Antiviral Effects of Plant-Derived Essential Oils and Their Components: An Updated Review
Abstract
1. Introduction
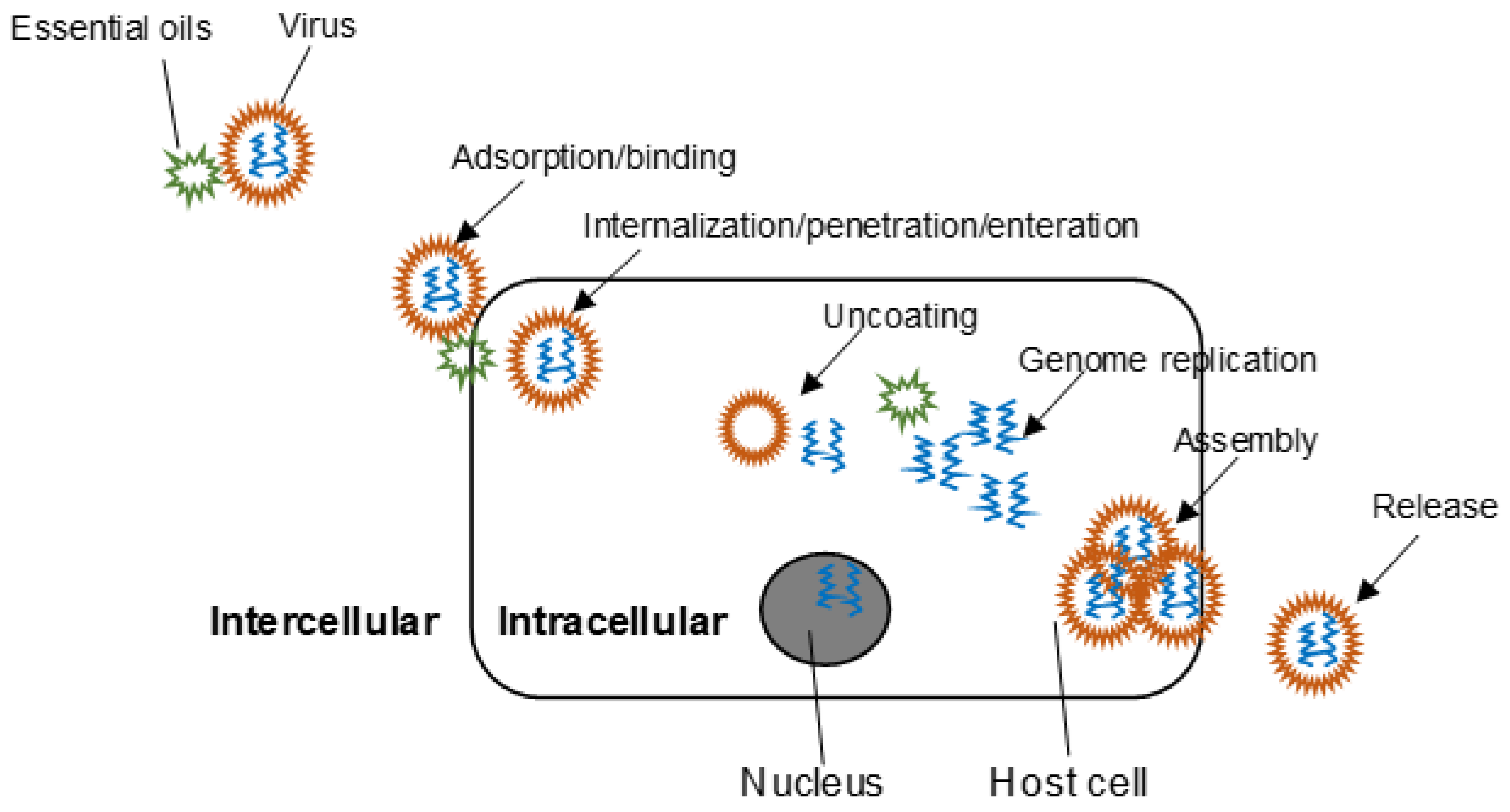
2. Mechanisms of Action
2.1. Time-of-Addition Experiment
2.2. Morphological Alteration
2.3. Protein Inhibition
2.4. Other Mechanisms of Action
3. In Vitro Studies of Antiviral Activities of Essential Oils
3.1. Human Herpes Virus
3.2. Influenza Virus
3.3. Non-Enveloped Viruses
3.4. Other Viruses
4. Effect of Polarity of the Components on Anti-Viral Activities
5. Activities of EOs Compared to That of Their Principal Components
6. Effects of Essential Oils Relative to Available Drugs
7. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Böhme, K.; Velázquez, J.B.; Calo-Mata, P.; Aubourg, S.P. Antibacterial, Antiviral and Antifungal Activity of Essential Oils: Mechanisms and Applications. In Antimicrobial Compounds; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2013; pp. 51–81. [Google Scholar]
- Cagno, V.; Sgorbini, B.; Sanna, C.; Cagliero, C.; Ballero, M.; Civra, A.; Donalisio, M.; Bicchi, C.; Lembo, D.; Rubiolo, P. In vitro anti-herpes simplex virus-2 activity of Salvia desoleana Atzei & V. Picci essential oil. PLoS ONE 2017, 12, e0172322. [Google Scholar] [CrossRef]
- Takeda, M.; Pekosz, A.; Shuck, K.; Pinto, L.H.; Lamb, R.A. Influenza A Virus M2 Ion Channel Activity Is Essential for Efficient Replication in Tissue Culture. J. Virol. 2002, 76, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Ghodke, Y.; Anderson, P.L.; Sangkuhl, K.; Lamba, J.; Altman, R.B.; Klein, T.E. PharmGKB summary: Zidovudine pathway. Pharmacogenet. Genom. 2012, 22, 891–894. [Google Scholar] [CrossRef]
- McKimm-Breschkin, J.L. Influenza neuraminidase inhibitors: Antiviral action and mechanisms of resistance. Influenza Other Respir. Viruses 2012, 7, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Schnitzler, P. Essential Oils for the Treatment of Herpes Simplex Virus Infections. Chemotherapy 2019, 64, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Luna, E.C.; Luna, I.S.; Scotti, L.; Monteiro, A.F.M.; Scotti, M.T.; De Moura, R.O.; De Araújo, R.S.A.; Monteiro, K.L.C.; De Aquino, T.M.; Ribeiro, F.F.; et al. Active Essential Oils and Their Components in Use against Neglected Diseases and Arboviruses. Oxid. Med. Cell. Longev. 2019, 2019, 6587150. [Google Scholar] [CrossRef]
