurobiol. 2019, 56, 3280–3294. [Google Scholar] [CrossRef]
- Kim, H.J.; Shin, E.J.; Lee, B.H.; Choi, S.H.; Jung, S.W.; Cho, I.K.; Hwang, S.H.; Kim, J.Y.; Han, J.S.; Chung, C.H.; et al. Oral administration of gintonin attenuates cholinergic impairments by scopolamine, amyloid-B protein, and mouse model of Alzheimer’s Disease. Mol. Cells 2015, 38, 796–805. [Google Scholar] [CrossRef]
- Calabrese, E.J. Alzheimer’s Disease drugs: An application of the hormetic dose-response model. Crit. Rev. Toxicol. 2008, 38, 419–452. [Google Scholar] [CrossRef]
- Kim, J.; Lee, H.; Kang, K.S.; Chun, K.H.; Hwang, G.S. Protective effect of Korean Red Ginseng against glucocorticoid-induced osteoporosis in vitro and in in vivo. J. Ginseng Res. 2015, 39, 46–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attele, A.S.; Wua, J.A.; Yuan, C.S. Ginseng pharmacology, multiple constituents and multiple actions. Biochem. Pharmacol. 1999, 58, 1685–1693. [Google Scholar] [CrossRef]
- Lee, D.; Kang, K.S.; Yu, J.S.; Woo, J.Y.; Hwang, G.S.; Eom, D.W.; Baek, S.H.; Lee, H.L.; Kim, K.H.; Yamabe, N. Protective effect of Korean Red Ginseng against FK506-induced damage in LLC-PKI cells. J. Ginseng Res. 2017, 41, 284–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, H.; Park, J.; Choi, K.O.; Lee, J.; Chen, J.; Park, H.J.; Yu, B.I.; Iida, M.; Rhyu, M.R.; Lee, Y.J. Ginsenoside Rf inhibits cyclooxygenase-2 induction via peroxisome proliferator-activated receptor gamma in A549 cells. J. Ginseng Res. 2019, 43, 319–325. [Google Scholar] [CrossRef]
