Zinc Phthalocyanine Photochemistry by Raman Imaging, Fluorescence Spectroscopy and Femtosecond Spectroscopy in Normal and Cancerous Human Colon Tissues and Single Cells
Abstract
:1. Introduction
2. Experimental Methods
2.1. Phthalocyanine Synthesis
2.2. Sample Preparation
2.3. Cell Culture
2.4. Raman Spectroscopy and Imaging
2.5. Chemical Compounds for Fluorescence Measurements
2.6. Femtosecond Spectroscopy
2.7. UV-VIS Spectroscopy
3. Results and Discussion
3.1. Absorption Spectra
3.2. Human Colon Tissue Without ZnPcS4
3.3. Human Colon Tissue After Adding ZnPcS4
3.4. Human Colon Cells Without ZnPcS4
3.5. Human Colon Cells After Adding ZnPcS4
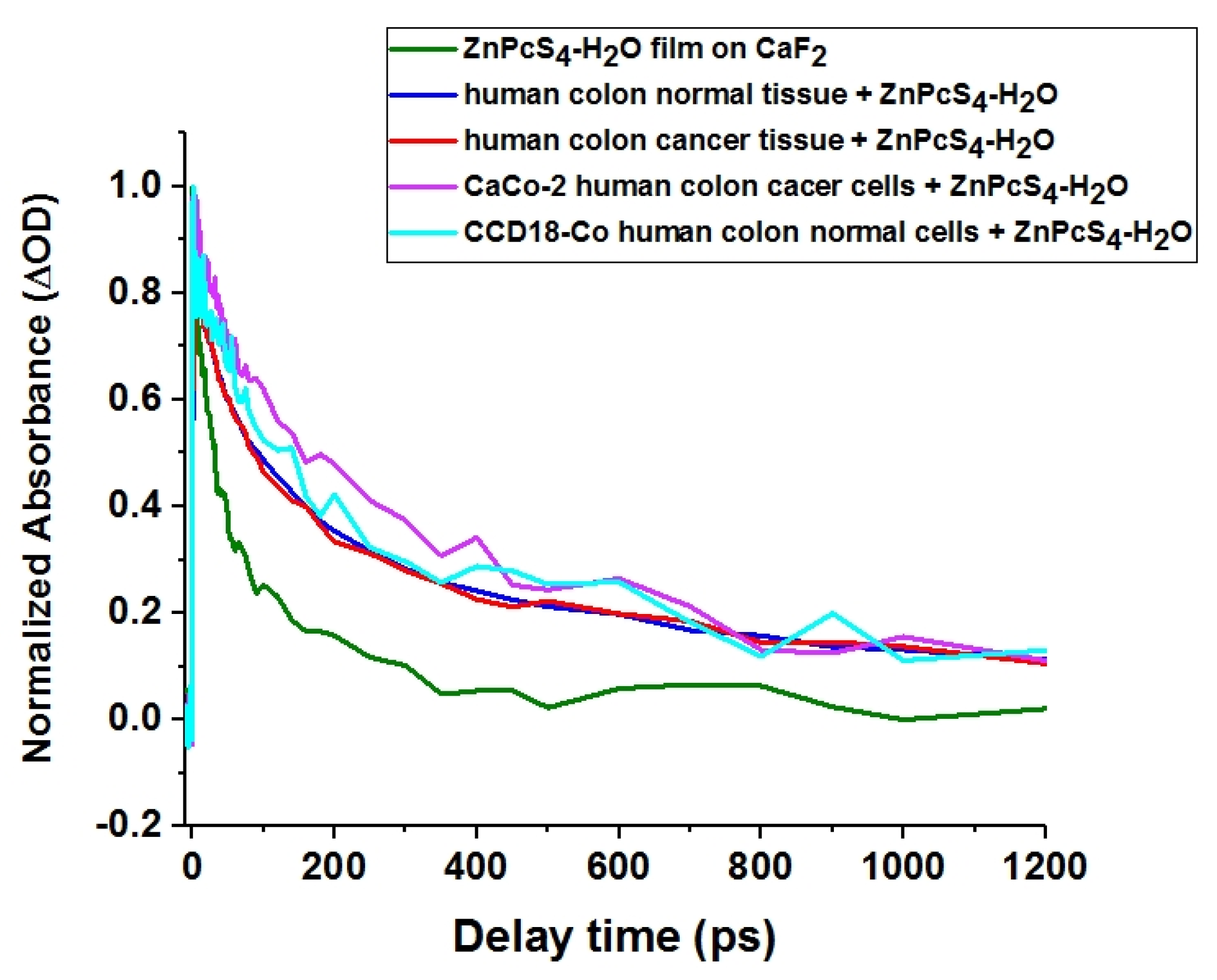
3.6. Transient Absorption
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lu, G.; Yan, S.; Shi, M.; Yu, W.; Li, J.; Zhu, W.; Ou, Z.; Kadish, K.M. A new class of rare earth tetrapyrrole sandwich complexes containing corrole and phthalocyanine macrocycles: Synthesis, physicochemical characterization and X-ray analysis. Chem. Commun. 2015, 51, 2411–2413. [Google Scholar] [CrossRef] [PubMed]
- Abramczyk, H.; Szymczyk, I.; Waliszewska, G.; Lebioda, A. Photoinduced Redox Processes in Phthalocyanine Derivatives by Resonance Raman Spectroscopy. J. Phys. Chem. A 2004, 108, 264–274. [Google Scholar] [CrossRef]
- Abramczyk, H.; Szymczyk, I. Novel Approaches to the Structure and Dynamics of Liquids: Experiments, Theories and Simulations; Springer Science & Business Media: Heidelberg, Germany, 2013; ISBN 978-1-4020-2384-2. [Google Scholar]
- Szymczyk, I.; Abramczyk, H. Peripheral substituent and solvent effects on the aggregation and photochemical properties of copper(II)phthalocyanine and copper(II)phthalocyanine-3,4’,4’’,4’’’-tetrasulfonic anion. Pure Appl. Chem. 2004, 76, 183–187. [Google Scholar] [CrossRef]
- Abramczyk, H.; Szymczyk, I. Aggregation of phthalocyanine derivatives in liquid solutions and human blood. J. Mol. Liq. 2004, 110, 51–56. [Google Scholar] [CrossRef]
- An, L.B.; Feng, L.J.; Lu, C.G. Mechanical Properties and Applications of Carbon Nanotubes. Adv. Mater. Res. 2011, 295, 1516–1521. [Google Scholar] [CrossRef]
- Kobayashi, N.; Sirai, H. Phthalocyanines - Chemistry and Functions; IPc: Tokyo, Japan, 1997. [Google Scholar]
- Prasad, D.R.; Ferraudi, G. Photochemistry of transition-metal phthalocyanines. Monophotonic and sequential biphotonic photochemical processes of copper(II) tetrakis(N-octadecylsulfamoyl)phthalocyanine in nonaqueous media. Inorg. Chem. 1982, 21, 2967–2971. [Google Scholar] [CrossRef]
- Ferraudi, G.; Muralidharan, S. Photochemistry of transition-metal phthalocyanines. Analysis of the photochemical and photophysical properties of the acido(phthalocyaninato)rhodium(III) complexes. Inorg. Chem. 1983, 22, 1369–1374. [Google Scholar] [CrossRef]
- Muralidharan, S.; Ferraudi, G. Sequential biphotonic processes in rhodium(III) phthalocyanines. J. Phys. Chem. 1983, 87, 4877–4881. [Google Scholar] [CrossRef]