- Al-Zubairi, A.; Al-Mamary, M.; Al-Ghasani, E. The antibacterial, antifungal, and antioxidant activities of essential oil from different aromatic plants. Glob. Adv. Res. J. Med. Sci. 2017, 6, 224–233. [Google Scholar]
- Valdivieso-Ugarte, M.; Gómez-Llorente, C.; Plaza-Diaz, J.; Gil, A. Antimicrobial, Antioxidant, and Immunomodulatory Properties of Essential Oils: A Systematic Review. Nutrients 2019, 11, 2786. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; De Feo, V. Essential Oils and Antifungal Activity. Pharmaceuticals 2017. [Google Scholar] [CrossRef]
- Nagy, M.M.; Almahdy, D.; El Aziz, O.M.A.; Kandil, A.M.; Tantawy, M.A.; El Alfy, T.S. Chemical Composition and Antiviral Activity of Essential Oils from Citrus reshni hort. ex Tanaka (Cleopatra mandarin) Cultivated in Egypt. J. Essent. Oil Bear. Plants 2018, 21, 264–272. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; El-Hawary, S.S.; Mohammed, M.M.; Farid, M.A.; Abdel-Wahed, N.; Ali, M.A.; El-Abd, E. Chemical composition, antiviral against avian influenza (H5N1) virus and antimicrobial activities of the essential oils of the leaves and fruits of Fortunella margarita, lour. swingle, growing in Egypt. J. Pharm. Sci. 2015. [Google Scholar] [CrossRef]
- Pajaro-Castro, N.; Flechas, M.C.; Ocazionez, R.; Stashenko, E.; Olivero-Verbel, J. Potential interaction of components from essential oils with dengue virus proteins. Bol. Latinoam. Caribe Plantas Med. Aromat. 2015, 14, 141–155. [Google Scholar]
- Sharifi-Rad, J.; Salehi, B.; Schnitzler, P.; Ayatollahi, S.A.; Kobarfard, F.; Fathi, M.; Eisazadeh, M.; Sharifi-Rad, M. Susceptibility of herpes simplex virus type 1 to monoterpenes thymol, carvacrol, p-cymene and essential oils of Sinapis arvensis L., Lallemantia royleana Benth. and Pulicaria vulgaris Gaertn. Cell. Mol. Boil. 2017, 63, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Gavanji, S.; Sayedipour, S.S.; Larki, B.; Bakhtari, A. Antiviral activity of some plant oils against herpes simplex virus type 1 in Vero cell culture. J. Acute Med. 2015, 5, 62–68. [Google Scholar] [CrossRef]