- Hisamura, F.; Kojima-Yuasa, A.; Kennedy, D.O.; Matsui-Yuasa, I. Protective effect of green tea extract and tea polyphenols against FK506-induced cytotoxicity in renal cells. Pharmacol. Toxicol. 2006, 98, 192–196. [Google Scholar] [CrossRef]
- Hisamura, F.; Kojima-Yuasa, A.; Huang, X.D.; Kennedy, D.O.; Opare, D.; Matsui-Yuasa, I. Synergistic effect of green tea polyphenols on their protection against FK506-induced cytotoxicity in renal cells. Am. J. Chin. Med. 2008, 36, 615–624. [Google Scholar] [CrossRef]
- Yokozawa, T.; Oura, H.; Sakanaka, S.; Ishigaki, S.; Kim, M. Depressor effect of tannin in green tea on rats with renal hypertension. Biosci. Biotechnol. Biochem. 1994, 58, 855–858. [Google Scholar] [CrossRef]
- Yokozawa, T.; Chung, H.Y.; Lin, A.H.; Oura, H. Effectiveness of green tea tannin on rats with chronic renal failure. Biosci. Biotechnol. Biochem. 1996, 60, 1000–1005. [Google Scholar] [CrossRef] [Green Version]
- Yokozawa, T.; Dong, E.; Oura, H.; Nonoka, G.; Nishioka, I. Magnesium lithospermate B ameliorates cisplatin-induced injury in cultured renal epithelial cells. Exp. Toxicol. Pathol 1997, 49, 343–346. [Google Scholar] [CrossRef]
- Yokozawa, T.; Cho, E.J.; Nakagawa, T. Influence of green tea polyphenols tea tannin on rats with chronic renal failure. J. Agric. Food Chem. 2003, 51, 2424–2425. [Google Scholar] [CrossRef]
- Lee, Y.K.; Chin, Y.W.; Choi, Y.H. Effects of Korean red ginseng extract on acute renal failure induced by gentamicin and pharmacokinetic changes by metformin in rats. Food Chem. Toxicol. 