- Kaneko, Y.; Arai, T.; Sakuragi, H.; Tokumaru, K.; Pac, C. Effect of excitation wavelength on photoreduction of metal-free and copper(II) 1,4,8,11,15,18,22,25-octabutoxyphthalocyanines with triethanolamine. J. Photochem. Photobiol. A Chem. 1996, 97, 155–162. [Google Scholar] [CrossRef]
- Kaneko, Y.; Nishimura, Y.; Arai, T.; Sakuragi, H.; Tokumaru, K.; Matsunaga, D. UV light and red light chemistry of metallophthalocyanine: Wavelength-dependent photochemical reduction of tetrasodium salts of Zn(II) and Cu(II) tetrasulphonatophthalocyanines with amines. J. Photochem. Photobiol. A Chem. 1995, 89, 37–44. [Google Scholar] [CrossRef]
- Kaneko, Y.; Nishimura, Y.; Takane, N.; Arai, T.; Sakuragi, H.; Kobayashi, N.; Matsunaga, D.; Pac, C.; Tokumaru, K. Violet emission observed from phthalocyanines. J. Photochem. Photobiol. A Chem. 1997, 106, 177–183. [Google Scholar] [CrossRef]
- Howe, L.; Zhang, J.Z. Ultrafast Studies of Excited-State Dynamics of Phthalocyanine and Zinc Phthalocyanine Tetrasulfonate in Solution. J. Phys. Chem. A 1997, 101, 3207–3213. [Google Scholar] [CrossRef]
- RuUckmann, I.; Zeug, A.; Herter, R.; Röder, B. On the Influence of Higher Excited States on the ISC Quantum Yield of Octa-aL-alkyloxy-substituted Zn-Phthalocyanine Molecules Studied by Nonlinear Absorption. Photochem. Photobiol. 1997, 66, 576–584. [Google Scholar] [CrossRef]
- Chahraoui, D.; Valat, P.; Kossanyi, J. Fluorescence of phthalocyanines: Emission from an upper excited state. Res. Chem. Intermed. 1992, 17, 219–232. [Google Scholar] [CrossRef]
- Ball, D.J.; Mayhew, S.; Wood, S.R.; Griffiths, J.; Vernon, D.I.; Brown, S.B. A Comparative Study of the Cellular Uptake and Photodynamic Efficacy of Three Novel Zinc Phthalocyanines of Differing Charge. Photochem. Photobiol. 2008, 69, 390–396. [Google Scholar] [CrossRef]
- Kobayashi, N.; Lever, A.B.P. Cation or solvent-induced supermolecular phthalocyanine formation: Crown ether substituted phthalocyanines. J. Am. Chem. Soc. 1987, 109, 7433–7441. [Google Scholar] [CrossRef]
- Kobayashi, N.; Lam, H.; Nevin, W.A.; Janda, P.; Leznoff, C.C.; Koyama, T.; Monden, A.; Shirai, H. Synthesis, spectroscopy, electrochemistry, spectroelectrochemistry, Langmuir-Blodgett film formation, and molecular orbital calculations of planar binuclear phthalocyanines. J. Am. Chem. Soc. 1994, 116, 879–890. [Google Scholar] [CrossRef]
- Kobayashi, N.; Togashi, M.; Osa, T.; Ishii, K.; Yamauchi, S.; Hino, H. Low Symmetrical Phthalocyanine Analogues Substituted with Three Crown Ether Voids and Their Cation-Induced Supermolecules. J. Am. Chem. Soc. 1996, 118, 1073–1085. [Google Scholar] [CrossRef]
- Lagorio, M.G.; Dicelio, L.E.; Roman, E.S. Visible and near-IR spectroscopic and photochemical characterization of substituted metallophthalocyanines. J. Photochem. Photobiol. A Chem. 1993, 72, 153–161. [Google Scholar] [CrossRef]
- Marino, J.; Vior, M.C.G.; Dicelio, L.E.; Roguin, L.P.; Awruch, J. Photodynamic effects of isosteric water-soluble phthalocyanines on human nasopharynx KB carcinoma cells. Eur. J. Med. Chem. 2010, 45, 4129–4139. [Google Scholar] [CrossRef] [PubMed]
