Xanthine Oxidase Inhibitory Activity, Chemical Composition, Antioxidant Properties and GC-MS Analysis of Keladi Candik (Alocasia longiloba Miq)
Abstract
1. Introduction
2. Results and Discussion
2.1. Qualitative Phytochemical Screening
2.2. Quantification of Total Phenolic and Flavonoid Content
2.3. DPPH and ABTS Free Radical Scavenging Properties of A. longiloba
2.4. Xanthine Oxidase Inhibitory Activity of A. longiloba Extracts
2.5. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
3. Materials and Methods
3.1. Chemicals and Equipment
3.2. Plant Material Collection
3.3. Preparation of Extracts
3.4. Qualitative Phytochemical Screening
3.5. Quantification of Total Phenolic and Total Flavonoid Content
3.6. Determination of Antioxidant Activity
3.6.1. DPPH (1,1-Diphenyl-2-picryl-hydrazyl) Assay
3.6.2. ABTS (2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonate) Radical Scavenging Assay
3.7. Xanthine Oxidase Inhibitory Activity In Vitro Assay
3.8. Gas Chromatography–Mass Spectrometry (GC–MS) Analysis
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Klemow, K.M.; Bartlow, A.; Crawford, J.; Kocher, N.; Shah, J.; Ritsick, M. Herbal Medicine: Biomolecular and Clinical Aspects; CRC Press: Boca Raton, FL, USA, 2011; pp. 211–228. [Google Scholar]
- Kostic, D.A.; Dimitrijevic, D.S.; Stojanovic, G.S.; Palic, I.R.; Dordevic, A.S.; Ickovski, J.D. Xanthine oxidase: Isolation, assays of activity, and inhibition. J. Chem. 2015, 8, 294858. [Google Scholar] [CrossRef]
- Mathur, R.; Velpandian, T. Medicinal plant-based health products: Where is the medicinal constituent? Indian J. Pharmacol. 2009, 41, 205. [Google Scholar] [CrossRef] [PubMed]
- Teke, G.N.; Kuete, V. Acute and subacute toxicities of African medicinal plants. In Toxicological Survey of African Medicinal Plants; Elsevier: Amsterdam, The Netherlands, 2014; pp. 63–98. [Google Scholar]
- Kabir, M.S.H.; Hossain, M.M.; Kabir, M.I.; Ahmad, S.; Chakrabarty, N.; Rahman, M.A.; Rahman, M.M. Antioxidant, antidiarrheal, hypoglycemic and thrombolytic activities of organic and aqueous extracts of Hopea odorata leaves and in silico PASS prediction of its isolated compounds. BMC Complem. Altern. Med. 2016, 16, 474. [Google Scholar] [CrossRef] [PubMed]
