Therapeutic Emergence of Rhein as a Potential Anticancer Drug: A Review of Its Molecular Targets and Anticancer Properties
Abstract
1. Introduction
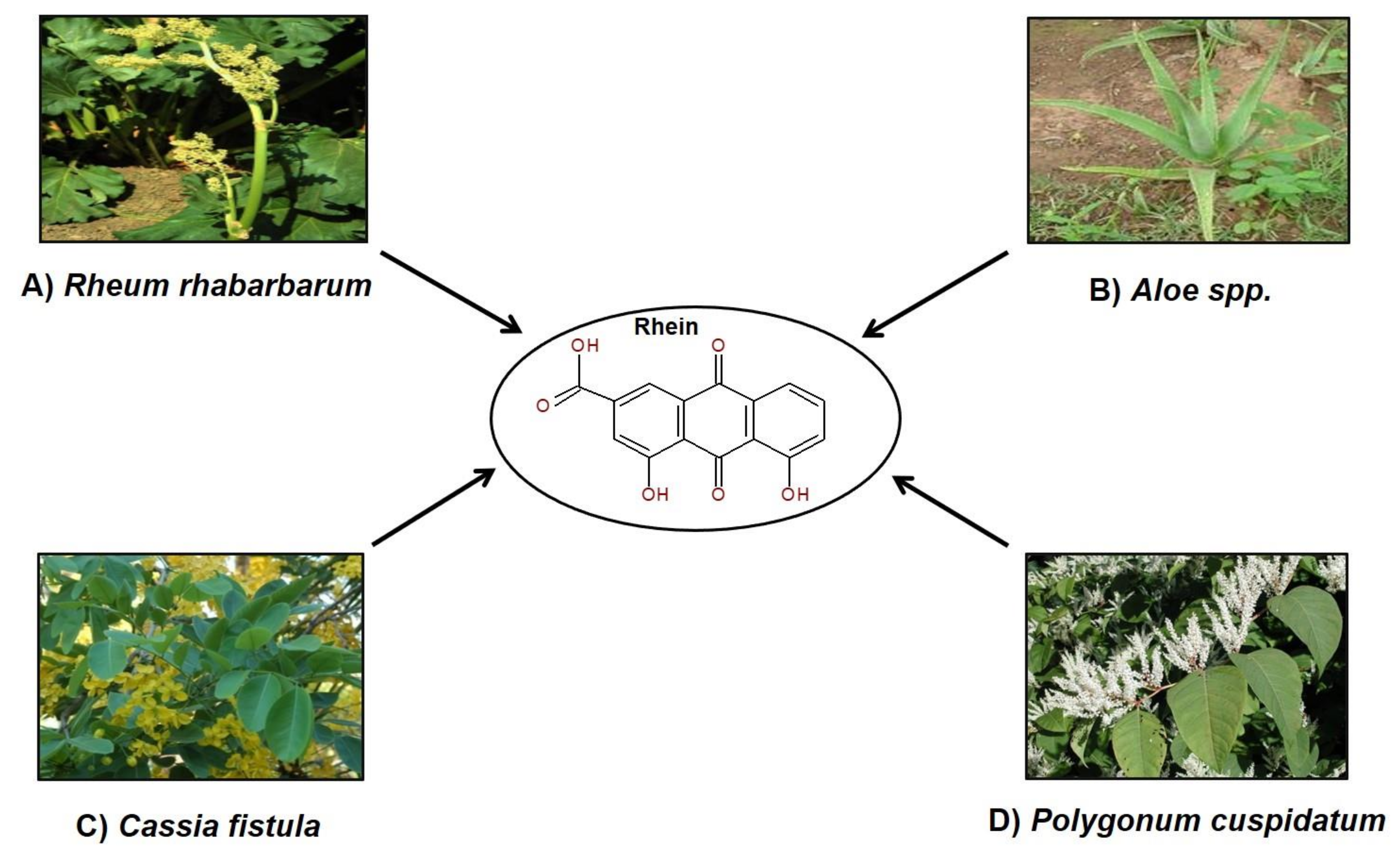
2. Rhein in Nature
3. Ethnopharmacological Uses of Plants Containing Rhein
4. Chemistry of Rhein
5. Biological Activities of Rhein
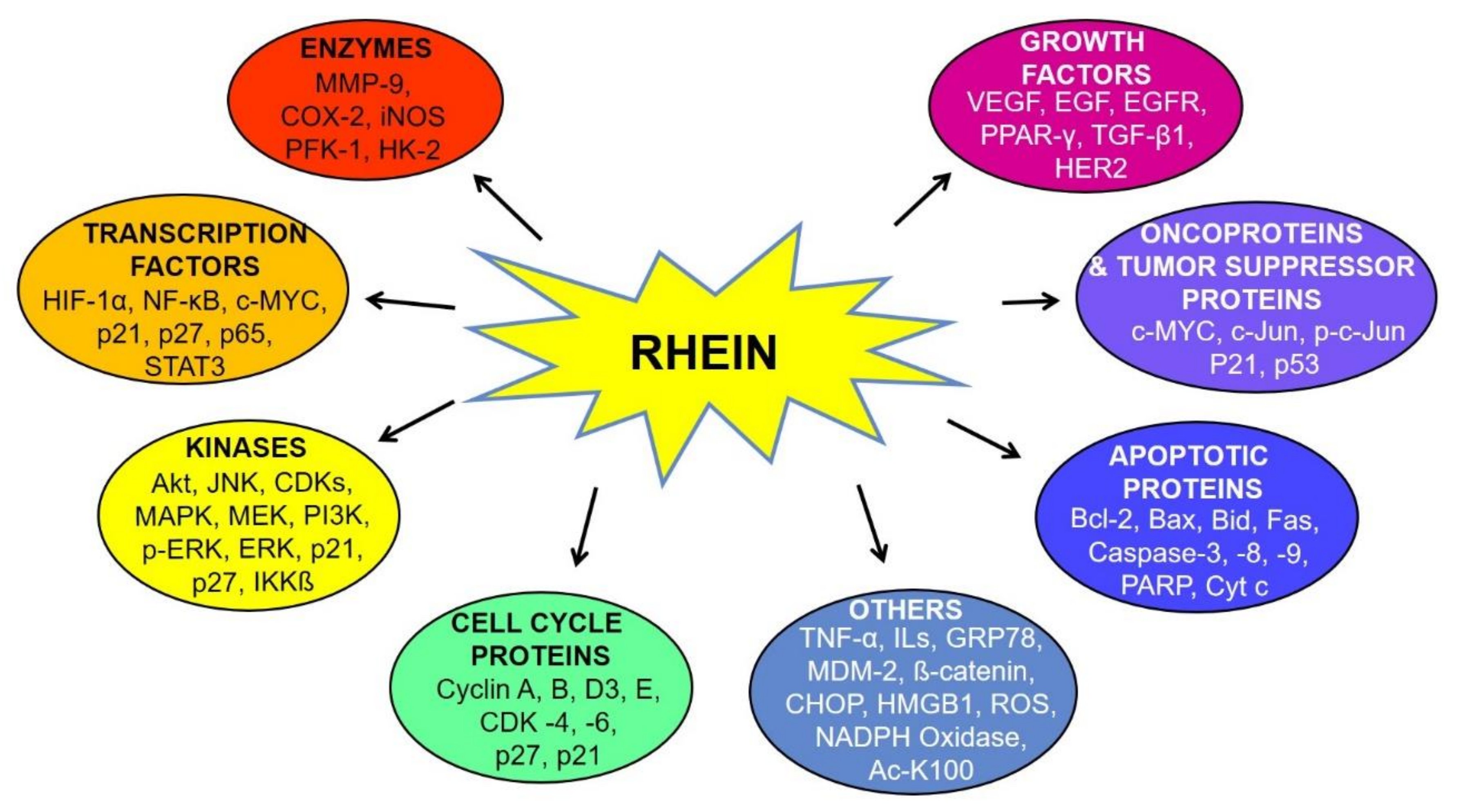
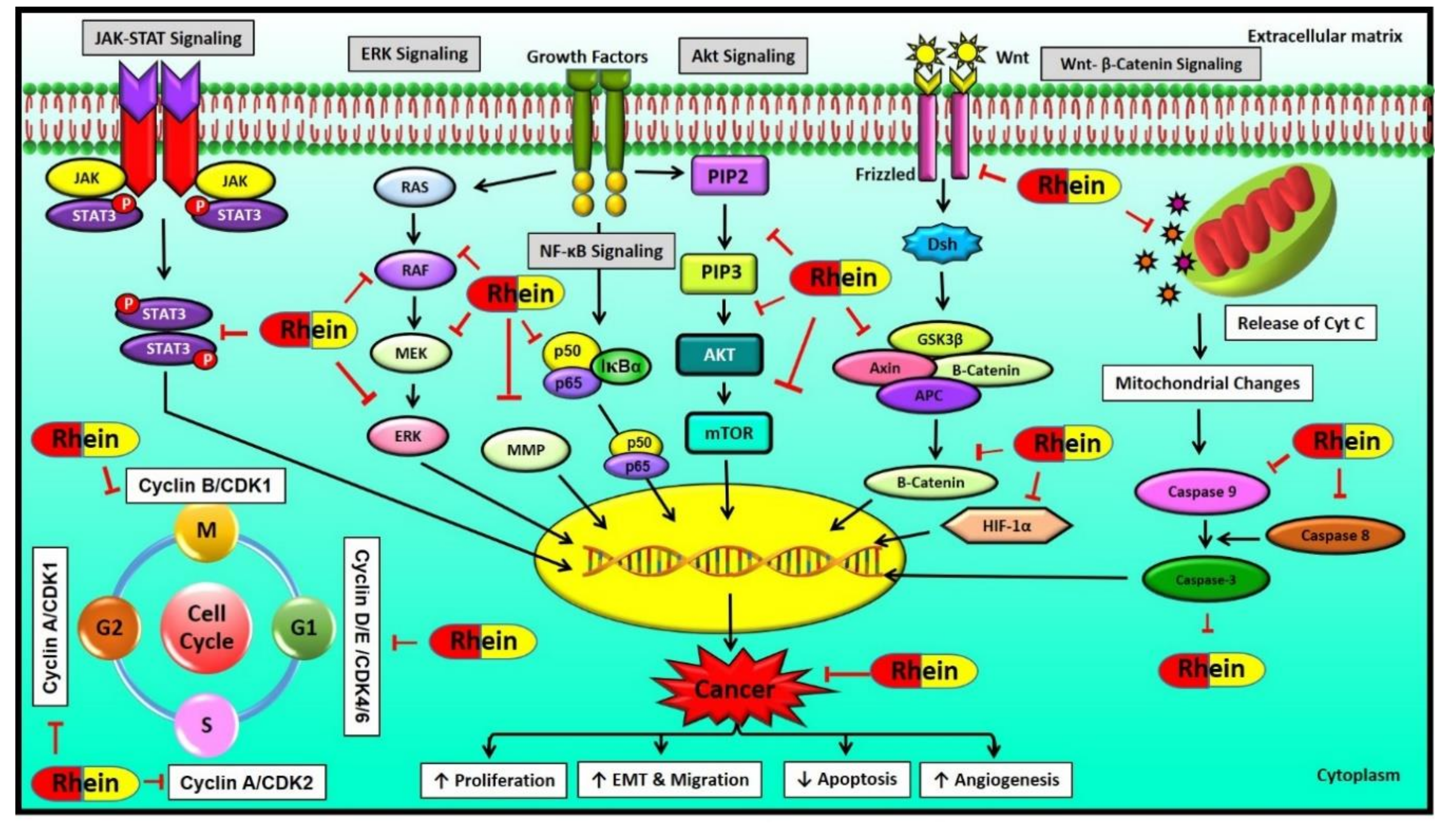
6. Molecular Targets of Rhein
6.1. MAPK Signaling Pathway
6.2. Wnt Signaling Pathway
6.3. NF-κB Signaling Pathway
6.4. HIF-1 Signaling Pathway
6.5. Other Signaling Pathways Regulated by Rhein
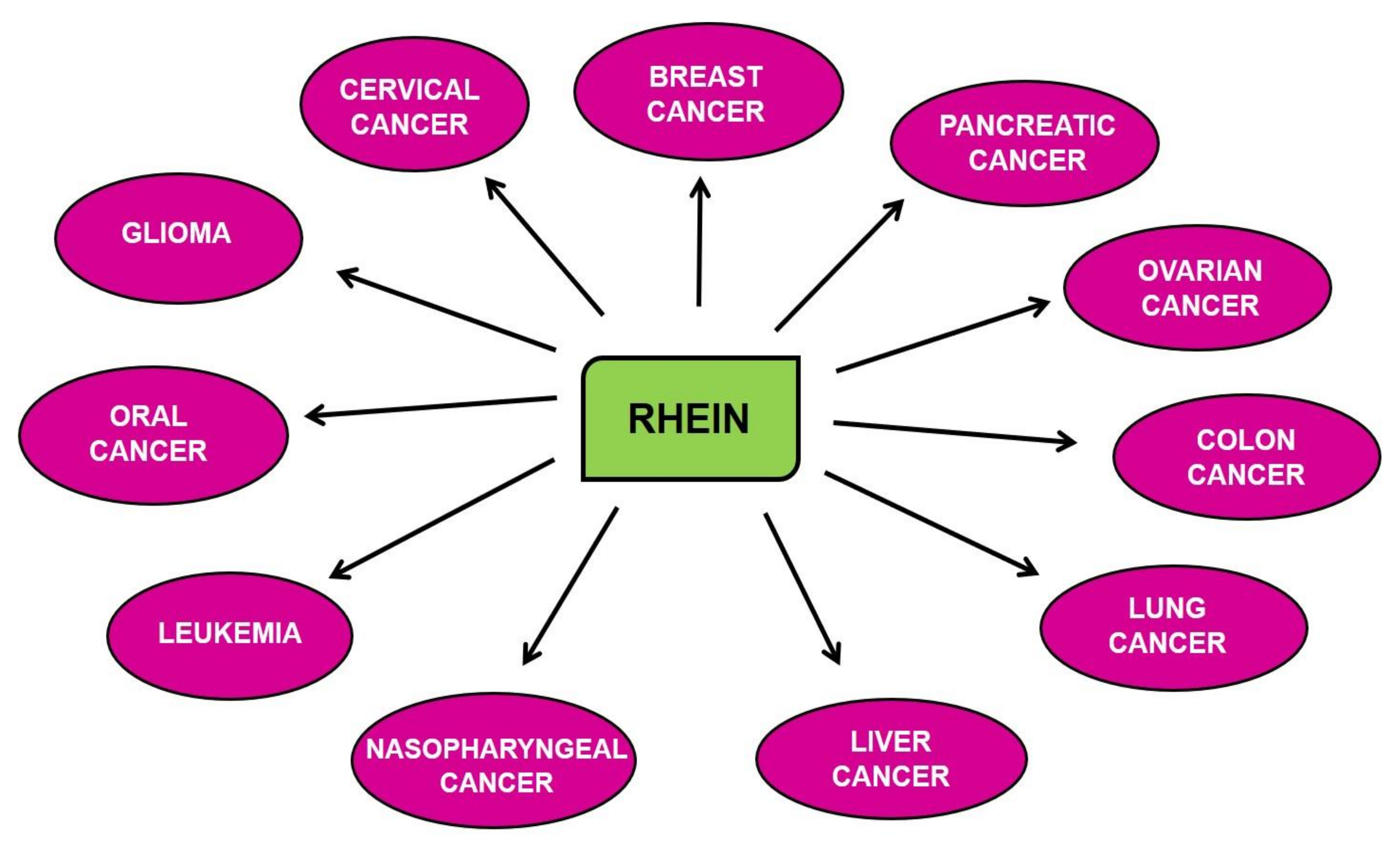
7. Chemopreventive and Therapeutic Properties of Rhein for Different Cancers
7.1. Breast Cancer
7.2. Cervical Cancer
7.3. Colon Cancer
7.4. Glioma
7.5. Leukemia
7.6. Liver Cancer
7.7. Lung Cancer
7.8. Nasopharyngeal Cancer (NPC)
7.9. Ovarian Cancer
7.10. Pancreatic Cancer (PC)
7.11. Oral Cancer
8. Toxicity of Rhein
9. Conclusions
Funding
Conflicts of Interest
Abbreviations
| Ac-K100 | Acetylated lysine |
| ATF6 | Activating transcription factor 6 |
| ATP | Adenosine triphosphate |
| Bax | Bcl-2-associated X protein |
| Bcl-2 | B cell lymphoma 2 |
| Bid | BH3 interacting domain death agonist |
| CD | Cluster of differentiation |
| c-PARP | cleaved- Poly ADP ribose polymerase |
| CHOP | CCAAT/enhancer-binding protein homologous protein |
| COX-2 | Cyclooxygenase 2 |
| EGFR | Epidermal growth factor receptor |
| ER | Endoplasmic reticulum |
| ERK | Extracellular signal-regulated kinase |
| FOXO | Class O of Forkhead box transcription factors |
| FOXO3a | Forkhead box O3a |
| Fas | FS-7-associated surface antigen |
| GADD153 | Growth arrest and DNA damage153 |
| Glut-1 | Glucose transporter1 |
| GRP78 | 78 kDa Glucose-regulated protein |
| HER-2 | Human epidermal growth factor receptor 2 |
| HIF-1α | Hypoxia-inducible factor 1 alpha |
| HK-II | Hexokinase2 |
| HMGB1 | High-mobility-group-box-1 |
| IL-6 | Interleukin-6 |
| IL-1β | Interleukin-1 beta |
| IKKβ | Inhibitor of nuclear factor kappa –B kinase subunit beta |
| JNK | c-Jun N-terminal kinase |
| mTOR | Mammalian target of rapamycin |
| MAPK | Mitogen-activated protein kinase |
| mCD95L | Membrane-bound CD95 ligand |
| MDM2 | Murine double minute 2 |
| MEK | Mitogen-activated protein kinase kinase |
| MMP | Matrix metalloproteinase |
| NO | Nitric oxide |
| NADPH oxidase | Nicotinamide adenine dinucleotide phosphate oxidase |
| NDRG1 | N-myc downstream regulated 1 |
| NF-κB | Nuclear factor- kappa light chain enhancer of activated B cells |
| p-Akt | Phosphorylated Akt |
| PARP | Poly ADP ribose polymerase |
| PFK-1 | Phosphofructokinase-1 |
| p-HER-2 | Phosphorylated human epidermal growth factor receptor 2 |
| p-EGFR | Phosphorylated epidermal growth factor receptor |
| p-c-Jun | Phosphorylated c-Jun. |
| p-eIF2α | Phosphorylated eukaryotic initiation factor 2 alpha |
| RAC1 | Ras-related C3 botulinum toxin substrate 1 |
| ROS | Reactive oxygen species |
| sCD95L | Soluble CD95 ligand |
| STAT3 | Signal transducer and activator of transcription 3 |
| TIMP | Tissue inhibitor of metalloproteinase |
| TNF-α | Tumor necrosis factor- alpha |
| UPR | Unfolded protein response |
| VEGF | Vascular endothelial growth factor |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Devi Khwairakpam, A.; Monisha, J.; Roy, N.K.; Bordoloi, D.; Padmavathi, G.; Banik, K.; Khatoon, E.; Kunnumakkara, A.B. Vietnamese coriander inhibits cell proliferation, survival and migration via suppression of Akt/mTOR pathway in oral squamous cell carcinoma. J. Basic Clin. Physiol. Pharmacol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Banik, K.; Harsha, C.; Bordoloi, D.; Lalduhsaki Sailo, B.; Sethi, G.; Leong, H.C.; Arfuso, F.; Mishra, S.; Wang, L.; Kumar, A.P.; et al. Therapeutic potential of gambogic acid, a caged xanthone, to target cancer. Cancer Lett. 2018, 416, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Padmavathi, G.; Roy, N.K.; Bordoloi, D.; Arfuso, F.; Mishra, S.; Sethi, G.; Bishayee, A.; Kunnumakkara, A.B. Butein in health and disease: A comprehensive review. Phytomed. Int. J. Phytother. Phytopharm. 2017, 25, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Roy, N.K.; Deka, A.; Bordoloi, D.; Mishra, S.; Kumar, A.P.; Sethi, G.; Kunnumakkara, A.B. The potential role of boswellic acids in cancer prevention and treatment. Cancer Lett. 2016, 377, 74–86. [Google Scholar] [CrossRef]
- Monisha, J.; Jaiswal, A.; Banik, K.; Choudhary, H.; Singh, A.K.; Bordoloi, D.; Kunnumakkara, A.B. Cancer Cell Chemoresistance: A Prime Obstacle in Cancer Therapy. In Cancer Cell Chemoresistance and Chemosensitization; World Scientific: Singapore, 2018; pp. 15–49. [Google Scholar]
- Khwairakpam, A.D.; Monisha, J.; Banik, K.; Choudhary, H.; Sharma, A.; Bordoloi, D.; Kunnumakkara, A.B. Chemoresistance in Brain Cancer and Different Chemosensitization Approaches. In Cancer Cell Chemoresistance and Chemosensitization; World Scientific: Singapore, 2018; pp. 107–127. [Google Scholar]
- Padmavathi, G.; Monisha, J.; Banik, K.; Thakur, K.K.; Choudhary, H.; Bordoloi, D.; Kunnumakkara, A.B. Different chemosensitization approaches to overcome chemoresistance in prostate cancer. In Cancer Cell Chemoresistance and Chemosensitization; World Scientific: Singapore, 2018; pp. 583–613. [Google Scholar]
- Javadi, M.; Roy, N.K.; Sharma, A.; Banik, K.; Ganesan, P.; Bordoloi, D.; Kunnumakkara, A.B. Chemoresistance and chemosensitization in Melanoma. In Cancer Cell Chemoresistance and Chemosensitization; World Scientific: Singapore, 2018; pp. 479–527. [Google Scholar]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; Chemical Industry Press: Beijing, China, 2005; p. 17. [Google Scholar]
- Kunnumakkara, A.B.; Banik, K.; Bordoloi, D.; Harsha, C.; Sailo, B.L.; Padmavathi, G.; Roy, N.K.; Gupta, S.C.; Aggarwal, B.B. Googling the Guggul (Commiphora and Boswellia) for Prevention of Chronic Diseases. Front. Pharmacol. 2018, 9, 686. [Google Scholar] [CrossRef]
- Khwairakpam, A.D.; Bordoloi, D.; Thakur, K.K.; Monisha, J.; Arfuso, F.; Sethi, G.; Mishra, S.; Kumar, A.P.; Kunnumakkara, A.B. Possible use of Punica granatum (Pomegranate) in cancer therapy. Pharmacol. Res. 2018, 133, 53–64. [Google Scholar] [CrossRef]
- Lu, K.; Zhang, C.; Wu, W.; Zhou, M.; Tang, Y.; Peng, Y. Rhubarb extract has a protective role against radiation-induced brain injury and neuronal cell apoptosis. Mol. Med. Rep. 2015, 12, 2689–2694. [Google Scholar] [CrossRef]
- Kashyap, D.; Tuli, H.S.; Yerer, M.B.; Sharma, A.; Sak, K.; Srivastava, S.; Pandey, A.; Garg, V.K.; Sethi, G.; Bishayee, A. Natural product-based nanoformulations for cancer therapy: Opportunities and challenges. Semin. Cancer Biol. 2019. [Google Scholar] [CrossRef]
- Merarchi, M.; Sethi, G.; Shanmugam, M.K.; Fan, L.; Arfuso, F.; Ahn, K.S. Role of Natural Products in Modulating Histone Deacetylases in Cancer. Molecules 2019, 24. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Warrier, S.; Kumar, A.P.; Sethi, G.; Arfuso, F. Potential Role of Natural Compounds as Anti-Angiogenic Agents in Cancer. Curr. Vasc. Pharmacol. 2017, 15, 503–519. [Google Scholar] [CrossRef] [PubMed]
- Tewari, D.; Nabavi, S.F.; Nabavi, S.M.; Sureda, A.; Farooqi, A.A.; Atanasov, A.G.; Vacca, R.A.; Sethi, G.; Bishayee, A. Targeting activator protein 1 signaling pathway by bioactive natural agents: Possible therapeutic strategy for cancer prevention and intervention. Pharmacol. Res. 2018, 128, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.S.; Yang, S.F.; Sethi, G.; Hu, D.N. Natural bioactives in cancer treatment and prevention. BioMed Res. Int. 2015, 2015, 182835. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.F.; Weng, C.J.; Sethi, G.; Hu, D.N. Natural bioactives and phytochemicals serve in cancer treatment and prevention. Evid. Based Complementary Altern. Med. Ecam 2013, 2013, 698190. [Google Scholar] [CrossRef]