- Nuzhat, T.; Vidyasagar, G. Antifungal investigations on plant essential oils. A review. Int. J. Pharm. Pharm. Sci. 2014, 5, 19–28. [Google Scholar]
- Raut, J.S.; Karuppayil, S.M. A status review on the medicinal properties of essential oils. Ind. Crop. Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Miguel, M.G. Antioxidant and Anti-Inflammatory Activities of Essential Oils: A Short Review. Molecules 2010, 15, 9252–9287. [Google Scholar] [CrossRef]
- Schnitzler, P.; Astani, A.; Reichling, J. Antiviral Effects of Plant-Derived Essential Oils and Pure Oil Components. In Lipids and Essential Oils as Antimicrobial Agents; John Wiley and Sons: Hoboken, NJ, USA, 2010; pp. 239–254. [Google Scholar]
- Kumari, C.B.C.; Nagaveni, H.C. Essential oils of aromatic plants with antibacterial, antifungal, antiviral, and cytotoxic properties—An overview. J. Pharmacogn. Phytochem. 2018, 7, 278–282. [Google Scholar]
- Gilling, D.; Kitajima, M.; Torrey, J.; Bright, K.R. Antiviral efficacy and mechanisms of action of oregano essential oil and its primary component carvacrol against murine norovirus. J. Appl. Microbiol. 2014, 116, 1149–1163. [Google Scholar] [CrossRef]
- Cliver, D.O. Capsid and Infectivity in Virus Detection. Food Environ. Virol. 2009, 1, 123–128. [Google Scholar] [CrossRef]
- Vimalanathan, S.; Hudson, J. Anti-influenza virus activity of essential oils and vapors. Am. J. Essent. Oil 2014, 2, 47–53. [Google Scholar]
- Vimalanathan, S.; Hudson, J. The Activity of Cedar Leaf oil Vapor Against Respiratory Viruses: Practical Applications. J. Pharm. Sci. 2013. [Google Scholar] [CrossRef]
- Feriotto, G.; Marchetti, N.; Costa, V.; Beninati, S.; Tagliati, F.; Mischiati, C.; Giordana, F.; Nicola, M.; Valentina, C.; Simone, B.; et al. Chemical Composition of Essential Oils from Thymus vulgaris, Cymbopogon citratus, and Rosmarinus officinalis, and Their Effects on the HIV-1 Tat Protein Function. Chem. Biodivers. 2018, 15. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.D.; Kaur, I. Eucalyptol (1,8 cineole) from Eucalyptus Essential Oil a Potential Inhibitor of COVID 19 Corona Virus Infection by Molecular Docking Studies. EuropePMC 2020. Available online: https://europepmc.org/article/ppr/ppr138242 (accessed on 31 March 2020).
