Preparation, Characterization, Morphological and Particle Properties of Crystallized Palm-Based Methyl Ester Sulphonates (MES) Powder
Abstract
1. Introduction
2. Results and Discussion
2.1. Crystallized MES Powder
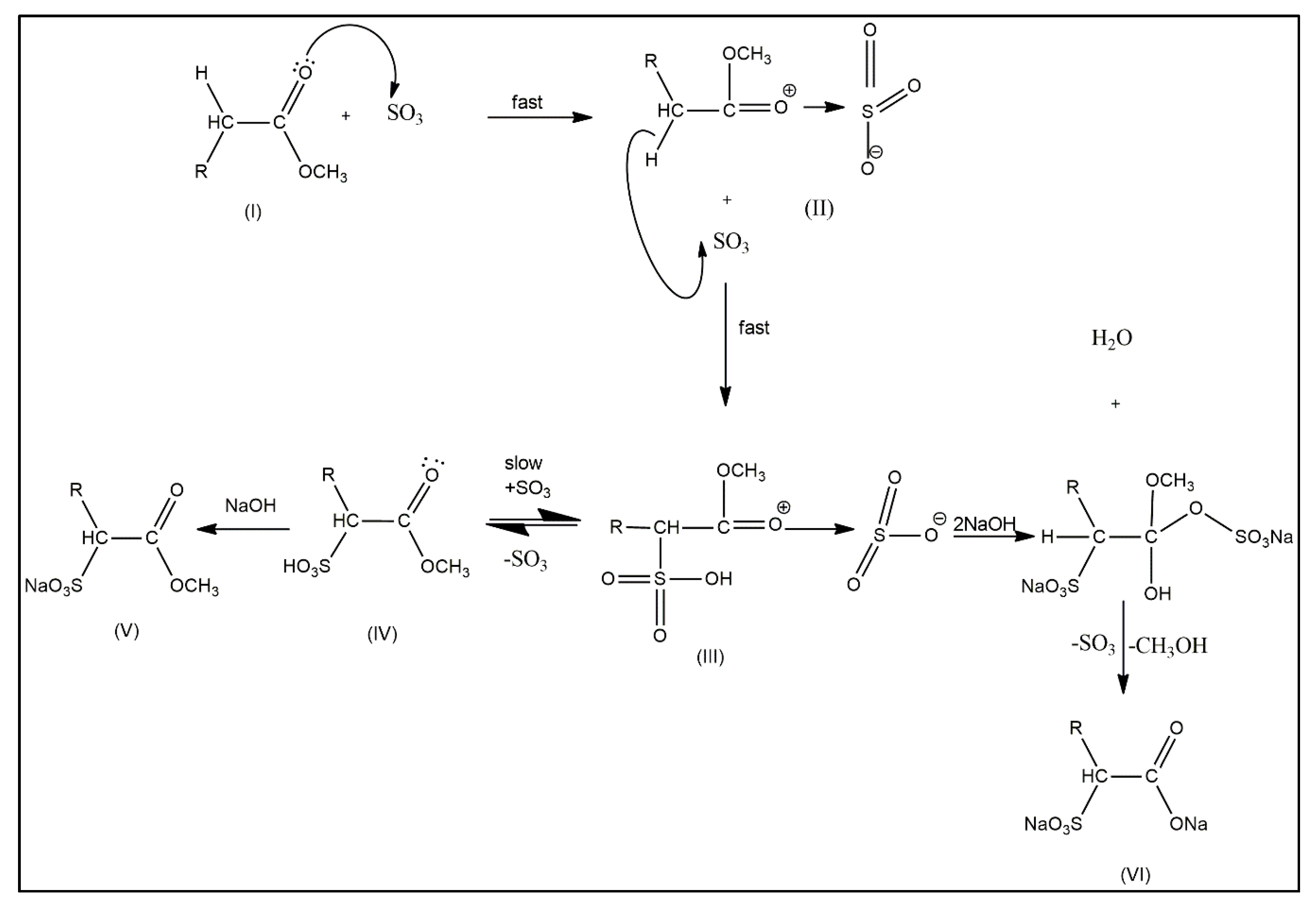
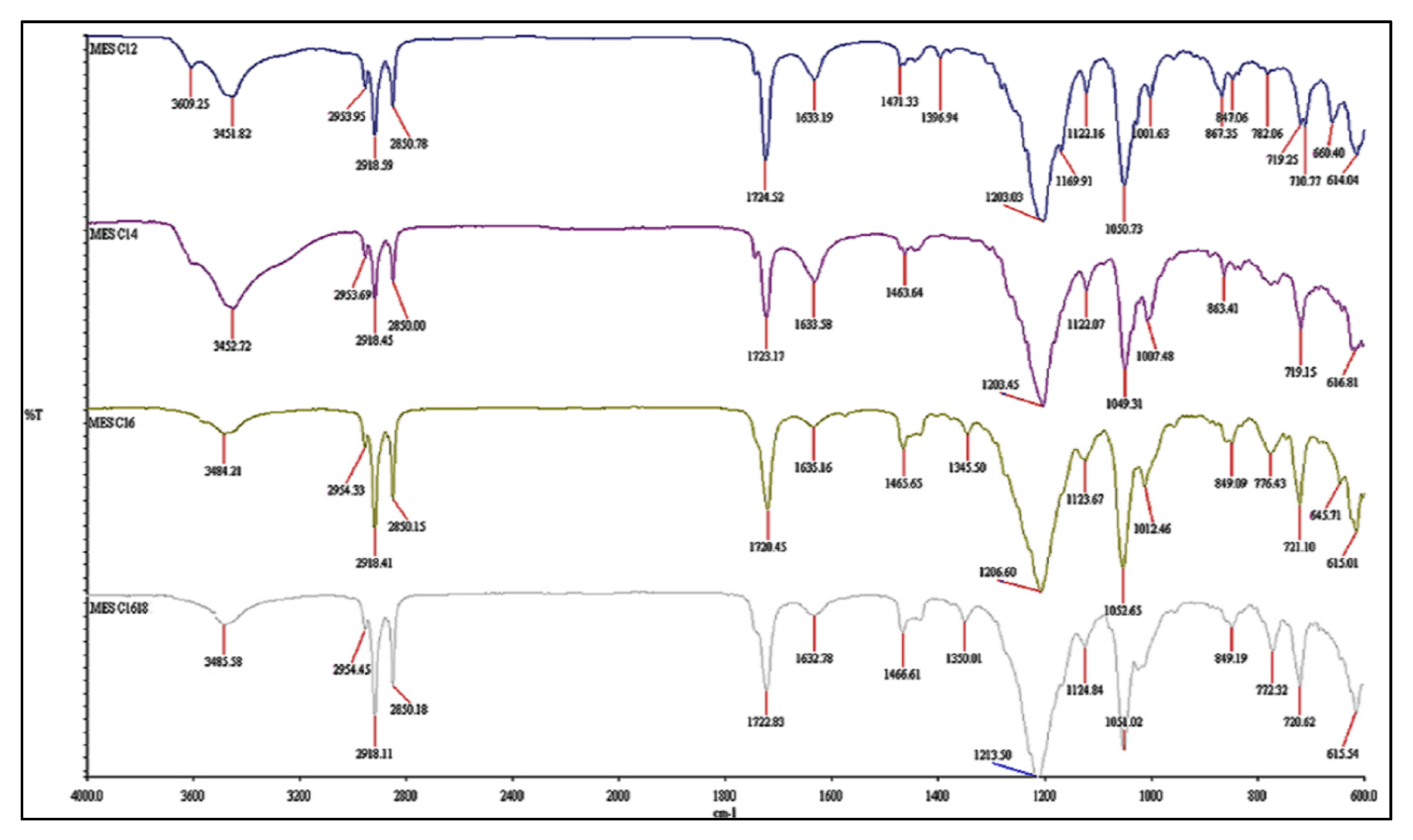
2.2. Structural Conformation of Crystallized MES Powder