2013, 59, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.S.; Yu, M.; Kim, M.; Choi, H.S.; Kang, D.H. Renoprotective effect of red ginseng in gentamicin-induced acute kidney injury. Lab. Investig. 2014, 94, 1147–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doh, K.C.; Lim, S.W.; Plao, S.G.; Jin, I.; Heo, S.B.; Zheng, Y.F.; Bae, S.K.; Hwang, C.H.; Min, K.I.; Chung, B.H.; et al. Ginseng treatment attenuates chronic cyclosporine nephropathy via reducing oxidative stress in an experimental mouse model. Am. J. Nephrol. 2013, 37, 421–433. [Google Scholar] [CrossRef]
- Zhang, Y.; Chi, X.; Wang, Z.; Bi, S.; Wang, Y.; Shi, F.; Hu, S.; Wang, H. Protective effects of Panax notoginseng saponins on PME-induced nephrotoxicity in mice. Biomed. Pharmacother. 2019, 116, 108970. [Google Scholar] [CrossRef] [PubMed]
- Petkov, V. Effect of ginseng on the brain biogenic monoamines and 3′,5′-AMP system. Arzneim. Forsch./Drug Res. 1978, 28, 388–393. [Google Scholar]
- Petkov, V.D.; Mosharrof, A.H. Effects of standardized ginseng extract on learning, memory and physical capabilities. Am. J. Chin. Med. 1987, 15, 19–29. [Google Scholar] [CrossRef]
- Yu, S.H.; Huang, H.Y.; Korivi, M.; Hsu, M.F.; Huang, C.Y.; Hou, C.W.; Chen, C.Y.; Kao, C.L.; Lee, R.P.; Lee, S.D.; et al. Oral Rg1 supplementation strengthens antioxidant defense system against exercise-induced oxidative stress in rat skeletal muscles. J. Int. Soc. Sports Nutr. 2012, 9, 23. [Google Scholar] [CrossRef] [Green Version]