- Abdelli, I.; Hassani, F.; Brikci, S.B.; Ghalem, S. In silico study the inhibition of angiotensin converting enzyme 2 receptor of COVID-19 by Ammoides verticillata components harvested from Western Algeria. J. Biomol. Struct. Dyn. 2020, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Guinea, R.; Carrasco, L. Requirement for vacuolar proton-ATPase activity during entry of influenza virus into cells. J. Virol. 1995, 69, 2306–2312. [Google Scholar] [CrossRef] [PubMed]
- Garozzo, A.; Timpanaro, R.; Stivala, A.; Bisignano, G.; Castro, A. Activity of Melaleuca alternifolia (tea tree) oil on Influenza virus A/PR/8: Study on the mechanism of action. Antivir. Res. 2011, 89, 83–88. [Google Scholar] [CrossRef]
- Civitelli, L.; Panella, S.; Marcocci, M.E.; De Petris, A.; Garzoli, S.; Pepi, F.; Vavala, E.; Ragno, R.; Nencioni, L.; Palamara, A.; et al. In vitro inhibition of herpes simplex virus type 1 replication by Mentha suaveolens essential oil and its main component piperitenone oxide. Phytomedicine 2014, 21, 857–865. [Google Scholar] [CrossRef]
- Mori, K.; Obossou, E.K.; Suwa, S.; Miura, S.; Oh, S.; Jinbo, N.; Ishibashi, Y.; Shikamoto, Y.; Hosono, T.; Toda, T. Human Immunodeficiency virus type 1 (hiv-1) reverse transcriptase inhibitory effect of Cymbopogon nardus essential oil. Int. J. Adv. Res. 2016, 2, 7–13. [Google Scholar]
- Ralambondrainy, M.; Belarbi, E.; Viranaicken, W.; Baranauskienė, R.; Venskutonis, P.R.; Desprès, P.; Roques, P.; El Kalamouni, C.; Sélambarom, J. In vitro comparison of three common essential oils mosquito repellents as inhibitors of the Ross River virus. PLoS ONE 2018, 13, e0196757. [Google Scholar] [CrossRef]
- Pilau, M.R.; Alves, S.H.; Weiblen, R.; Arenhart, S.; Cueto, A.P.; Lovato, L.T. Antiviral activity of the Lippia graveolens (Mexican oregano) essential oil and its main compound carvacrol against human and animal viruses. Braz. J. Microbiol. 2011, 42, 1616–1624. [Google Scholar] [CrossRef]
- Cos, P.; Vlietinck, A.J.; Berghe, D.V.; Maes, L. Anti-infective potential of natural products: How to develop a stronger in vitro ‘proof-of-concept’. J. Ethnopharmacol. 2006, 106, 290–302. [Google Scholar] [CrossRef]
- Stránská, R.; Schuurman, R.; Nienhuis, E.; Goedegebuure, I.W.; Polman, M.; Weel, J.F.; Dillen, P.M.W.-V.; Berkhout, R.J.; Van Loon, A.M. Survey of acyclovir-resistant herpes simplex virus in the Netherlands: Prevalence and characterization. J. Clin. Virol. 2005, 32, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Orhan, İ.E.; Özçelik, B.; Kartal, M.; Kan, Y. Antimicrobial and antiviral effects of essential oils from selected Umbelliferae and Labiatae plants and individual essential oil components. Turk. J. Biol. 2012, 36, 239–246. [Google Scholar]
- Francomano, F.; Caruso, A.; Barbarossa, A.; Fazio, A.; La Torre, C.; Ceramella, J.; Mallamaci, R.; Saturnino, C.; Iacopetta, D.; Sinicropi, M.S. β-Caryophyllene: A Sesquiterpene with Countless Biological Properties. Appl. Sci. 2019, 9, 5420. [Google Scholar] [CrossRef]