- Hafez, R.M.; Abdel-Rahman, T.M.; Naguib, R.M. Uric acid in plants and microorganisms: Biological applications and genetics—A review. J. Adv. Res. 2017, 8, 475–486. [Google Scholar] [CrossRef]
- Gaur, V.; Malik, P.; Paul, O. Prevalence of Hyperuricemia & relation of Serum uric Acid in Burn Patients amongst Different Gender and Age Groups. Int. J. Biochem. 2018, 1, 16–19. [Google Scholar]
- Mahmoudi Moghaddam, H.; Beitollahi, H.; Tajik, S.; Soltani, H. Fabrication of a nanostructure based electrochemical sensor for voltammetric determination of epinephrine, uric acid and folic acid. Electroanalysis 2015, 27, 2620–2628. [Google Scholar] [CrossRef]
- Aroor, A.R.; Jia, G.; Habibi, J.; Sun, Z.; Ramirez-Perez, F.I.; Brady, B.; Whaley-Connell, A.T. Uric acid promotes vascular stiffness, maladaptive inflammatory responses and proteinuria in western diet fed mice. Metabolism 2017, 74, 32–40. [Google Scholar] [CrossRef]
- Paul, B.J.; James, R. Gout: An Asia-pacific update. Int. J. Rheum. Dis. 2017, 20, 407–416. [Google Scholar] [CrossRef]
- Hille, R.; Massey, V. Tight binding inhibitors of xanthine oxidase. Pharmacol. Ther. 1981, 14, 249–263. [Google Scholar] [CrossRef]
- Skibo, E.B. Noncompetitive and irreversible inhibition of xanthine oxidase by benzimidazole analogs acting at the functional flavin adenine dinucleotide cofactor. Biochemistry 1986, 25, 4189–4194. [Google Scholar] [CrossRef]
- Santi, M.D.; Zunini, M.P.; Vera, B.; Bouzidi, C.; Dumontet, V.; Abin-Carriquiry, A.; Ortega, M.G. Xanthine oxidase inhibitory activity of natural and hemisynthetic flavonoids from Gardenia oudiepe (Rubiaceae) in vitro and molecular docking studies. Eur. J. Med. Chem. 2018, 143, 577–582. [Google Scholar] [CrossRef]
- Latif, M.A.; Zaki, M.Z.M.; Leng, T.M.; Rahman, N.H.A.; Arshad, S.A.; Hamid, A. Alocasia denudata Engler treatment enhance open wound healing activities in Wistar rat’s skin. J. Ethnopharmacol. 2015, 176, 258–267. [Google Scholar] [CrossRef]