- Hui, J.; Gao, J.; Wang, Y.; Zhang, J.; Han, Y.M.; Wei, L.; Wu, J. Panax notoginseng saponins ameliorates experimental hepatic fibrosis and hepatic stellae cell proliferation by inhibiting the Jak2.State3 pathways. J. Tradit. Chin. Med. 2016, 36, 217–224. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, C.; Feng, Z.; Liu, Z.; Ahu, H.; Zhou, X. Triptolide-induced hepatotoxicity can be alleviated when combined with Panax notoginseng saponins and Catapol. J. Ethnopharmacol. 2018, 214, 232–329. [Google Scholar] [CrossRef]
- Ding, R.B.; Tian, K.; Cao, Y.W.; Bao, J.L.; Wang, M.; He, C.; Hu, Y.; Su, H.; Wan, J.B. Protective effect of Panax notoginseng saponins on acute ethanol-induced liver injury is associated with ameliorating hepatic lipid accumulation and reducing ethanol-mediated oxidative stress. J. Agric. Food Chem. 2015, 63, 2413–2422. [Google Scholar] [CrossRef]
- Zhong, H.; Wu, H.; Bai, H.; Wang, M.; Wen, J.; Gong, J.; Miao, M.; Yuan, F. Panax notoginseng saponins promote liver regeneration through activation of the P13/AKTmTOR cell proliferation pathway and upregulation of the AKT/BAD cell survival pathway in mice. BMC Complement. Altern. Med. 2019, 19, 22. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Blain, R.B. The occurrence of hormestic dose responses in the toxicological literature. The hormesis database: An overview. Toxicol. Appl. Pharmacol. 2005, 202, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Zhou, P.; Sun, Y.; Meng, X.; Dai, Z.; Sun, G.; Sun, X. Protective effects and target network analysis of Ginsenoside Rg1 in cerebral ischemia and reperfusion injury: A comprehensive overview of experimental studies. Cells 2018, 7, 270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calabrese, E.J.; Blain, R.B. Hormesis and plant biology. Environ. Pollut. 