2.3. Properties of Crystallized MES Powder
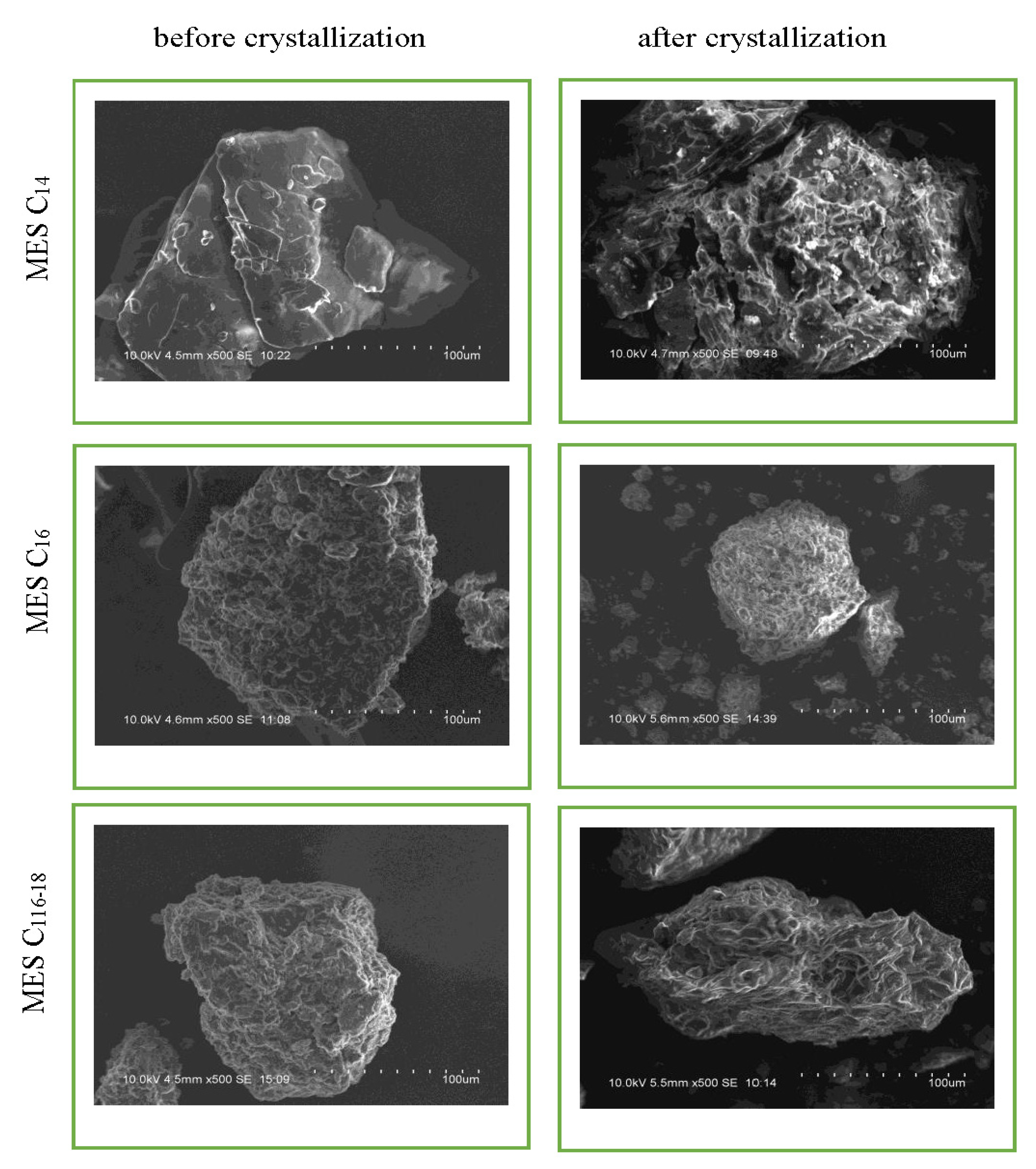
2.3.1. Surface Morphology
2.3.2. Particle Size (Dynamic Light Scattering)
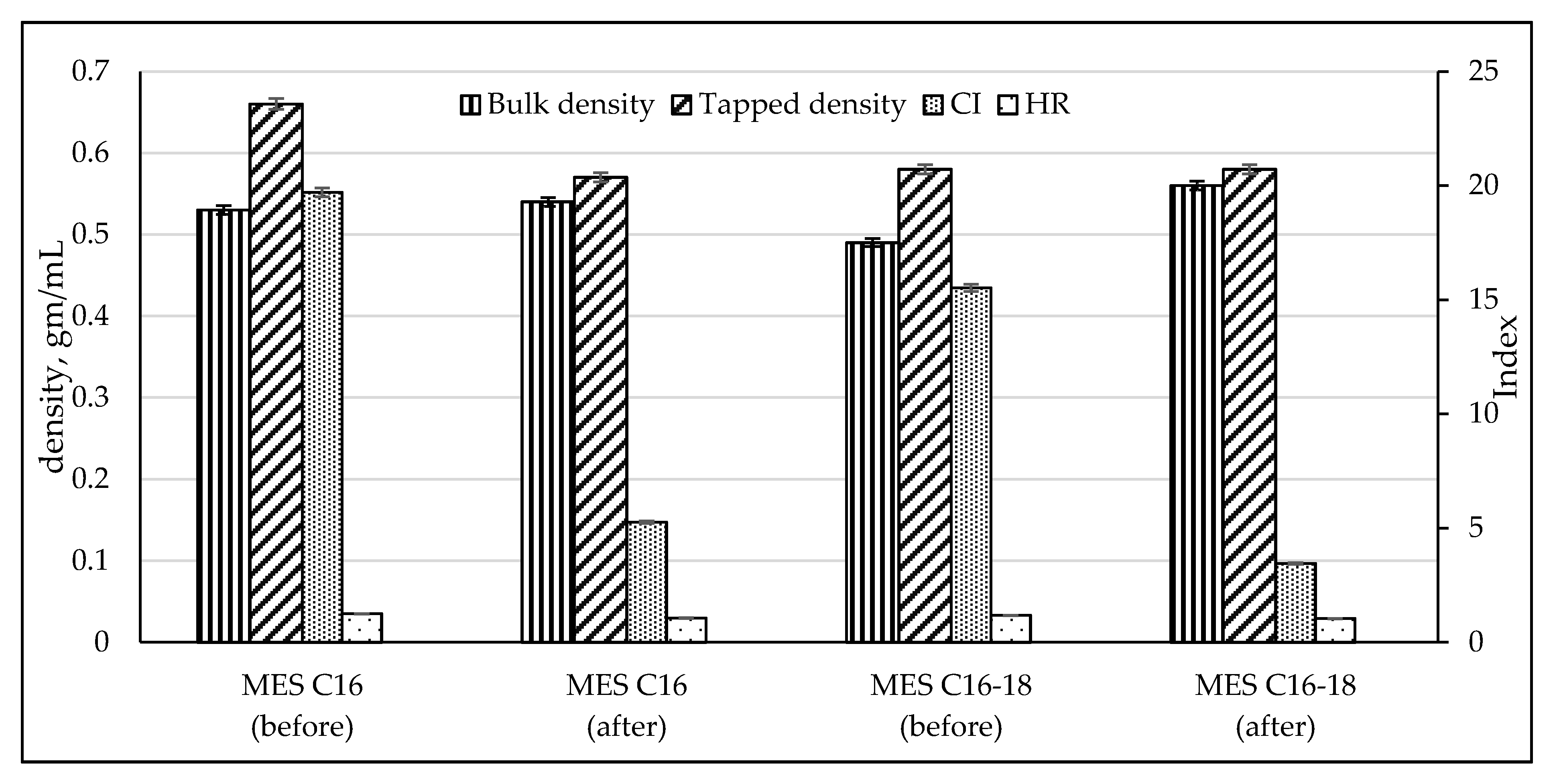
2.3.3. Flow Behavior
2.3.4. Crystallinity
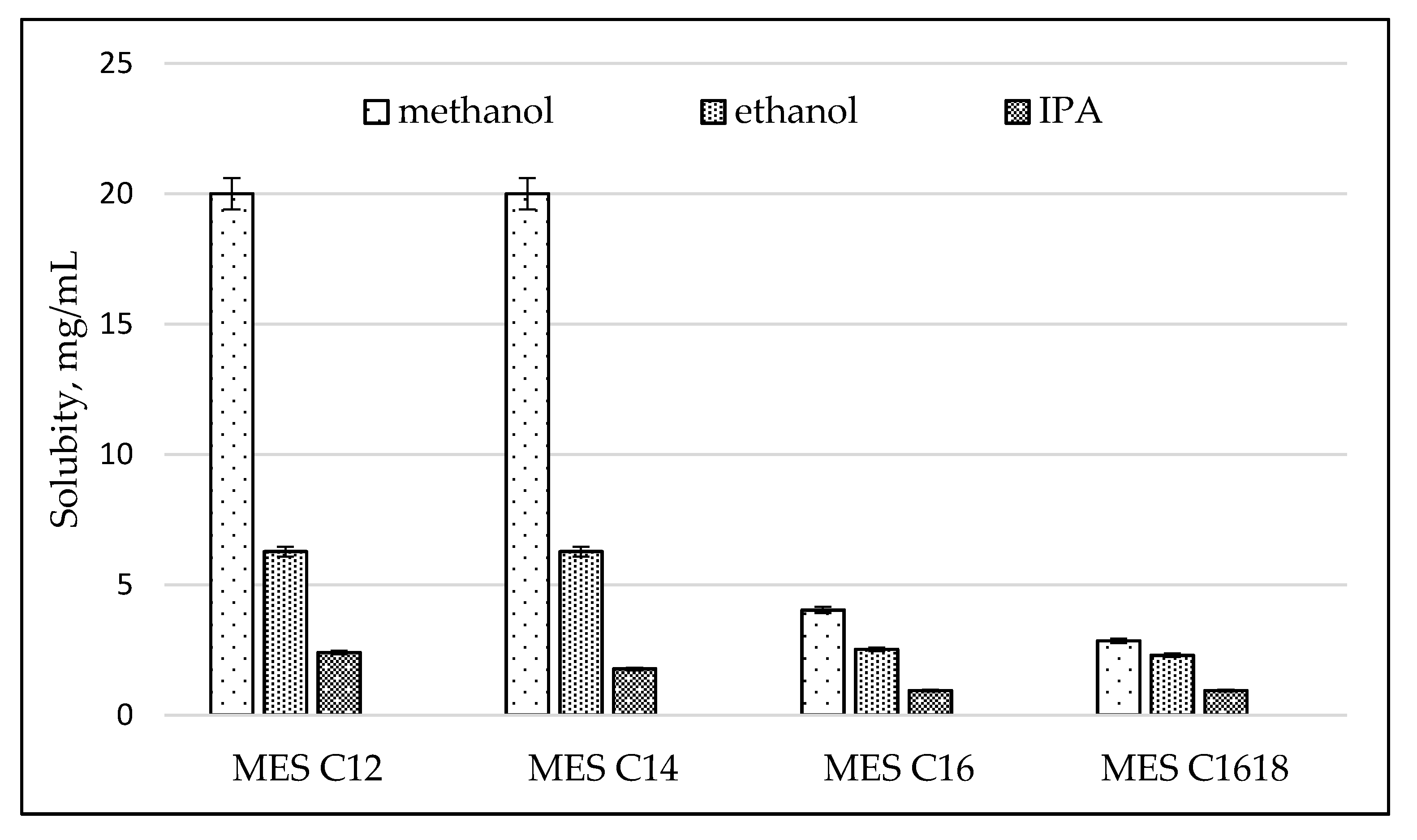
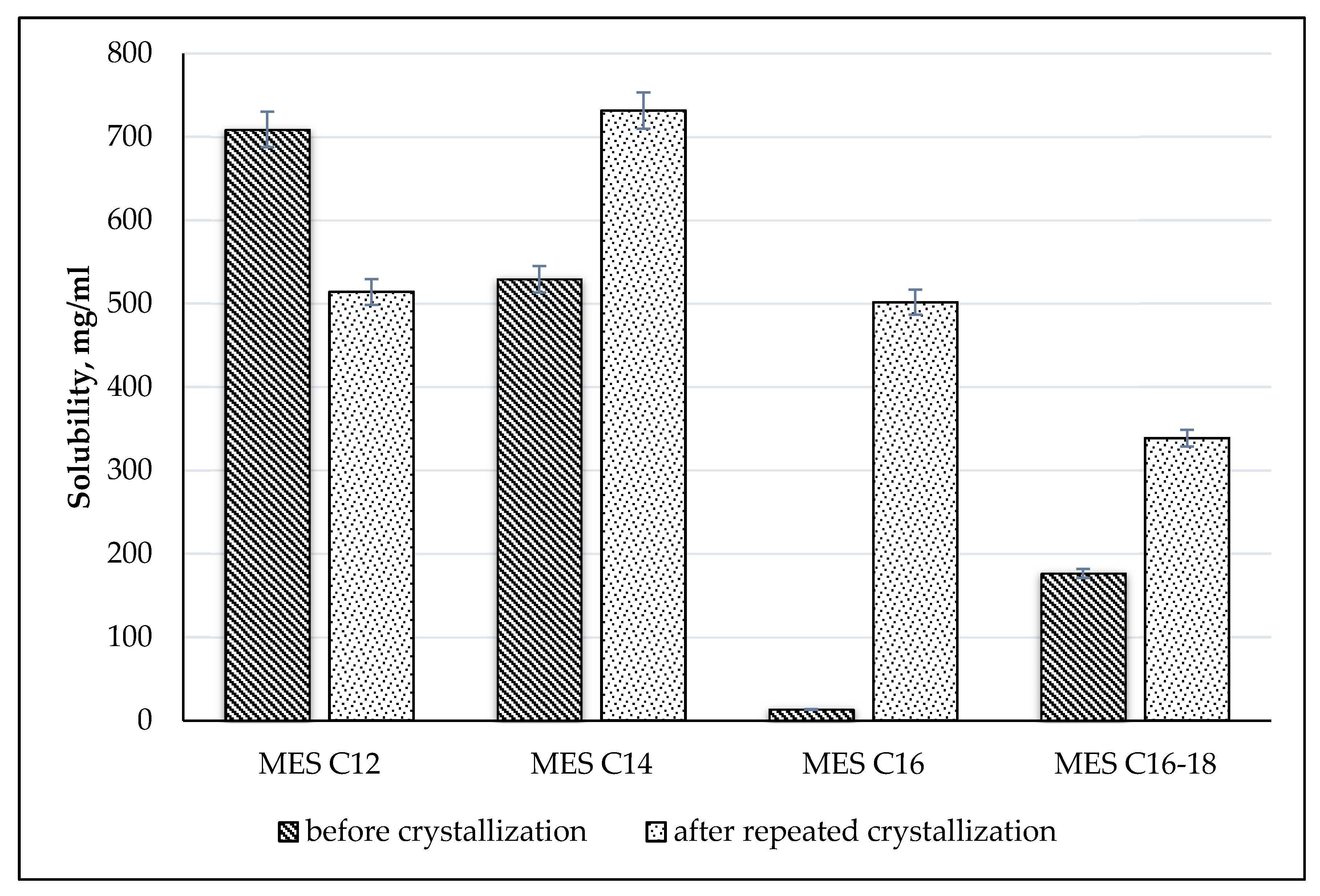
2.3.5. Solubility
2.3.6. Melting Point
3. Materials and Methods