- Prasannan, R.; Kalesh, K.A.; Shanmugam, M.K.; Nachiyappan, A.; Ramachandran, L.; Nguyen, A.H.; Kumar, A.P.; Lakshmanan, M.; Ahn, K.S.; Sethi, G. Key cell signaling pathways modulated by zerumbone: Role in the prevention and treatment of cancer. Biochem. Pharmacol. 2012, 84, 1268–1276. [Google Scholar] [CrossRef]
- Ramachandran, L.; Manu, K.A.; Shanmugam, M.K.; Li, F.; Siveen, K.S.; Vali, S.; Kapoor, S.; Abbasi, T.; Surana, R.; Smoot, D.T.; et al. Isorhamnetin inhibits proliferation and invasion and induces apoptosis through the modulation of peroxisome proliferator-activated receptor gamma activation pathway in gastric cancer. J. Biol. Chem. 2012, 287, 38028–38040. [Google Scholar] [CrossRef]
- Yarla, N.S.; Bishayee, A.; Sethi, G.; Reddanna, P.; Kalle, A.M.; Dhananjaya, B.L.; Dowluru, K.S.; Chintala, R.; Duddukuri, G.R. Targeting arachidonic acid pathway by natural products for cancer prevention and therapy. Semin. Cancer Biol. 2016, 40–41, 48–81. [Google Scholar] [CrossRef]
- Thakur, K.K.; Bordoloi, D.; Prakash, J.; Javadi, M.; Roy, N.K.; Kunnumakkara, A.B. Different Chemosensitization Approaches for the Effective Management of HNSCC. In Cancer Cell Chemoresistance and Chemosensitization; World Scientific: Singapore, 2018; pp. 399–423. [Google Scholar]
- Padmavathi, G.; Bordoloi, D.; Banik, K.; Javadi, M.; Singh, A.K.; Kunnumakkara, A.B. Mechanism of Chemoresistance in Bone Cancer and Different Chemosensitization Approaches. In Cancer Cell Chemoresistance and Chemosensitization; World Scientific: Singapore, 2018; pp. 81–106. [Google Scholar]
- Bordoloi, D.; Monisha, J.; Roy, N.K.; Padmavathi, G.; Banik, K.; Harsha, C.; Wang, H.; Kumar, A.P.; Arfuso, F.; Kunnumakkara, A.B. An Investigation on the Therapeutic Potential of Butein, A Tretrahydroxychalcone Against Human Oral Squamous Cell Carcinoma. Asian Pac. J. Cancer Prev. APJCP 2019, 20, 3437–3446. [Google Scholar] [CrossRef]
- Girisa, S.; Shabnam, B.; Monisha, J.; Fan, L.; Halim, C.E.; Arfuso, F.; Ahn, K.S.; Sethi, G.; Kunnumakkara, A.B. Potential of Zerumbone as an Anti-Cancer Agent. Molecules 2019, 24. [Google Scholar] [CrossRef]
- Sailo, B.L.; Banik, K.; Padmavathi, G.; Javadi, M.; Bordoloi, D.; Kunnumakkara, A.B. Tocotrienols: The promising analogues of vitamin E for cancer therapeutics. Pharmacol. Res. 2018, 130, 259–272. [Google Scholar] [CrossRef]
- Ranaware, A.M.; Banik, K.; Deshpande, V.; Padmavathi, G.; Roy, N.K.; Sethi, G.; Fan, L.; Kumar, A.P.; Kunnumakkara, A.B. Magnolol: A Neolignan from the Magnolia Family for the Prevention and Treatment of Cancer. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Monisha, J.; Padmavathi, G.; Roy, N.K.; Deka, A.; Bordoloi, D.; Anip, A.; Kunnumakkara, A.B. NF-kappaB Blockers Gifted by Mother Nature: Prospectives in Cancer Cell Chemosensitization. Curr. Pharm. Des. 2016, 22, 4173–4200. [Google Scholar] [CrossRef] [PubMed]
- Padmavathi, G.; Rathnakaram, S.R.; Monisha, J.; Bordoloi, D.; Roy, N.K.; Kunnumakkara, A.B. Potential of butein, a tetrahydroxychalcone to obliterate cancer. Phytomed. Int. J. Phytother. Phytopharm. 2015, 22, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Liskova, A.; Stefanicka, P.; Samec, M.; Smejkal, K.; Zubor, P.; Bielik, T.; Biskupska-Bodova, K.; Kwon, T.K.; Danko, J.; Büsselberg, D.; et al. Dietary phytochemicals as the potential protectors against carcinogenesis and their role in cancer chemoprevention. Clin. Exp. Med. 2020, 20, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Sung, B.; Ravindran, J.; Diagaradjane, P.; Deorukhkar, A.; Dey, S.; Koca, C.; Yadav, V.R.; Tong, Z.; Gelovani, J.G.; et al. γ-Tocotrienol inhibits pancreatic tumors and sensitizes them to gemcitabine treatment by modulating the inflammatory microenvironment. Cancer Res. 2010, 70, 8695–8705. [Google Scholar] [CrossRef]
- Bordoloi, D.; Roy, N.K.; Monisha, J.; Padmavathi, G.; Kunnumakkara, A.B. Multi-Targeted Agents in Cancer Cell Chemosensitization: What We Learnt from Curcumin Thus Far. Recent Pat. Anti-Cancer Drug Discov. 2016, 11, 67–97. [Google Scholar] [CrossRef]
- Banik, K.; Ranaware, A.M.; Harsha, C.; Nitesh, T.; Girisa, S.; Deshpande, V.; Fan, L.; Nalawade, S.P.; Sethi, G.; Kunnumakkara, A.B. Piceatannol: A natural stilbene for the prevention and treatment of cancer. Pharmacol. Res. 2020, 153, 104635. [Google Scholar] [CrossRef]
- Banik, K.; Sailo, B.L.; Thakur, K.K.; Jaiswal, A.; Monisha, J.; Bordoloi, D.; Kunnumakkara, A.B. Potential of different chemosensitizers to overcome chemoresistance in cervical cancer. In Cancer Cell Chemoresistance and Chemosensitization; World Scientific: Singapore, 2018; pp. 163–179. [Google Scholar]
- Sailo, B.L.; Bordoloi, D.; Banik, K.; Khwairakpam, A.D.; Roy, N.K.; Prakash, J.; Kunnumakkara, A.B. Therapeutic strategies for chemosensitization of renal cancer. In Cancer Cell Chemoresistance and Chemosensitization; World Scientific: Singapore, 2018; pp. 615–639. [Google Scholar]
- Sailo, B.L.; Javadi, M.; Jaiswal, A.; Prakash, J.; Roy, N.K.; Thakur, K.K.; Banik, K.; Bordoloi, D.; Kunnumakkara, A.B. Molecular Alterations Involved in Pancreatic Cancer Chemoresistance and Chemosensitization Strategies. In Cancer Cell Chemoresistance and Chemosensitization; World Scientific: Singapore, 2018; pp. 557–581. [Google Scholar]
- Choudhary, H.; Thakur, K.K.; Sharma, A.; Roy, N.K.; Khwairakpam, A.D.; Bordoloi, D.; Kunnumakkara, A.B. Strategies to Overcome Chemoresistance in Ovarian Cancer. In Cancer Cell Chemoresistance and Chemosensitization; World Scientific: Singapore, 2018; pp. 529–555. [Google Scholar]
- Mohammed, A.; Ibrahim, M.A.; Tajuddeen, N.; Aliyu, A.B.; Isah, M.B. Antidiabetic potential of anthraquinones: A review. Phytother. Res. 2020, 34, 486–504. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Xia, W.; Yue, W.; Peng, C.; Rahman, K.; Zhang, H. Rhein: A Review of Pharmacological Activities. Evid. Based Complementary Altern. Med. 2015, 2015, 578107. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, S.; Hu, N.; Gu, M.; Chu, C.; Li, Y.; Lu, X.; Huang, C. Rhein Reduces Fat Weight in db/db Mouse and Prevents Diet-Induced Obesity in C57Bl/6 Mouse through the Inhibition of PPARgamma Signaling. PPAR Res. 2012, 2012, 374936. [Google Scholar] [CrossRef]
- Sheng, X.; Wang, M.; Lu, M.; Xi, B.; Sheng, H.; Zang, Y.Q. Rhein ameliorates fatty liver disease through negative energy balance, hepatic lipogenic regulation, and immunomodulation in diet-induced obese mice. Am. J. Physiol. Endocrinol. Metab. 2011, 300, e886–e893. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.W.; Yang, J.S.; Lai, K.C.; Kuo, C.L.; Hsu, C.K.; Wang, C.K.; Chang, C.Y.; Lin, J.J.; Tang, N.Y.; Chen, P.Y.; et al. Rhein induced apoptosis through the endoplasmic reticulum stress, caspase- and mitochondria-dependent pathways in SCC-4 human tongue squamous cancer cells. In Vivo 2009, 23, 309–316. [Google Scholar]
- Zheng, J.M.; Zhu, J.M.; Li, L.S.; Liu, Z.H. Rhein reverses the diabetic phenotype of mesangial cells over-expressing the glucose transporter (GLUT1) by inhibiting the hexosamine pathway. Br. J. Pharmacol. 2008, 153, 1456–1464. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liang, C.; Chen, Q.; Yang, Z. Rhein: A potential biological therapeutic drug for intervertebral disc degeneration. Med. Hypotheses 2011, 77, 1105–1107. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.J.; Zhen, Y.S. Rhein lysinate suppresses the growth of breast cancer cells and potentiates the inhibitory effect of Taxol in athymic mice. Anti-Cancer Drugs 2009, 20, 65–72. [Google Scholar] [CrossRef]