2009, 157, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.W.; Wang, C.Z.; Yuan, C.S. Isolation and analysis of ginseng: Advances and challenges. Nat. Prod. Rep. 2011, 28, 467–495. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Wang, H.; Qu, C.; Xie, L.; Wicks, S.M.; Xie, J. Ginsenoside Re: Its chemistry, metabolism and pharmacokinetics. Chin. Med. 2012, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Dai, Y.-L.; Zheng, F.; Xu, C.-E.; Feng, L.; Wang, X.-Y.; Zheng, B.-S.; Yu, S.-S. Oral absorption and in vivo biotransformation of ginsenosides. Chin. J. Biol. 2014, 27, 1633–1636. [Google Scholar]
- Yu, S.E.; Mwesige, B.; Yi, Y.S.; Yoo, B.C. Ginsenosides: The need to move forward from bench to clinical trials. J. Ginseng Res. 2019, 43, 361–367. [Google Scholar]
- Ki, S.H.; Yang, J.H.; Ku, S.K.; Kim, S.C.; Kim, S.C.; Kim, Y.W.; Cho, I.J. Red ginseng extract protects against carbon tetrachloride-induced liver fibrosis. J. Ginseng Res. 2013, 37, 45–53. [Google Scholar] [CrossRef] [Green Version]
- Hou, J.; Xue, J.; Lee, M.; Yu, J.; Sung, C. Long-term administration of ginsenoside Rh1 enhances learning and memory b promoting cell survival in the mouse hippocampus. Int. J. Mol. Med. 2014, 33, 234–240. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Ji, L.L. Chronic ginseng consumption attenuates age-associated oxidative stress in rats. J. Nutr. 2003, 133, 3603–3609. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.J.; So, H.K.; Jo, A.; Kim, H.B.; Lee, S.J.; Bae, G.U.; Kang, J.S. Ginsenoside Rg1 augments oxidative metabolism and anabolic response to skeletal muscle in mice. J. Ginseng Res. 2019, 43, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Dong, G.; Liu, L.; Zhou, H. The long-term consumption of ginseng extract reduces the susceptibility of intermediate-aged heart to acute ischemia reperfusion injury. PLoS ONE 2015, 10, e0144733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
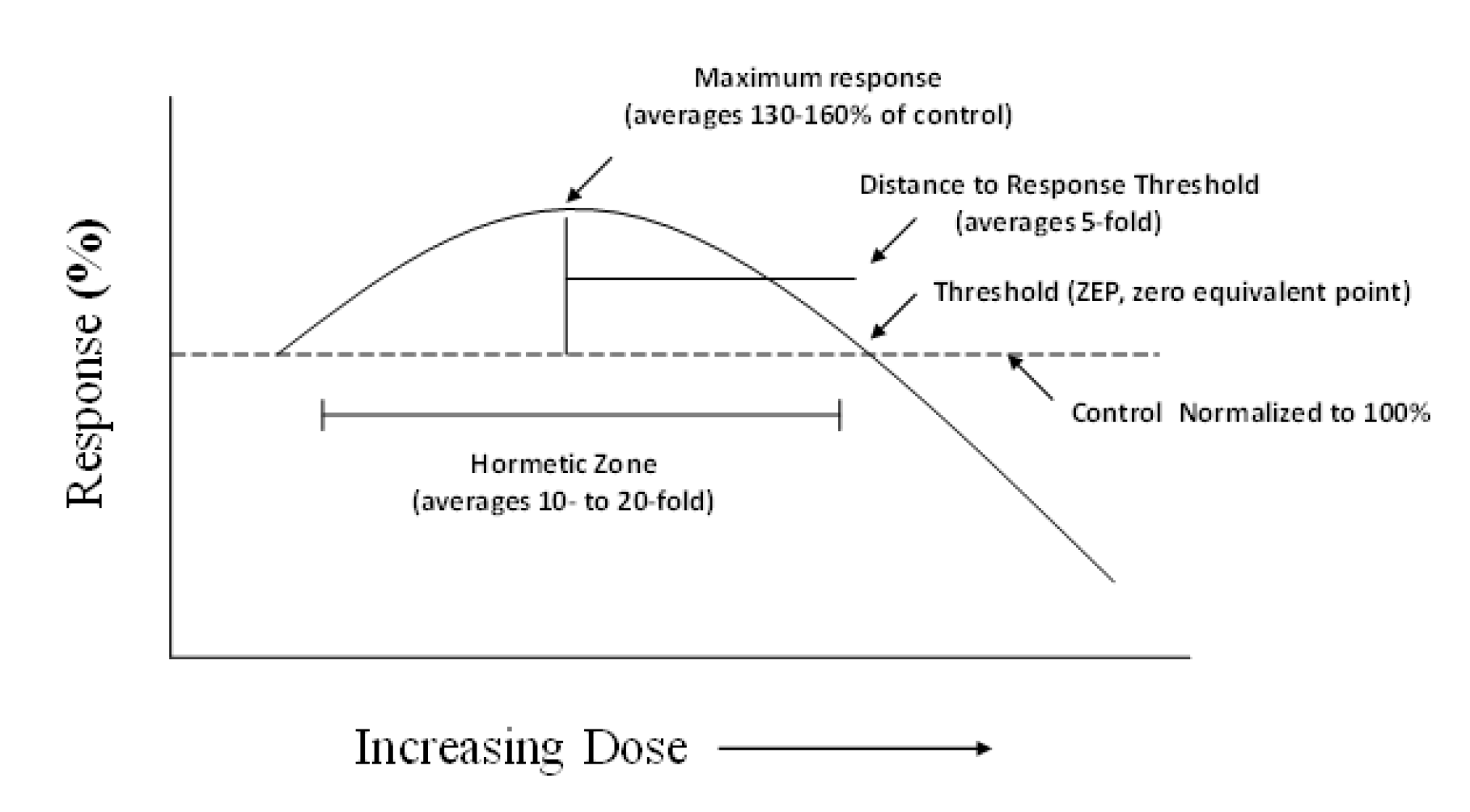


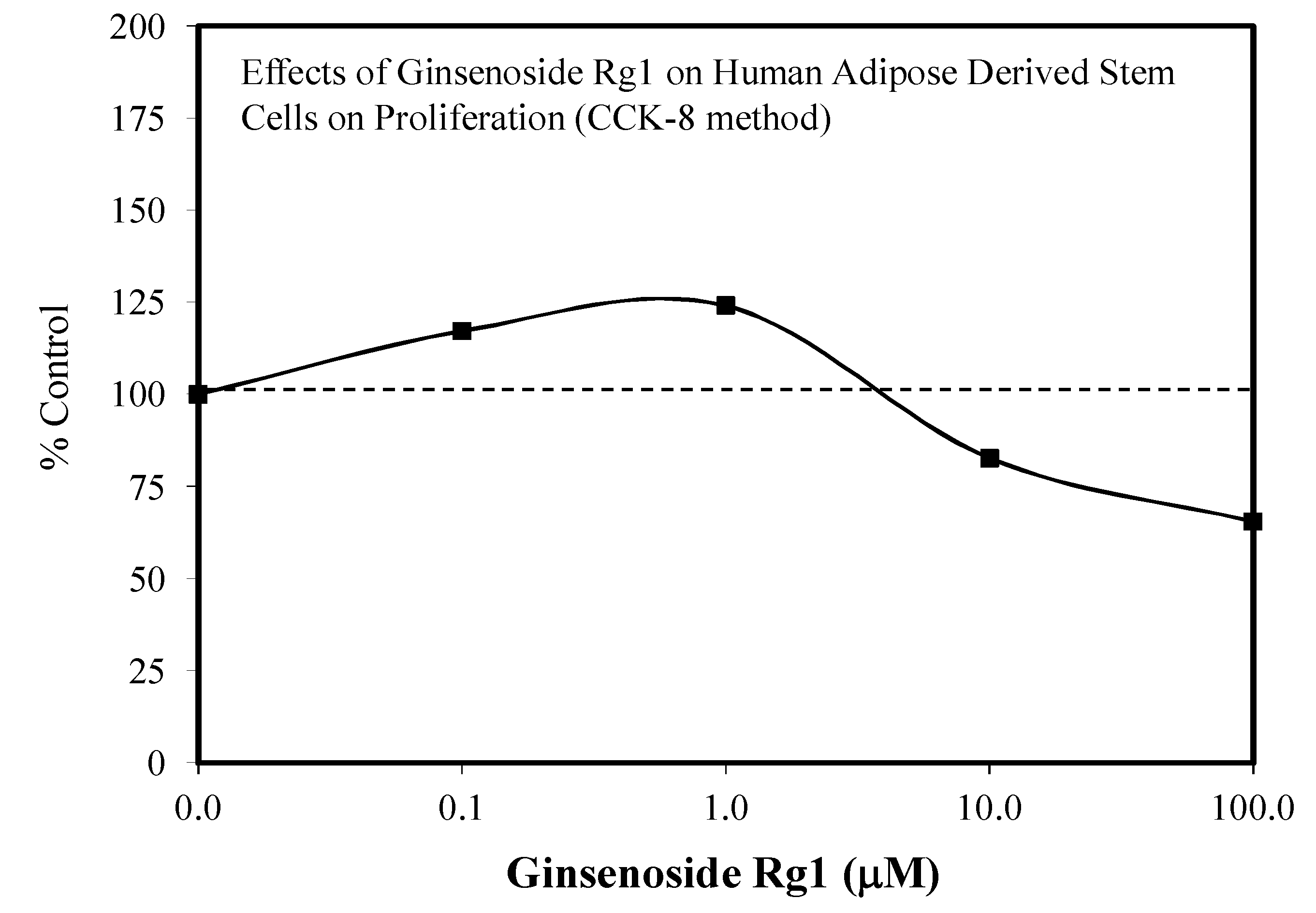
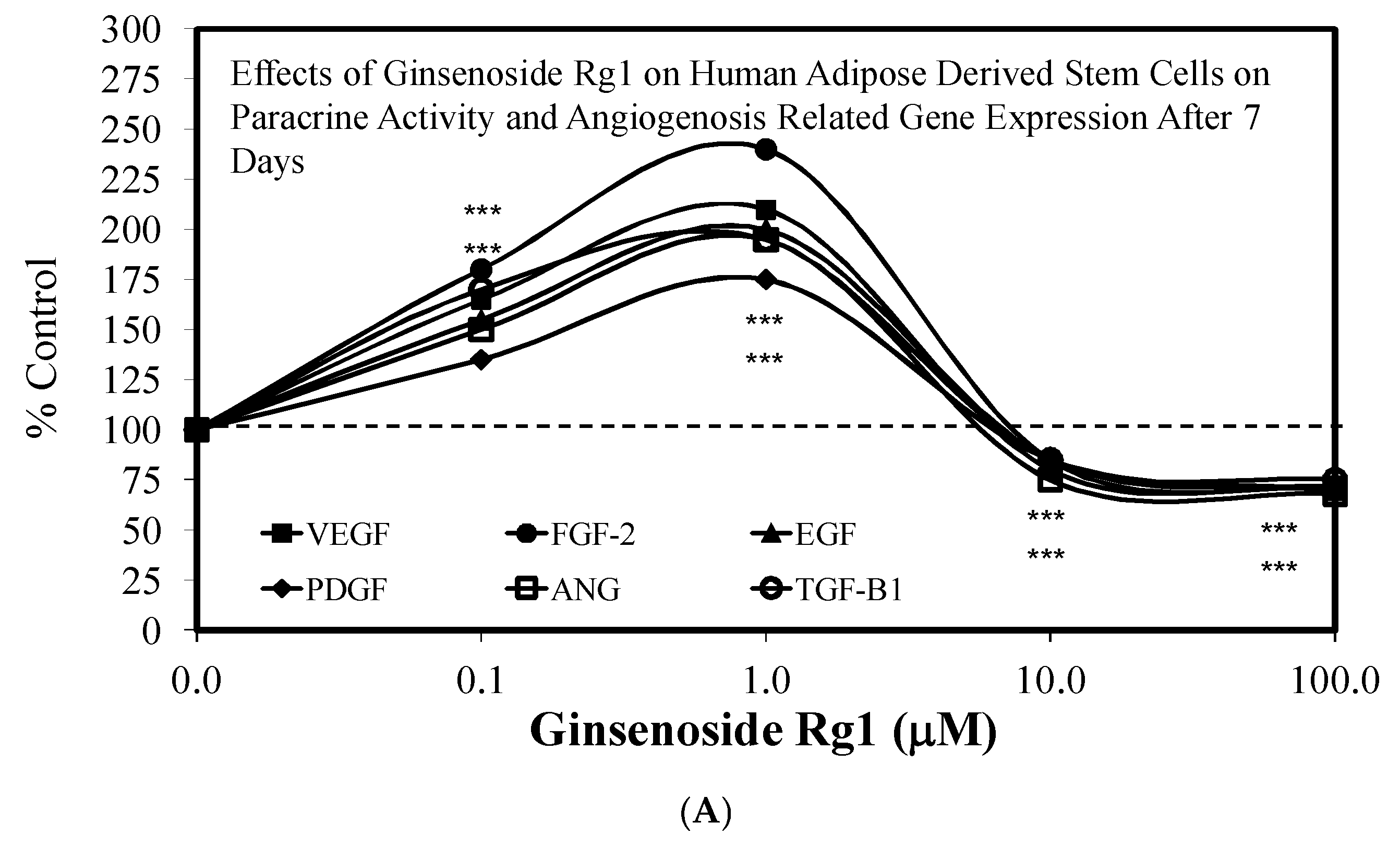
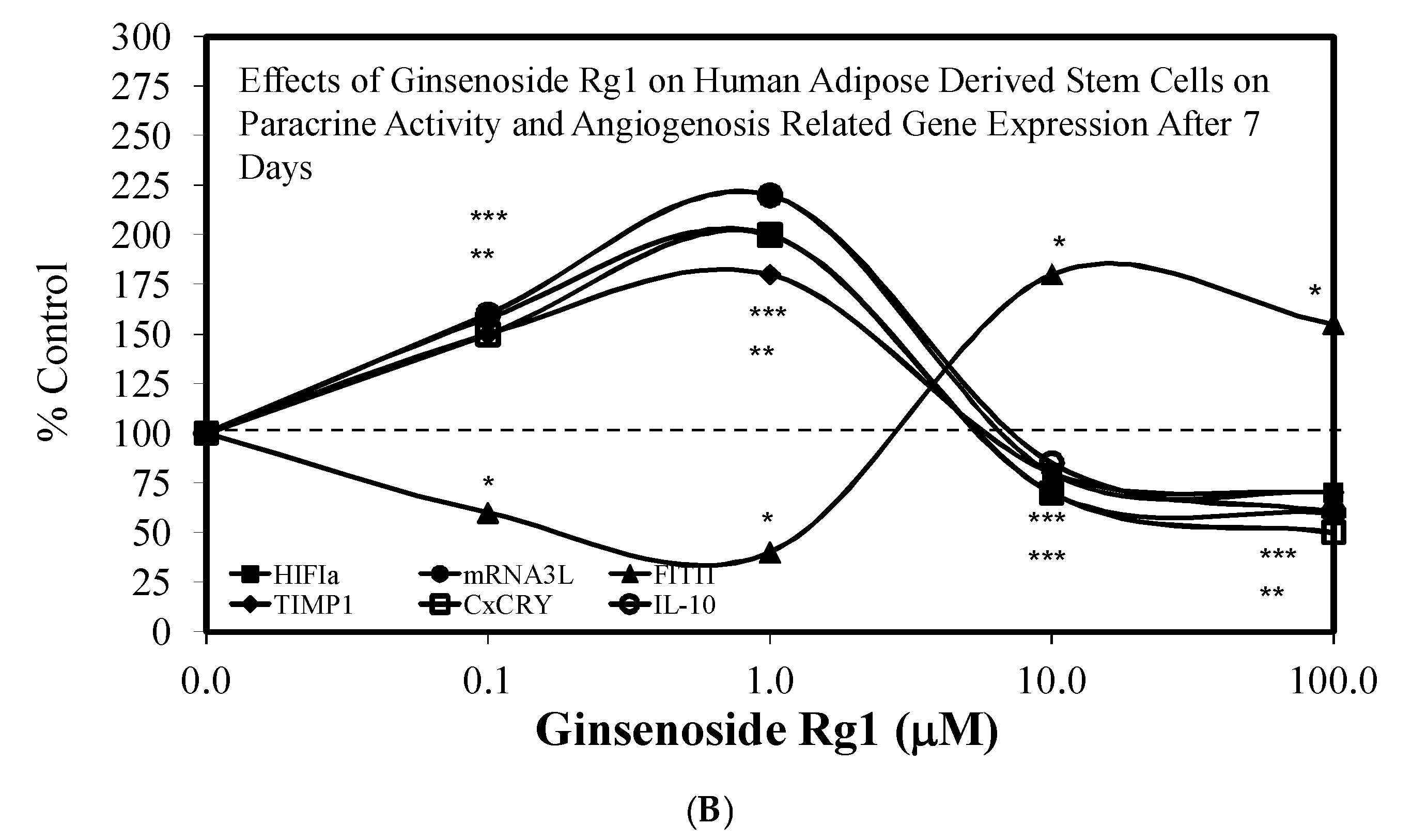
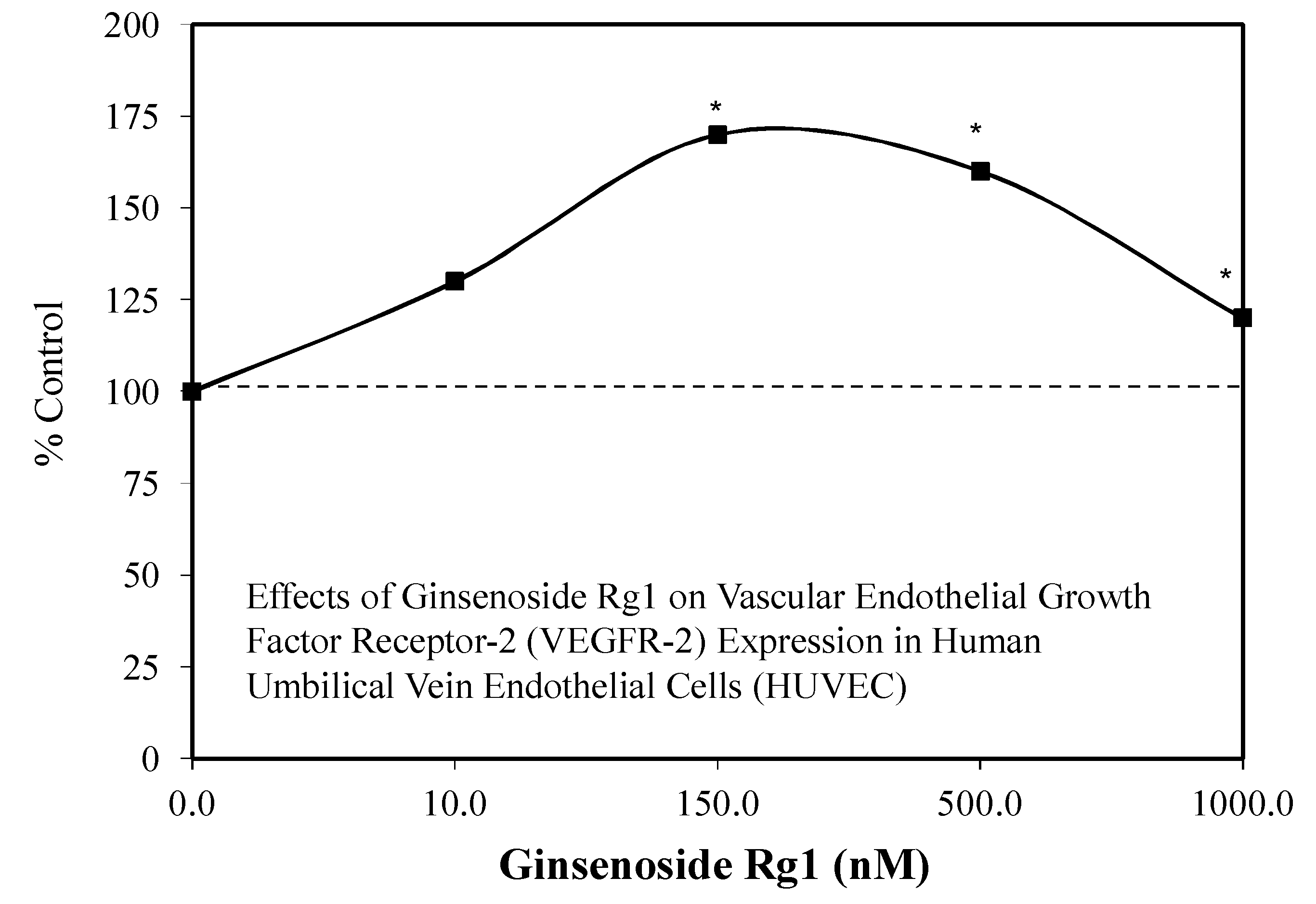
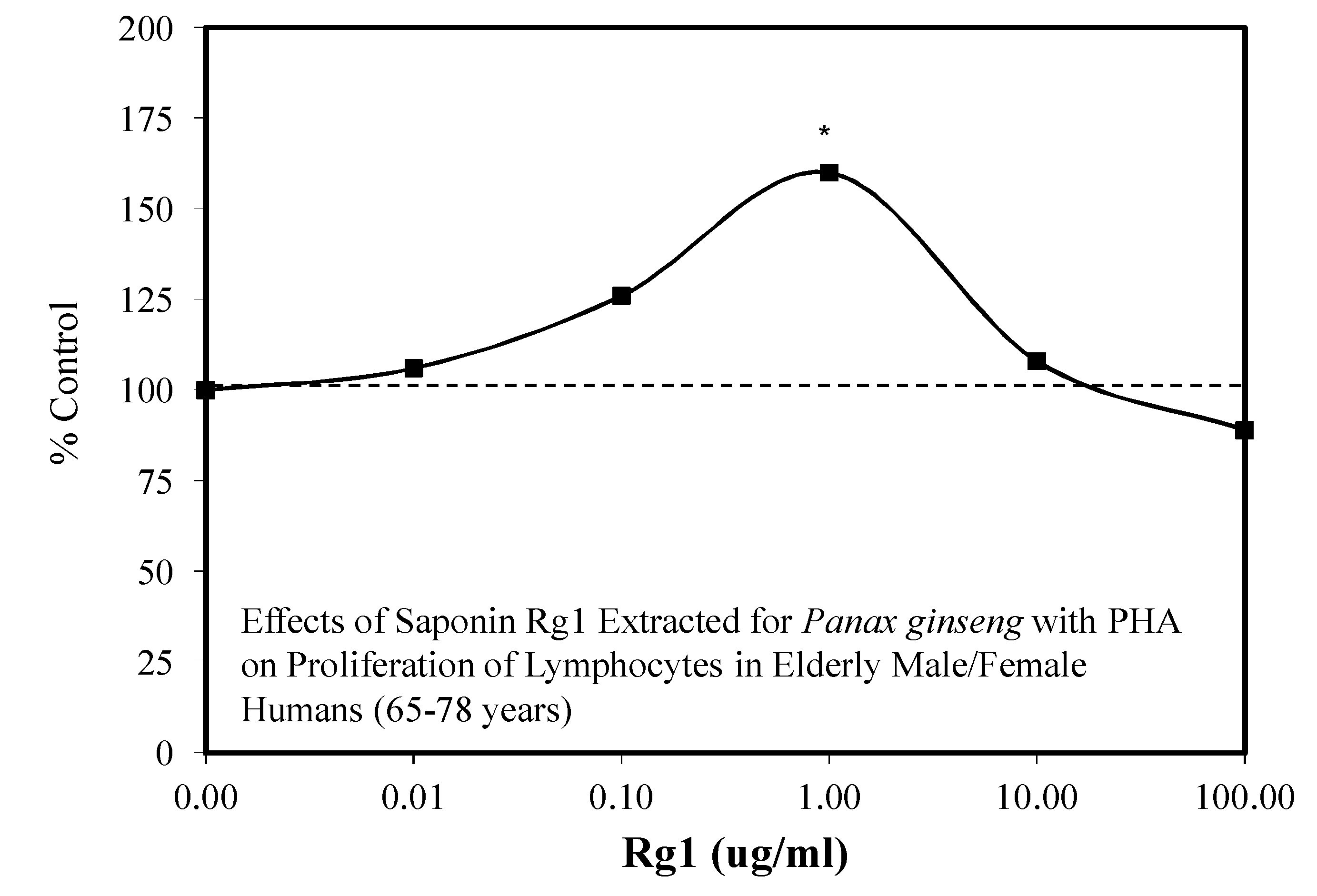
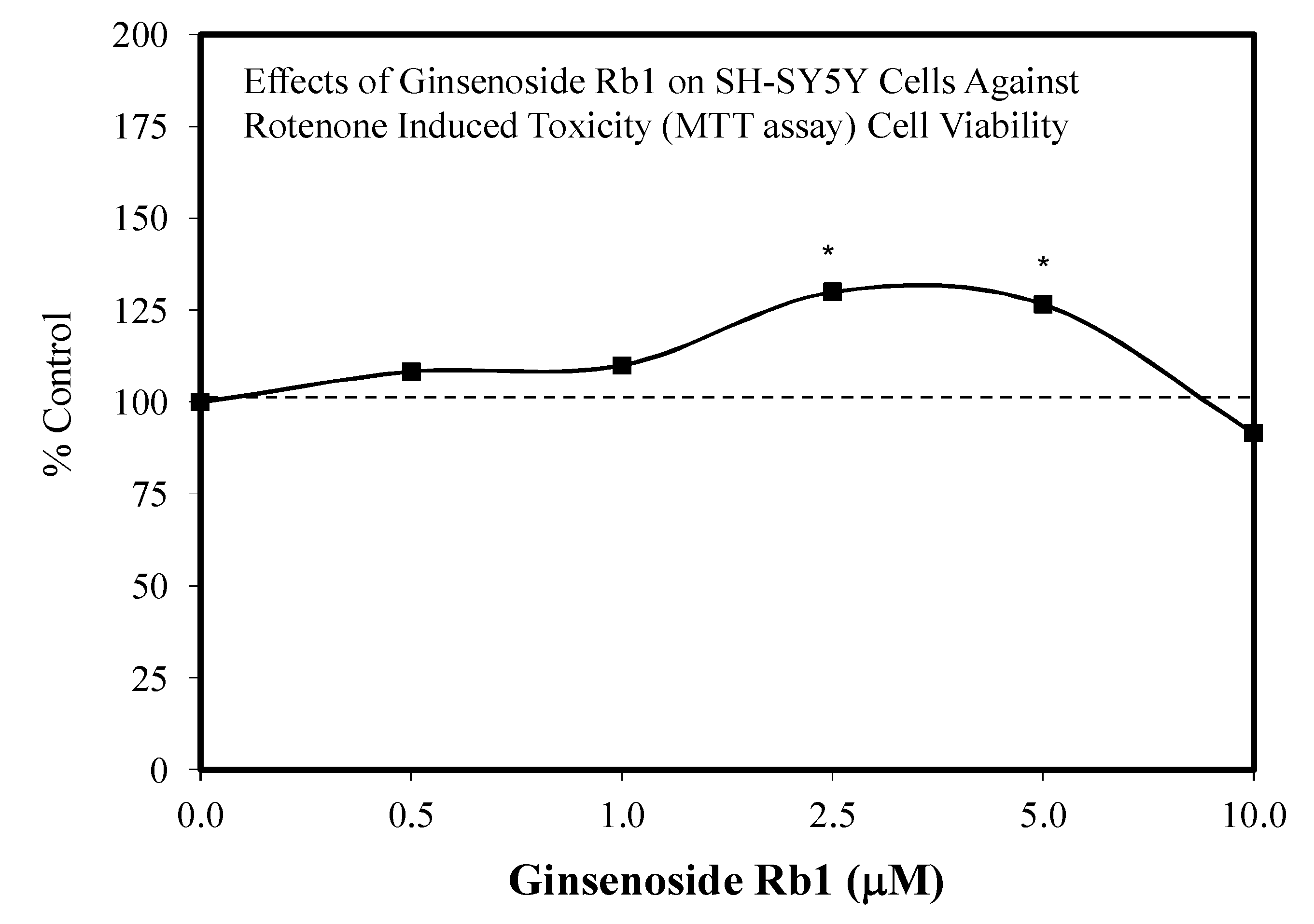
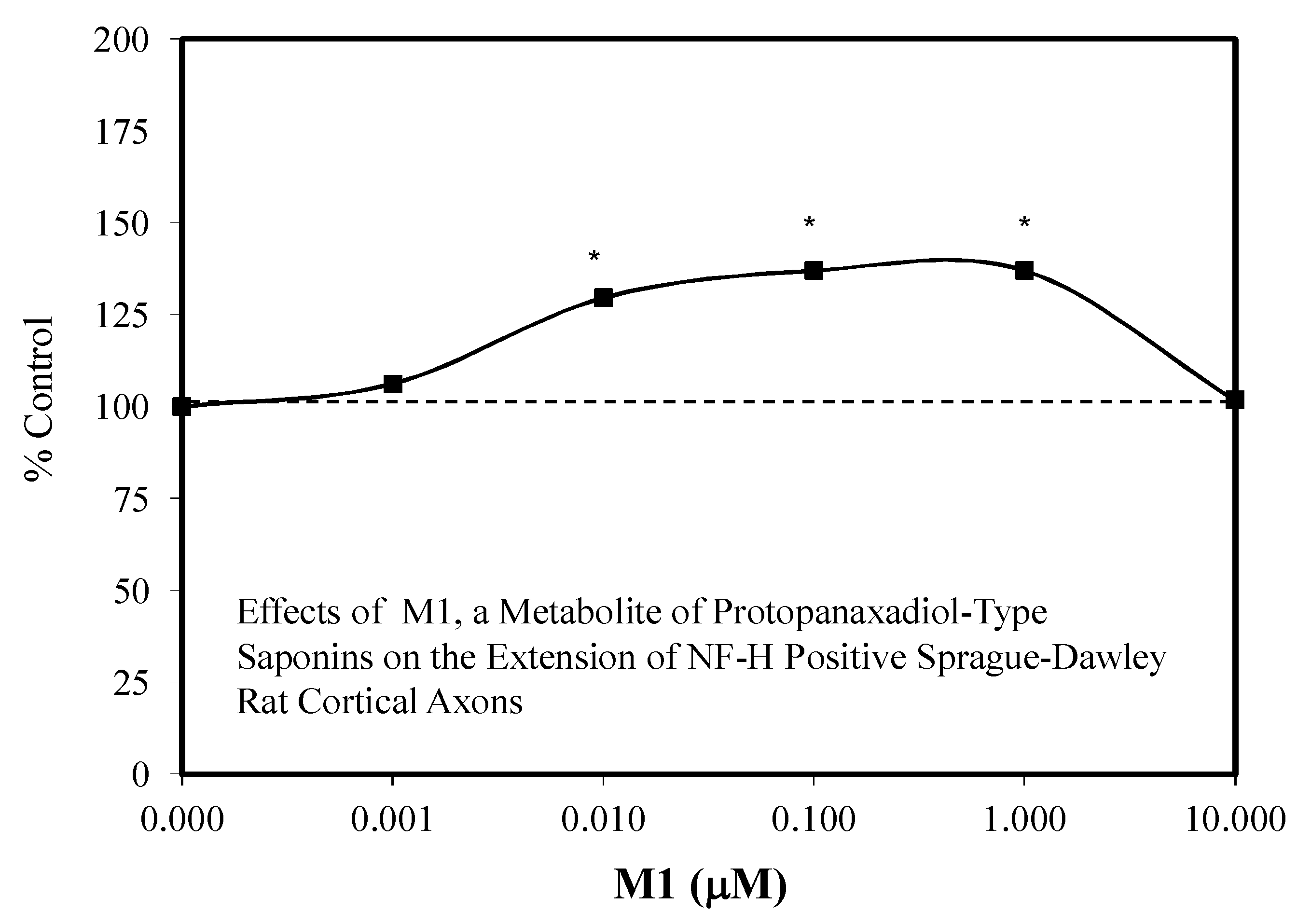
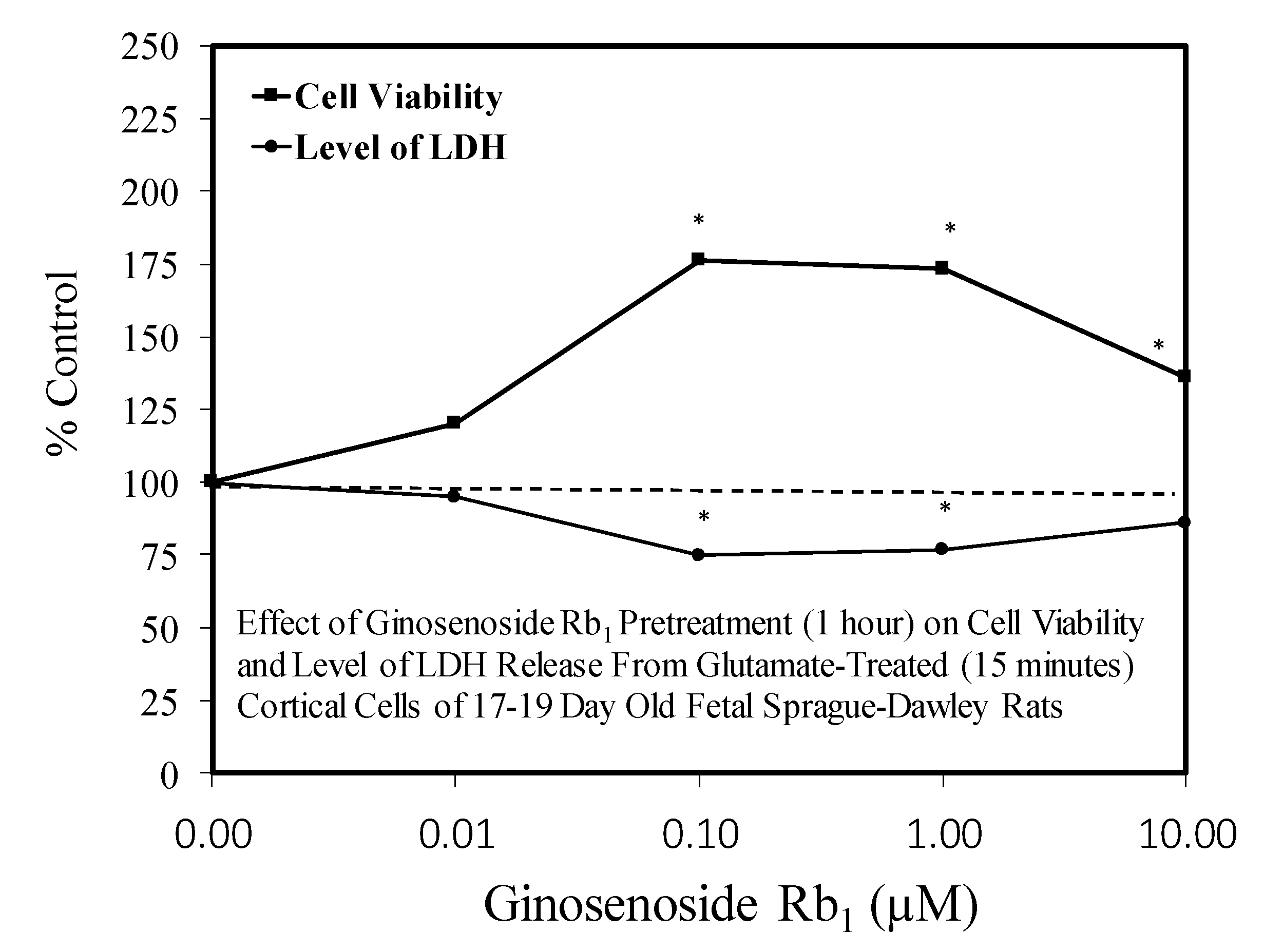
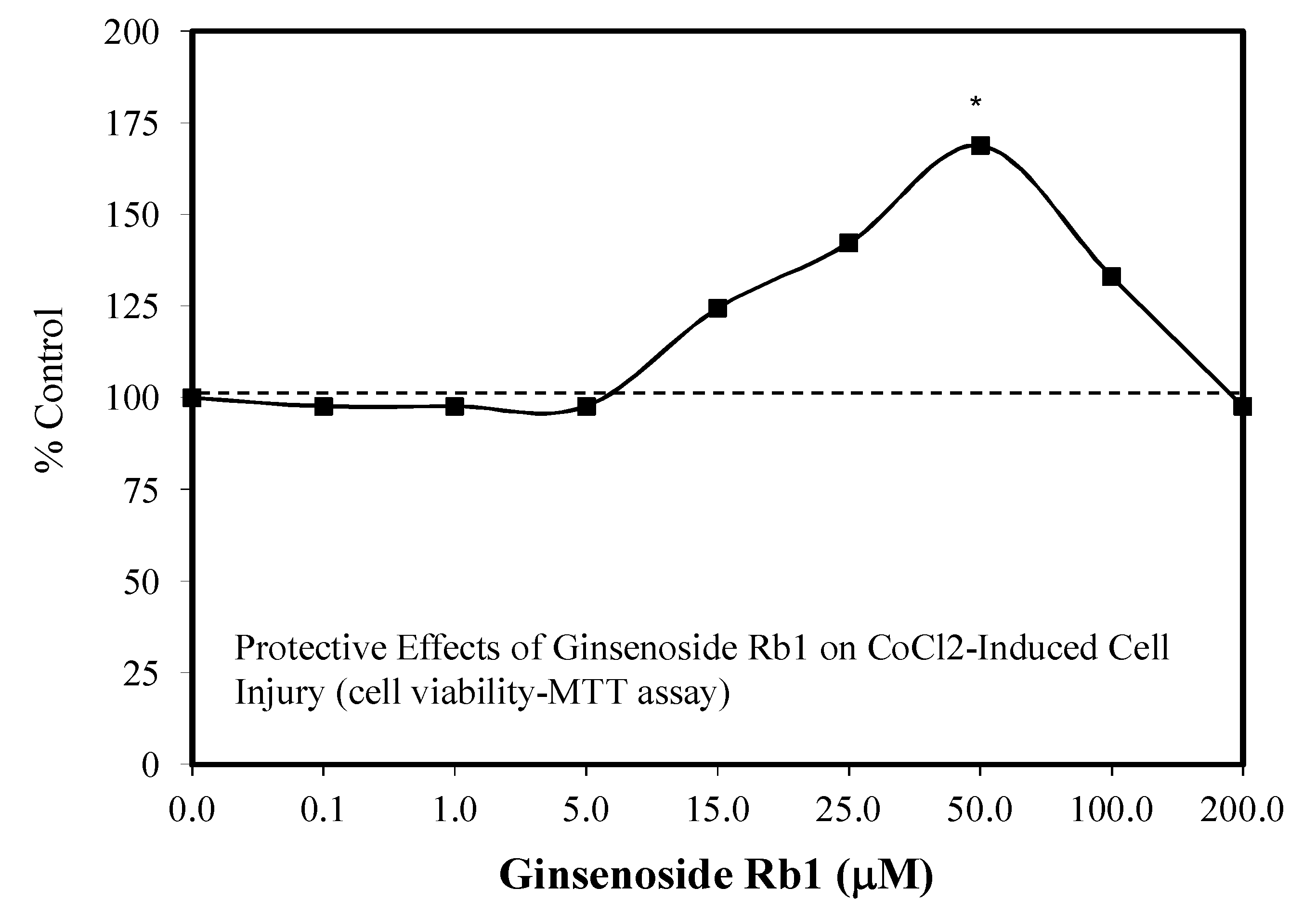

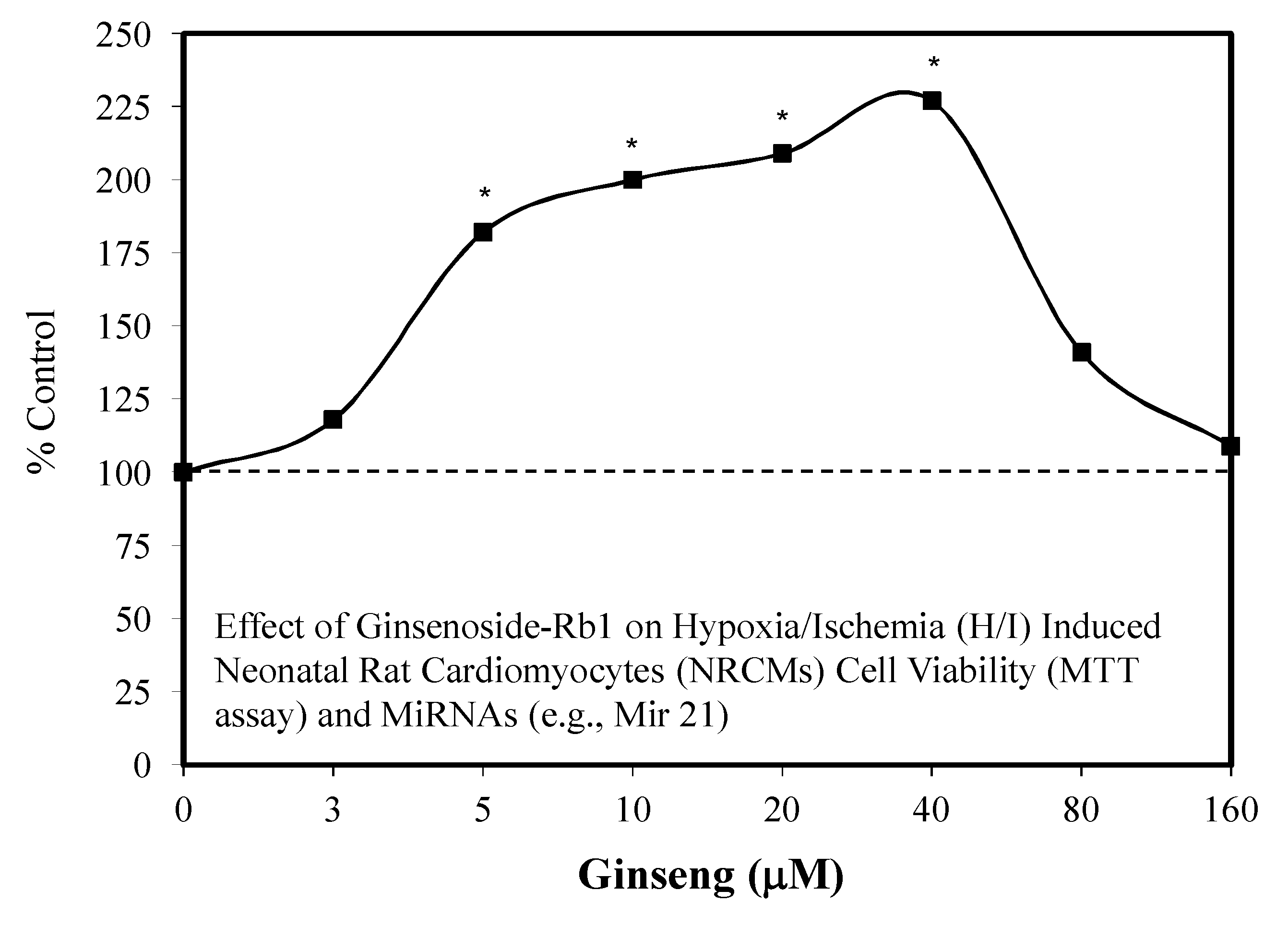

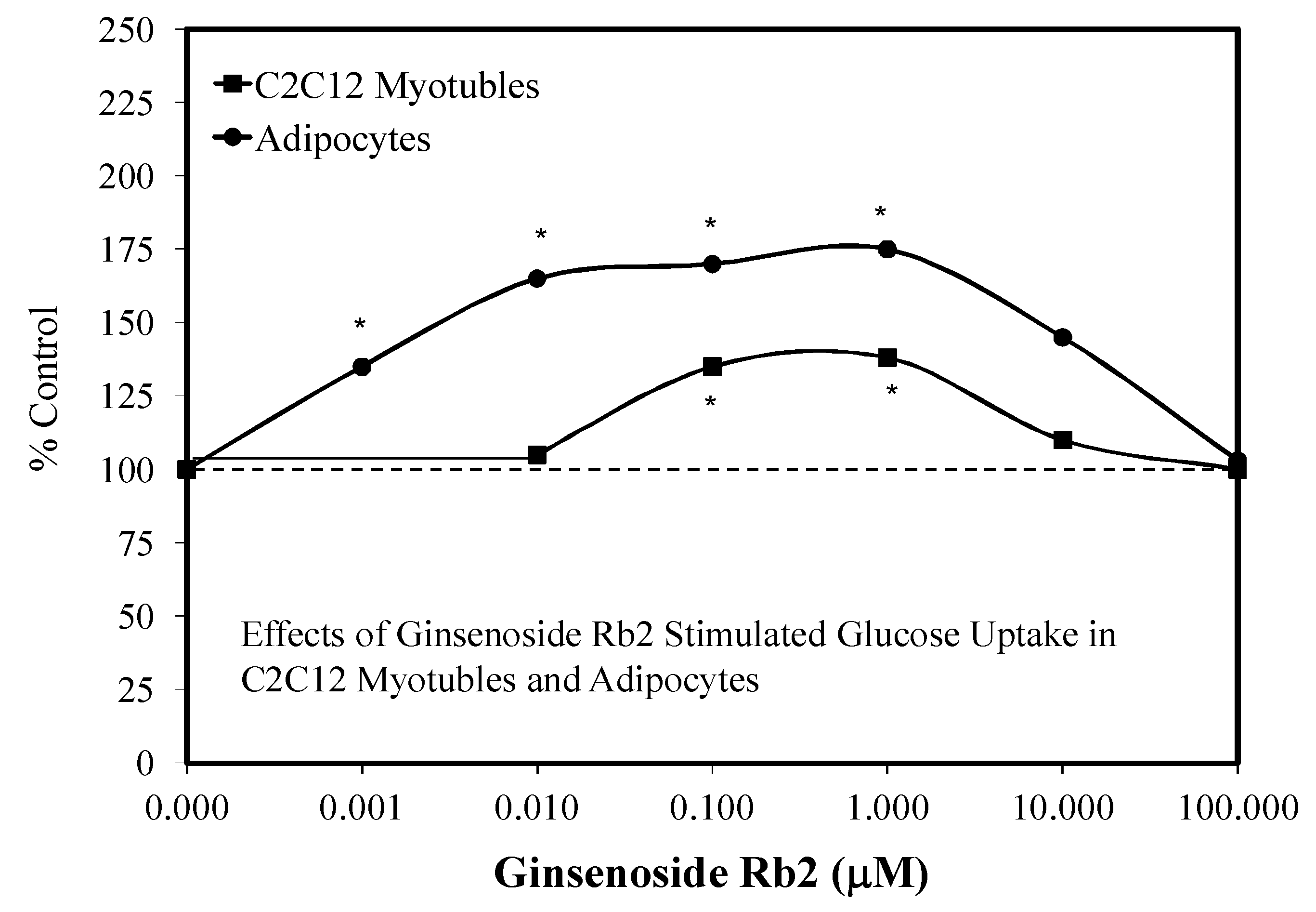


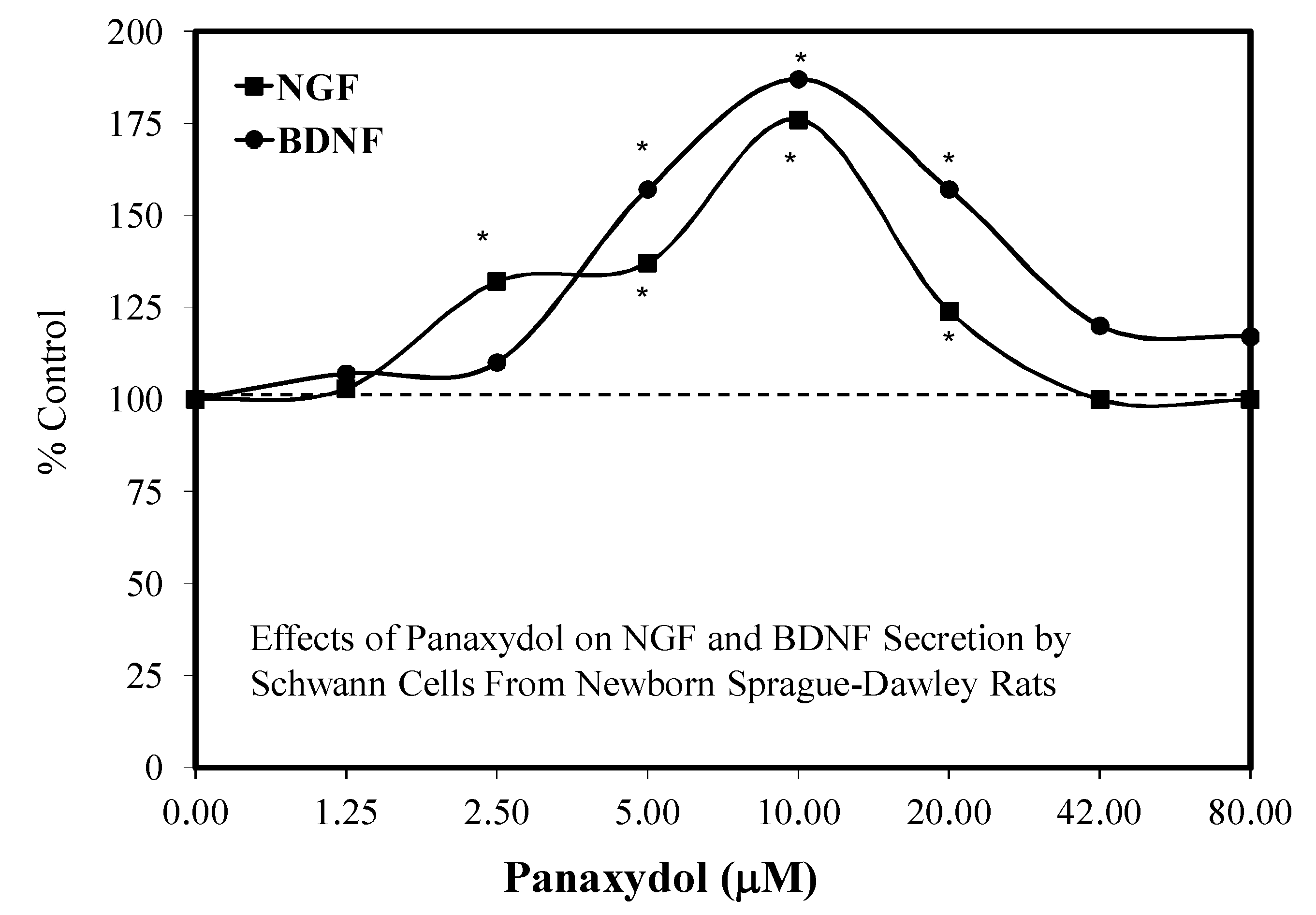
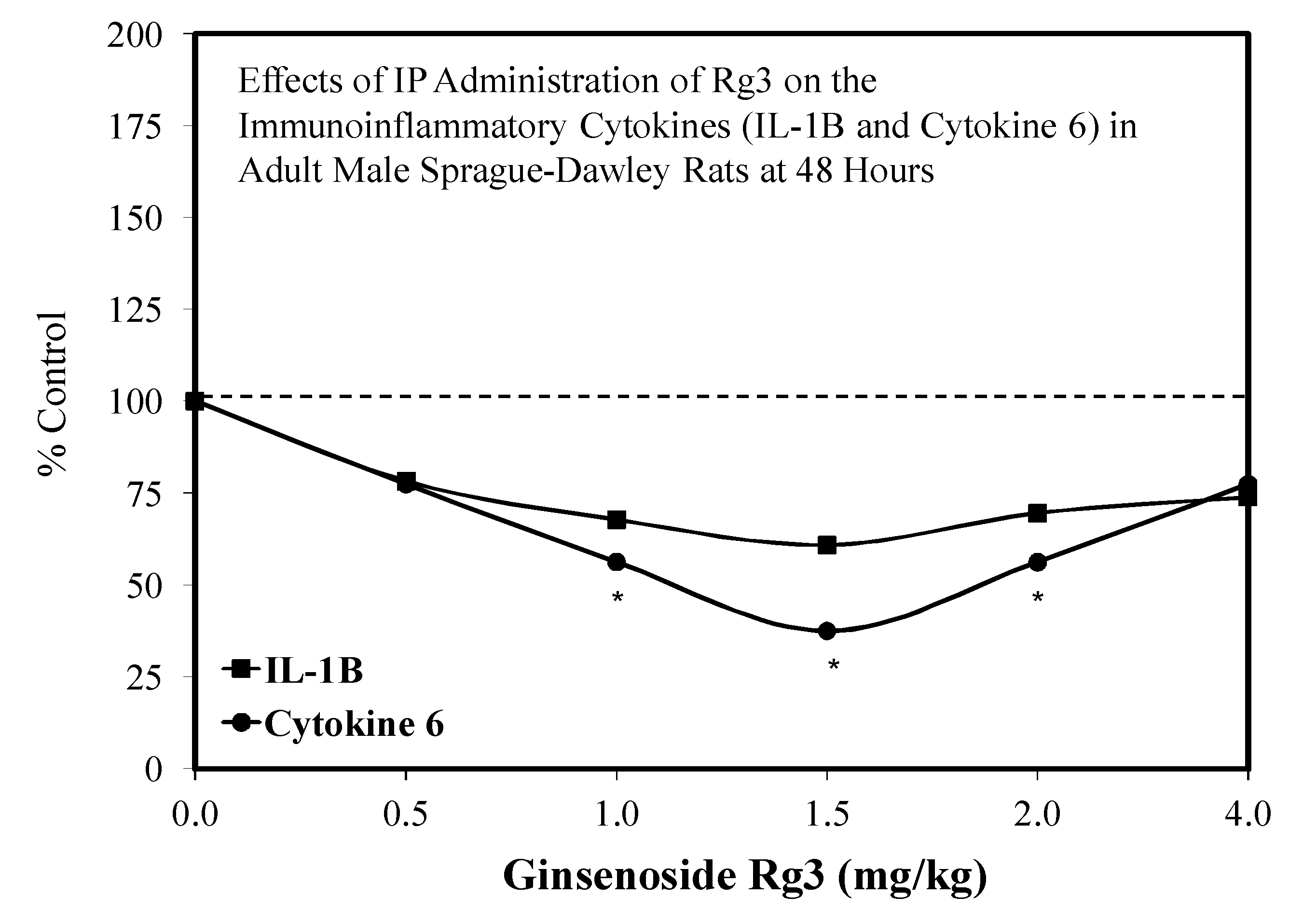
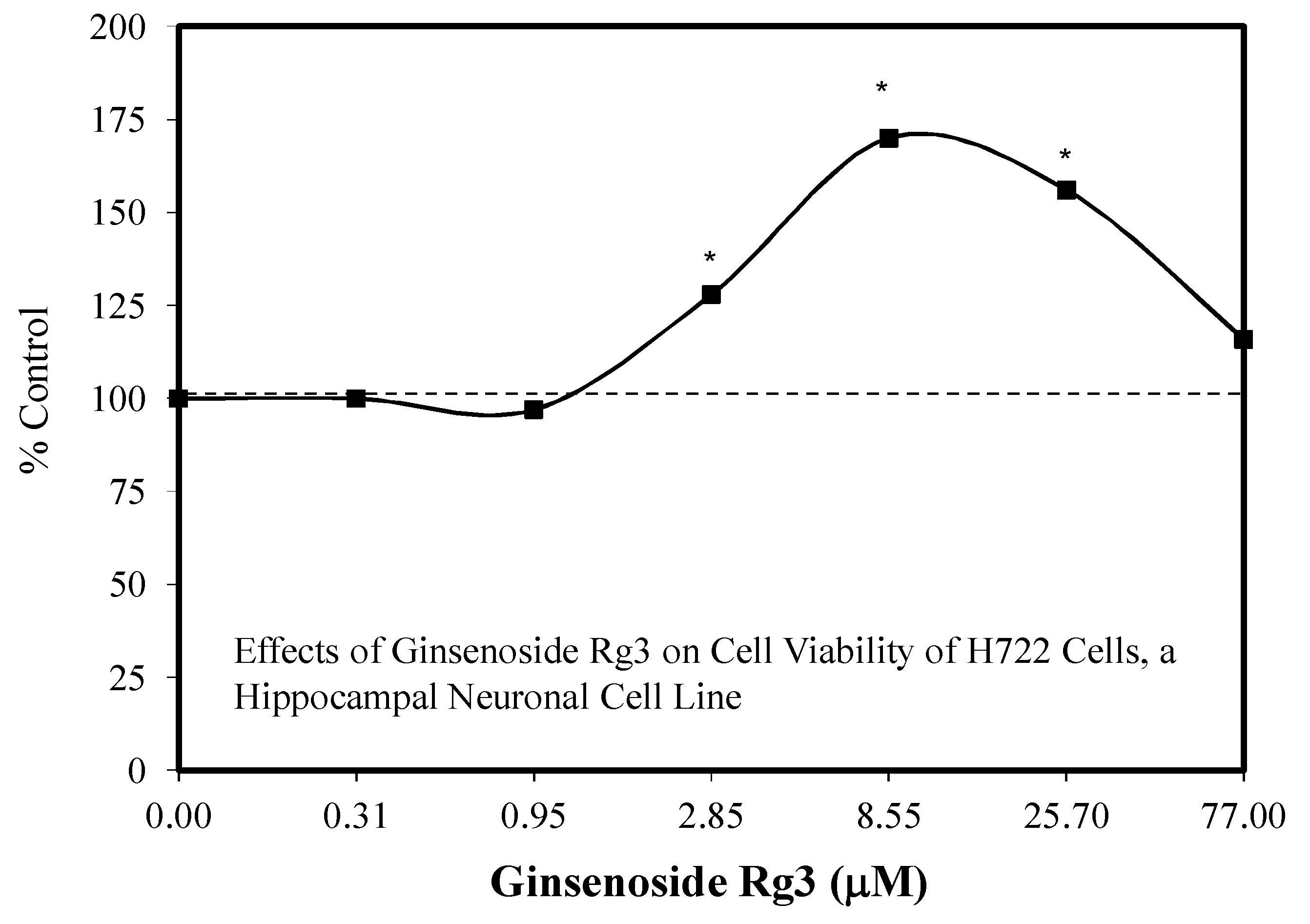
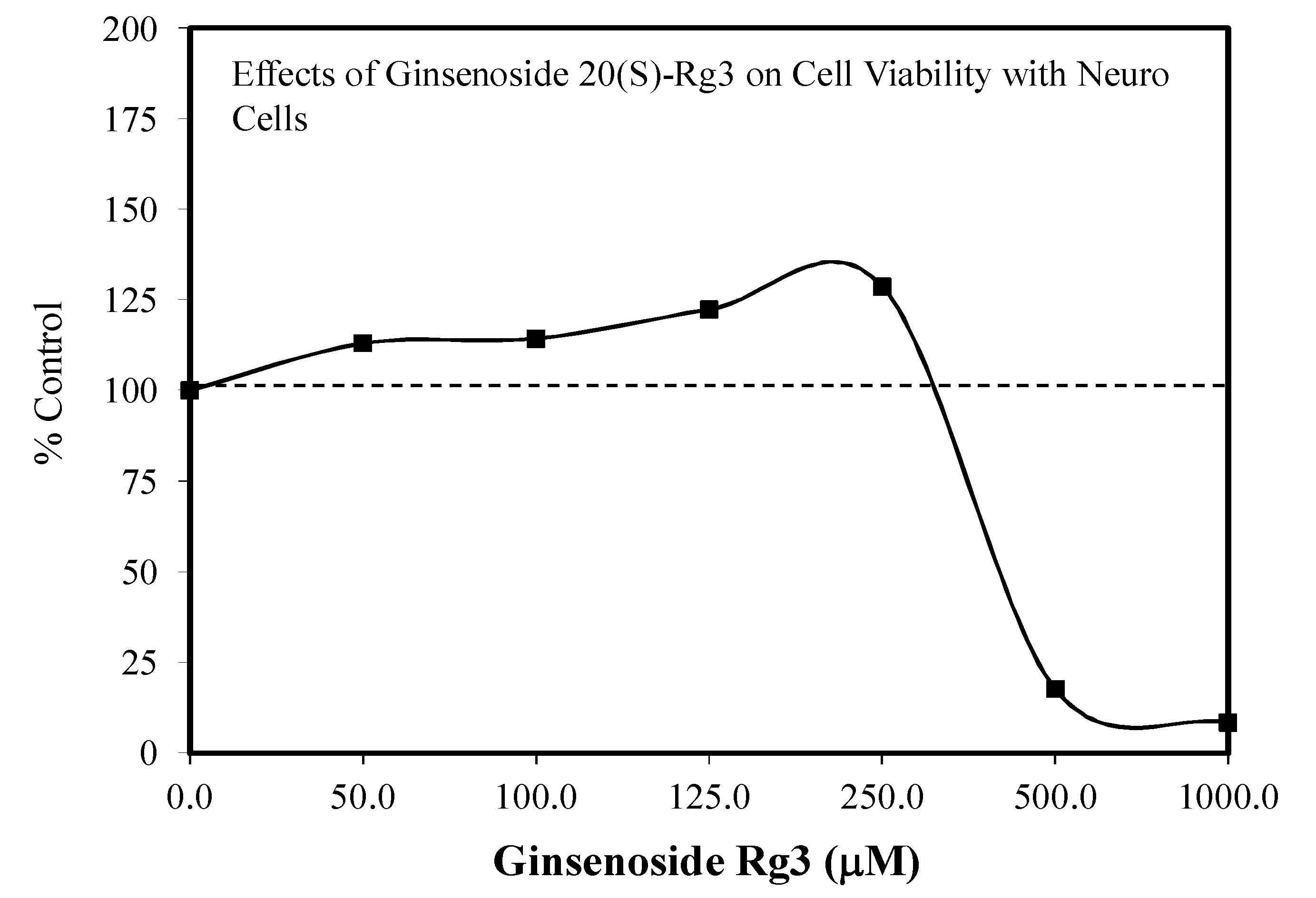




| Non-Neural Models | |
| References | |
| Muscle fiber damage-exercise | Voces et al. [12,13] |
| HEPG2 cell proliferation | Li et al. [14] |
| HUVEC | Sung et al. [15] |
| A549-human pulmonary epithelial cells | Son et al. [16] |
| Ovary-hormone production | Seghinsara et al. [17] |
| Diabetes-STZ-model | Lim et al. [18] |
| Human skin fibroblasts | Kanzaki et al. [19] |
| PAI-1- PAI-1 (Plasminogen activator inhibitor) | Morisaki et al. [20] |
| Neural Models | |