- Hamzah, N.H.C.; Mohammed, A.; Sirajudeen, K.; Asari, M.A.; Hamzah, Z.; Shaik, I.K. Keladi candik (Alocasia longiloba Miq.) petiole extracts promote wound healing in a full thickness excision wound model in rats. Asian Pac. J. Trop. Biomed. 2019, 9, 140. [Google Scholar]
- Jing, L.; Ma, H.; Fan, P.; Gao, R.; Jia, Z. Antioxidant potential, total phenolic and total flavonoid contents of Rhododendron anthopogonoides and its protective effect on hypoxia-induced injury in PC12 cells. BMC Complem. Altern. Med. 2015, 15, 287. [Google Scholar] [CrossRef]
- Hudaib, M.M.; Tawaha, K.A.; Mohammad, M.K.; Assaf, A.M.; Issa, A.Y.; Alali, F.Q.; Bustanji, Y.K. Xanthine oxidase inhibitory activity of the methanolic extracts of selected Jordanian medicinal plants. Pharmacogn. Mag. 2011, 7, 320. [Google Scholar] [CrossRef]
- Isa, M.; Putri, S.S.; Ablat, A.; Mohamad, J. The antioxidant and xanthine oxidase inhibitory activity of Plumeria rubra flowers. Molecules 2018, 23, 400. [Google Scholar]
- Yao, Y.; Sang, W.; Zhou, M.; Ren, G. Phenolic composition and antioxidant activities of 11 celery cultivars. J. Food Sci. 2010, 75, C9–C13. [Google Scholar] [CrossRef]
- Rawat, S.; Bhatt, I.D.; Rawal, R.S. Total phenolic compounds and antioxidant potential of Hedychium spicatum Buch. Ham. ex D. Don in west Himalaya. India J. Food Compos. Anal. 2011, 24, 574–579. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Yang, K.; Zhou, Y.X.; Wang, C.F.; Du, S.S.; Deng, Z.W.; Liu, Q.Z.; Liu, Z.L. Toxicity of Rhododendron anthopogonoides essential oil and its constituent compounds towards Sitophilus zeamais. Molecules 2011, 16, 7320–7330. [Google Scholar] [CrossRef]
- Hyun, T.K.; Kim, H.C.; Kim, J.S. Antioxidant and antidiabetic activity of Thymus quinquecostatus Celak. Ind. Crops Prod. 2014, 52, 611–616. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Jayawardena, M.H.S.; De Alwis, N.M.W.; Hettigoda, V.; Fernando, D.J.S. A double blind randomised placebo controlled cross over study of a herbal preparation containing Salacia reticulata in the treatment of type 2 diabetes. J. Ethnopharmacol. 2005, 97, 215–218. [Google Scholar] [CrossRef]
- Dailey, A.; Vuong, Q.V. Effect of extraction solvents on recovery of bioactive compounds and antioxidant properties from macadamia (Macadamia tetraphylla) skin waste. Cogent Food Agric. 2015, 1, 1115646. [Google Scholar] [CrossRef]