- Jori, G. Far-red-absorbing photosensitizers: Their use in the photodynamic therapy of tumours. J. Photochem. Photobiol. A Chem. 1992, 62, 371–378. [Google Scholar] [CrossRef]
- Kuznetsova, N.A.; Gretsova, N.S.; Derkacheva, V.M.; Kaliya, O.L.; Lukyanets, E.A. Sulfonated phthalocyanines: Aggregation and singlet oxygen quantum yield in aqueous solutions. J. Porphyrins Phthalocyanines 2003, 7, 147–154. [Google Scholar] [CrossRef]
- Wang, S.; Gao, R.; Zhou, F.; Selke, M. Nanomaterials and singlet oxygen photosensitizers: Potential applications in photodynamic therapy. J. Mater. Chem. 2004, 14, 487. [Google Scholar] [CrossRef]
- Zhang, Y.; Lovell, J.F. Recent Applications of Phthalocyanines and Naphthalocyanines for Imaging and Therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 9, e1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, S.B.; A Brown, E.; Walker, I. The present and future role of photodynamic therapy in cancer treatment. Lancet Oncol. 2004, 5, 497–508. [Google Scholar] [CrossRef]
- Zhang, J.; Fan, Y.; He, M.; Ma, X.; Song, Y.; Liu, M.; Xu, J. Accuracy of Raman spectroscopy in differentiating brain tumor from normal brain tissue. Oncotarget 2017, 8, 36824–36831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalkanis, S.N.; Kast, R.; Rosenblum, M.L.; Mikkelsen, T.; Yurgelevic, S.M.; Nelson, K.M.; Raghunathan, A.; Poisson, L.M.; Auner, G.W. Raman spectroscopy to distinguish grey matter, necrosis, and glioblastoma multiforme in frozen tissue sections. J. Neuro Oncol. 2014, 116, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Hollon, T.; Lewis, S.; Freudiger, C.W.; Xie, X.S.; Orringer, D.A. Improving the accuracy of brain tumor surgery via Raman-based technology. Neurosurg. Focus 2016, 40, E9. [Google Scholar] [CrossRef] [PubMed]
- Anna, I.; Bartosz, P.; Lech, P.; Halina, A. Novel strategies of Raman imaging for brain tumor research. Oncotarget 2017, 8, 85290–85310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abramczyk, H.; Brozek-Pluska, B.; Surmacki, J.M.; Jablonska-Gajewicz, J.; Kordek, R. Raman ‘optical biopsy’ of human breast cancer. Prog. Biophys. Mol. Boil. 2012, 108, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Gao, Q.; Zhang, G. Classification for breast cancer diagnosis with Raman spectroscopy. Biomed. Opt. Express 2014, 5, 2435–2445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Hao, C.; Xu, Z. Diagnosis of Breast Cancer Tissues Using 785 nm Miniature Raman Spectrometer and Pattern Regression. Sensors 2017, 17, 627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abramczyk, H.; Brozek-Pluska, B. Apical-basal polarity of epithelial cells imaged by Raman microscopy and Raman imaging: Capabilities and challenges for cancer research. J. Mol. Liq. 2017, 245, 52–61. [Google Scholar] [CrossRef]
- Brozek-Pluska, B.; Kopeć, M.; Abramczyk, H. Development of a new diagnostic Raman method for monitoring epigenetic modifications in the cancer cells of human breast tissue. Anal. Methods 2016, 8, 8542–8553. [Google Scholar] [CrossRef]
