Cytotoxic Activity of Christia vespertilionis Root and Leaf Extracts and Fractions against Breast Cancer Cell Lines
Abstract
1. Introduction
2. Results
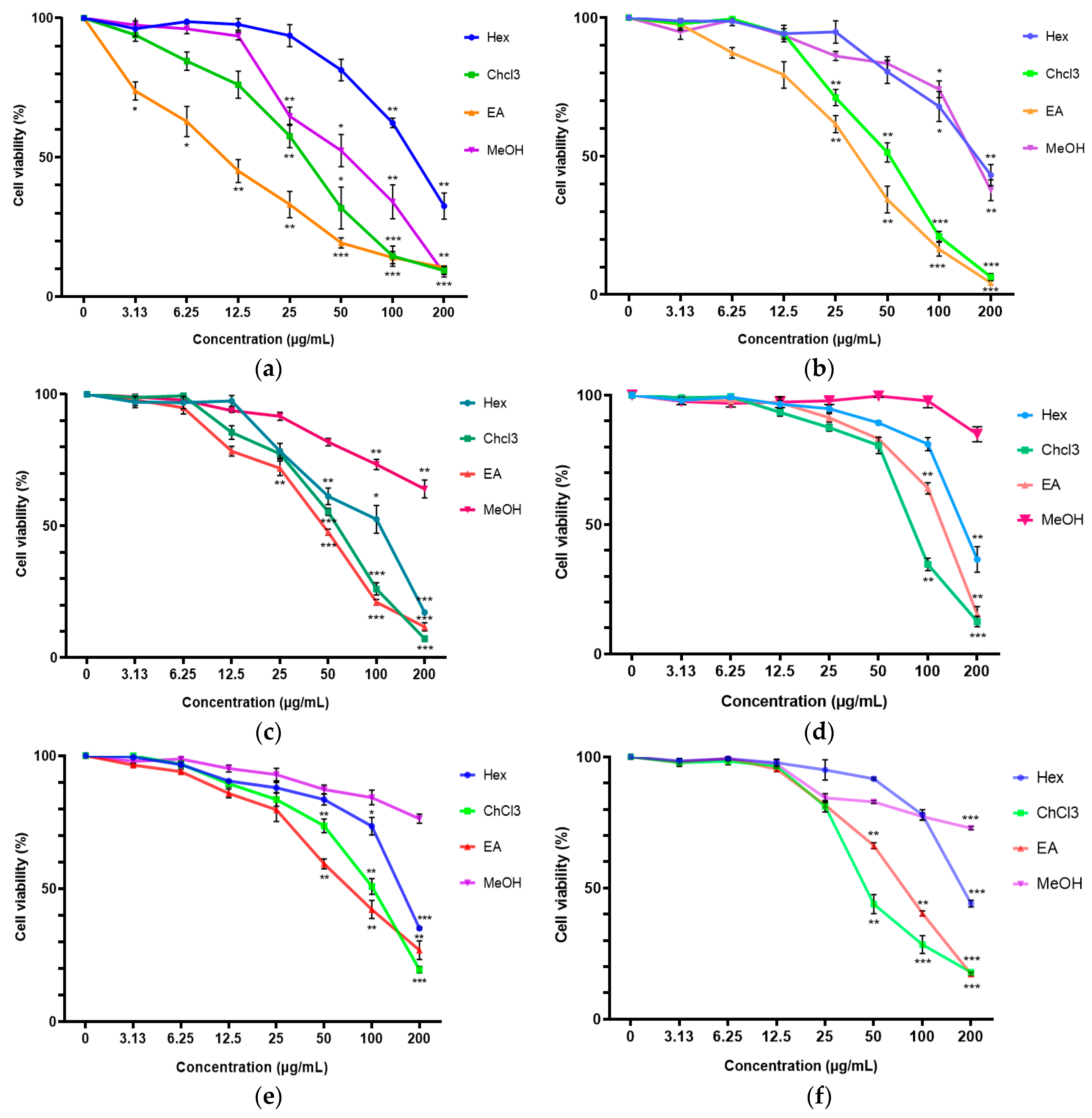
2.1. Cytotoxicity of C. vespertilionis Crude Extracts