- Yumita, A.; Suganda, A.G.; Sukandar, E.Y. Xanthine oxidase inhibitory activity of some Indonesian medicinal plants and active fraction of selected plants. Int. J. Pharm. Pharm. Sci. 2013, 5, 293–296. [Google Scholar]
- Alsultanee, I.R.; Ewadh, M.J.; Mohammed, M.F. Novel natural anti-gout medication extract from Momordica charantia. J. Nat. Sci. Res. 2014, 4, 16–23. [Google Scholar]
- Hameed, I.H.; Hussein, H.J.; Kareem, M.A.; Hamad, N.S. Identification of five newly described bioactive chemical compounds in methanolic extract of Mentha viridis by using gas chromatography-mass spectrometry (GC-MS). J. Pharmacogn. Phytother. 2015, 7, 107–125. [Google Scholar]
- Hadi, M.Y.; Mohammed, G.J.; Hameed, I.H. Analysis of bioactive chemical compounds of Nigella sativa using gas chromatography-mass spectrometry. J. Pharmacogn. Phytother. 2016, 8, 8–24. [Google Scholar]
- Alakurtti, S.; Makela, T.; Koskimies, S.; Yli-Kauhaluoma, J. Pharmacological properties of the ubiquitous natural product betulin. Eur. J. Pharm. Sci. 2006, 29, 1–13. [Google Scholar] [CrossRef]
- Davidson, R.N.; Den Boer, M.; Ritmeijer, K. Paromomycin. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 653–660. [Google Scholar] [CrossRef]
- Kadhim, M.J.; Sosa, A.A.; Hameed, I.H. Evaluation of anti-bacterial activity and bioactive chemical analysis of Ocimum basilicum using Fourier transform infrared (FT-IR) and gas chromatography-mass spectrometry (GC-MS) techniques. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 127–146. [Google Scholar]
- Salem, M.Z.M.; Elansary, H.O.; Elkelish, A.A.; Zeidler, A.; Ali, H.M.; Mervat, E.H.; Yessoufou, K. In vitro bioactivity and antimicrobial activity of Picea abies and Larix decidua wood and bark extracts. BioResources 2016, 11, 9421–9437. [Google Scholar] [CrossRef]
- Al-Marzoqi, A.H.; Al-Khafaji, N.M.S.; Hussein, H.J. In vitro Antibacterial Activity Assessment of the crude Phenolic, Alkaloid and Terpenoid compounds extracts of Lepidium sativum L. on Human Pathogenic Bacteria. Int. J. ChemTech Res. 2016, 9, 529–532. [Google Scholar]