- Lin, Y.J.; Zhen, Y.Z.; Shang, B.Y.; Zhen, Y.S. Rhein lysinate suppresses the growth of tumor cells and increases the anti-tumor activity of Taxol in mice. Am. J. Chin. Med. 2009, 37, 923–931. [Google Scholar] [CrossRef]
- Zhen, Y.Z.; Lin, Y.J.; Gao, J.L.; Zhao, Y.F.; Xu, A.J. Rhein lysinate inhibits cell growth by modulating various mitogen-activated protein kinases in cervical cancer cells. Oncol. Lett. 2011, 2, 129–133. [Google Scholar] [CrossRef]
- Lin, M.L.; Chen, S.S.; Lu, Y.C.; Liang, R.Y.; Ho, Y.T.; Yang, C.Y.; Chung, J.G. Rhein induces apoptosis through induction of endoplasmic reticulum stress and Ca2+-dependent mitochondrial death pathway in human nasopharyngeal carcinoma cells. Anticancer Res. 2007, 27, 3313–3322. [Google Scholar]
- Chen, Y.Y.; Chiang, S.Y.; Lin, J.G.; Ma, Y.S.; Liao, C.L.; Weng, S.W.; Lai, T.Y.; Chung, J.G. Emodin, aloe-emodin and rhein inhibit migration and invasion in human tongue cancer SCC-4 cells through the inhibition of gene expression of matrix metalloproteinase-9. Int. J. Oncol. 2010, 36, 1113–1120. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Chiang, S.Y.; Lin, J.G.; Yang, J.S.; Ma, Y.S.; Liao, C.L.; Lai, T.Y.; Tang, N.Y.; Chung, J.G. Emodin, aloe-emodin and rhein induced DNA damage and inhibited DNA repair gene expression in SCC-4 human tongue cancer cells. Anticancer Res. 2010, 30, 945–951. [Google Scholar]
- Yang, L.; Lin, S.; Kang, Y.; Xiang, Y.; Xu, L.; Li, J.; Dai, X.; Liang, G.; Huang, X.; Zhao, C. Rhein sensitizes human pancreatic cancer cells to EGFR inhibitors by inhibiting STAT3 pathway. J. Exp. Clin. Cancer Res. 2019, 38, 31. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Peng, F.; Zhong, Y.; Chen, Y.; Tang, M.; Li, D. Rhein suppresses matrix metalloproteinase production by regulating the Rac1/ROS/MAPK/AP-1 pathway in human ovarian carcinoma cells. Int. J. Oncol. 2017, 50, 933–941. [Google Scholar] [CrossRef]
- Shi, P.; Huang, Z.; Chen, G. Rhein induces apoptosis and cell cycle arrest in human hepatocellular carcinoma BEL-7402 cells. Am. J. Chin. Med. 2008, 36, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Jiang, H.; Liu, Y.; Chen, J.; Zhou, X.; Zhao, C.; Chen, X.; Lin, M. Rhein induces liver cancer cells apoptosis via activating ROS-dependent JNK/Jun/caspase-3 signaling pathway. J. Cancer 2020, 11, 500–507. [Google Scholar] [CrossRef]
- He, Z.H.; He, M.F.; Ma, S.C.; But, P.P. Anti-angiogenic effects of rhubarb and its anthraquinone derivatives. J. Ethnopharmacol. 2009, 121, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Chai, S.; To, K.K.; Lin, G. Circumvention of multi-drug resistance of cancer cells by Chinese herbal medicines. Chin. Med. 2010, 5, 26. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Lu, Y.; Zhang, T.; Liu, K.; Liu, L.; He, Z.; Xu, B.; Wu, X. Characterization of binding interactions of anthraquinones and bovine beta-lactoglobulin. Food Chem. 2019, 281, 28–35. [Google Scholar] [CrossRef]
- Feng, H.; Zhu, Y.; Fu, Z.; Li, D. Preparation, characterization, and in vivo study of rhein solid lipid nanoparticles for oral delivery. Chem. Biol. Drug Des. 2017, 90, 867–872. [Google Scholar] [CrossRef]
- Gómez-Gaete, C.; Retamal, M.; Chávez, C.; Bustos, P.; Godoy, R.; Torres-Vergara, P. Development, characterization and in vitro evaluation of biodegradable rhein-loaded microparticles for treatment of osteoarthritis. Eur. J. Pharm. Sci. 2017, 96, 390–397. [Google Scholar] [CrossRef]
- Yuan, Z.; Gu, X. Preparation, characterization, and in vivo study of rhein-loaded poly (lactic-co-glycolic acid) nanoparticles for oral delivery. Drug Des. Dev. 2015, 9, 2301. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Sailo, B.L.; Banik, K.; Harsha, C.; Prasad, S.; Gupta, S.C.; Bharti, A.C.; Aggarwal, B.B. Chronic diseases, inflammation, and spices: How are they linked? J. Transl. Med. 2018, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Khwairakpam, A.D.; Damayenti, Y.D.; Deka, A.; Monisha, J.; Roy, N.K.; Padmavathi, G.; Kunnumakkara, A.B. Acorus calamus: A bio-reserve of medicinal values. J. Basic Clin. Physiol. Pharmacol. 2018, 29, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Sung, B.; Ravindran, J.; Diagaradjane, P.; Deorukhkar, A.; Dey, S.; Koca, C.; Tong, Z.; Gelovani, J.G.; Guha, S.; et al. Zyflamend suppresses growth and sensitizes human pancreatic tumors to gemcitabine in an orthotopic mouse model through modulation of multiple targets. Int. J. Cancer 2012, 131, E292–E303. [Google Scholar] [CrossRef] [PubMed]
- Rokaya, M.B.; Munzbergova, Z.; Timsina, B.; Bhattarai, K.R. Rheum australe D. Don: A review of its botany, ethnobotany, phytochemistry and pharmacology. J. Ethnopharmacol. 2012, 141, 761–774. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhang, T.; Xu, G.; Chou, F.E.; Ito, Y. pH-MODULATED STEPWISE ELUTION CCC AND ITS APPLICATION TO THE PREPARATIVE SEPARATION OF HYDROXYANTHRAQUINONE COMPOUNDS FROM TRADITIONAL CHINESE MEDICINAL HERBS. J. Liq. Chromatogr. Relat. Technol. 2001, 24, 1617–1628. [Google Scholar] [CrossRef]
- Yang, D.Y.; Fushimi, H.; Cai, S.Q.; Komatsu, K. Molecular analysis of Rheum species used as Rhei Rhizoma based on the chloroplast matK gene sequence and its application for identification. Biol. Pharm. Bull. 2004, 27, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Cojocaru, A.; Vlase, L.; Munteanu, N.; Stan, T.; Teliban, G.C.; Burducea, M.; Stoleru, V. Dynamic of phenolic compounds, antioxidant activity, and yield of rhubarb under chemical, organic and biological fertilization. Plants 2020, 9, 355. [Google Scholar] [CrossRef]
- Duraipandiyan, V.; Baskar, A.A.; Ignacimuthu, S.; Muthukumar, C.; Al-Harbi, N.A. Anticancer activity of Rhein isolated from Cassia fistula L. flower. Asian Pac. J. Trop. Dis. 2012, 2, S517–S523. [Google Scholar] [CrossRef]
- Duraipandiyan, V.; Ignacimuthu, S.; Gabriel Paulraj, M. Antifeedant and larvicidal activities of Rhein isolated from the flowers of Cassia fistula L. Saudi J. Biol. Sci. 2011, 18, 129–133. [Google Scholar] [CrossRef]
- Yang, F.; Xu, Y.; Xiong, A.; He, Y.; Yang, L.; Wan, Y.J.; Wang, Z. Evaluation of the protective effect of Rhei Radix et Rhizoma against alpha-naphthylisothiocyanate induced liver injury based on metabolic profile of bile acids. J. Ethnopharmacol. 2012, 144, 599–604. [Google Scholar] [CrossRef]
- Harsha, C.; Banik, K.; Bordoloi, D.; Kunnumakkara, A.B. Antiulcer properties of fruits and vegetables: A mechanism based perspective. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2017, 108, 104–119. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; Chemical Industry Press: Beijing, China, 1997; pp. 149–150. [Google Scholar]
- Choi, R.Y.; Lee, H.I.; Ham, J.R.; Yee, S.T.; Kang, K.Y.; Lee, M.K. Heshouwu (Polygonum multiflorum Thunb.) ethanol extract suppresses pre-adipocytes differentiation in 3T3-L1 cells and adiposity in obese mice. Biomed. Pharmacother. 2018, 106, 355–362. [Google Scholar] [CrossRef]
- Lin, L.; Ni, B.; Lin, H.; Zhang, M.; Li, X.; Yin, X.; Qu, C.; Ni, J. Traditional usages, botany, phytochemistry, pharmacology and toxicology of Polygonum multiflorum Thunb.: A review. J. Ethnopharmacol. 2015, 159, 158–183. [Google Scholar] [CrossRef]
- Zhou, H.; Jiao, D. 312 cases of gastric and duodenal ulcer bleeding treated with 3 kinds of alcoholic extract rhubarb tablets. Zhong Xi Yi Jie He Za Zhi = Chin. J. Mod. Dev. Tradit. Med. 1990, 10, 150-1–131-2. [Google Scholar]