- Surmacki, J.M.; Brozek-Pluska, B.; Kordek, R.; Abramczyk, H. The lipid-reactive oxygen species phenotype of breast cancer. Raman spectroscopy and mapping, PCA and PLSDA for invasive ductal carcinoma and invasive lobular carcinoma. Molecular tumorigenic mechanisms beyond Warburg effect. Analyst 2015, 140, 2121–2133. [Google Scholar] [CrossRef] [PubMed]
- Ishigaki, M.; Maeda, Y.; Taketani, A.; Andriana, B.B.; Ishihara, R.; Wongravee, K.; Ozaki, Y.; Sato, H. Diagnosis of early-stage esophageal cancer by Raman spectroscopy and chemometric techniques. Analyst 2016, 141, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Shetty, G.; Kendall, C.; Shepherd, N.; Stone, N.; Barr, H. Raman spectroscopy: Elucidation of biochemical changes in carcinogenesis of oesophagus. Br. J. Cancer 2006, 94, 1460–1464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teh, S.K.; Zheng, W.; Ho, K.Y.; Teh, M.; Yeoh, K.G.; Huang, Z. Diagnosis of gastric cancer using near-infrared Raman spectroscopy and classification and regression tree techniques. J. Biomed. Opt. 2008, 13, 034013. [Google Scholar] [CrossRef] [PubMed]
- Brozek-Pluska, B.; Morawiec-Sztandera, A.; Kopeć, M.; Niedzwiecka, I. Label-free determination of lipid composition and secondary protein structure of human salivary noncancerous and cancerous tissues by Raman microspectroscopy. Analyst 2015, 140, 2107–2113. [Google Scholar] [CrossRef] [PubMed]
- Kanter, E.M.; Majumder, S.; Vargis, E.; Robichaux-Viehoever, A.; Kanter, G.J.; Shappell, H.; Jones, H.W.; Mahadevan-Jansen, A. Multiclass discrimination of cervical precancers using Raman spectroscopy. J. Raman Spectrosc. 2009, 40, 205–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Wang, H.; Du, J.; Jing, C. Raman microspectroscopy of nucleus and cytoplasm for human colon cancer diagnosis. Biosens. Bioelectron. 2017, 97, 70–74. [Google Scholar] [CrossRef] [PubMed]
- A Jenkins, C.; Lewis, P.D.; Dunstan, P.; A Harris, D. Role of Raman spectroscopy and surface enhanced Raman spectroscopy in colorectal cancer. World J. Gastrointest. Oncol. 2016, 8, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Ryu, S.; Kim, Y.-H.; Lee, C.K.; Eun, C.; Han, D.-S.; Lee, S.-H. Comparison of a Guaiac-Based and a Quantitative Immunochemical Fecal Occult Blood Testing in Average-Risk Population for Colorectal Cancer Screening. Gastrointest. Endosc. 2009, 69, AB278–AB279. [Google Scholar] [CrossRef]
- Brozek-Pluska, B.; Miazek, K.; Musiał, J.; Kordek, R. Label-free diagnostics and cancer surgery Raman spectra guidance for the human colon at different excitation wavelengths. RSC Adv. 2019, 9, 40445–40454. [Google Scholar] [CrossRef] [Green Version]
- Brozek-Pluska, B.; Orlikowski, M.; Abramczyk, H.; Kadish, K.M.; Smith, K.M.; Guilard, R. Phthalocyanines: From Dyes to Photosensitizers in Diagnostics and Treatment of Cancer. Spectroscopy and Raman Imaging Studies of Phthalocyanines in Human Breast Tissues. In Handbook of Porphyrin Science (Volumes 36–40); World Scientific Pub Co Pte Lt: Hackensack, NJ, USA, 2016; Volume 40, pp. 1–49. [Google Scholar]