| SH-SY-5Y-Neural | Shi et al. [21] |
| Brain-TBI studies-Neural | Hu et al. [22]; Xia et al. [23] |
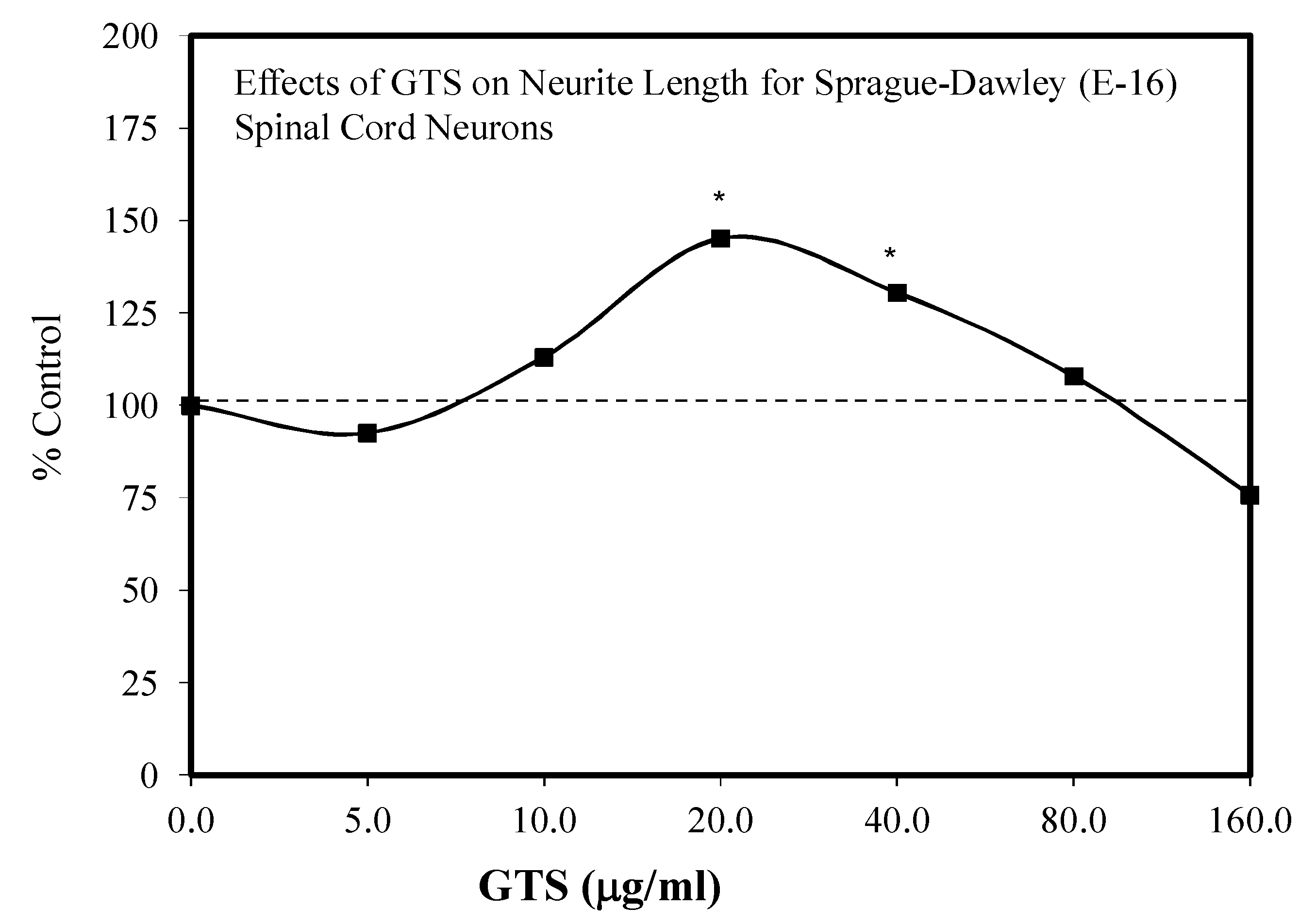
| Neurite outgrowth-Sprague Dawley- E-16-Neural | Liao et al. [24] |
| Zebrafish-neuron loss/behavior-Neural | Zhang et al. [25] |
| Hippocampus, dentate gryus-Neural | Hu et al. [22]; Lim et al. [18] |
| Astrocytes, preconditioning-Neural | Naval et al. [26] |
| NSC-Cell proliferation/differentiation-Neural | Gao et al. [27] |
| IRC mice/female-behavior-Neural-prefrontal cortex | Boonlert et al. [28] |
| Spinal cord survival | Liao et al. [24] |
| Rg1 | Rb1 | Rd | Rg3 | Polyacetylenes | Gentonin | Ginseng Mixtures |
|---|---|---|---|---|---|---|
| Stem cells | Neuroprotection | Neuroprotection | Neuronal injury | Nerve repair | Wound healing | Neuroprotection |
| Proliferation/differentiation | AD/PD | PD | Neuronal regeneration | AD | Corneal damage | Learning/memory |
| Neuroprotection | Stroke | Stroke | Atherosclerosis | AD | Behaviors | |
| AD/PD | Neuronal Repair | Neurosphere production | Glycogenolysis | Hippocampal cell | ||
| Stroke | Other: Heart, Diabetes | Glutamate toxicity. | Neurite outgrowth | |||
| Neonatal brain hypoxia | Spinal Cord | NSC proliferation | ||||
| Neuronal wound healing | Other: Heart-cardiomyocytes, Kidney-FK506 | Brain Injury | ||||
| Nerve regeneration | Other: Muscle injury, Osteogenesis, Kidney damage, Hormone production | |||||
| Other: Heart cardiomyocytes, Immune enhancement, Diabetes |
| Alzheimer′s Disease Prevention: Rg1 |
| Parkinson’s Disease Prevention: Rg1 |