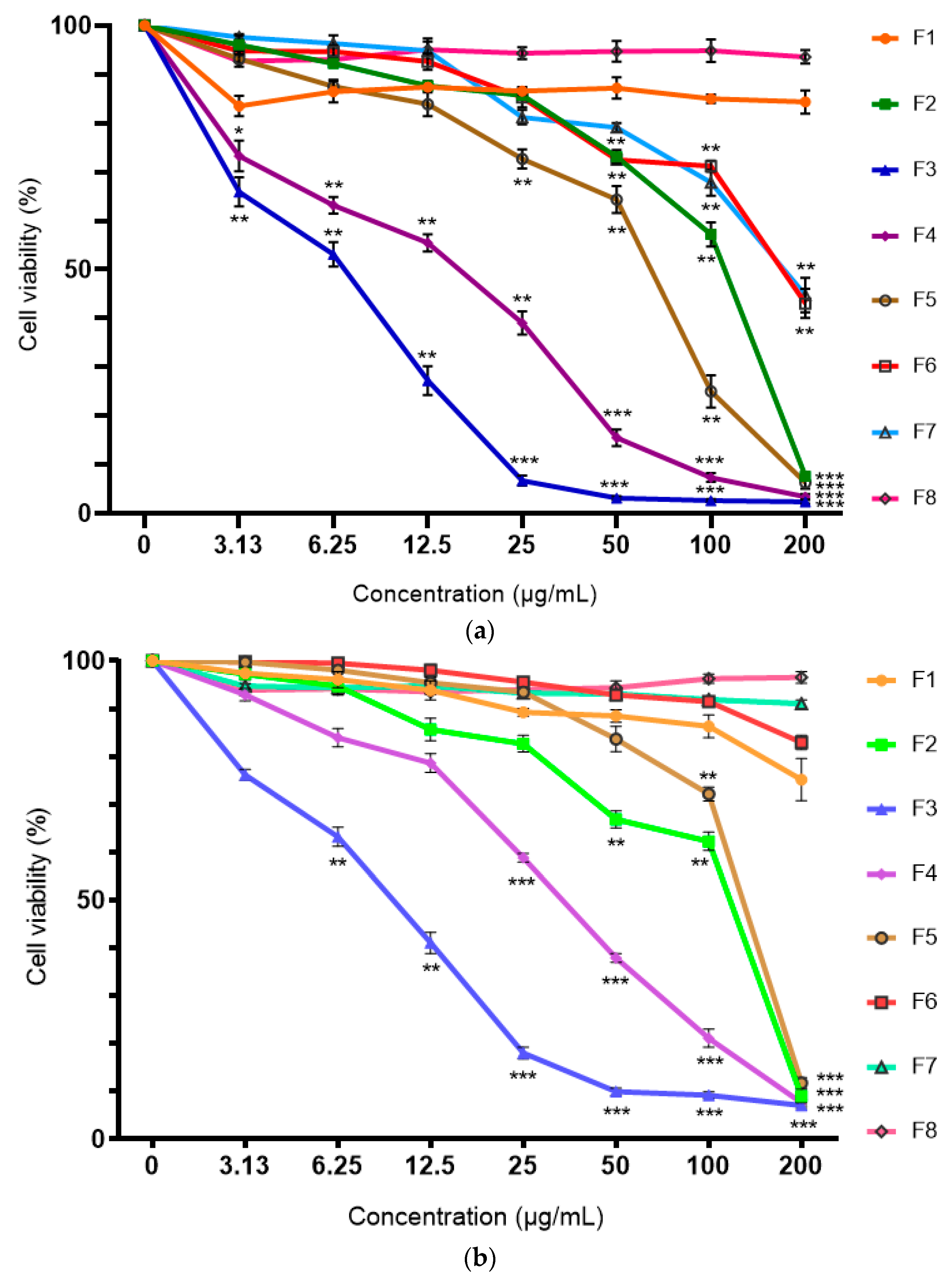
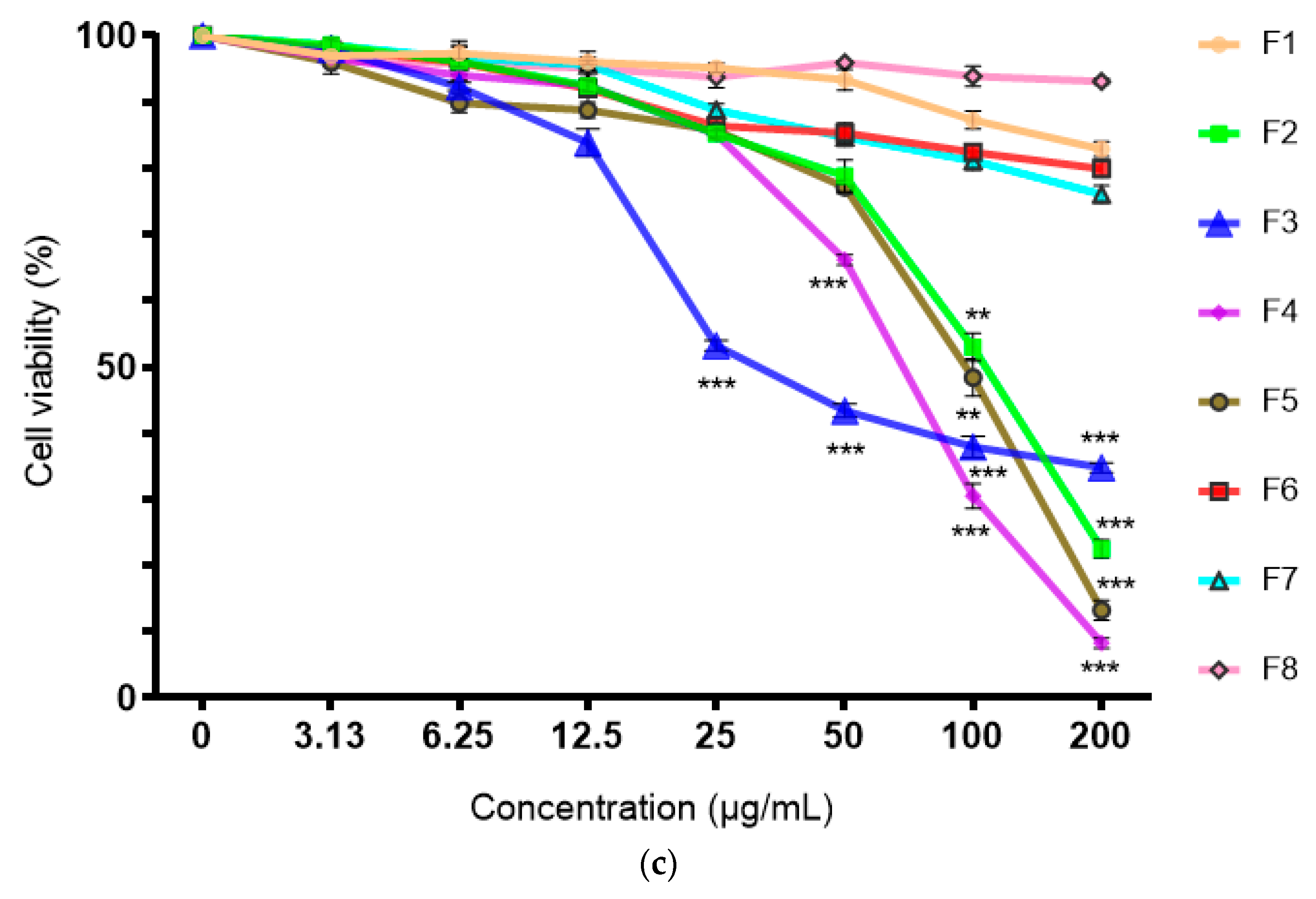
2.2. Cytotoxicity of Ethyl Acetate Root Fraction