- Brozek-Pluska, B.; Jarota, A.; Kurczewski, K.; Abramczyk, H. Photochemistry of tetrasulphonated zinc phthalocyanine in water and DMSO solutions by absorption, emission, Raman spectroscopy and femtosecond transient absorption spectroscopy. J. Mol. Struct. 2009, 924, 338–346. [Google Scholar] [CrossRef]
- Brozek-Pluska, B.; Szymczyk, I.; Abramczyk, H. Raman spectroscopy of phthalocyanines and their sulfonated derivatives. J. Mol. Struct. 2005, 744, 481–485. [Google Scholar] [CrossRef]
- Brozek-Pluska, B.; Jarota, A.; Jablonska-Gajewicz, J.; Kordek, R.; Czajkowski, W.; Abramczyk, H. Distribution of phthalocyanines and Raman reporters in human cancerous and noncancerous breast tissue as studied by Raman imaging. Technol. Cancer Res. Treat. 2012, 11, 317–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abramczyk, H.; Brozek-Pluska, B.; Surmacki, J.M.; Musial, J.; Kordek, R. Oncologic photodynamic diagnosis and therapy: Confocal Raman/fluorescence imaging of metal phthalocyanines in human breast cancer tissue in vitro. Analyst 2014, 139, 5547–5559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abramczyk, H.; Brozek-Pluska, B.; Tondusson, M.; Freysz, E. Ultrafast Dynamics of Metal Complexes of Tetrasulfonated Phthalocyanines at Biological Interfaces: Comparison between Photochemistry in Solutions, Films, and Noncancerous and Cancerous Human Breast Tissues. J. Phys. Chem. C 2013, 117, 4999–5013. [Google Scholar] [CrossRef] [Green Version]
- Jarota, A.; Tondusson, M.; Galle, G.; Freysz, E.; Abramczyk, H. Ultrafast Dynamics of Metal Complexes of Tetrasulphonated Phthalocyanines. J. Phys. Chem. A 2012, 116, 4000–4009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howe, L.; Zhang, J.Z. The Effect of Biological Substrates on the Ultrafast Excited-state Dynamics of Zinc Phthalocyanine Tetrasulfonate in Solution. Photochem. Photobiol. 1998, 67, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Mack, J.; Stillman, M.J. Transition assignments in the ultraviolet-visible absorption and magnetic circular dichroism spectra of phthalocyanines. Inorg. Chem. 2001, 40, 812–814. [Google Scholar] [CrossRef] [PubMed]
- Movasaghi, Z.; Rehman, S.; Rehman, I.U. Raman Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2007, 42, 493–541. [Google Scholar] [CrossRef]
- Abramczyk, H.; Imiela, A.; Brozek-Pluska, B.; Kopeć, M.; Surmacki, J.M.; Sliwinska, A. Aberrant Protein Phosphorylation in Cancer by Using Raman Biomarkers. Cancers 2019, 11, 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dukor, R.K. Vibrational Spectroscopy in the Detection of Cancer. In Handbook of Vibrational Spectroscopy; Wiley: Hoboken, NJ, USA, 2006. [Google Scholar]
- Kopec, M.; Imiela, A.; Abramczyk, H. Monitoring glycosylation metabolism in brain and breast cancer by Raman imaging. Sci. Rep. 2019, 9, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brozek-Pluska, B.; Musial, J.; Kordek, R.; Abramczyk, H.; Pluska, B. Analysis of Human Colon by Raman Spectroscopy and Imaging-Elucidation of Biochemical Changes in Carcinogenesis. Int. J. Mol. Sci. 2019, 20, 3398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasha, M. Energy Transfer Mechanisms and the Molecular Exciton Model for Molecular Aggregates. Radiat. Res. 1963, 20, 55. [Google Scholar] [CrossRef] [PubMed]