- Wu, J.; Hu, Y.; Xiang, L.; Li, S.; Yuan, Y.; Chen, X.; Zhang, Y.; Huang, W.; Meng, X.; Wang, P. San-Huang-Xie-Xin-Tang constituents exert drug-drug interaction of mutual reinforcement at both pharmacodynamics and pharmacokinetic level: A review. Front. Pharmacol. 2016, 7, 448. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.L.; Ma, Y.M.; Yan, D.M.; Zhou, H.; Shi, R.; Wang, T.M.; Yang, Y.; Wang, C.H.; Zhang, N. Effective constituents in Xiexin Decoction for anti-inflammation. J. Ethnopharmacol. 2009, 125, 151–156. [Google Scholar] [CrossRef]
- Yan, S.; Yue, Y.; Wang, J.; Li, W.; Sun, M.; Zeng, L.; Wang, X. Banxia Xiexin decoction, a traditional Chinese medicine, alleviates colon cancer in nude mice. Ann. Transl. Med. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Lee, G.S.; Kim, M.S.; Ryu, D.G.; So, H.S.; Moon, H.C.; Lee, Y.R.; Yang, S.H.; Kwon, K.B. Effects of Banxia Xiexin Decoction (半夏泻心汤) on Cisplatin-Induced Apoptosis of Human A549 Lung Cancer Cells. Chin. J. Integr. Med. 2018, 24, 436–441. [Google Scholar] [CrossRef]
- Zhang, A.; Sun, H.; Yuan, Y.; Sun, W.; Jiao, G.; Wang, X. An in vivo analysis of the therapeutic and synergistic properties of Chinese medicinal formula Yin-Chen-Hao-Tang based on its active constituents. Fitoterapia 2011, 82, 1160–1168. [Google Scholar] [CrossRef]
- Ibrahim, M.; Khan, A.A.; Tiwari, S.K.; Habeeb, M.A.; Khaja, M.N.; Habibullah, C.M. Antimicrobial activity of Sapindus mukorossi and Rheum emodi extracts against H pylori: In vitro and in vivo studies. World J. Gastroenterol. 2006, 12, 7136–7142. [Google Scholar] [CrossRef]
- Xu, W.; Hu, M.; Zhang, Q.; Yu, J.; Su, W. Effects of anthraquinones from Cassia occidentalis L. on ovalbumin-induced airways inflammation in a mouse model of allergic asthma. J. Ethnopharmacol. 2018, 221, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhang, T.; Ito, Y. Preparative separation of rhein from Chinese traditional herb by repeated high-speed counter-current chromatography. J. Chromatogr. A 2003, 1017, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Petralito, S.; Zanardi, I.; Memoli, A.; Annesini, M.C.; Travagli, V. Solubility, spectroscopic properties and photostability of Rhein/cyclodextrin inclusion complex. Spectrochim. Acta. Part Amol. Biomol. Spectrosc. 2009, 74, 1254–1259. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Jiang, C.; Gao, M.; Zhang, D.; Yao, N.; Feng, Y.; Wu, T.; Zhang, J. Target exploration of rhein as a small-molecule necrosis avid agent by post-treatment click modification. New J. Chem. 2019, 43, 6121–6125. [Google Scholar] [CrossRef]
- Zhang, A.; Wu, T.; Bian, L.; Li, P.; Liu, Q.; Zhang, D.; Jin, Q.; Zhang, J.; Huang, G.; Song, S. Synthesis and Evaluation of Ga-68-Labeled Rhein for Early Assessment of Treatment-Induced Tumor Necrosis. Mol. Imaging Biol. 2019. [Google Scholar] [CrossRef]
- Hwang, D.S.; Gu, P.S.; Kim, N.; Jang, Y.P.; Oh, M.S. Effects of Rhei Undulati Rhizoma on lipopolysaccharide-induced neuroinflammation in vitro and in vivo. Environ. Toxicol. 2018, 33, 23–31. [Google Scholar] [CrossRef]
- Liu, Z.; Lang, Y.; Li, L.; Liang, Z.; Deng, Y.; Fang, R.; Meng, Q. Effect of emodin on chondrocyte viability in an in vitro model of osteoarthritis. Exp. Ther. Med. 2018, 16, 5384–5389. [Google Scholar] [CrossRef]
- Qin, F.; Huang, J.; Huang, X.; Ren, P. Simultaneous determination and pharmacokinetic comparisons of aloe-emodin, rhein, emodin, and chrysophanol after oral administration of these monomers, rhei rhizoma and chaiqin-chengqi-tang, to rats. J. Liq. Chromatogr. Relat. Technol. 2011, 34, 1381–1390. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, X.; Chen, A.; Cheng, X.; Zhang, G.; Sun, J.; Zhao, Y.; Huang, Y.; Zhu, Y. Rhein protects against cerebral ischemic-/reperfusion-induced oxidative stress and apoptosis in rats. Int. J. Mol. Med. 2018, 41, 2802–2812. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, L.; Lin, W.; Yin, S.; Duan, A.; Liu, Z.; Cao, W. Rhein reverses Klotho repression via promoter demethylation and protects against kidney and bone injuries in mice with chronic kidney disease. Kidney Int. 2017, 91, 144–156. [Google Scholar] [CrossRef]
- Cyong, J.; Matsumoto, T.; Arakawa, K.; Kiyohara, H.; Yamada, H.; Otsuka, Y. Anti-Bacteroides fragilis substance from rhubarb. J. Ethnopharmacol. 1987, 19, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Vargas, F.; Díaz, Y.; Carbonell, K. Antioxidant and Scavenging Activity of Emodin, Aloe-Emodin, and Rhein on Free-Radical and Reactive Oxygen Species. Pharm. Biol. 2004, 42, 342–348. [Google Scholar] [CrossRef]
- Mendes, A.F.; Caramona, M.M.; De Carvalho, A.P.; Lopes, M.C. Diacerhein and rhein prevent interleukin-1beta-induced nuclear factor-kappaB activation by inhibiting the degradation of inhibitor kappaB-alpha. Pharmacol. Toxicol. 2002, 91, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.L.; Hsu, Y.L.; Ng, L.T.; Lin, C.C. Rhein inhibits the growth and induces the apoptosis of Hep G2 cells. Planta Med. 2004, 70, 12–16. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Dong, X.; Yin, X.; Yang, C.; Leng, X.; Wang, W.; Ni, J. Rhein Induces Cell Death in HepaRG Cells through Cell Cycle Arrest and Apoptotic Pathway. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef]
- Yuan, X.; Tian, W.; Hua, Y.; Hu, L.; Yang, J.; Xie, J.; Hu, J.; Wang, F. Rhein enhances the cytotoxicity of effector lymphocytes in colon cancer under hypoxic conditions. Exp. Ther. Med. 2018, 16, 5350–5358. [Google Scholar] [CrossRef]
- Pearson, G.; Robinson, F.; Beers Gibson, T.; Xu, B.E.; Karandikar, M.; Berman, K.; Cobb, M.H. Mitogen-activated protein (MAP) kinase pathways: Regulation and physiological functions. Endocr. Rev. 2001, 22, 153–183. [Google Scholar] [CrossRef]
- Morrison, D.K. MAP kinase pathways. Cold Spring Harb. Perspect. Biol. 2012, 4. [Google Scholar] [CrossRef]
- Dai, X.; Wang, L.; Deivasigamni, A.; Looi, C.Y.; Karthikeyan, C.; Trivedi, P.; Chinnathambi, A.; Alharbi, S.A.; Arfuso, F.; Dharmarajan, A.; et al. A novel benzimidazole derivative, MBIC inhibits tumor growth and promotes apoptosis via activation of ROS-dependent JNK signaling pathway in hepatocellular carcinoma. Oncotarget 2017, 8, 12831–12842. [Google Scholar] [CrossRef]
- Kim, S.M.; Kim, C.; Bae, H.; Lee, J.H.; Baek, S.H.; Nam, D.; Chung, W.S.; Shim, B.S.; Lee, S.G.; Kim, S.H.; et al. 6-Shogaol exerts anti-proliferative and pro-apoptotic effects through the modulation of STAT3 and MAPKs signaling pathways. Mol. Carcinog. 2015, 54, 1132–1146. [Google Scholar] [CrossRef]
- Kannaiyan, R.; Manu, K.A.; Chen, L.; Li, F.; Rajendran, P.; Subramaniam, A.; Lam, P.; Kumar, A.P.; Sethi, G. Celastrol inhibits tumor cell proliferation and promotes apoptosis through the activation of c-Jun N-terminal kinase and suppression of PI3 K/Akt signaling pathways. Apoptosis Int. J. Program. Cell Death 2011, 16, 1028–1041. [Google Scholar] [CrossRef] [PubMed]
- Woo, C.C.; Hsu, A.; Kumar, A.P.; Sethi, G.; Tan, K.H. Thymoquinone inhibits tumor growth and induces apoptosis in a breast cancer xenograft mouse model: The role of p38 MAPK and ROS. PLoS ONE 2013, 8, e75356. [Google Scholar] [CrossRef]
- Fu, M.; Wang, C.; Li, Z.; Sakamaki, T.; Pestell, R.G. Minireview: Cyclin D1: Normal and abnormal functions. Endocrinology 2004, 145, 5439–5447. [Google Scholar] [CrossRef] [PubMed]
- Bhuvanalakshmi, G.; Gamit, N.; Patil, M.; Arfuso, F.; Sethi, G.; Dharmarajan, A.; Kumar, A.P.; Warrier, S. Stemness, Pluripotentiality, and Wnt Antagonism: sFRP4, a Wnt antagonist Mediates Pluripotency and Stemness in Glioblastoma. Cancers 2018, 11. [Google Scholar] [CrossRef] [PubMed]