2.3. Selectivity Index of C. vespertilionis
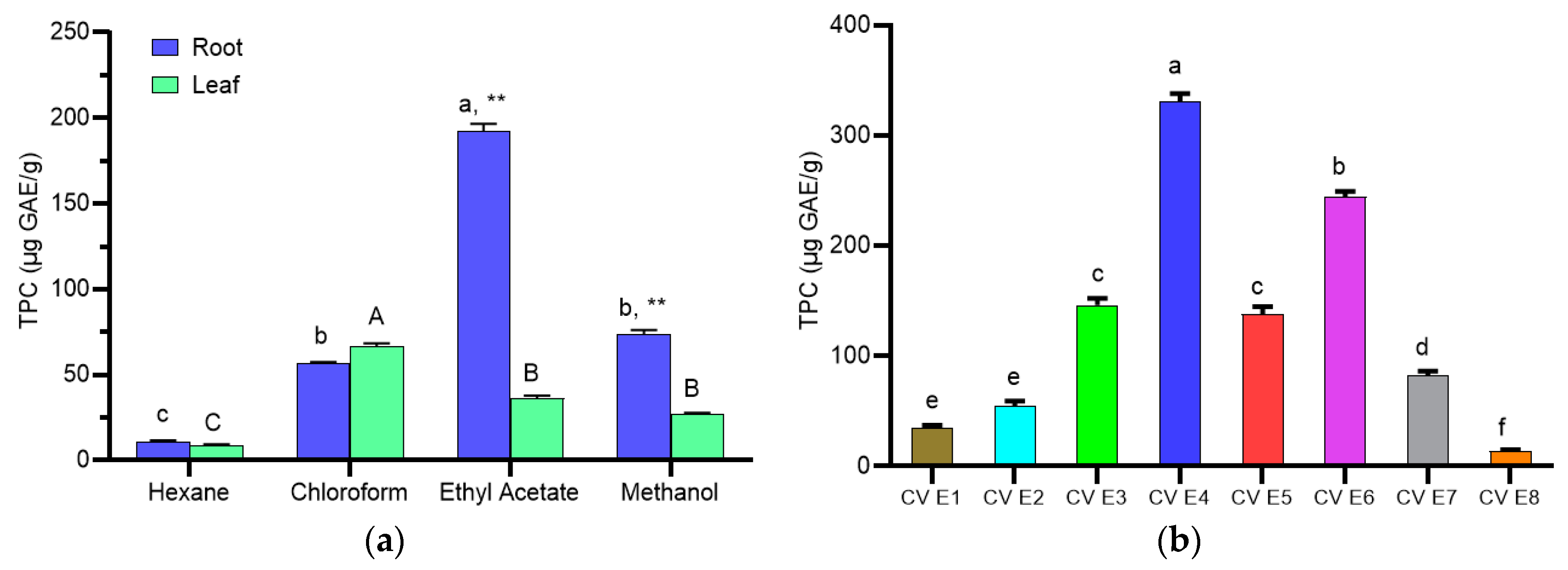
2.4. Total Phenolic Content (TPC)