- Ke, M.-R.; Chen, S.-F.; Peng, X.-H.; Zheng, Q.-F.; Zheng, B.-Y.; Yeh, C.-K.; Huang, J.-D. A tumor-targeted activatable phthalocyanine-tetrapeptide-doxorubicin conjugate for synergistic chemo-photodynamic therapy. Eur. J. Med. Chem. 2017, 127, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Damania, D.; Subramanian, H.; Backman, V.; Anderson, E.C.; Wong, M.H.; Mccarty, O.J.T.; Phillips, K.G. Network signatures of nuclear and cytoplasmic density alterations in a model of pre and postmetastatic colorectal cancer. J. Biomed. Opt. 2014, 19, 16016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bronkhorst, A.J.; Ungerer, V.; Holdenrieder, S. The emerging role of cell-free DNA as a molecular marker for cancer management. Biomol. Detect. Quantif. 2019, 17, 100087. [Google Scholar] [CrossRef] [PubMed]
- Abramczyk, H.; Imiela, A.; Śliwińska, A. Novel strategies of Raman imaging for exploring cancer lipid reprogramming. J. Mol. Liq. 2019, 274, 52–59. [Google Scholar] [CrossRef]
- Tynga, I.; Houreld, N.N.; Abrahamse, H. The primary subcellular localization of Zinc phthalocyanine and its cellular impact on viability, proliferation and structure of breast cancer cells (MCF-7). J. Photochem. Photobiol. B Biol. 2013, 120, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.R.; Holroyd, J.A.; Brown, S.B. The Subcellular Localization of Zn(ll) Phthalocyanines and Their Redistribution on Exposure to Light. Photochem. Photobiol. 1997, 65, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Manoto, S.L.; Houreld, N.N.; Abrahamse, H. Phototoxic effect of photodynamic therapy on lung cancer cells grown as a monolayer and three dimensional multicellular spheroids. Lasers Surg. Med. 2013, 45, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Tynga, I.; Houreld, N.N.; Abrahamse, H. Induced Cell Death Pathway Post Photodynamic Therapy Using a Metallophthalocyanine Photosensitizer in Breast Cancer Cells. Photomed. Laser Surg. 2014, 32, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Muli, D.K.; Rajaputra, P.; You, Y.; McGrath, D.V. Asymmetric ZnPc–rhodamine B conjugates for mitochondrial targeted photodynamic therapy. Bioorg. Med. Chem. Lett. 2014, 24, 4496–4500. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.-L.; Liu, F.-R.; Ke, M.-R.; Lo, P.-C.; Fong, W.-P.; Ng, D.; Huang, J.-D. The effects of formulation and serum albumin on the in vitro photodynamic activity of zinc(II) phthalocyanines substituted with sulfonated quinolineoxy groups. Dye. Pigment. 2016, 128, 215–225. [Google Scholar] [CrossRef]