- Bhuvanalakshmi, G.; Basappa; Rangappa, K.S.; Dharmarajan, A.; Sethi, G.; Kumar, A.P.; Warrier, S. Breast Cancer Stem-Like Cells Are Inhibited by Diosgenin, a Steroidal Saponin, by the Attenuation of the Wnt beta-Catenin Signaling via the Wnt Antagonist Secreted Frizzled Related Protein-4. Front. Pharmacol. 2017, 8, 124. [Google Scholar] [CrossRef]
- Panda, P.K.; Naik, P.P.; Praharaj, P.P.; Meher, B.R.; Gupta, P.K.; Verma, R.S.; Maiti, T.K.; Shanmugam, M.K.; Chinnathambi, A.; Alharbi, S.A.; et al. Abrus agglutinin stimulates BMP-2-dependent differentiation through autophagic degradation of beta-catenin in colon cancer stem cells. Mol. Carcinog. 2018, 57, 664–677. [Google Scholar] [CrossRef]
- Fang, D.; Hawke, D.; Zheng, Y.; Xia, Y.; Meisenhelder, J.; Nika, H.; Mills, G.B.; Kobayashi, R.; Hunter, T.; Lu, Z. Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J. Biol. Chem. 2007, 282, 11221–11229. [Google Scholar] [CrossRef]
- Hsia, T.C.; Yang, J.S.; Chen, G.W.; Chiu, T.H.; Lu, H.F.; Yang, M.D.; Yu, F.S.; Liu, K.C.; Lai, K.C.; Lin, C.C.; et al. The roles of endoplasmic reticulum stress and Ca2+ on rhein-induced apoptosis in A-549 human lung cancer cells. Anticancer Res. 2009, 29, 309–318. [Google Scholar]
- Liu, S.; Wang, J.; Shao, T.; Song, P.; Kong, Q.; Hua, H.; Luo, T.; Jiang, Y. The natural agent rhein induces beta-catenin degradation and tumour growth arrest. J. Cell. Mol. Med. 2018, 22, 589–599. [Google Scholar] [CrossRef]
- Puar, Y.R.; Shanmugam, M.K.; Fan, L.; Arfuso, F.; Sethi, G.; Tergaonkar, V. Evidence for the Involvement of the Master Transcription Factor NF-kappaB in Cancer Initiation and Progression. Biomedicines 2018, 6. [Google Scholar] [CrossRef]
- Shin, E.M.; Hay, H.S.; Lee, M.H.; Goh, J.N.; Tan, T.Z.; Sen, Y.P.; Lim, S.W.; Yousef, E.M.; Ong, H.T.; Thike, A.A.; et al. DEAD-box helicase DP103 defines metastatic potential of human breast cancers. J. Clin. Investig. 2014, 124, 3807–3824. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.S.; Sethi, G.; Chaturvedi, M.M.; Aggarwal, B.B. Simvastatin, 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor, suppresses osteoclastogenesis induced by receptor activator of nuclear factor-kappaB ligand through modulation of NF-kappaB pathway. Int. J. Cancer 2008, 123, 1733–1740. [Google Scholar] [CrossRef] [PubMed]
- Sethi, G.; Ahn, K.S.; Sung, B.; Aggarwal, B.B. Pinitol targets nuclear factor-kappaB activation pathway leading to inhibition of gene products associated with proliferation, apoptosis, invasion, and angiogenesis. Mol. Cancer Ther. 2008, 7, 1604–1614. [Google Scholar] [CrossRef]
- Sawhney, M.; Rohatgi, N.; Kaur, J.; Shishodia, S.; Sethi, G.; Gupta, S.D.; Deo, S.V.; Shukla, N.K.; Aggarwal, B.B.; Ralhan, R. Expression of NF-kappaB parallels COX-2 expression in oral precancer and cancer: Association with smokeless tobacco. Int. J. Cancer 2007, 120, 2545–2556. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.S.; Sethi, G.; Jain, A.K.; Jaiswal, A.K.; Aggarwal, B.B. Genetic deletion of NAD(P)H:quinone oxidoreductase 1 abrogates activation of nuclear factor-kappaB, IkappaBalpha kinase, c-Jun N-terminal kinase, Akt, p38, and p44/42 mitogen-activated protein kinases and potentiates apoptosis. J. Biol. Chem. 2006, 281, 19798–19808. [Google Scholar] [CrossRef] [PubMed]
- Monisha, J.; Roy, N.K.; Bordoloi, D.; Kumar, A.; Golla, R.; Kotoky, J.; Padmavathi, G.; Kunnumakkara, A.B. Nuclear Factor Kappa B: A Potential Target to Persecute Head and Neck Cancer. Curr. Drug Targets 2017, 18, 232–253. [Google Scholar] [CrossRef]
- Ahn, K.S.; Sethi, G.; Aggarwal, B.B. Reversal of chemoresistance and enhancement of apoptosis by statins through down-regulation of the NF-kappaB pathway. Biochem. Pharmacol. 2008, 75, 907–913. [Google Scholar] [CrossRef]
- Manna, S.K.; Aggarwal, R.S.; Sethi, G.; Aggarwal, B.B.; Ramesh, G.T. Morin (3,5,7,2’,4’-Pentahydroxyflavone) abolishes nuclear factor-kappaB activation induced by various carcinogens and inflammatory stimuli, leading to suppression of nuclear factor-kappaB-regulated gene expression and up-regulation of apoptosis. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2007, 13, 2290–2297. [Google Scholar] [CrossRef]
- Chua, A.W.; Hay, H.S.; Rajendran, P.; Shanmugam, M.K.; Li, F.; Bist, P.; Koay, E.S.; Lim, L.H.; Kumar, A.P.; Sethi, G. Butein downregulates chemokine receptor CXCR4 expression and function through suppression of NF-kappaB activation in breast and pancreatic tumor cells. Biochem. Pharmacol. 2010, 80, 1553–1562. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Manu, K.A.; Ong, T.H.; Ramachandran, L.; Surana, R.; Bist, P.; Lim, L.H.; Kumar, A.P.; Hui, K.M.; Sethi, G. Inhibition of CXCR4/CXCL12 signaling axis by ursolic acid leads to suppression of metastasis in transgenic adenocarcinoma of mouse prostate model. Int. J. Cancer 2011, 129, 1552–1563. [Google Scholar] [CrossRef]
- Siveen, K.S.; Mustafa, N.; Li, F.; Kannaiyan, R.; Ahn, K.S.; Kumar, A.P.; Chng, W.J.; Sethi, G. Thymoquinone overcomes chemoresistance and enhances the anticancer effects of bortezomib through abrogation of NF-kappaB regulated gene products in multiple myeloma xenograft mouse model. Oncotarget 2014, 5, 634–648. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Guha, S.; Krishnan, S.; Diagaradjane, P.; Gelovani, J.; Aggarwal, B.B. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-κB–regulated gene products. Cancer Res. 2007, 67, 3853–3861. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Nair, A.S.; Ahn, K.S.; Pandey, M.K.; Yi, Z.; Liu, M.; Aggarwal, B.B. Gossypin, a pentahydroxy glucosyl flavone, inhibits the transforming growth factor beta-activated kinase-1-mediated NF-kappaB activation pathway, leading to potentiation of apoptosis, suppression of invasion, and abrogation of osteoclastogenesis. Blood 2007, 109, 5112–5121. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Shanmugam, M.K.; Siveen, K.S.; Wang, F.; Ong, T.H.; Loo, S.Y.; Swamy, M.M.; Mandal, S.; Kumar, A.P.; Goh, B.C.; et al. Garcinol sensitizes human head and neck carcinoma to cisplatin in a xenograft mouse model despite downregulation of proliferative biomarkers. Oncotarget 2015, 6, 5147–5163. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Shanmugam, M.K.; Chen, L.; Chatterjee, S.; Basha, J.; Kumar, A.P.; Kundu, T.K.; Sethi, G. Garcinol, a polyisoprenylated benzophenone modulates multiple proinflammatory signaling cascades leading to the suppression of growth and survival of head and neck carcinoma. Cancer Prev. Res. 2013, 6, 843–854. [Google Scholar] [CrossRef]
- Manu, K.A.; Shanmugam, M.K.; Ramachandran, L.; Li, F.; Fong, C.W.; Kumar, A.P.; Tan, P.; Sethi, G. First evidence that gamma-tocotrienol inhibits the growth of human gastric cancer and chemosensitizes it to capecitabine in a xenograft mouse model through the modulation of NF-kappaB pathway. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012, 18, 2220–2229. [Google Scholar] [CrossRef]
- Manu, K.A.; Shanmugam, M.K.; Li, F.; Chen, L.; Siveen, K.S.; Ahn, K.S.; Kumar, A.P.; Sethi, G. Simvastatin sensitizes human gastric cancer xenograft in nude mice to capecitabine by suppressing nuclear factor-kappa B-regulated gene products. J. Mol. Med. 2014, 92, 267–276. [Google Scholar] [CrossRef]
- Manu, K.A.; Shanmugam, M.K.; Ramachandran, L.; Li, F.; Siveen, K.S.; Chinnathambi, A.; Zayed, M.E.; Alharbi, S.A.; Arfuso, F.; Kumar, A.P.; et al. Isorhamnetin augments the anti-tumor effect of capecitabine through the negative regulation of NF-kappaB signaling cascade in gastric cancer. Cancer Lett. 2015, 363, 28–36. [Google Scholar] [CrossRef]