- Astani, A.; Reichling, J.; Schnitzler, P. Comparative study on the antiviral activity of selected monoterpenes derived from essential oils. Phytother. Res. 2009, 24, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Astani, A.; Reichling, J.; Schnitzler, P. Screening for Antiviral Activities of Isolated Compounds from Essential Oils. Evid. Based Complement. Altern. Med. 2011, 2011, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Toujani, M.M.; Rittà, M.; Civra, A.; Genovese, S.; Epifano, F.; Ghram, A.; Lembo, D.; Donalisio, M. Inhibition of HSV-2 infection by pure compounds from Thymus capitatus extract in vitro. Phytother. Res. 2018, 32, 1555–1563. [Google Scholar] [CrossRef]
- Wei, X.; Cheng, P.; Feng, W. Study on the effect of anti-respiratory viruses of Patchouli Oil in vitro. J. Pharm. Clin. Chin. Mater. Med. 2012, 6, 65–68. [Google Scholar]
- El Mokni, R.; Youssef, F.S.; Jmii, H.; Khmiri, A.; Bouazzi, S.; Jlassi, I.; Jaidane, H.; Dhaouadi, H.; Ashour, M.L.; Hammami, S. The Essential Oil of Tunisian Dysphania ambrosioides and its Antimicrobial and Antiviral Properties. J. Essent. Oil Bear. Plants 2019, 22, 282–294. [Google Scholar] [CrossRef]
- Bouazzi, S.; Jmii, H.; El Mokni, R.; Faidi, K.; Falconieri, D.; Piras, A.; Jaïdane, H.; Porcedda, S.; Hammami, S. Cytotoxic and antiviral activities of the essential oils from Tunisian Fern, Osmunda regalis. S. Afr. J. Bot. 2018, 118, 52–57. [Google Scholar] [CrossRef]
- Elaissi, A.; Rouis, Z.; Ben Salem, N.A.; Mabrouk, S.; Ben Salem, Y.; Salah, K.B.H.; Aouni, M.; Farhat, F.; Chemli, R.; Harzallah-Skhiri, F.; et al. Chemical composition of 8 eucalyptus species’ essential oils and the evaluation of their antibacterial, antifungal and antiviral activities. BMC Complement. Altern. Med. 2012, 12. [Google Scholar] [CrossRef]
- Kubiça, T.F.; Alves, S.H.; Weiblen, R.; Lovato, L.T. In vitro inhibition of the bovine viral diarrhoea virus by the essential oil of Ocimum basilicum (basil) and monoterpenes. Braz. J. Microbiol. 2014, 45, 209–214. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gómez, L.A.; Stashenko, E.E.; Ocazionez, R.E. Comparative study on in vitro activities of citral, limonene and essential oils from Lippia citriodora and L. alba on yellow fever virus. Nat. Prod. Commun. 2013, 8, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Haddad, J.G.; Picard, M.; Bénard, S.; Desvignes, C.; Desprès, P.; Diotel, N.; El Kalamouni, C. Ayapana triplinervis Essential Oil and Its Main Component Thymohydroquinone Dimethyl Ether Inhibit Zika Virus at Doses Devoid of Toxicity in Zebrafish. Molecules 2019, 24, 3447. [Google Scholar] [CrossRef] [PubMed]
- Hammami, S.; Jmii, H.; El Mokni, R.; Khmiri, A.; Faidi, K.; Dhaouadi, H.; El Aouni, M.H.; Aouni, M.; Joshi, R.K. Essential Oil Composition, Antioxidant, Cytotoxic and Antiviral Activities of Teucrium pseudochamaepitys Growing Spontaneously in Tunisia. Molecules 2015, 20, 20426–20433. [Google Scholar] [CrossRef]
- Lai, W.-L.; Chuang, H.-S.; Lee, M.-H.; Wei, C.-L.; Lin, C.-F.; Tsai, Y.-C. Inhibition of Herpes Simplex Virus Type 1 by Thymol-Related Monoterpenoids. Planta Med. 2012, 78, 1636–1638. [Google Scholar] [CrossRef]
- Astani, A.; Schnitzler, P. Antiviral activity of monoterpenes beta-pinene and limonene against herpes simplex virus in vitro. Iran. J. Microbiol. 2014, 6, 149–155. [Google Scholar]