- Ayoola, G.A.; Coker, H.A.; Adesegun, S.A.; Adepoju-Bello, A.A.; Obaweya, K.; Ezennia, E.C.; Atangbayila, T.O. Phytochemical screening and antioxidant activities of some selected medicinal plants used for malaria therapy in Southwestern Nigeria. Trop. J. Pharm. Res. 2008, 7, 1019–1024. [Google Scholar]
- Njoku, V.O.; Obi, C. Phytochemical constituents of some selected medicinal plants. Afr. J. Pure Appl. Chem. 2009, 3, 228–233. [Google Scholar] [CrossRef]
- Azmi, S.M.N.; Jamal, P.; Amid, A. Xanthine oxidase inhibitory activity from potential Malaysian medicinal plant as remedies for gout. Int. Food Res. J. 2012, 19, 159. [Google Scholar]
- Sermakkani, M.; Thangapandian, V. GC-MS analysis of Cassia italica leaf methanol extract. Asian J. Pharm. Clin. Res. 2012, 5, 90–94. [Google Scholar]
- Edeoga, H.O.; Okwu, D.E.; Mbaebie, B.O. Phytochemical constituents of some Nigerian medicinal plants. Afr. J. Biotechnol. 2005, 4, 685–688. [Google Scholar] [CrossRef]
Sample Availability: Samples of the Alocasia longiloba extracts are available from the authors. |
| Total Amount/Scavenging Activity | Extracts | |
|---|---|---|
| Petiole | Fruit | |
| Total Phenolic Content mg GAE/g | 288.14 ± 4.91 b | 512.84 ± 2.03 a |
| Total Flavonoids Content mg QCE/g | 453.18 ± 2.52 a | 438.18 ± 5.63 a |
| DPPH, IC50 (μg/mL) | 126.23 ± 0.52 a | 137.66 ± 0.09 a |
| ABTS, IC50 (μg/mL) | 88.30 ± 0.05 a | 83.40 ± 0.057 a |
| Concentration (μg/mL) | Percent of Inhibition (%) of Plant Extracts | |
|---|---|---|
| Petiole | Fruit | |
| 50 | 54.63 ± 1.44 | 50.33 ± 0.56 |
| 100 | 70.40 ± 0.05 | 61.44 ± 1.24 |
| 150 | 73.76 ± 0.95 | 65.53 ± 0.86 |
| 200 | 77.03 ± 0.95 | 70.40 ± 0.10 |
| 250 | 85.76 ± 1.08 | 75.96 ± 2.41 |
| IC50 (μg/mL) | 42.71 * | 51.32 * |
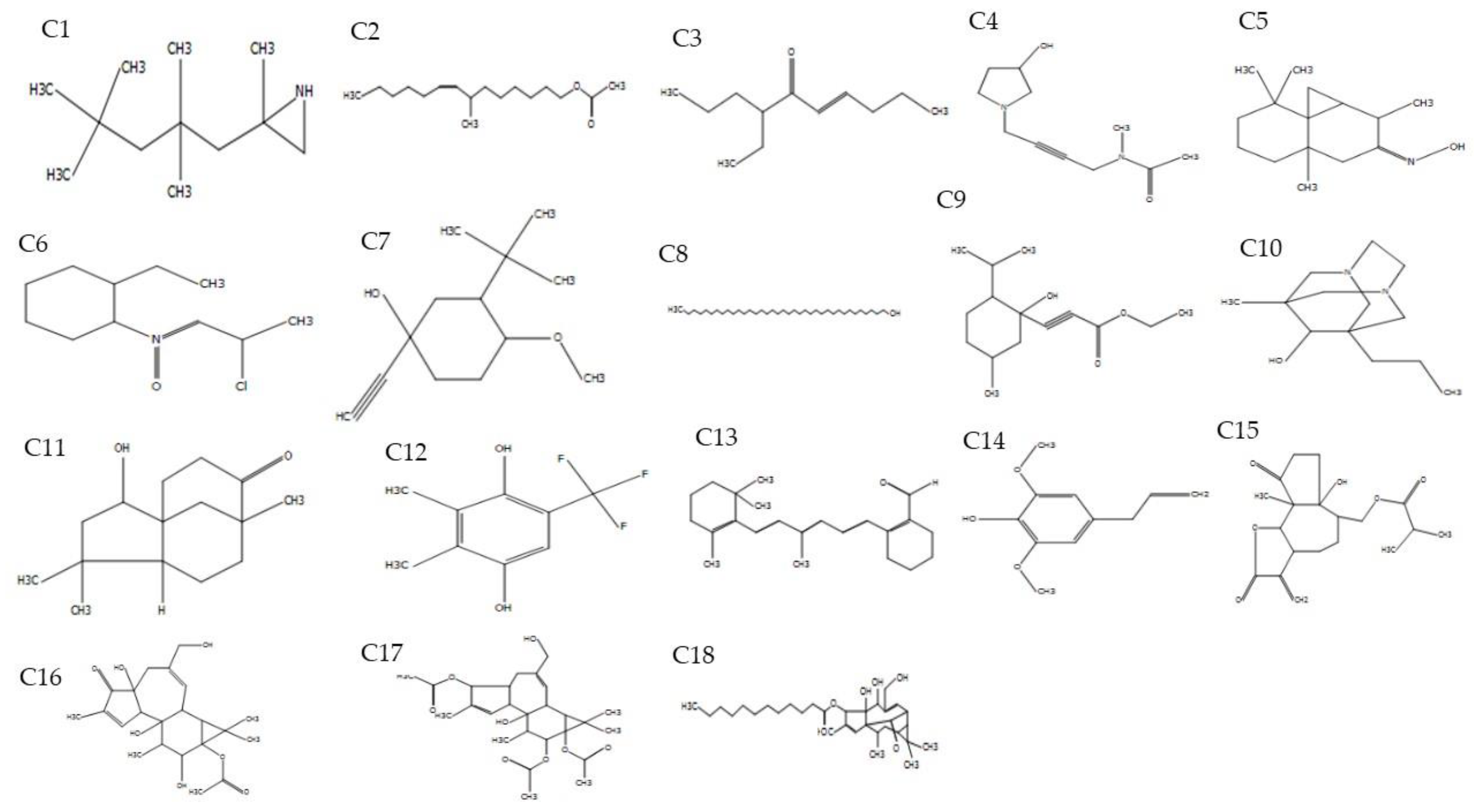
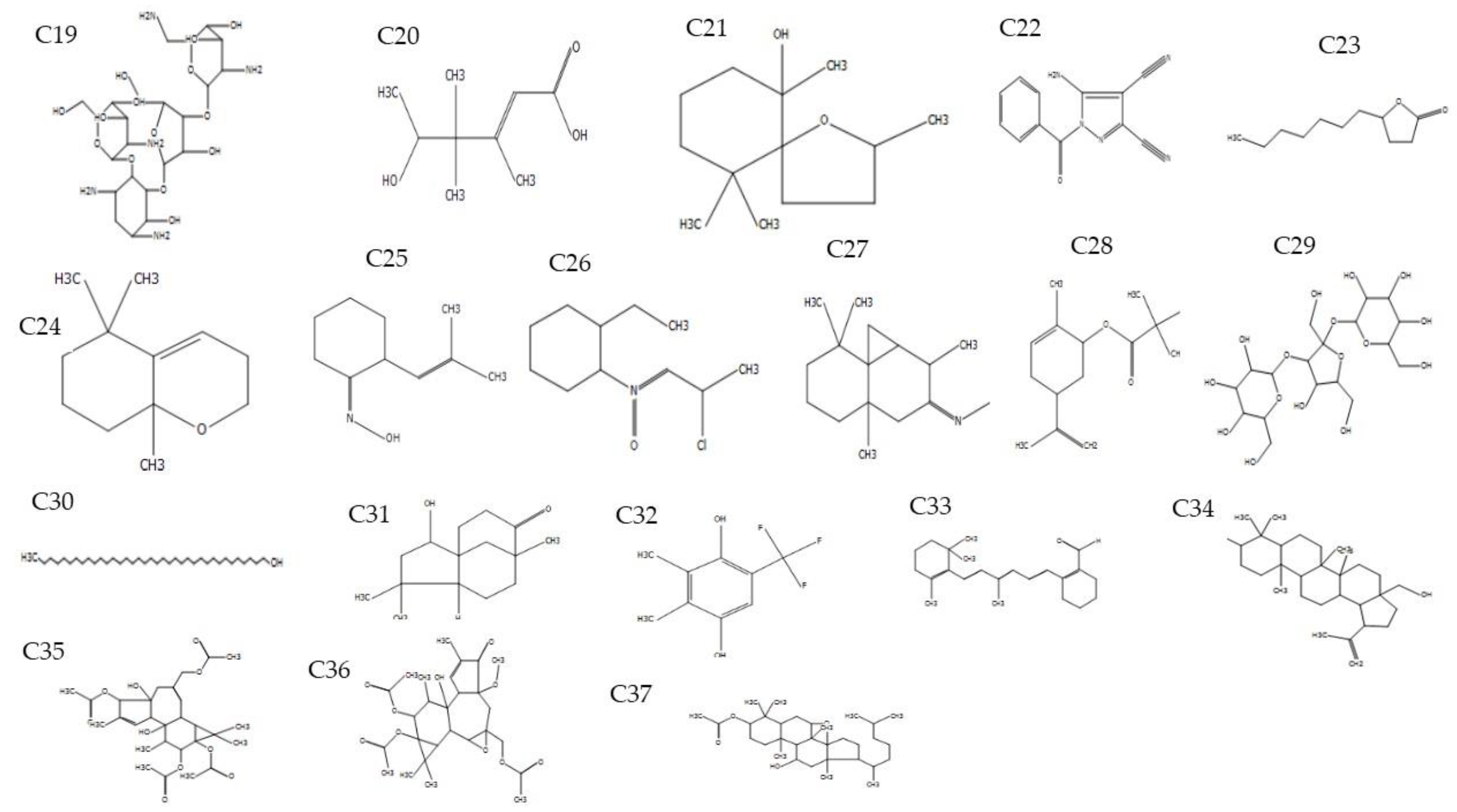
| Compound | Name of Compound | Plant Part | RT (min) | Peak Area % | Ion Mass (m/z) | M.F | M.W | Biological Activity |
|---|---|---|---|---|---|---|---|---|
| 1 | Aziridine,2-methyl-2-(2,2,4,4-tetramethylpentyl)- | Petiole | 3.52 | 3.25 | 124.0 | C12H25N | 183 | Not reported |