- Ghosh, S.; May, M.J.; Kopp, E.B. NF-kappa B and Rel proteins: Evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 1998, 16, 225–260. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Diagaradjane, P.; Guha, S.; Deorukhkar, A.; Shentu, S.; Aggarwal, B.B.; Krishnan, S. Curcumin sensitizes human colorectal Cancer xenografts in nude mice to γ-radiation by targeting nuclear factor-κB–regulated gene products. Clin. Cancer Res. 2008, 14, 2128–2136. [Google Scholar] [CrossRef]
- Lin, Y.G.; Kunnumakkara, A.B.; Nair, A.; Merritt, W.M.; Han, L.Y.; Armaiz-Pena, G.N.; Kamat, A.A.; Spannuth, W.A.; Gershenson, D.M.; Lutgendorf, S.K.; et al. Curcumin inhibits tumor growth and angiogenesis in ovarian carcinoma by targeting the nuclear factor-κB pathway. Clin. Cancer Res. 2007, 13, 3423–3430. [Google Scholar] [CrossRef] [PubMed]
- Heymach, J.V.; Shackleford, T.J.; Tran, H.T.; Yoo, S.Y.; Do, K.A.; Wergin, M.; Saintigny, P.; Vollmer, R.T.; Polascik, T.J.; Snyder, D.C.; et al. Effect of low-fat diets on plasma levels of NF-kappaB-regulated inflammatory cytokines and angiogenic factors in men with prostate cancer. Cancer Prev. Res. 2011, 4, 1590–1598. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Chen, X.; Fang, L.; Liu, F.; Cai, R.; Peng, C.; Qi, Y. Rhein exerts pro- and anti-inflammatory actions by targeting IKKbeta inhibition in LPS-activated macrophages. Free Radic. Biol. Med. 2014, 72, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Y.; Chen, T.T.; Xia, L.; Guo, M.; Xu, Y.; Yue, F.; Jiang, Y.; Chen, G.Q.; Zhao, K.W. Hypoxia inducible factor-1 mediates expression of galectin-1: The potential role in migration/invasion of colorectal cancer cells. Carcinogenesis 2010, 31, 1367–1375. [Google Scholar] [CrossRef]
- Clambey, E.T.; McNamee, E.N.; Westrich, J.A.; Glover, L.E.; Campbell, E.L.; Jedlicka, P.; De Zoeten, E.F.; Cambier, J.C.; Stenmark, K.R.; Colgan, S.P.; et al. Hypoxia-inducible factor-1 alpha-dependent induction of FoxP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa. Proc. Natl. Acad. Sci. USA 2012, 109, E2784–E2793. [Google Scholar] [CrossRef]
- Fernand, V.E.; Losso, J.N.; Truax, R.E.; Villar, E.E.; Bwambok, D.K.; Fakayode, S.O.; Lowry, M.; Warner, I.M. Rhein inhibits angiogenesis and the viability of hormone-dependent and -independent cancer cells under normoxic or hypoxic conditions in vitro. Chem. Biol. Interact. 2011, 192, 220–232. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Diagaradjane, P.; Anand, P.; Kuzhuvelil, H.B.; Deorukhkar, A.; Gelovani, J.; Guha, S.; Krishnan, S.; Aggarwal, B.B. Curcumin sensitizes human colorectal cancer to capecitabine by modulation of cyclin D1, COX-2, MMP-9, VEGF and CXCR4 expression in an orthotopic mouse model. Int. J. Cancer 2009, 125, 2187–2197. [Google Scholar] [CrossRef]
- Roy, N.K.; Bordoloi, D.; Monisha, J.; Padmavathi, G.; Kotoky, J.; Golla, R.; Kunnumakkara, A.B. Specific Targeting of Akt Kinase Isoforms: Taking the Precise Path for Prevention and Treatment of Cancer. Curr. Drug Targets 2017, 18, 421–435. [Google Scholar] [CrossRef]
- Bao, L.; Kimzey, A.; Sauter, G.; Sowadski, J.M.; Lu, K.P.; Wang, D.G. Prevalent overexpression of prolyl isomerase Pin1 in human cancers. Am. J. Pathol. 2004, 164, 1727–1737. [Google Scholar] [CrossRef]
- Cho, J.H.; Chae, J.I.; Shim, J.H. Rhein exhibits antitumorigenic effects by interfering with the interaction between prolyl isomerase Pin1 and c-Jun. Oncol. Rep. 2017, 37, 1865–1872. [Google Scholar] [CrossRef]
- Banik, K.; Ranaware, A.M.; Deshpande, V.; Nalawade, S.P.; Padmavathi, G.; Bordoloi, D.; Sailo, B.L.; Shanmugam, M.K.; Fan, L.; Arfuso, F.; et al. Honokiol for cancer therapeutics: A traditional medicine that can modulate multiple oncogenic targets. Pharmacol. Res. 2019, 144, 192–209. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, S.; Yin, Y.; Li, M.; Wang, B.; Yang, L.; Jiang, Y. FOXO3-mediated up-regulation of Bim contributes to rhein-induced cancer cell apoptosis. Apoptosis Int. J. Program. Cell Death 2015, 20, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Fujii, M.; Hou, D.X. Rhein induces apoptosis in HL-60 cells via reactive oxygen species-independent mitochondrial death pathway. Arch. Biochem. Biophys. 2003, 418, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Zhu, B.; Li, J.; Qin, L. Rhein Augments Antiproliferative Effects of Atezolizumab Based on Breast Cancer (4T1) Regression. Planta Med. 2019, 85, 1143–1149. [Google Scholar] [CrossRef]
- Lin, Y.J.; Huang, Y.H.; Zhen, Y.Z.; Liu, X.J.; Zhen, Y.S. Rhein lysinate induces apoptosis in breast cancer SK-Br-3 cells by inhibiting HER-2 signal pathway. Yao Xue Xue Bao = Acta Pharm. Sin. 2008, 43, 1099–1105. [Google Scholar]
- Liu, Y.; Zhong, Y.; Tian, W.; Lan, F.; Kang, J.; Pang, H.; Hou, H.; Li, D. An autophagy-dependent cell death of MDA-MB-231 cells triggered by a novel Rhein derivative 4F. Anti-Cancer Drugs 2019, 30, 1038–1047. [Google Scholar] [CrossRef]
- Ip, S.W.; Weng, Y.S.; Lin, S.Y.; Mei, D.; Tang, N.Y.; Su, C.C.; Chung, J.G. The role of Ca+2 on rhein-induced apoptosis in human cervical cancer Ca Ski cells. Anticancer. Res. 2007, 27, 379–389. [Google Scholar]
- Aviello, G.; Rowland, I.; Gill, C.I.; Acquaviva, A.M.; Capasso, F.; McCann, M.; Capasso, R.; Izzo, A.A.; Borrelli, F. Anti-proliferative effect of rhein, an anthraquinone isolated from Cassia species, on Caco-2 human adenocarcinoma cells. J. Cell. Mol. Med. 2010, 14, 2006–2014. [Google Scholar] [CrossRef]
- Zhuang, Y.; Bai, Y.; Hu, Y.; Guo, Y.; Xu, L.; Hu, W.; Yang, L.; Zhao, C.; Li, X.; Zhao, H. Rhein sensitizes human colorectal cancer cells to EGFR inhibitors by inhibiting STAT3 pathway. Oncotargets Ther. 2019, 12, 5281–5291. [Google Scholar] [CrossRef]
- Tang, N.; Chang, J.; Lu, H.C.; Zhuang, Z.; Cheng, H.L.; Shi, J.X.; Rao, J. Rhein induces apoptosis and autophagy in human and rat glioma cells and mediates cell differentiation by ERK inhibition. Microb. Pathog. 2017, 113, 168–175. [Google Scholar] [CrossRef]
- Chen, J.; Luo, B.; Wen, S.; Pi, R. Discovery of a novel rhein-SAHA hybrid as a multi-targeted anti-glioblastoma drug. Investig. New Drugs 2019. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Zhang, H.; Liu, T.; Draganov, A.; Yi, S.; Wang, B.; Zhou, M. Inhibition of MDM2 by a Rhein-Derived Compound AQ-101 Suppresses Cancer Development in SCID Mice. Mol. Cancer Ther. 2018, 17, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.K.; Noh, E.K.; Kim, J.Y.; Jegal, S.; Jeong, Y.; Cheon, J.; Koh, S.; Baek, J.H.; Min, Y.J.; Choi, Y.; et al. Rhein augments ATRA-induced differentiation of acute promyelocytic leukemia cells. Phytomed. Int. J. Phytother. Phytopharm. 2018, 49, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Cao, K.; Ni, Z.; Wang, S.; Li, W.; Liu, X.; Chen, Z. Rhein reverses doxorubicin resistance in SMMC-7721 liver cancer cells by inhibiting energy metabolism and inducing mitochondrial permeability transition pore opening. Biofactors 2019, 45, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, J.; Xu, L.; Lin, S.; Xiang, Y.; Dai, X.; Liang, G.; Huang, X.; Zhu, J.; Zhao, C. Rhein shows potent efficacy against non-small-cell lung cancer through inhibiting the STAT3 pathway. Cancer Manag. Res. 2019, 11, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Bu, T.; Wang, C.; Jin, H.; Meng, Q.; Huo, X.; Sun, H.; Sun, P.; Wu, J.; Ma, X.; Liu, Z.; et al. Organic anion transporters and PI3K-AKT-mTOR pathway mediate the synergistic anticancer effect of pemetrexed and rhein. J. Cell. Physiol. 2020, 235, 3309–3319. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Guo, W.; Tang, Y.; Zhang, J.; Xiao, N.; Zhang, L.; Li, W. Rhein Inhibits the Migration of Ovarian Cancer Cells through Down-Regulation of Matrix Metalloproteinases. Biol. Pharm. Bull. 2019, 42, 568–572. [Google Scholar] [CrossRef]