- Vimalanathan Anti-Influenza Virus Activities of Commercial Oregano oils and their Carriers. J. Appl. Pharm. Sci. 2012, 2, 214–218. [CrossRef]
- Paulpandi, M.; Kannan, S.; Thangam, R.; Kaveri, K.; Gunasekaran, P.; Rejeeth, C. In vitro anti-viral effect of β-santalol against influenza viral replication. Phytomedicine 2012, 19, 231–235. [Google Scholar] [CrossRef]
- Liao, Q.; Qian, Z.; Liu, R.; An, L.; Chen, X. Germacrone inhibits early stages of influenza virus infection. Antivir. Res. 2013, 100, 578–588. [Google Scholar] [CrossRef]
- Choi, H.-J. Chemical Constituents of Essential Oils Possessing Anti-Influenza A/WS/33 Virus Activity. Osong Public Heal. Res. Perspect. 2018, 9, 348–353. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Y.; Zu, S.; Sun, X.; Liu, C.; Liu, D.; Zhang, X.; Tian, J.; Qu, L. In vitro antiviral effect of germacrone on feline calicivirus. Arch. Virol. 2016, 161, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lai, Y.; Wang, Y.; Liu, N.; Zhang, F.; Xu, P. 1, 8-Cineol Protect Against Influenza-Virus-Induced Pneumonia in Mice. Inflammation 2016, 39, 1582–1593. [Google Scholar] [CrossRef] [PubMed]
- Camero, M.; Lanave, G.; Catella, C.; Capozza, P.; Gentile, A.; Fracchiolla, G.; Britti, M.; Martella, V.; Buonavoglia, C.; Tempesta, M. Virucidal activity of ginger essential oil against caprine alphaherpesvirus-1. Veter. Microbiol. 2019, 230, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Venturi, C.R.; Danielli, L.J.; Klein, F.; Apel, M.A.; Montanha, J.A.; Bordignon, S.A.L.; Roehe, P.; Fuentefria, A.M.; Henriques, A.T. Chemical analysis and in vitro antiviral and antifungal activities of essential oils from Glechon spathulata and Glechon marifolia. Pharm. Boil. 2014, 53, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Pourghanbari, G.; Nili, H.; Moattari, A.; Mohammadi, A.; Iraji, A. Antiviral activity of the oseltamivir and Melissa officinalis L. essential oil against avian influenza A virus (H9N2). VirusDisease 2016, 27, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef]
- Duijker, G.; Bertsias, A.; Symvoulakis, E.; Moschandreas, J.; Malliaraki, N.; Derdas, S.; Tsikalas, G.; Katerinopoulos, H.; Pirintsos, S.; Sourvinos, G.; et al. Reporting effectiveness of an extract of three traditional Cretan herbs on upper respiratory tract infection: Results from a double-blind randomized controlled trial. J. Ethnopharmacol. 2015, 163, 157–166. [Google Scholar] [CrossRef]
- Nikakhtar, Z.; Hasanzadeh, M.; Hamedi, S.S.; Najafi, M.N.; Tavassoli, A.P.; Feyzabadi, Z.; Meshkat, Z.; Saki, A. The efficacy of vaginal suppository based on myrtle in patients with cervicovaginal human papillomavirus infection: A randomized, double-blind, placebo trial. Phytother. Res. 2018, 32, 2002–2008. [Google Scholar] [CrossRef]
| No | Source of Essential Oils | Viruses | IC50 | SI | Intercellular or Intracellular Mechanisms | References |
|---|---|---|---|---|---|---|
| 1 | Star Anise | HSV-1 | 1 μg/mL | 160 | Intercellular | [39] |
| 2 | Mentha suaveolens | HSV-1 | 5.1 μg/mL | 67 | Intracellular | [30] |