| 2 | 7-Methyl-Z-tetradecen-1-ol acetate | Petiole | 4.06 | 1.65 | 126.02 | C17H32O2 | 268 | Anti-cancer, anti-inflammatory, [29] |
| 3 | 7-Ethyl-4-decen-6-one | Petiole | 5.55 | 100 | 110.0 | C12H22O | 182 | Not reported |
| 4 | Acetamide,N-methyl-N-[4-(3-hydroxypyrrolidinyl)-2-butynyl]- | Petiole | 6.15 | 17.85 | 124.01 | C11H18N2O2 | 210 | Anti-fungal activity [33] |
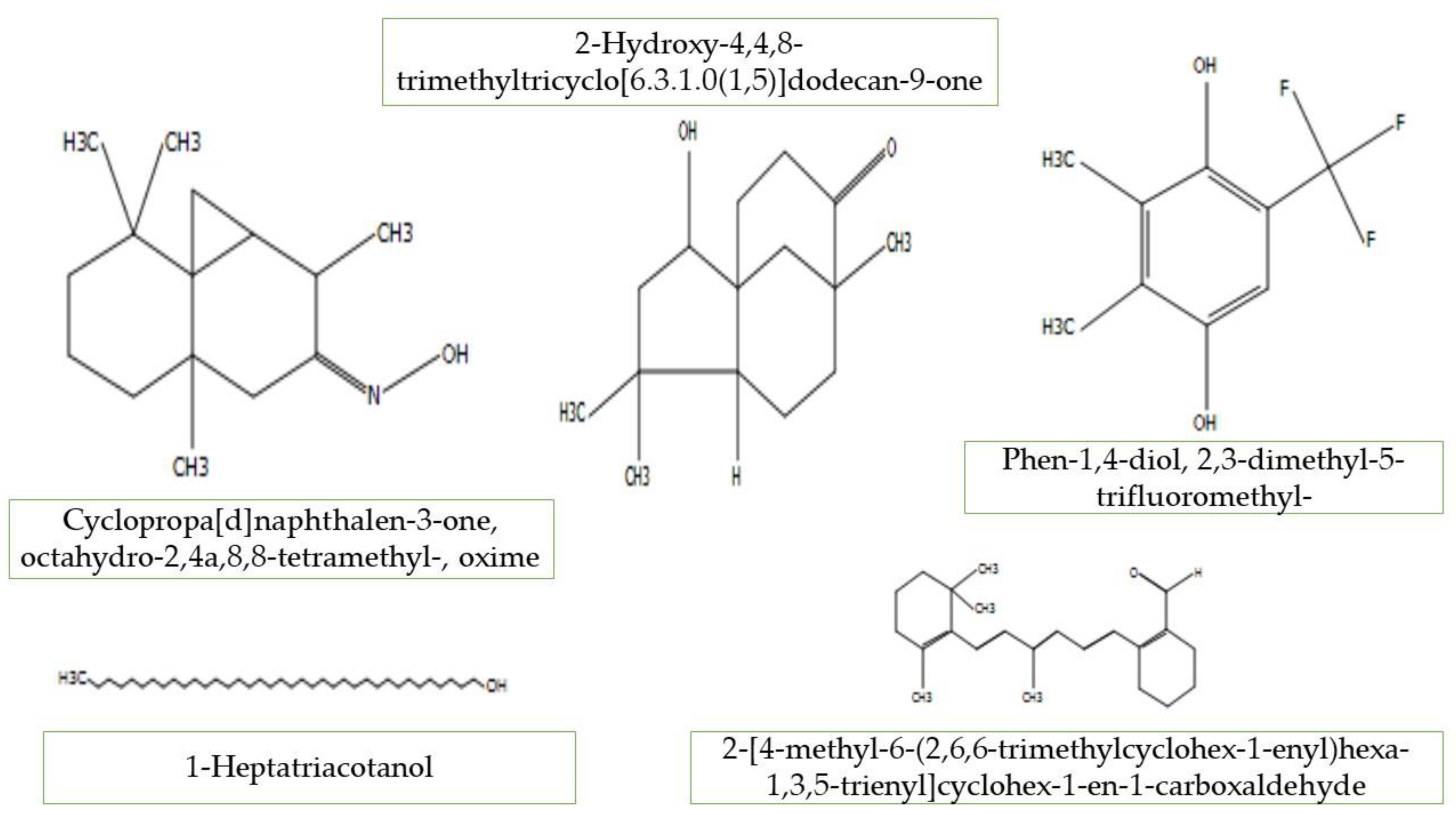
| 5 | Cyclopropa[d]naphthalen-3-one,octahydro-2,4a,8,8-tetramethyl-,oxime | Petiole | 6.67 | 33.18 | 124.02 | C15H25NO | 235 | Not reported |
| 6 | 2-Ethylcyclohexylamine,N-(2-chloropropylidene)-,N-oxide | Petiole | 7.16 | 17.28 | 154.08 | C11H20ClNO | 217 | Not reported |
| 7 | 1-Ethynyl-3,trans(1,1-dimethylethyl)-4,cis-methoxycyclohexan-1-ol | Petiole | 7.50 | 5.91 | 97.0 | C13H22O2 | 210 | Anti-Candida, anti-inflammatory [29] |
| 8 | 1-Heptatriacotanol | Petiole | 7.76 | 8.14 | 123.02 | C37H76O | 536 | Antioxidant, anticancer and, anti inflammatory [30] |
| 9 | Propiolic acid, 3-(1-hydroxy-2-isopropyl-5-methylcyclohexyl)-, ethyl ester | Petiole | 9.12 | 4.42 | 191.11 | C15H24O3 | 252 | Antiangiogenic activity [30] |
| 10 | 1-Methyl-8-propyl-3,6-diazahomoadamantan-9-ol | Petiole | 9.30 | 5.19 | 137.04 | C13H24N2O | 224 | Not reported |
| 11 | 2-Hydroxy-4,4,8-trimethyltricyclo[6.3.1.0(1,5)]dodecan-9-one | Petiole | 9.79 | 8.85 | 180.10 | C15H24O2 | 236 | Not reported |
| 12 | Phen-1,4-diol, 2,3-dimethyl-5-trifluoromethyl- | Petiole | 10.12 | 5.22 | 149.0 | C9H9F3O2 | 206 | Antioxidant, antithrombotic and anti-tuberculosis [29] |
| 13 | 2-[4-methyl-6-(2,6,6-trimethylcyclohex-1-enyl)hexa-1,3,5-trienyl]cyclohex-1-en-1-carboxaldehyde | Petiole | 11.15 | 4.09 | 73.0 | C23H32O | 324 | Not reported |
| 14 | Phenol, 2,6-dimethoxy-4-(2-propenyl)- | Petiole | 11.36 | 4.45 | 194.10 | C11H14O3 | 194 | Anti-fungal and Anti-helminthic |
| 15 | Propanoic acid, 2-methyl-, (dodecahydro-6a-hydroxy-9a-methyl-3-methylene-2,9-dioxoazuleno[4,5-b]furan-6-yl)methyl ester, | Petiole | 12.57 | 6.01 | 135.04 | C19H26O6 | 350 | Anti-biotic |
| 16 | 5H-Cyclopropa[3,4]benz[1,2-e]azulen-5-one, | Petiole | 17.63 | 1.69 | 81.04 | C28H36O11 | 548 | Not reported [29] |