- Hu, L.; Cui, R.; Liu, H.; Wang, F. Emodin and rhein decrease levels of hypoxia-inducible factor-1alpha in human pancreatic cancer cells and attenuate cancer cachexia in athymic mice carrying these cells. Oncotarget 2017, 8, 88008–88020. [Google Scholar] [CrossRef][Green Version]
- Shanmugam, M.K.; Ahn, K.S.; Hsu, A.; Woo, C.C.; Yuan, Y.; Tan, K.H.B.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A.; Koh, A.P.F.; et al. Thymoquinone Inhibits Bone Metastasis of Breast Cancer Cells Through Abrogation of the CXCR4 Signaling Axis. Front. Pharmacol. 2018, 9, 1294. [Google Scholar] [CrossRef]
- Liu, L.; Ahn, K.S.; Shanmugam, M.K.; Wang, H.; Shen, H.; Arfuso, F.; Chinnathambi, A.; Alharbi, S.A.; Chang, Y.; Sethi, G.; et al. Oleuropein induces apoptosis via abrogating NF-kappaB activation cascade in estrogen receptor-negative breast cancer cells. J. Cell. Biochem. 2019, 120, 4504–4513. [Google Scholar] [CrossRef]
- Wang, C.; Kar, S.; Lai, X.; Cai, W.; Arfuso, F.; Sethi, G.; Lobie, P.E.; Goh, B.C.; Lim, L.H.K.; Hartman, M.; et al. Triple negative breast cancer in Asia: An insider’s view. Cancer Treat. Rev. 2018, 62, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Mohan, C.D.; Srinivasa, V.; Rangappa, S.; Mervin, L.; Mohan, S.; Paricharak, S.; Baday, S.; Li, F.; Shanmugam, M.K.; Chinnathambi, A.; et al. Trisubstituted-Imidazoles Induce Apoptosis in Human Breast Cancer Cells by Targeting the Oncogenic PI3K/Akt/mTOR Signaling Pathway. PLoS ONE 2016, 11, e0153155. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.Y.; Shanmugam, M.K.; Sethi, G.; Bishayee, A. Potential role of targeted therapies in the treatment of triple-negative breast cancer. Anti-Cancer Drugs 2016, 27, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Ningegowda, R.; Shivananju, N.S.; Rajendran, P.; Basappa; Rangappa, K.S.; Chinnathambi, A.; Li, F.; Achar, R.R.; Shanmugam, M.K.; Bist, P.; et al. A novel 4,6-disubstituted-1,2,4-triazolo-1,3,4-thiadiazole derivative inhibits tumor cell invasion and potentiates the apoptotic effect of TNFalpha by abrogating NF-kappaB activation cascade. Apoptosis Int. J. Program. Cell Death 2017, 22, 145–157. [Google Scholar] [CrossRef]
- Bhojwani, D.; Yang, J.J.; Pui, C.H. Biology of childhood acute lymphoblastic leukemia. Pediatric Clin. N. Am. 2015, 62, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Stewart, B.W.; Wild, C. World Cancer Report 2014; The International Agency for Research on Cancer-IARC Publications: Lyon, France, 2014. [Google Scholar]
- Sethi, G.; Chatterjee, S.; Rajendran, P.; Li, F.; Shanmugam, M.K.; Wong, K.F.; Kumar, A.P.; Senapati, P.; Behera, A.K.; Hui, K.M.; et al. Inhibition of STAT3 dimerization and acetylation by garcinol suppresses the growth of human hepatocellular carcinoma in vitro and in vivo. Mol. Cancer 2014, 13, 66. [Google Scholar] [CrossRef]
- Siveen, K.S.; Ahn, K.S.; Ong, T.H.; Shanmugam, M.K.; Li, F.; Yap, W.N.; Kumar, A.P.; Fong, C.W.; Tergaonkar, V.; Hui, K.M.; et al. Y-tocotrienol inhibits angiogenesis-dependent growth of human hepatocellular carcinoma through abrogation of AKT/mTOR pathway in an orthotopic mouse model. Oncotarget 2014, 5, 1897–1911. [Google Scholar] [CrossRef]
- Mohan, C.D.; Bharathkumar, H.; Bulusu, K.C.; Pandey, V.; Rangappa, S.; Fuchs, J.E.; Shanmugam, M.K.; Dai, X.; Li, F.; Deivasigamani, A.; et al. Development of a novel azaspirane that targets the Janus kinase-signal transducer and activator of transcription (STAT) pathway in hepatocellular carcinoma in vitro and in vivo. J. Biol. Chem. 2014, 289, 34296–34307. [Google Scholar] [CrossRef]
- Dai, X.; Ahn, K.S.; Kim, C.; Siveen, K.S.; Ong, T.H.; Shanmugam, M.K.; Li, F.; Shi, J.; Kumar, A.P.; Wang, L.Z.; et al. Ascochlorin, an isoprenoid antibiotic inhibits growth and invasion of hepatocellular carcinoma by targeting STAT3 signaling cascade through the induction of PIAS3. Mol. Oncol. 2015, 9, 818–833. [Google Scholar] [CrossRef]
- Rajendran, P.; Li, F.; Shanmugam, M.K.; Vali, S.; Abbasi, T.; Kapoor, S.; Ahn, K.S.; Kumar, A.P.; Sethi, G. Honokiol inhibits signal transducer and activator of transcription-3 signaling, proliferation, and survival of hepatocellular carcinoma cells via the protein tyrosine phosphatase SHP-1. J. Cell. Physiol. 2012, 227, 2184–2195. [Google Scholar] [CrossRef]
- Rajendran, P.; Li, F.; Manu, K.A.; Shanmugam, M.K.; Loo, S.Y.; Kumar, A.P.; Sethi, G. gamma-Tocotrienol is a novel inhibitor of constitutive and inducible STAT3 signalling pathway in human hepatocellular carcinoma: Potential role as an antiproliferative, pro-apoptotic and chemosensitizing agent. Br. J. Pharmacol. 2011, 163, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.M.; Li, F.; Rajendran, P.; Kumar, A.P.; Hui, K.M.; Sethi, G. Identification of beta-escin as a novel inhibitor of signal transducer and activator of transcription 3/Janus-activated kinase 2 signaling pathway that suppresses proliferation and induces apoptosis in human hepatocellular carcinoma cells. J. Pharmacol. Exp. Ther. 2010, 334, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Roy, N.K.; Anip, A.; Banik, K.; Monisha, J.; Bordoloi, D.; Kunnumakkara, A.B. Different methods to inhibit chemoresistance in Hepatocellular carcinoma. In Cancer Cell Chemoresistance and Chemosensitization; World Scientific: Singapore, 2018; pp. 378–398. [Google Scholar]
- Wang, L.; Syn, N.L.; Subhash, V.V.; Any, Y.; Thuya, W.L.; Cheow, E.S.H.; Kong, L.; Yu, F.; Peethala, P.C.; Wong, A.L.; et al. Pan-HDAC inhibition by panobinostat mediates chemosensitization to carboplatin in non-small cell lung cancer via attenuation of EGFR signaling. Cancer Lett. 2018, 417, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.Y.; Shanmugam, M.K.; Narula, A.S.; Kim, C.; Lee, J.H.; Namjoshi, O.A.; Blough, B.E.; Sethi, G.; Ahn, K.S. Oxymatrine Attenuates Tumor Growth and Deactivates STAT5 Signaling in a Lung Cancer Xenograft Model. Cancers 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Mohan, C.D.; Basappa, S.; Rangappa, S.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A.; Kumar, A.P.; Sethi, G.; Ahn, K.S.; et al. The IkappaB Kinase Inhibitor ACHP Targets the STAT3 Signaling Pathway in Human Non-Small Cell Lung Carcinoma Cells. Biomolecules 2019, 9. [Google Scholar] [CrossRef]
- Lee, J.H.; Chinnathambi, A.; Alharbi, S.A.; Shair, O.H.M.; Sethi, G.; Ahn, K.S. Farnesol abrogates epithelial to mesenchymal transition process through regulating Akt/mTOR pathway. Pharmacol. Res. 2019, 150, 104504. [Google Scholar] [CrossRef]
- Baek, S.H.; Ko, J.H.; Lee, J.H.; Kim, C.; Lee, H.; Nam, D.; Lee, J.; Lee, S.G.; Yang, W.M.; Um, J.Y.; et al. Ginkgolic Acid Inhibits Invasion and Migration and TGF-beta-Induced EMT of Lung Cancer Cells Through PI3K/Akt/mTOR Inactivation. J. Cell. Physiol. 2017, 232, 346–354. [Google Scholar] [CrossRef]
- Ong, P.S.; Wang, L.; Chia, D.M.; Seah, J.Y.; Kong, L.R.; Thuya, W.L.; Chinnathambi, A.; Lau, J.Y.; Wong, A.L.; Yong, W.P.; et al. A novel combinatorial strategy using Seliciclib((R)) and Belinostat((R)) for eradication of non-small cell lung cancer via apoptosis induction and BID activation. Cancer Lett. 2016, 381, 49–57. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, C.; Sethi, G.; Ahn, K.S. Brassinin inhibits STAT3 signaling pathway through modulation of PIAS-3 and SOCS-3 expression and sensitizes human lung cancer xenograft in nude mice to paclitaxel. Oncotarget 2015, 6, 6386–6405. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, C.; Lee, S.G.; Sethi, G.; Ahn, K.S. Ophiopogonin D, a Steroidal Glycoside Abrogates STAT3 Signaling Cascade and Exhibits Anti-Cancer Activity by Causing GSH/GSSG Imbalance in Lung Carcinoma. Cancers 2018, 10. [Google Scholar] [CrossRef]