| 3 | Australian tea tree | HSV-1 | 13.2 μg/mL | 43 | Intracellular | [30] |
| 4 | Sinapis arvensis | HSV-1 | 0.035% | 1.5 | Intercellular | [14] |
| 5 | Lallemantia royleana | HSV-1 | 0.011% | 6.4 | Intercellular | [14] |
| 6 | Pulicaria vulgaris | HSV-1 | 0.001% | 1 | Intercellular | [14] |
| 7 | Mexican oregano (Lippia graveolens) | HSV-1 | 99.6 μg/mL | 7.4 | Intercellular | [33] |
| 8 | Zataria multiflora | HSV-1 | 0.003% | 55.4 | ND | [15] |
| 9 | Eucalyptus caesia | HSV-1 | 0.007% | 38.8 | ND | [15] |
| 10 | Artemisia kermanensis | HSV-1 | 0.004% | 66.4 | ND | [15] |
| 11 | Satureja hotensis | HSV-1 | 0.008% | 32.2 | ND | [15] |
| 12 | Rosmarinus officinalis | HSV-1 | 0.006% | 46.1 | ND | [15] |
| 13 | Thymus capitatus | HSV-1 | 17.6 μg/mL | 6.9 | Intercellular | [40] |
| 14 | Thymus capitatus | HSV-2 | 18.6 μg/mL | 6.0 | Intercellular | [40] |
| 15 | Salvia desoleana | Acyclovir-resistant HSV-2 | 28.6 μg/mL | 55.2 | Intercellular and intracellular a | [2] |
| 16 | Mexican oregano (Lippia graveolens) | Acyclovir-resistant HSV-1 | 55.9 μg/mL | 13.1 | Intercellular | [33] |
| 17 | Patchouli | IFV-A (H1N1) | 0.088 mg/mL | 1.15 | ND | [41] |
| 18 | Cinnamomum zeylanicum | IFV-A (H1N1) | <3.1 μL/mL | >4 | Intercellular | [23] |
| 19 | Citrus bergamia | IFV-A (H1N1) | <3.1 μL/mL | >5 | Intercellular | [23] |
| 20 | Cymbopogon flexuosus | IFV-A (H1N1) | <3.1 μL/mL | >4 | ND | [23] |
| 21 | Thymus vulgaris | IFV-A (H1N1) | <3.1 μL/mL | >4 | Intercellular | [23] |
| 22 | Lavandula officinalis | IFV-A (H1N1) | <3.1 μL/mL | >8 | Intercellular | [23] |
| 23 | Eucalyptus globulus | IFV-A (H1N1) | <50 μL/mL | >0.5 | Intercellular | [23] |
| 24 | Pelargonium graveolens | IFV-A (H1N1) | <3.1 μL/mL | >21 | Intercellular | [23] |
| 25 | Citrus reshni ripe fruit peel | Avian influenza virus A (H5N1) | 2.5 μg/mL | 8.7 | ND | [11] |
| 26 | Fortunella margarita fruit | Avian influenza virus A (H5N1) | 6.8 μg/mL | ND | ND | [12] |
| 27 | Thymus vulgaris vulgaris, Cymbopogon citratus, Rosmarinus officinalis | HIV-1 | 0.05–0.83 μg/mL | 1.13–3.6 | ND | [25] |
| 28 | Cymbopogon nardus | HIV-1 | 1.2 mg/mL | ND | ND | [31] |
| 29 | Dysphania ambrosioides | Coxsackie virus B4 | 21.7 μg/mL | 74.3 | ND | [42] |
| 30 | Osmunda regalis (Tunisian fern) | Coxsackie virus B4 | 2.2 μg/mL | 789.8 | ND | [43] |
| 31 | Eucalyptus globulus bicostata | Coxsackie virus B3 | 0.7 mg/mL | 22.8 | Intercellular | [44] |
| 32 | Patchouli | Coxsackie virus B3 | 0.081 mg/mL | 1.2 | ND | [41] |
| 33 | Mexican oregano (Lippia graveolens) | Bovine viral diarrhoea virus | 78 μg/mL | 7.2 | Intracellular | [33] |
| 34 | Ocimum basilicum | Bovine viral diarrhoea virus | 474.3 μg/mL | 3.7 | ND | [45] |
| 35 | Patchouli | Respiratory syncytial virus | 0.092 mg/mL | 1.1 | ND | [41] |
| 36 | Mexican oregano (Lippia graveolens) | Respiratory syncytial virus | 68 μg/mL | 10.8 | Intercellular | [33] |
| 37 | Lippia alba | Yellow fever virus | 4.3 μg/mL | 30.6 | Intercellular and intracellular | [46] |
| 38 | Patchouli | Adenovirus-3 | 0.084 mg/mL | 1.2 | ND | [38] |
| 39 | Mexican oregano (Lippia graveolens) | Bovine herpes virus 2 | 58.4 μg/mL | 9.7 | Intercellular and intracellular | [33] |