3.1. Materials
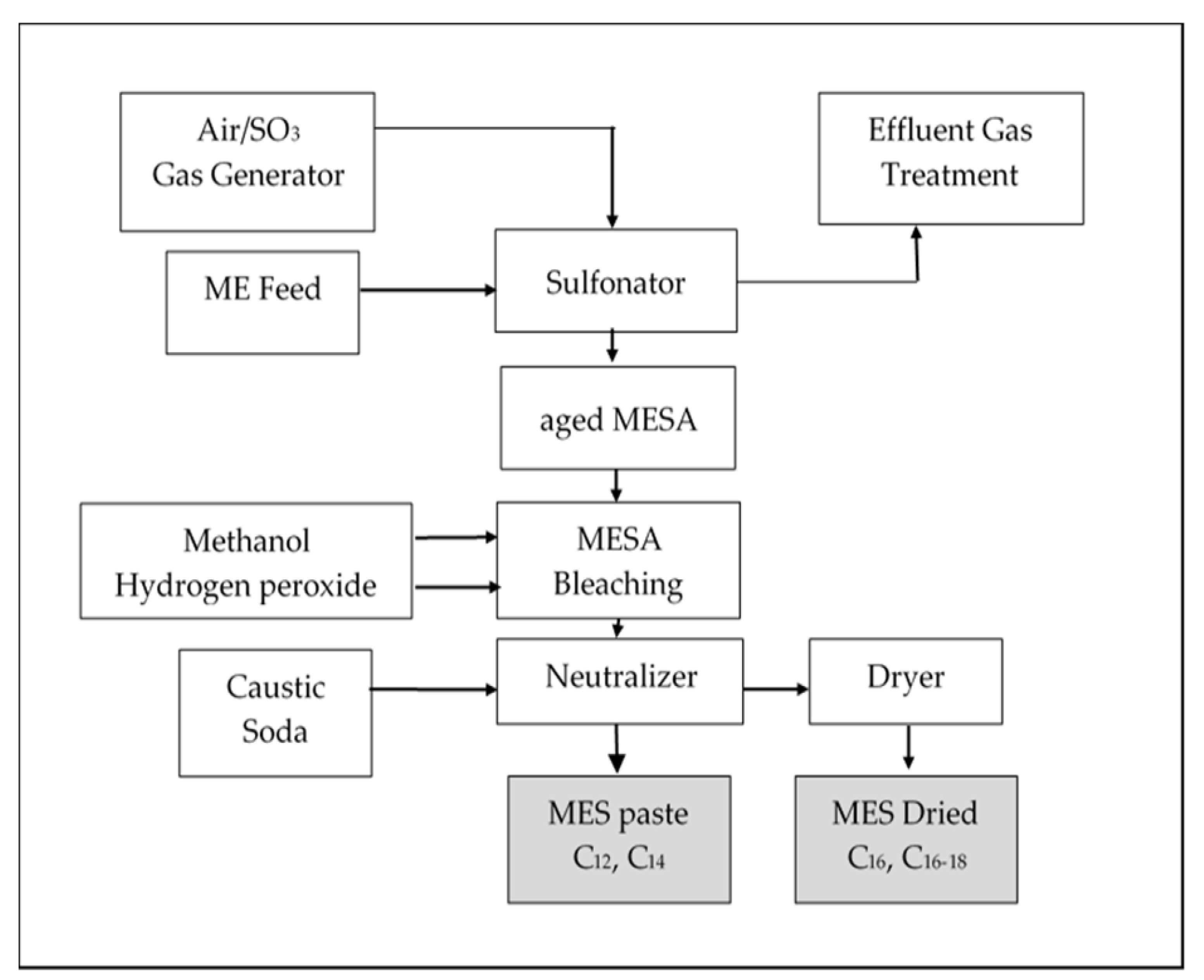
3.2. Preparation of Crystallized MES Powder
3.3. Analyses
3.3.1. MES Specifications
3.3.2. Active Content
3.3.3. Moisture Content
3.3.4. Fourier Transform—Infrared Analysis
3.3.5. Nuclear Magnetic Resonance Analysis (NMR)
3.3.6. Scanning Electron Microscopy Analysis
3.3.7. Particle Size Distribution Analysis
3.3.8. Flow behavior
3.3.9. X-ray Diffraction
3.3.10. Melting Point
4. Conclusions
5. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kushairi, A.; Loh, S.K.; Azman, I.; Hishamuddin, E.; Ong-Abdullah, M.; Izuddin, Z. Oil palm economic performance in Malaysia and R&D progress in 2017. J. Oil Palm Res. 2018, 30, 163–195. [Google Scholar]
- Maurad, Z.A.; Idris, Z.; Ghazali, R. Performance of Palm-Based C16/18 Methyl Ester Sulphonate (MES) in Liquid Detergent Formulation. J. OLEO Sci. 2017, 66, 677–687. [Google Scholar] [CrossRef]
- Market, A. Global Industry Analysis, Size, Share, Growth, Trends and Forecast 2016–2024. 2018. Available online: https://www.transparencymarketresearch.com/logistics-market.html (accessed on 20 February 2020).