- Xianghong, S.; Yuwei, S.; Hong, L.; Wei, S. Influence of main component of Heshouwu such as emodin, rhein and toluylene glycoside on hepatic cells and hepatoma carcinoma cell. Mod. J. Integr. Tradit. Chin. West. Med. 2010, 19, 1315–1319. [Google Scholar]
- Mao, Y.; Zhang, M.; Yang, J.; Sun, H.; Wang, D.; Zhang, X.; Yu, F.; Li, J. The UCP2-related mitochondrial pathway participates in rhein-induced apoptosis in HK-2 cells. Toxicol. Res. 2017, 6, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Bounda, G.A.; Zhou, W.; Wang, D.D.; Yu, F. Rhein elicits in vitro cytotoxicity in primary human liver HL-7702 cells by inducing apoptosis through mitochondria-mediated pathway. Evid. Based Complementary Altern. Med. 2015. [Google Scholar] [CrossRef] [PubMed]
- Roy, N.K.; Parama, D.; Banik, K.; Bordoloi, D.; Devi, A.K.; Thakur, K.K.; Padmavathi, G.; Shakibaei, M.; Fan, L.; Sethi, G.; et al. An Update on Pharmacological Potential of Boswellic Acids against Chronic Diseases. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef]
- Abotaleb, M.; Kubatka, P.; Caprnda, M.; Varghese, E.; Zolakova, B.; Zubor, P.; Opatrilova, R.; Kruzliak, P.; Stefanicka, P.; Büsselberg, D. Chemotherapeutic agents for the treatment of metastatic breast cancer: An update. Biomed. Pharmacother. 2018, 101, 458–477. [Google Scholar] [CrossRef] [PubMed]
- Kapinova, A.; Stefanicka, P.; Kubatka, P.; Zubor, P.; Uramova, S.; Kello, M.; Mojzis, J.; Blahutova, D.; Qaradakhi, T.; Zulli, A.; et al. Are plant-based functional foods better choice against cancer than single phytochemicals? A critical review of current breast cancer research. Biomed. Pharmacother. 2017, 96, 1465–1477. [Google Scholar] [CrossRef]
- Harikumar, K.B.; Kunnumakkara, A.B.; Sethi, G.; Diagaradjane, P.; Anand, P.; Pandey, M.K.; Gelovani, J.; Krishnan, S.; Guha, S.; Aggarwal, B.B. Resveratrol, a multitargeted agent, can enhance antitumor activity of gemcitabine in vitro and in orthotopic mouse model of human pancreatic cancer. Int. J. Cancer 2010, 127, 257–268. [Google Scholar] [CrossRef]
- Sung, B.; Kunnumakkara, A.B.; Sethi, G.; Anand, P.; Guha, S.; Aggarwal, B.B. Curcumin circumvents chemoresistance in vitro and potentiates the effect of thalidomide and bortezomib against human multiple myeloma in nude mice model. Mol. Cancer. Ther. 2009, 8, 959–970, Erratum in 2009, 8, 1398. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Bordoloi, D.; Sailo, B.L.; Roy, N.K.; Thakur, K.K.; Banik, K.; Shakibaei, M.; Gupta, S.C.; Aggarwal, B.B. Cancer drug development: The missing links. Exp. Biol. Med. 2019, 244, 663–689. [Google Scholar] [CrossRef]
| Cancer | In Vitro/In Vivo /Ex Vivo | Model | Mechanism of Action | References |
|---|---|---|---|---|
| Breast cancer | In vivo | 4T1 xenograft mice | Caspase-3, -8, -9↑, TNF-α↑, IL-6↑ | [146] |
| In vitro and in vivo | MCF-7, SK-Br-3, and MDA-MB-231 cells | p-EGFR↓, p-ΜΕΚ↓, p-ERK↓ | [46] | |
| MCF-7 injected BALB/c athymic mice | ||||
| In vitro | SK-Br-3 | p-HER-2↓, NF-κB↓, p53↑, p21↑ | [147] | |
| In vitro | MCF-7 | Cleaved caspase↑, p-Akt↓, FOXO3a↑, Bim↑ | [144] | |
| In vitro | MCF-7, MDA-MB-435s | PI3K↓, p-Akt↓, p-ERK↓, NF-κB↓, HIF-1α↓, EGF↓ | [138] | |
| Hsp90α↓, COX-2↓, HER-2↓, VEGF(165)↓, p-I-κB↓ | ||||
| In vitro | MDA-MB-231 | Beclin-1↑, LC3-II/LC3-I↑, p62↓ | [148] | |
| Cervical cancer | In vitro | HeLa | MAPK↑, JNK↑, p-ERK↑, | [48] |
| cleaved PARP↑, Caspase-3, -7↑ | ||||
| In vitro | HeLa | β-catenin↓, S phase arrest↑ | [111] | |
| In vitro | CaSki | Cytc↑, Caspase-3, -8, -9↑, Fas↑, p53↑, p21↑, Bcl-2↓, | [149] | |
| ΔΨm↓, cleaved Bid↑, cleaved PARP↑ | ||||
| Colon cancer | In vitro | Caco-2 | p-ERK1/2↑ (at higher concentrations of rhein) | [150] |
| In vitro | HT29, HCT116, Colo205, SW620 | HIF-1α↓, PD-L1↓, VEGF↓, COX-2↓, galectin-1↓ | [98] | |
| In vitro | HCT116, SW620 | p-STAT3↓ | [151] | |
| Glioma | In vitro | F98 | ERK1/2↓ | [152] |
| In vitro | T98G, U87, U251 | Ac-K100↑, NDRG1↑ | [153] | |
| Leukemia | In vivo | EU-1 injected SCID mice | MDM2↓, p53↑ | [154] |
| In vitro | HL-60 | Cleaved caspase↑, cleaved PARP↑, cleaved Bid↑, ΔΨm↓ | [145] | |
| In vitro | NB4 | p-ERK↑, Caspase-3↑ | [155] | |
| Liver cancer | In vitro and in vivo | HepG2, HepG2 injected BALB/c-nu mice | β-catenin↓, S phase arrest↑ | [111] |
| In vitro | HepG2 | CD95↑, p53↑, p21/WAF↑, mCD95L↑, sCD95L↑ | [96] | |
| In vitro | BEL-7402 | c-Myc↓, Caspase-3↑, S phase arrest↑ | [54] | |
| In vitro | HepG2 | p-Akt↓, FOXO↑, Bim↑, CHOP↑, p-eIF2α↑, p-ERK↓, | [144] | |
| Caspase-3, -8, -9↑ | ||||
| In vitro | HepaRG | ROS↑, ΔΨm↓, Bcl-2↓, Cyclin A↓, S-phase arrest↑ | [97] | |
| In vitro | SMMC-7721, SMMC-7721/DOX | ATP synthesis↓, inner ΔΨm↓ | [156] | |
| In vitro | HepG2, Huh7 | ROS↑, p-c-Jun↑, Caspase-3↑ | [55] | |
| Lung cancer | In vitro and in vivo | PC-9, H460, A549, H460 xenograft mice | STAT3↓, Bax↑, Bcl-2↓, G2/M phase arrest↑ | [157] |
| In vitro | A549 | p-PI3K↓, Akt↓, mTOR↓, Bcl-2↓ | [158] | |
| In vitro | A549 | G0/G1 phase arrest↑, GADD153↑, GRP78↑, Cyt c↑, | [110] | |
| Caspase-8↑, Bax↑,Bcl-2↓, Cleaved Bid↑, Cyclin D3↓, | ||||
| Cyclin E↓, CDK-4↓, CDK-6↓, ROS↑, p53↑, p21↑, ΔΨm↓ | ||||
| Nasopharyngeal cancer | In vitro | NPC | GRP78↑, ATF6↑, CHOP↑, ROS↑, | [49] |
| Caspase-3, -8,-9↑ | ||||
| Ovarian cancer | In vitro | SKOV3-PM4 | Rac1↓, ROS↓, MAPK↓, | [53] |
| TIMP-1↑, TIMP-2↑,AP-1↓ | ||||
| In vitro | A2780, OV2008 | MMP↓ | [159] | |
| Pancreatic cancer | In vitro and in vivo | AsPC-1, Patu8988T, | p-STAT3↓ | [52] |
| BxPC-3,PANC-1 injected BALB/c athymic mice | ||||
| In vitro and in vivo | AsPC-1, BxPC-3, HPAF-2, MiaPaCa2, Panc-1, | HIF-1α↓, PFK-1↓, HK-II↓, Glut-1↓ | [160] | |
| MiaPaCa2 injected athymic Balb/c mice | ||||
| Oral cancer | In vivo | SCC-4 | p53↓, cyclin A & E↓, ER Ca2+↑, ROS↑, | [43] |
| Caspase-3, -8, -9↑, Bcl-2↓, Cyt c↑ | ||||
| In vitro | SCC-4 | MMP-9↓ | [50] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henamayee, S.; Banik, K.; Sailo, B.L.; Shabnam, B.; Harsha, C.; Srilakshmi, S.; VGM, N.; Baek, S.H.; Ahn, K.S.; Kunnumakkara, A.B. Therapeutic Emergence of Rhein as a Potential Anticancer Drug: A Review of Its Molecular Targets and Anticancer Properties. Molecules 2020, 25, 2278. https://doi.org/10.3390/molecules25102278
Henamayee S, Banik K, Sailo BL, Shabnam B, Harsha C, Srilakshmi S, VGM N, Baek SH, Ahn KS, Kunnumakkara AB. Therapeutic Emergence of Rhein as a Potential Anticancer Drug: A Review of Its Molecular Targets and Anticancer Properties. Molecules. 2020; 25(10):2278. https://doi.org/10.3390/molecules25102278
Chicago/Turabian StyleHenamayee, Sahu, Kishore Banik, Bethsebie Lalduhsaki Sailo, Bano Shabnam, Choudhary Harsha, Satti Srilakshmi, Naidu VGM, Seung Ho Baek, Kwang Seok Ahn, and Ajaikumar B Kunnumakkara. 2020. "Therapeutic Emergence of Rhein as a Potential Anticancer Drug: A Review of Its Molecular Targets and Anticancer Properties" Molecules 25, no. 10: 2278. https://doi.org/10.3390/molecules25102278
APA StyleHenamayee, S., Banik, K., Sailo, B. L., Shabnam, B., Harsha, C., Srilakshmi, S., VGM, N., Baek, S. H., Ahn, K. S., & Kunnumakkara, A. B. (2020). Therapeutic Emergence of Rhein as a Potential Anticancer Drug: A Review of Its Molecular Targets and Anticancer Properties. Molecules, 25(10), 2278. https://doi.org/10.3390/molecules25102278