| 40 | Ayapana triplinervis | Zika virus | 38 μg/mL | 12.5 | Intercellular | [47] |
| 41 | Teucrium pseudochamaepitys | Coxsackievirus B | 589.6 μg/mL | 1.11 | ND | [48] |
| No | Components | Viruses | IC50 | SI | Intercellular or Intracellular Mechanisms | References |
|---|---|---|---|---|---|---|
| 1 | β-Caryophyllene | HSV-1 | 0.25 μg/mL | 140 | Intercellular | [39] |
| 2 | Farnesol | HSV-1 | 3.5 μg/mL | 11.4 | Intercellular | [39] |
| 3 | β-Eudesmol | HSV-1 | 6 μg/mL | 5.8 | Intercellular | [39] |
| 4 | Trans-anethole | HSV-1 | 20 μg/mL | 5 | Intercellular | [39] |
| 5 | Eugenol | HSV-1 | 35 μg/mL | 2.4 | Intercellular | [39] |
| 6 | Thymol | HSV-1 | 0.002% | 7 | Intercellular | [14] |
| 7 | Carvacrol | HSV-1 | 0.037% | 1.4 | Intercellular | [14] |
| 8 | p-Cymene | HSV-1 | >0.1% | ND | Intercellular | [14] |
| 9 | Carvacrol | HSV-1 | 48.6 μg/mL | 5.1 | Intracellular | [33] |
| 10 | Thymol | HSV-1 | 7 µM | 43 | Intercellular | [49] |
| 11 | Carvacrol | HSV-1 | 7 µM | 43 | Intercellular | [49] |
| 12 | β-Pinene | HSV-1 | 3.5 μg/mL | 24.3 | Intercellular | [50] |
| 13 | Limonene | HSV-1 | 5.9 μg/mL | 10.2 | Intercellular | [50] |
| 14 | Carvacrol | Acyclovir-resistant HSV-1 | 28.6 μg/mL | 8.7 | Intracellular | [33] |
| 15 | Carvacrol | IFV-A (H1N1) | 2.6 μg/mL | <0.15 | ND | [51] |
| 16 | Eugenol | IFV-A (H1N1) | <3.1 μL/mL | ND | ND | [23] |
| 17 | β-Santalol | IFV-A (H3N2) | 10–100 μg/mL | Intracellular a | [52] | |
| 18 | Germacrone | IFV-A (H1N1) | 6.03 μM | >41 | Intercellular and intracellular b | [53] |
| 19 | 1, 8-Cineole | BVDV | 331.17 μg/mL | 9.1 | Intercellular | [45] |
| 20 | Camphor | BVDV | 318.51 μg/mL | 13.9 | Intercellular | [45] |
| 21 | Thymol | BVDV | 248.56 μg/mL | 5.6 | Intercellular | [45] |
| 22 | Carvacrol | BVDV | 50.7 μg/mL | 4.2 | Intracellular | [33] |
| 23 | Carvacrol | Bovine herpes virus 2 | 663 μg/mL | 0.3 | Intracellular | [33] |
| 24 | Carvacrol | Respiratory syncytial virus | 62 μg/mL | 4.1 | Intracellular | [33] |
| 25 | Carvacrol | Human rotavirus | 27.9 μg/mL | 33 | Intracellular | [33] |
| 26 | Citral | Yellow fever virus | 17.6–25 μg/mL | 1.1–1.5 | ND | [46] |
| 27 | β-Caryophyllene | Dengue virus | 22.5 μM | 71.1 | Intercellular and intracellular b | [13] |
| 28 | Thymohydroquinone dimethyl ether | Zika virus | 45 μg/mL | 9.1 | Intercellular | [47] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, L.; Yao, L. Antiviral Effects of Plant-Derived Essential Oils and Their Components: An Updated Review. Molecules 2020, 25, 2627. https://doi.org/10.3390/molecules25112627
Ma L, Yao L. Antiviral Effects of Plant-Derived Essential Oils and Their Components: An Updated Review. Molecules. 2020; 25(11):2627. https://doi.org/10.3390/molecules25112627
Chicago/Turabian StyleMa, Li, and Lei Yao. 2020. "Antiviral Effects of Plant-Derived Essential Oils and Their Components: An Updated Review" Molecules 25, no. 11: 2627. https://doi.org/10.3390/molecules25112627
APA StyleMa, L., & Yao, L. (2020). Antiviral Effects of Plant-Derived Essential Oils and Their Components: An Updated Review. Molecules, 25(11), 2627. https://doi.org/10.3390/molecules25112627