| Stroke Damage Prevention: Rg1 |
| Kidney Damage Prevention: Rd |
| Heart Related Damage Prevention: Rg1 |
| Nerve Cell Damage Prevention/Regeneration: Rg1 |
| Diabetes Prevention: Rg1, Rb1, Rg3 |
| Stem Cell Proliferation/Differential: Rg1 and mixtures. |
| Brain Traumatic Injury: Mixtures |
| Mixtures | Rb1 | Rd | Rg1 | Gintonin | Re | |
|---|---|---|---|---|---|---|
| Max Stim | 155.5 | 169.6 | 146/59.5 (J) | 180 | 172.5 | 133 |
| Stim Range | 7 | 53.3 | 10 | 10 | 20 | 25 |
| Sample Size | 29 | 26 | 6 | 50 | 8 | 7 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calabrese, E.J. Hormesis and Ginseng: Ginseng Mixtures and Individual Constituents Commonly Display Hormesis Dose Responses, Especially for Neuroprotective Effects. Molecules 2020, 25, 2719. https://doi.org/10.3390/molecules25112719
Calabrese EJ. Hormesis and Ginseng: Ginseng Mixtures and Individual Constituents Commonly Display Hormesis Dose Responses, Especially for Neuroprotective Effects. Molecules. 2020; 25(11):2719. https://doi.org/10.3390/molecules25112719
Chicago/Turabian StyleCalabrese, Edward J. 2020. "Hormesis and Ginseng: Ginseng Mixtures and Individual Constituents Commonly Display Hormesis Dose Responses, Especially for Neuroprotective Effects" Molecules 25, no. 11: 2719. https://doi.org/10.3390/molecules25112719
APA StyleCalabrese, E. J. (2020). Hormesis and Ginseng: Ginseng Mixtures and Individual Constituents Commonly Display Hormesis Dose Responses, Especially for Neuroprotective Effects. Molecules, 25(11), 2719. https://doi.org/10.3390/molecules25112719