| 17 | 1H-Cyclopropa[3,4]benz[1,2-e]azulene-5,7b,9,9a-tetrol, 1a,1b,4,4a,5,7a,8,9-octahydro-3-(hydroxymethyl)-1,1,6,8-tetramethyl-, | Petiole | 18.36 | 4.51 | 91.08 | C26H36O8 | 476 | Not reported |
| 18 | Dodecanoic acid, 1a,2,5,5a,6,9,10,10a-octahydro-5,5a-dihydroxy-4-(hydroxymethyl)-1,1,7,9-tetramethyl-11-oxo-1H-2,8a-methanocyclopenta | Petiole | 19.65 | 1.95 | 105.06 | C32H50O6 | 530 | Flavor [34] |
| 19 | Paromomycin | Fruit | 3.13 | 3.31 | 112.02 | C23H45N5O14 | 615 | Antibiotic [32] |
| 20 | 2-Hexenoic acid, 5-hydroxy-3,4,4-trimethyl-, (E)- | Fruit | 3.52 | 7.91 | 128.0 | C9H16O3 | 172 | Not reported |
| 21 | 2,6,10,10-Tetramethyl-1-oxaspiro[4.5] decan-6-ol | Fruit | 4.21 | 3.18 | 85.0 | C13H24O2 | 212 | Not reported |
| 22 | 5-Amino-1-benzoyl-1H-pyrazole-3,4-dicarbonitrile | Fruit | 4.97 | 3.18 | 122.0 | C12H7N5O | 237 | Not reported |
| 23 | 2(3H)-Furanone, 5-heptyldihydro | Fruit | 5.16 | 1.8 | 85.0 | C11H20O2 | 184 | Not reported |
| 24 | 5,5,8a-Trimethyl-3,5,6,7,8,8a-hexahydro-2H-chromene | Fruit | 6.16 | 5.18 | 124.03 | C12H20O | 180 | Not reported |
| 25 | 2-(2-Methyl-propenyl)-cyclohexanone oxime | Fruit | 6.67 | 2.85 | 124.02 | C10H17NO | 167 | Not reported |
| 26 | 2-Ethylcyclohexylamine, N-(2-chloropropylidene)-, N-oxide | Fruit | 7.00 | 1.21 | 154.08 | Not found | Not reported | |
| 27 | Cyclopropa[d]naphthalen-3-one, octahydro-2,4a,8,8-tetramethyl-, oxime | Fruit | 7.75 | 4.34 | 142.01 | C15H25NO | 235 | Not reported |
| 28 | Limonen-6-ol, pivalate | Fruit | 8.87 | 11.42 | 133.02 | C15H24O2 | 236 | Antioxidant and anti-inflammatory [30] |
| 29 | α-DGlucopyranoside,O-α-d-glucopyranosyl-(1.fwdarw.3)-β-d-fructofuranosyl | Fruit | 9.15 | 19.39 | 137.06 | C18H32O16 | 504 | Anticarcinogenic, and anti-microbial [30] |
| 30 | 1-Heptatriacotanol | Fruit | 9.32 | 1.68 | 163.08 | C37H76O | 536 | Antioxidant, anticancer and inflammatory [30] |
| 31 | 2-Hydroxy-4,4,8-trimethyltricyclo[6.3.1.0(1,5)]dodecan-9-one | Fruit | 9.80 | 3.28 | 180.10 | C15H24O2 | 236 | Not reported |
| 32 | Phen-1,4-diol, 2,3-dimethyl-5-trifluoromethyl- | Fruit | 10.12 | 1.95 | 149.0 | C9H9F3O2 | 206 | Antimicrobial activity [35] |