- Shao, J.-W.; Dai, Y.; Zhao, W.; Xie, J.; Xue, J.; Ye, J.; Jia, L. Intracellular distribution and mechanisms of actions of photosensitizer Zinc(II)-phthalocyanine solubilized in Cremophor EL against human hepatocellular carcinoma HepG2 cells. Cancer Lett. 2013, 330, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Manoto, S.L.; Sekhejane, P.R.; Houreld, N.N.; Abrahamse, H. Localization and phototoxic effect of zinc sulfophthalocyanine photosensitizer in human colon (DLD-1) and lung (A549) carcinoma cells (in vitro). Photodiagnosis Photodyn. Ther. 2012, 9, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Ongarora, B.G.; Hu, X.; Verberne-Sutton, S.D.; Garno, J.C.; Vicente, M.G.H. Syntheses and Photodynamic Activity of Pegylated Cationic Zn(II)-Phthalocyanines in HEp2 Cells. Theranostics 2012, 2, 850–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doustvandi, M.A.; Mohammadnejad, F.; Mansoori, B.; Mohammadi, A.; Navaeipour, F.; Baradaran, B.; Tajalli, H. The interaction between the light source dose and caspase-dependent and -independent apoptosis in human SK-MEL-3 skin cancer cells following photodynamic therapy with zinc phthalocyanine: A comparative study. J. Photochem. Photobiol. B Biol. 2017, 176, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Chiarante, N.; Vior, M.C.G.; Rey, O.; Marino, J.; Roguin, L.P. Lysosomal permeabilization and endoplasmic reticulum stress mediate the apoptotic response induced after photoactivation of a lipophilic zinc(II) phthalocyanine. Int. J. Biochem. Cell Biol. 2018, 103, 89–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuzyniak, W.; Schmidt, J.; Glac, W.; Berkholz, J.; Steinemann, G.; Nitzsche, B.; Hoffmann, B.; Ermilov, E.A.; Gürek, A.G.; Ahsen, V.; et al. Novel zinc phthalocyanine as a promising photosensitizer for photodynamic treatment of esophageal cancer. Int. J. Oncol. 2017, 50, 953–963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machado, A.H.A.; Braga, F.M.P.; Soares, C.P.; Pelisson, M.M.M.; Beltrame, M.; Da Silva, N.S. Photodynamic Therapy with a New Photosensitizing Agent. Photomed. Laser Surg. 2007, 25, 220–228. [Google Scholar] [CrossRef] [PubMed]
- He, L.; He, X.; Lim, L.P.; De Stanchina, E.; Xuan, Z.; Liang, Y.; Xue, W.; Zender, L.; Magnus, J.; Ridzon, D.; et al. A microRNA component of the p53 tumour suppressor network. Nature 2007, 447, 1130–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raver-Shapira, N.; Marciano, E.; Meiri, E.; Spector, Y.; Rosenfeld, N.; Moskovits, N.; Bentwich, Z.; Oren, M. Transcriptional Activation of miR-34a Contributes to p53-Mediated Apoptosis. Mol. Cell 2007, 26, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Candé, C.; Cecconi, F.; Dessen, P.; Kroemer, G. Apoptosis-inducing factor (AIF): Key to the conserved caspase-independent pathways of cell death? J. Cell Sci. 2002, 115, 4727–4734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matlaba, P.M. Synthesis of Zinc Phthalocyanine Derivatives for Possible Use in Photodynamic Therapy. Masters’ Thesis, Rhodes University, Grahamstown, South Africa, 2002. [Google Scholar]
- Abramczyk, H.; Brozek-Pluska, B.; Surmacki, J.M.; Tondusson, M.; Freysz, E. Photostability of biological systems—Femtosecond dynamics of zinc tetrasulfonated phthalocyanine at cancerous and noncancerous human Breast tissues. J. Photochem. Photobiol. A Chem. 2017, 332, 10–24. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compound are not available from the authors. |