- Foster, N.C.; MacArthur, B.W.; Sheats, W.B.; Shea, M.C.; Trivedi, S.N. Production of Methyl Ester Sulfonates. Handbook of Detergents, Part F: Production; CRC Press: Boca Raton, FL, USA, 2008; Volume 142, p. 201. [Google Scholar]
- Kapur, B.; Solomon, J.; Bluestein, B. Summary of the technology for the manufacture of higher alpha-sulfo fatty acid esters. J. Am. Oil Chem. Soc. 1978, 55, 549–557. [Google Scholar] [CrossRef]
- Ortega, J.A.T.; Medina, G.M.; Palacios, O.Y.S.; Castellanos, F.J.S. Mathematical Model of a Falling film Reactor for Methyl Ester Sulfonation. Chem. Prod. Process Modeling 2009, 4. [Google Scholar] [CrossRef]
- Roberts, D.; Giusti, L.; Forcella, A. Chemistry of methyl ester sulfonates. Biorenewable Resour. 2008, 5, 2–19. [Google Scholar]
- Afida, S.; Razmah, G.; Zulina, A. Biodegradation of Various Homologues of Palm-based Methyl Ester Sulphonates (MES). Sains Malays. 2016, 45, 949–954. [Google Scholar]
- Kang, Y.; Ahmad Zahariah, I. Palm-based surfactants synergy in soap applications. In Proceedings of the 2001 PIPOC International Palm Oil Congress (Oleochemicals), Kuala Lumpur, Malaysia, 20–23 August 2001. [Google Scholar]
- Pletnev, M. Vegetable-Derived Surfactants as a Reply to the Natural Trend in the Household and Personal Care. SOFW J. 2004, 130, 34–43. [Google Scholar]
- Zolkarnain, N.; Maurad, Z.A.; Razmah, G.; Hazimah, A. Environmental Performance of Palm-Based Methyl Ester Sulphonates Production Using Life Cycle Approach. J. Oil Palm Res. 2016, 28, 104–113. [Google Scholar] [CrossRef][Green Version]
- Maurad, Z.A.; Ghazali, R.; Siwayanan, P.; Ismail, Z.; Ahmad, S. Alpha-sulfonated methyl ester as an active ingredient in palm-based powder detergents. J. Surfactants Deterg. 2006, 9, 161–167. [Google Scholar] [CrossRef]
- Fatty Methyl Ester Sulfonate (FMES) Market Analysis By End-Use (Personal Care, Detergents), By Region (North America, Europe, Asia Pacific, Central & South America, Middle East & Africa), Competitive Landscape, And Segment Forecasts, 2018–2025; Grand View Research: San Francisco, CA, USA, 2017.
- Yusof, N.Z.; Azizul Hasan, Z.A.; Abd Maurad, Z.; Idris, Z. Eye irritation potential: Palm-based methyl ester sulphonates. Cutan. Ocul. Toxicol. 2018, 37, 103–111. [Google Scholar] [CrossRef]
- Dreger, E.; Klein, G.; Miles, G.; Shedlovsky, L.; Ross, J. Sodium Alcohol Sulfates. Properties Involving Surface Activity. Ind. Eng. Chem. 1944, 36, 610–617. [Google Scholar] [CrossRef]
- Lim, W.H.; Ramle, R.A. The Behavior of Methyl Esters Sulphonate at the Water–Oil Interface: Straight-Chained Methyl Ester from Lauryl to Stearyl as an Oil Phase. J. Dispers. Sci. Technol. 2009, 30, 131–136. [Google Scholar] [CrossRef]
- MacArthur, B.W.; Brooks, B.; Sheats, W.B.; Foster, N.C. Meeting the challenge of methylester sulfonation. In Proceedings of the 1999 PORIM International Palm Oil Congress: Emerging technologies and Opportunities in the Next Millennium, Kuala Lumpur, Malaysia, 1–6 February 1999; pp. 229–250. [Google Scholar]
- Demirbaş, A. Bioethanol from cellulosic materials: A renewable motor fuel from biomass. Energy Sources 2005, 27, 327–337. [Google Scholar] [CrossRef]
- Li, L.; Zhu, Y.-C. Research and application progress of fatty acid methyl ester sulfonates. Deterg. Cosmet. J. 2012, 4. [Google Scholar]
- Hongping, H.; Ray, F.L.; Jianxi, Z. Infrared study of HDTMA+ intercalated montmorillonite. Spectrochimica Acta Part A Mol. Biomol. Spectrosc. 2020, 60, 2853–2859. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Bassler, G.C. Spectrometric identification of organic compounds. J. Chem. Educ. 1962, 39, 546. [Google Scholar] [CrossRef]
- Elraies, K.A.; Tan, I.; Awang, M.; Saaid, I. The synthesis and performance of sodium methyl ester sulfonate for enhanced oil recovery. Pet. Sci. Technol. 2010, 28, 1799–1806. [Google Scholar] [CrossRef]
- Jin, Y.; Tian, S.; Guo, J.; Ren, X.; Li, X.; Gao, S. Synthesis, characterization and exploratory application of anionic surfactant fatty acid methyl ester sulfonate from waste cooking oil. J. Surfactants Deterg. 2016, 19, 467–475. [Google Scholar] [CrossRef]
- Babu, K.; Maurya, N.; Mandal, A.; Saxena, V. Synthesis and characterization of sodium methyl ester sulfonate for chemically-enhanced oil recovery. Braz. J. Chem. Eng. 2015, 32, 795–803. [Google Scholar] [CrossRef]