| 33 | 2-[4-methyl-6-(2,6,6-trimethylcyclohex-1-enyl)hexa-1,3,5-trienyl]cyclohex-1-en-1-carboxaldehyde | Fruit | 12.66 | 3.99 | 123.10 | C23H32O | 324 | Antimicrobials and antiviral [35] |
| 34 | Betulin | Fruit | 14.77 | 1.29 | 267.10 | C30H50O2 | 442 | Anti-inflammatory, and cytotoxicity [31] |
| 35 | 1H-Cyclopropa[3,4]benz[1,2-e]azulene-4a,5,7b,9,9a(1aH)-pentol, 3-[(acetyloxy)methyl]-1b,4,5,7a,8,9-hexahydro- | Fruit | 15.63 | 1.55 | 69.10 | C28H38O10 | 534 | Not reported |
| 36 | 4H-Cyclopropa[5′,6′]benz[1′,2′:7,8]azuleno[5,6]oxiren-4-one, 8,8a-bis(acetyloxy)-2a-[(acetyloxy)methyl]-1,1a,1b,1c,2a,3,3a,6a | Fruit | 19.04 | 1.23 | 69.10 | C27H36O10 | 520 | Not reported |
| 37 | 7,8-Epoxylanostan-11-ol, 3-acetoxy- | Fruit | 20.37 | 3.66 | 139.10 | C32H54O4 | 502 | Not reported |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdulhafiz, F.; Mohammed, A.; Kayat, F.; Bhaskar, M.; Hamzah, Z.; Podapati, S.K.; Reddy, L.V. Xanthine Oxidase Inhibitory Activity, Chemical Composition, Antioxidant Properties and GC-MS Analysis of Keladi Candik (Alocasia longiloba Miq). Molecules 2020, 25, 2658. https://doi.org/10.3390/molecules25112658
Abdulhafiz F, Mohammed A, Kayat F, Bhaskar M, Hamzah Z, Podapati SK, Reddy LV. Xanthine Oxidase Inhibitory Activity, Chemical Composition, Antioxidant Properties and GC-MS Analysis of Keladi Candik (Alocasia longiloba Miq). Molecules. 2020; 25(11):2658. https://doi.org/10.3390/molecules25112658
Chicago/Turabian StyleAbdulhafiz, Ferid, Arifullah Mohammed, Fatimah Kayat, Matcha Bhaskar, Zulhazman Hamzah, Sanjay Kumar Podapati, and Lebaka Veeranjaneya Reddy. 2020. "Xanthine Oxidase Inhibitory Activity, Chemical Composition, Antioxidant Properties and GC-MS Analysis of Keladi Candik (Alocasia longiloba Miq)" Molecules 25, no. 11: 2658. https://doi.org/10.3390/molecules25112658
APA StyleAbdulhafiz, F., Mohammed, A., Kayat, F., Bhaskar, M., Hamzah, Z., Podapati, S. K., & Reddy, L. V. (2020). Xanthine Oxidase Inhibitory Activity, Chemical Composition, Antioxidant Properties and GC-MS Analysis of Keladi Candik (Alocasia longiloba Miq). Molecules, 25(11), 2658. https://doi.org/10.3390/molecules25112658