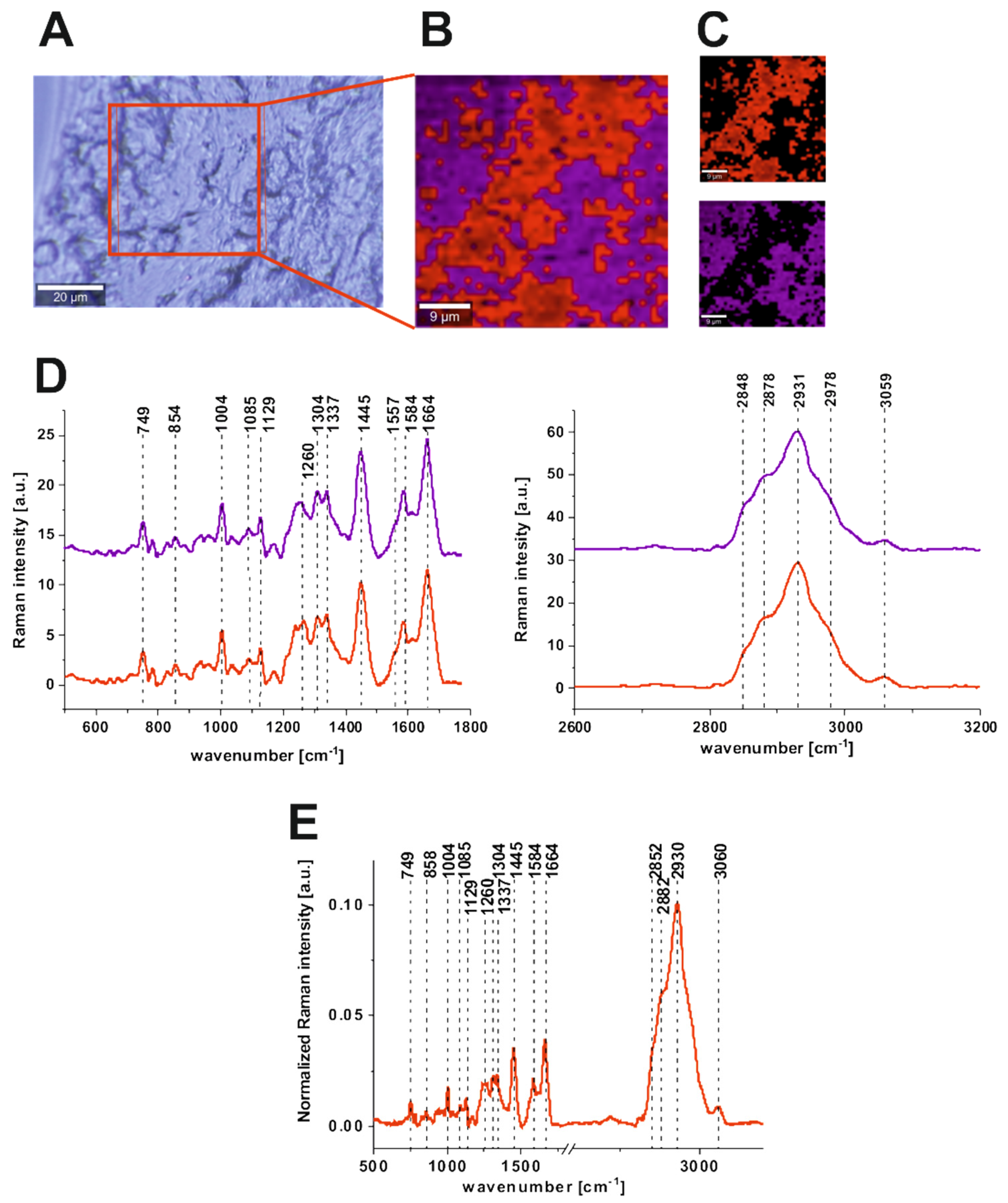

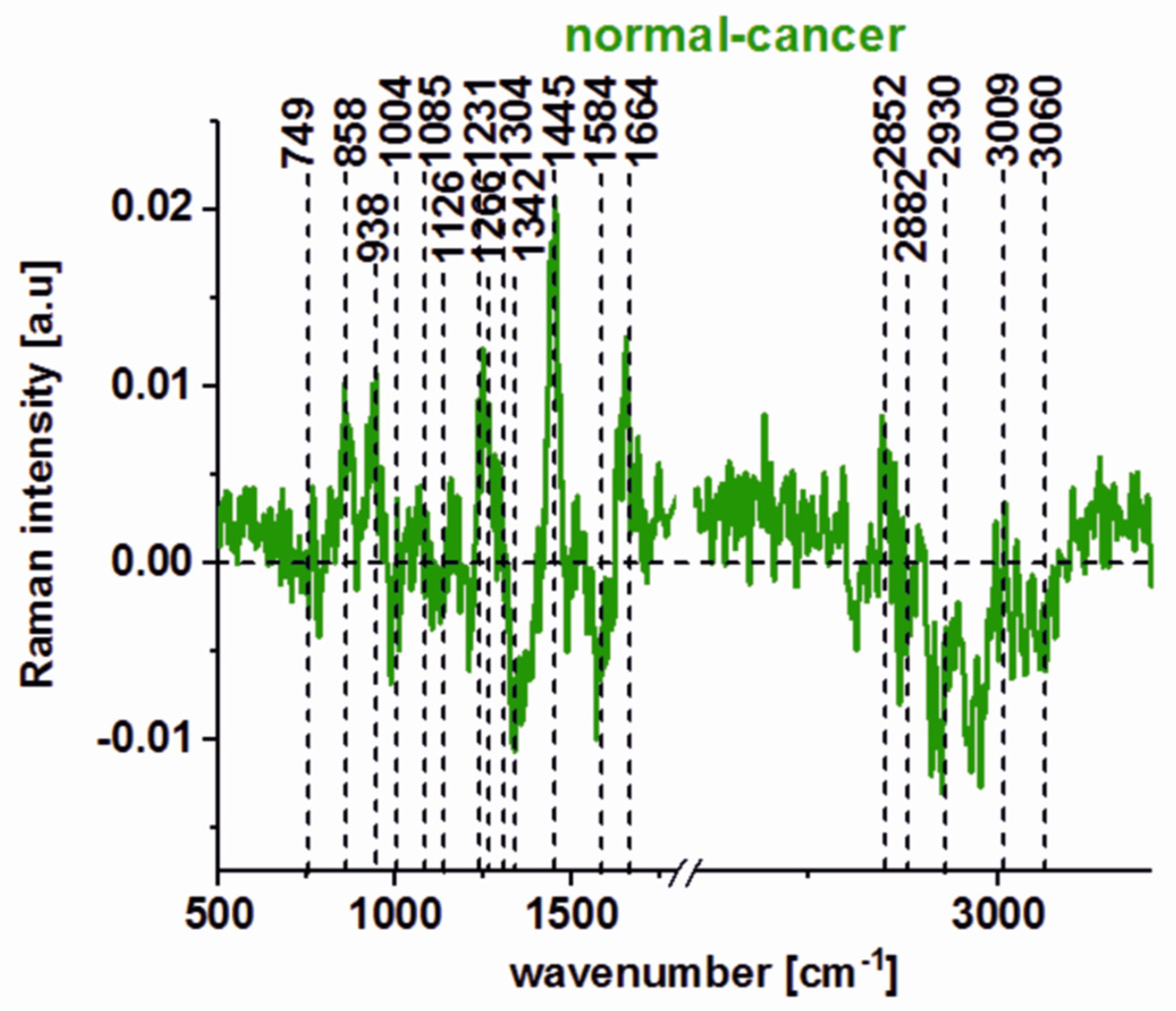

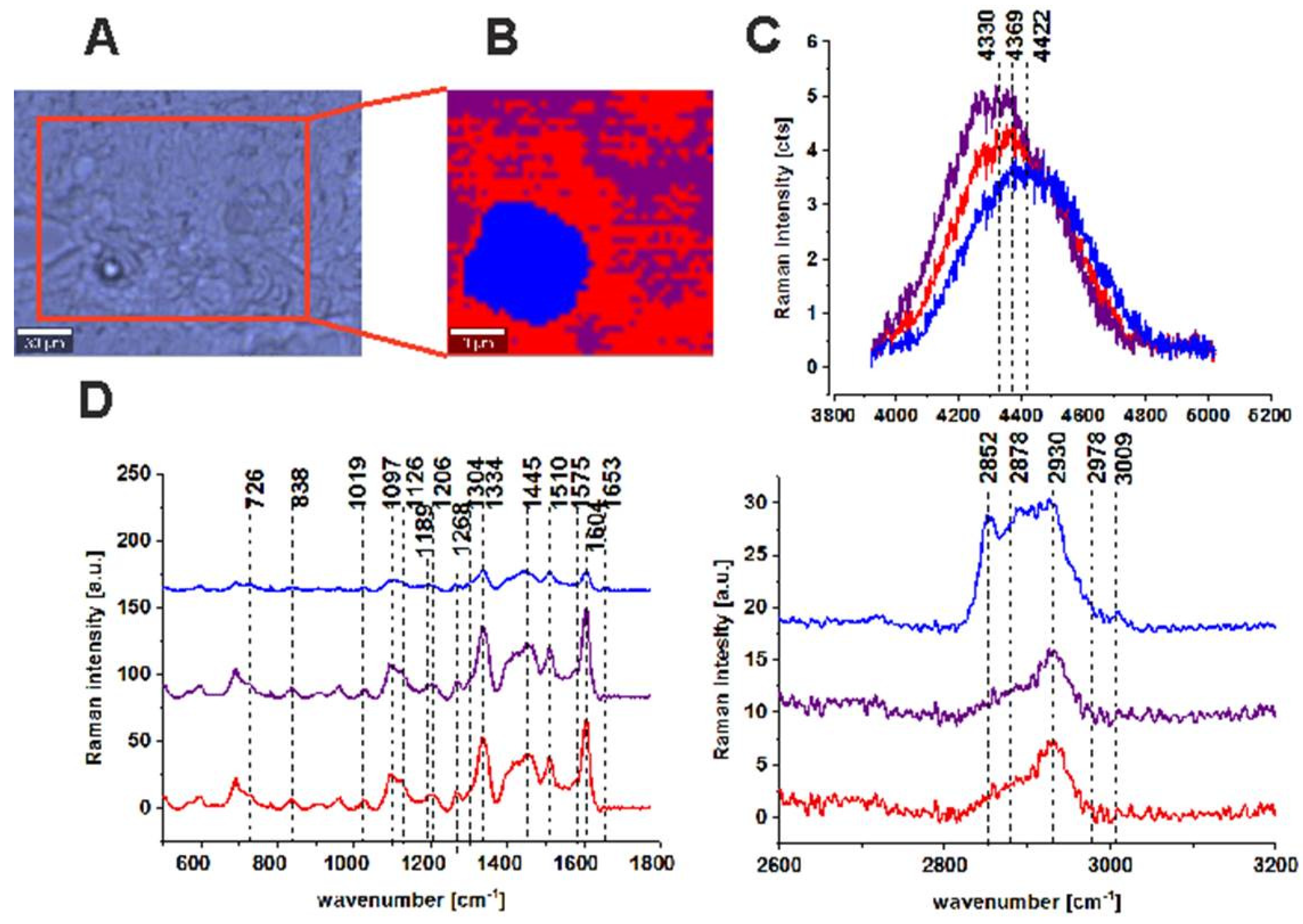
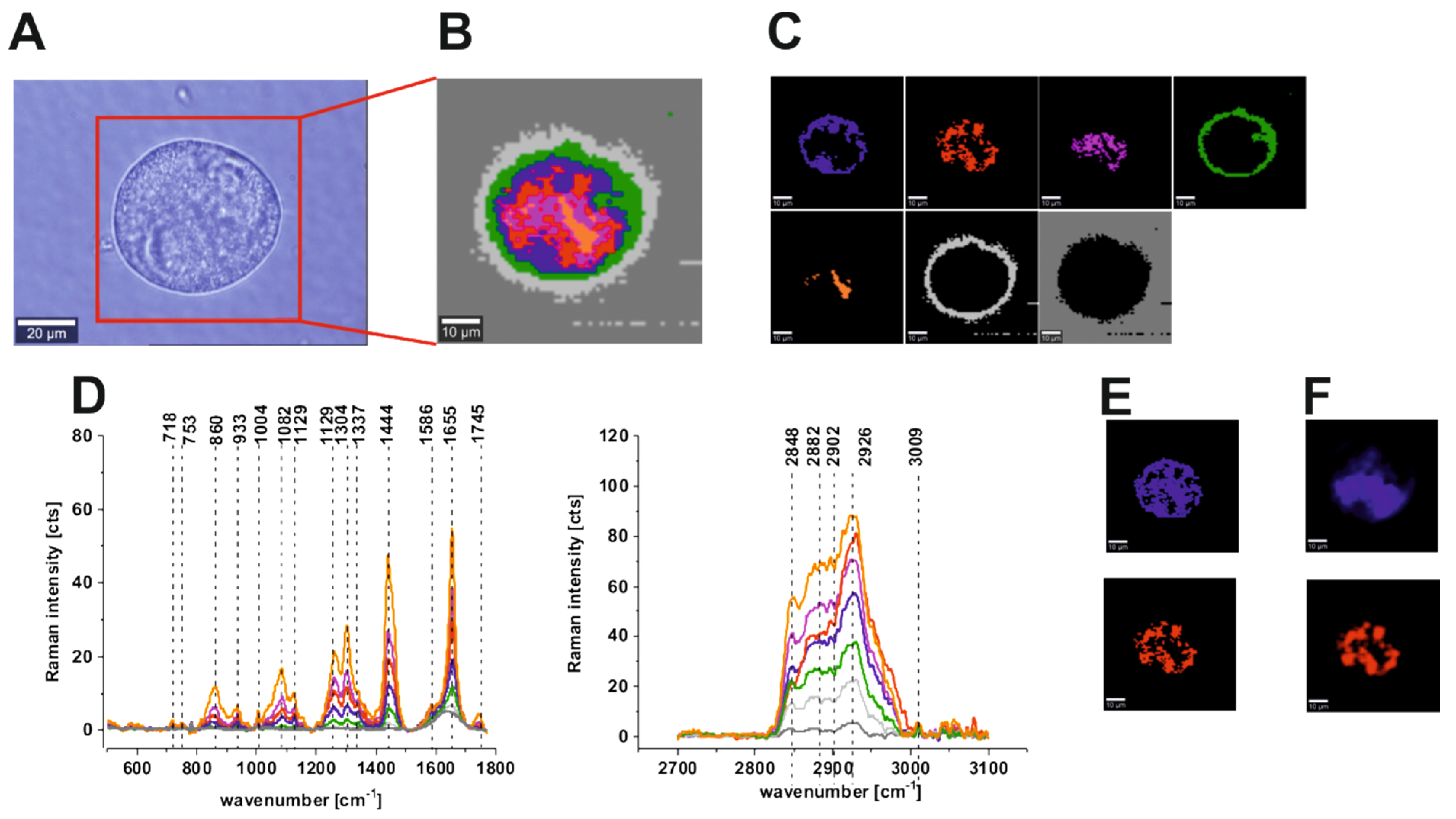


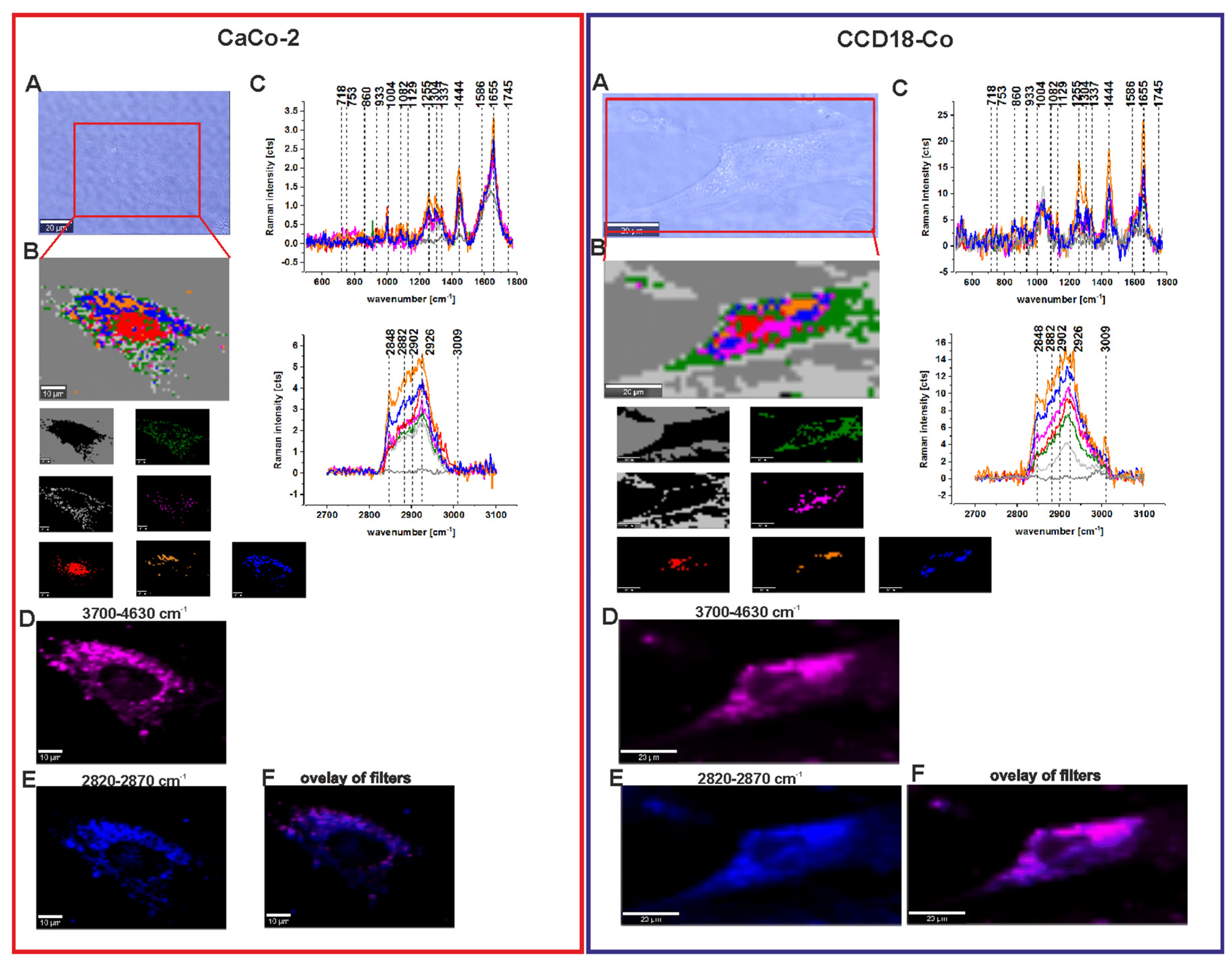
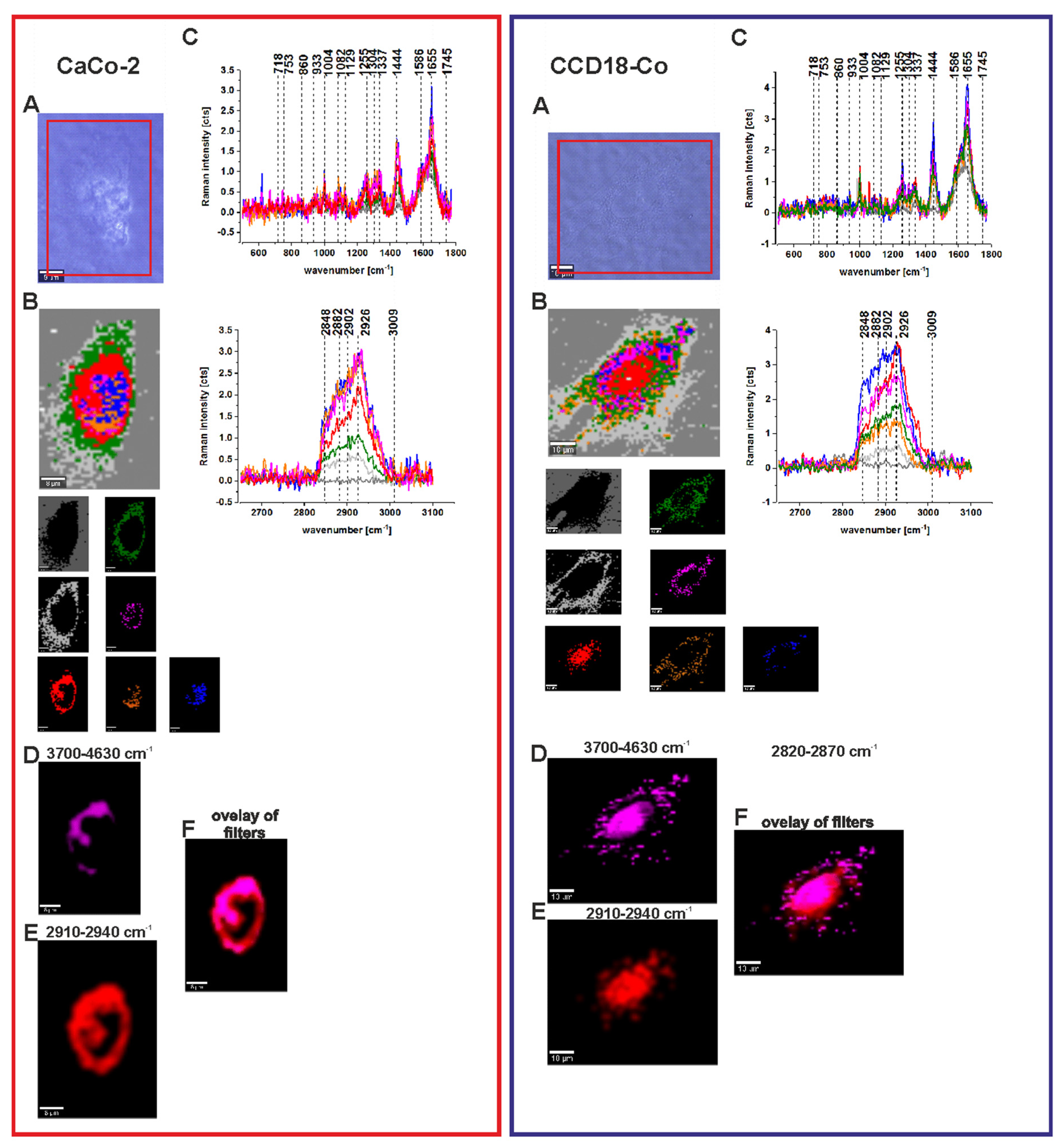
| Wavenumber [cm−1] | Tentative Assignments | Type of Human Colon Tissue |
|---|---|---|
| 749 | nucleic acids, DNA, tryptophan, and nucleoproteins | C ↑ |
| 829/849 | tyrosine | N ↑ |
| 858 | stretching mode of the phosphate group | N ↑ |
| 872/938 | stretching vibrations for the amino acids proline and valine and polysaccharides | N ↑ |
| 1004 | phenylalanine | C ↑ |
| 1085 | phosphodiester groups in nucleic acids | C ↑ |
| 1129 | saturated fatty acids | C ↑ |
| 1260 | Amide III (C–N stretch + N–H bend) | N ↑ |
| 1337 | CH3CH2 wagging vibrations of collagen | C ↑ |
| 1445 | lipids and proteins | N ↑ |
| 1557 | Amide II (N–H bend + C–N stretch) | C ↑ |
| 1585 | CN2 scissoring and NH2 rock of mitochondria and phosphorylated proteins | C ↑ |
| 1655 | Amide I (C=O stretch) | N ↑ |
| 1745 | C=O group of lipids and lipids esters. | N ↑ |
| 2848, 3009 | C–H bonds of lipids, glycogen, proteins, RNA, and DNA. | N ↑ |
| 2931 | Proteins | C ↑ |
| Sample | Time Constants |
|---|---|
| ZnPcS4 film on CaF2 | 3.97 ± 1.2 ps (28%), 32.3 ± 6.3 ps (44%), 190 ps ±46 (28%) |
| CaCo-2 cells incubated with ZnPcS4 | 4.4 ± 3.1 ps (7%), 65 ± 32 ps (28%), 424 ± 138 ps (65%) |
| CCD18-Co cells incubated with ZnPcS4 | 3.0 ± 2.5 ps (7%), 70 ± 32 ps (31%), 340 ± 89 ps (62%) |
| Cancerous colon tissue soaked with ZnPcS4 solution | 3.6 ± 0.25 ps (25%), 74.0 ± 9.0 ps (37%), 432.0 ± 98 ps (38%) |
| Normal colon tissue soaked with ZnPcS4 solution | 3.0 ± 0.25 ps (21%), 53.5 ± 5.7 ps (36%), 346.0 ± 38 ps (43%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brozek-Pluska, B.; Jarota, A.; Kania, R.; Abramczyk, H. Zinc Phthalocyanine Photochemistry by Raman Imaging, Fluorescence Spectroscopy and Femtosecond Spectroscopy in Normal and Cancerous Human Colon Tissues and Single Cells. Molecules 2020, 25, 2688. https://doi.org/10.3390/molecules25112688
Brozek-Pluska B, Jarota A, Kania R, Abramczyk H. Zinc Phthalocyanine Photochemistry by Raman Imaging, Fluorescence Spectroscopy and Femtosecond Spectroscopy in Normal and Cancerous Human Colon Tissues and Single Cells. Molecules. 2020; 25(11):2688. https://doi.org/10.3390/molecules25112688
Chicago/Turabian StyleBrozek-Pluska, Beata, Arkadiusz Jarota, Rafal Kania, and Halina Abramczyk. 2020. "Zinc Phthalocyanine Photochemistry by Raman Imaging, Fluorescence Spectroscopy and Femtosecond Spectroscopy in Normal and Cancerous Human Colon Tissues and Single Cells" Molecules 25, no. 11: 2688. https://doi.org/10.3390/molecules25112688
APA StyleBrozek-Pluska, B., Jarota, A., Kania, R., & Abramczyk, H. (2020). Zinc Phthalocyanine Photochemistry by Raman Imaging, Fluorescence Spectroscopy and Femtosecond Spectroscopy in Normal and Cancerous Human Colon Tissues and Single Cells. Molecules, 25(11), 2688. https://doi.org/10.3390/molecules25112688