- Kramer, H.; van Rosmalen, G. Crystallization; Academic Press: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Brunsteiner, M.; Jones, A.G.; Pratola, F.; Price, S.L.; Simons, S.J. Toward a molecular understanding of crystal agglomeration. Cryst. Growth Des. 2005, 5, 3–16. [Google Scholar] [CrossRef]
- Guo, A.; Beddow, J.; Vetter, A. A simple relationship between particle shape effects and density, flow rate and Hausner ratio. Powder Technol. 1985, 43, 279–284. [Google Scholar] [CrossRef]
- Larsson, K. Classification of glyceride crystal forms. Acta Chem. Scand. 1966, 20, 2255–2260. [Google Scholar] [CrossRef]
- Lida, H.; Ali, A.R.M. Physico-chemical characteristics of palm-based oil blends for the production of reduced fat spreads. J. Am. Oil Chem. Soc. 1998, 75, 1625–1631. [Google Scholar] [CrossRef]
- Marangoni, A.G. Structure-Function Analysis of Edible Fats; AOCS Press: Urbana, IL, USA, 2018. [Google Scholar]
- AOCS Cj 2–95. X-Ray Diffraction Analysis of Fats. Sampling and Analysis of Commercial Fats and Oils; AOCS: Chicago, IL, USA, 1997. [Google Scholar]
- Watanabe, H.; Morigaki, A.; Kaneko, Y.; Tobori, N.; Aramaki, K. Effects of temperature and humidity history on brittleness of α-sulfonated fatty acid methyl ester salt crystals. J. Oleo Sci. 2016, 65, 143–150. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.; Martin, A., Jr.; Conrad, C. An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Xu, H.; Li, P.; Ma, K.; Welbourn, R.J.; Penfold, J.; Roberts, D.W. Adsorption of methyl ester sulfonate at the air–water interface: Can limitations in the application of the Gibbs equation be overcome by computer purification? Langmuir 2017, 33, 9944–9953. [Google Scholar] [CrossRef] [PubMed]
- Stirton, A.; Wei, J.; Bistline, R. Sodium salts of alkyl α-sulfopalmitates and stearates. J. Am. Chem. Soc. 1953, 75, 4659–4960. [Google Scholar]
- Weil, J.; Bistline, R.; Stirton, A. Sodium Salts of Alkyl Alpha-Sulfopalmitates and Stearates; Amer Chemical Soc 1155 16TH ST, NW 20036; Amer Chemical Soc: Washington, DC, USA, 1953; pp. 4859–4860. [Google Scholar]
- Battaglini, G.T.; Larsen-Zobus, J.L.; Baker, T.G. Analytical methods for alpha sulfo methyl tallowate. J. Am. Oil Chem. Soc. 1986, 63, 1073–1077. [Google Scholar] [CrossRef]
- Sheat, W. Chemithon Analytical Methods; The Chemithon Corporation: Seattle, WA, USA, 1994. [Google Scholar]
- ASTM D. 3049 Standard Test Method for Synthetic Anionic Ingredient by Cationic Titration; American Society for Testing and Materials: West Conshohocken, PA, USA, 1989. [Google Scholar]
- Scholz, E. Karl Fischer Titration: Determination of Water; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Nikolic, A. Fourier Transform Infrared Spectroscopy (FTIR); Kvalitet voda; Departman za hemiju, Prirodno-matematički fakultet Novi Sad: Novi Sad, Serbia, 2007. [Google Scholar]
- Carr, R.L. Evaluating flow properties of solids. Chem Eng. 1965, 18, 163–168. [Google Scholar]
- Hausner, H.H. Friction Conditions in a Mass of Metal Powder; Polytechnic Inst. of Brooklyn. Univ. of California: Los Angeles, CA, USA, 1967. [Google Scholar]



| Product/Parameter | MES C12 | MES C14 | MES C16 | MES C16–18 |
|---|---|---|---|---|
| Molecular weight | 316.47 | 344.07 | 372.46 | 382.71 |
| Active (%) | 98.96 ± 0.04 | 97.76 ± 0.02 | 99.32 ± 0.04 | 99.18 ± 0.06 |
| Di-salt/active (%) | 0.42 ± 0.03 | 4.46 ± 0.03 | 4.76 ± 0.04 | 4.72 ± 0.01 |
| 5% Klett | 11.95 ± 0.23 | 2.03 ± 0.45 | 18.08 ± 0.12 | 25.20 ± 0.34 |
| pH (10%) | 5.42 ± 0.02 | 3.89 ± 0.04 | 5.26 ± 0.01 | 5.44 ± 0.01 |
| Moisture (%) | 2.32 ± 0.12 | 1.03 ± 0.43 | 1.74 ± 0.31 | 2.20 ± 0.09 |
| Appearance | -----------------------Powder crystals-------------------- | |||
| MES | Acyl Chain Length | Assignment (δ ppm) | ||||
|---|---|---|---|---|---|---|
| Terminal CH3– | –(CH2) n– | CH2– Linked with –CH– | –OCH3 | S–CH– | ||
| Before Crystallization | ||||||
| C12 | 12 | 0.74–0.76 | 1.17 | 1.92–1.97 | 3.71 | 3.87 |
| (t, 3H) | (d, 16H) | (d, 2H) | (t, 3H) | (s, H) | ||
| C14 | 14 | 0.73–0.76 | 1.16 | 1.90–1.95 | 3.71 | 3.87 |
| (t, 3H) | (d, 16H) | (d, 2H) | (t, 3H) | (s, H) | ||
| C16 | 16 | 0.73–0.76 | 1.17 | 1.91–1.96 | 3.71 | 3.87 |
| (t, 3H) | (d, 16H) | (d, 2H) | (t, 3H) | (s, H) | ||
| C16–18 | 16–18 | 0.73–0.77 | 1.18 | 1.92–1.97 | 3.71 | 3.87 |
| (t, 3H) | (d, 16H) | (d, 2H) | (t, 3H) | (s, H) | ||
| After Repeated Crystallization | ||||||
| C12 | 12 | 0.78–0.80 | 1.21 | 2.00 | 3.75 | 3.89 |
| (t, 3H) | (d, 16H) | (d, 2H) | (t, 3H) | (s, H) | ||
| C14 | 14 | 0.74–0.76 | 1.17 | 1.95 | 3.74 | 3.89 |
| (t, 3H) | (d, 16H) | (d, 2H) | (t, 3H) | (s, H) | ||
| C16 | 16 | 0.74–0.76 | 1.17 | 1.99 | 3.73 | 3.89 |
| (t, 3H) | (d, 16H) | (d, 2H) | (t, 3H) | (s, H) | ||
| C16–18 | 16–18 | 0.74–0.76 | 1.17 | 2.00 | 3.74 | 3.89 |
| (t, 3H) | (d, 16H) | (d, 2H) | (t, 3H) | (s, H) | ||
| MES | Assignment (δ ppm) | |||||
|---|---|---|---|---|---|---|
| Terminal CH3– | Adjacent (CH2)– | (CH2)n– | –OCH3 | –CH– | C=O | |
| Before Crystallization | ||||||
| C12 | 13.86 | 22.68 | 27.39–32.06 | 53.04 | 66.09 | 170.54 |
| C14 | 13.86 | 22.71 | 27.43–32.10 | 53.00 | 66.20 | 170.52 |
| C16 | 13.93 | 22.74 | 27.47–32.13 | 52.97 | 66.04 | 170.52 |
| C16–18 | 13.93 | 22.72 | 27.49–32.16 | 52.96 | 66.02 | 170.52 |
| After Repeated Crystallization | ||||||
| C12 | 13.88 | 22.71 | 27.43–32.09 | 53.06 | 66.14 | 170.59 |
| C14 | 13.89 | 22.74 | 27.48–32.13 | 53.03 | 66.12 | 170.58 |
| C16 | 13.89 | 22.76 | 27.52–32.15 | 52.99 | 66.07 | 170.57 |
| C16–18 | 13.95 | 22.79 | 27.56–32.17 | 52.98 | 66.06 | 170.56 |
| MES | Elemental Analyses, % | |||||
|---|---|---|---|---|---|---|
| Before Crystallization | After Crystallization | |||||
| O | Na | S | O | Na | S | |
| C14 | 16.44 ± 4.83 | 11.95 ± 2.67 | 14.64 ± 0.16 | 18.76 ± 4.89 | 15.22 ± 4.75 | 17.05 ± 1.65 |
| C16 | 16.72 ± 3.99 | 12.15 ± 1.82 | 15.69 ± 1.32 | 13.97 ± 13.62 | 12.18 ± 10.92 | 17.23 ± 1.12 |
| C16–18 | 19.20 ± 5.76 | 13.62 ± 3.70 | 14.65 ± 0.43 | 10.98 ± 8.13 | 8.84 ± 4.97 | 13.50 ± 0.87 |
| Parameter | Mean Size Diameter (µm) | |||
|---|---|---|---|---|
| MES C12 | MES C14 | MES C16 | MES C16–18 | |
| MES before crystallization | liquid | 72.59 ± 0.44 | 9.99 ± 0.25 | 404.87 ± 1.16 |
| MES after crystallization | 5.45 ± 1.11 | 73.74 ± 0.11 | 12.52 ± 0.35 | 447.18 ± 1.25 |
| Parameter | Melting Point Capillary Tube, °C | |||
|---|---|---|---|---|
| MES C12 | MES C14 | MES C16 | MES C16–18 | |
| Before crystallization | 131.0 ± 0.4 | 116.3 ± 0.2 | 186.5 ± 0.1 | 209.2 ± 0.3 |
| After repeated crystallization | 203.1 ± 0.2 | 217.8 ± 0.4 | 197.8 ± 0.0 | 229.5 ± 0.4 |
| Product/Parameter | MES C12 | MES C14 | MES C16 | MES C16–18 |
|---|---|---|---|---|
| Molecular weight | 316.47 | 344.07 | 372.46 | 382.71 |
| Active (%) | 68.46 ± 0.07 | 83.67 ± 0.05 | 83.42 ± 0.06 | 85.82 ± 0.09 |
| Di-salt/active (%) | 1.03 ± 0.05 | 4.18 ± 0.07 | 4.30 ± 0.10 | 4.02 ± 0.07 |
| 5% Klett colour | 7.14 ± 0.11 | 20.23 ± 0.15 | 21.89 ± 0.09 | 24.11 ± 0.12 |
| pH (10%) | 4.88 ± 0.03 | 3.29 ± 0.02 | 5.38 ± 0.03 | 5.52 ± 0.02 |
| Moisture (%) | 21.95 ± 0.24 | 14.03 ± 0.25 | 9.20 ± 0.19 | 8.92 ± 0.22 |
| Appearance | semi-liquid | paste | flakes | flakes |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd Maurad, Z.; Abdullah, L.C.; Anuar, M.S.; Abdul Karim Shah, N.N.; Idris, Z. Preparation, Characterization, Morphological and Particle Properties of Crystallized Palm-Based Methyl Ester Sulphonates (MES) Powder. Molecules 2020, 25, 2629. https://doi.org/10.3390/molecules25112629
Abd Maurad Z, Abdullah LC, Anuar MS, Abdul Karim Shah NN, Idris Z. Preparation, Characterization, Morphological and Particle Properties of Crystallized Palm-Based Methyl Ester Sulphonates (MES) Powder. Molecules. 2020; 25(11):2629. https://doi.org/10.3390/molecules25112629
Chicago/Turabian StyleAbd Maurad, Zulina, Luqman Chuah Abdullah, Mohd Shamsul Anuar, Nor Nadiah Abdul Karim Shah, and Zainab Idris. 2020. "Preparation, Characterization, Morphological and Particle Properties of Crystallized Palm-Based Methyl Ester Sulphonates (MES) Powder" Molecules 25, no. 11: 2629. https://doi.org/10.3390/molecules25112629
APA StyleAbd Maurad, Z., Abdullah, L. C., Anuar, M. S., Abdul Karim Shah, N. N., & Idris, Z. (2020). Preparation, Characterization, Morphological and Particle Properties of Crystallized Palm-Based Methyl Ester Sulphonates (MES) Powder. Molecules, 25(11), 2629. https://doi.org/10.3390/molecules25112629