2.5. Antioxidant Properties of Crude Extracts and Fractions
2.5.1. DPPH Free Radical Scavenging Activity
2.5.2. β-Carotene Bleaching Assay
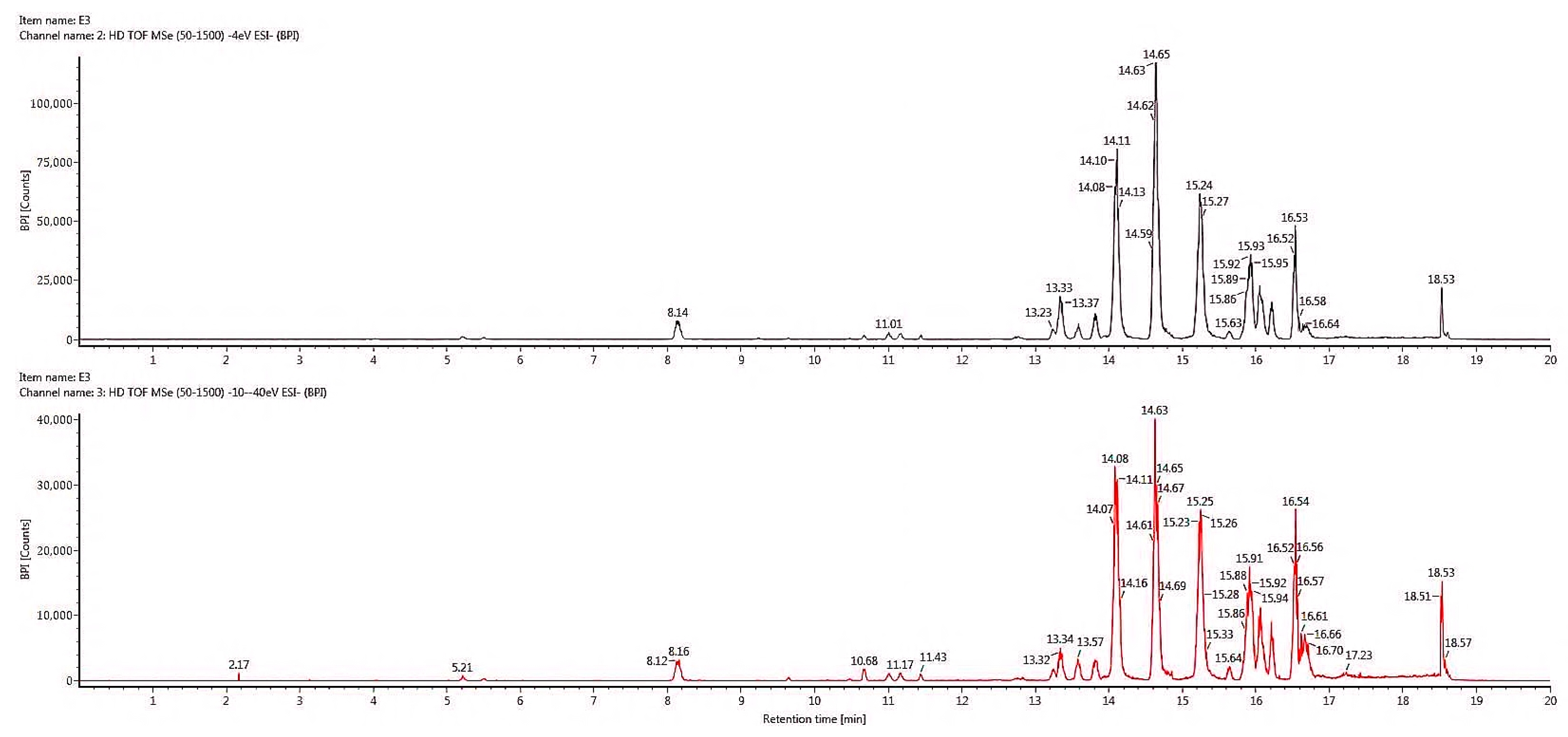
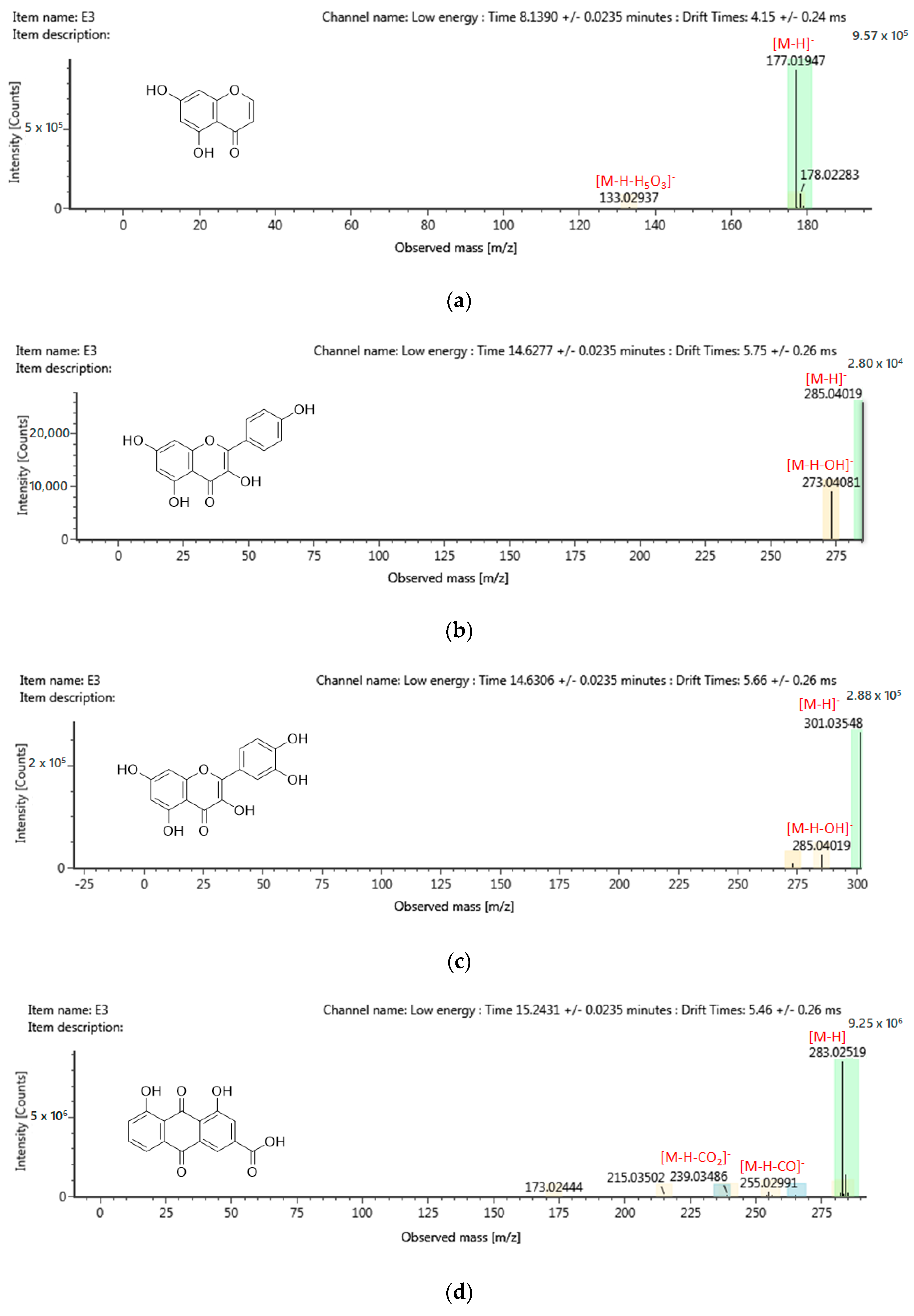
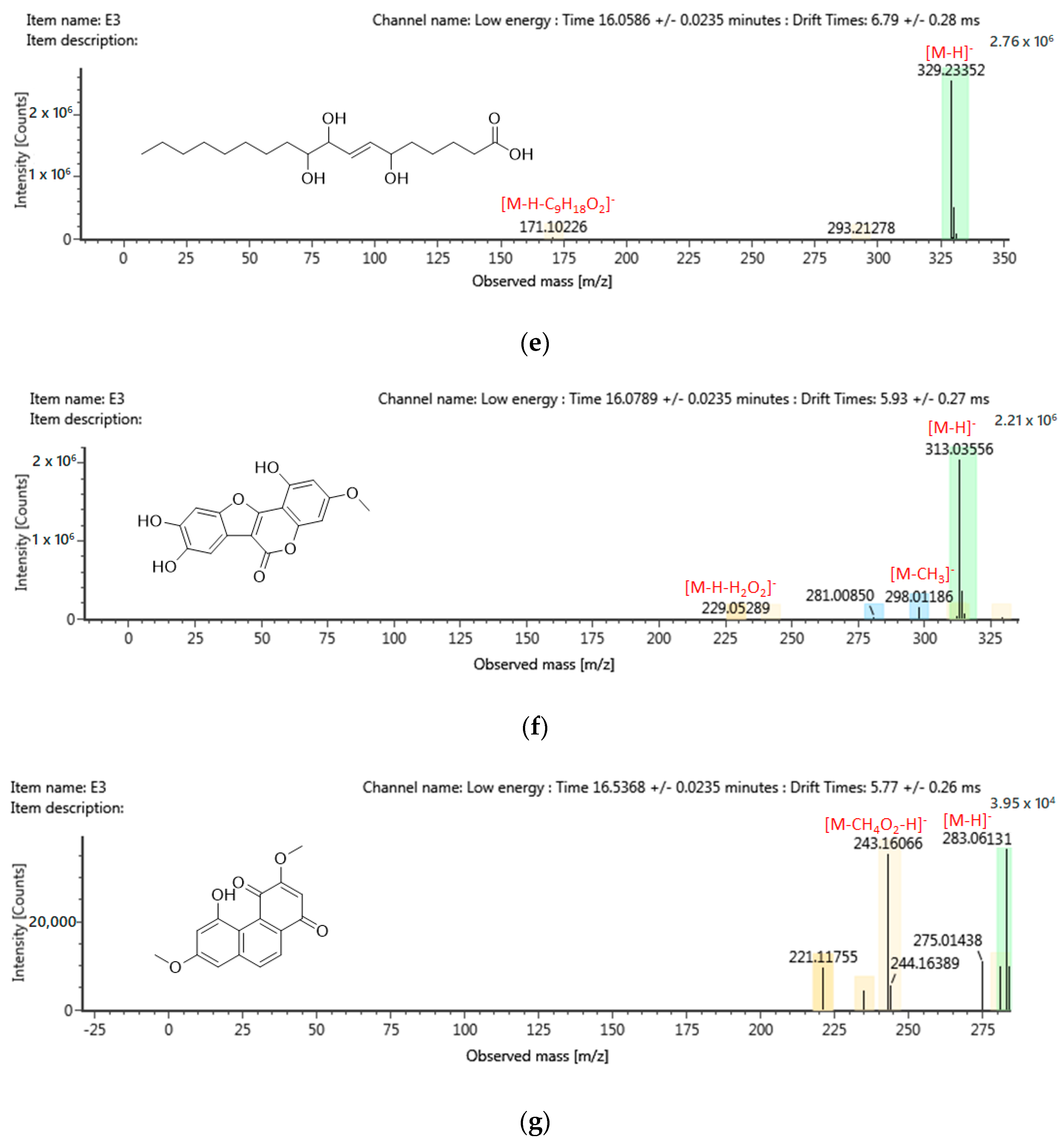
2.6. Chemical Profiling of Active Fraction Using LC-MS/MS
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Chemical Reagents
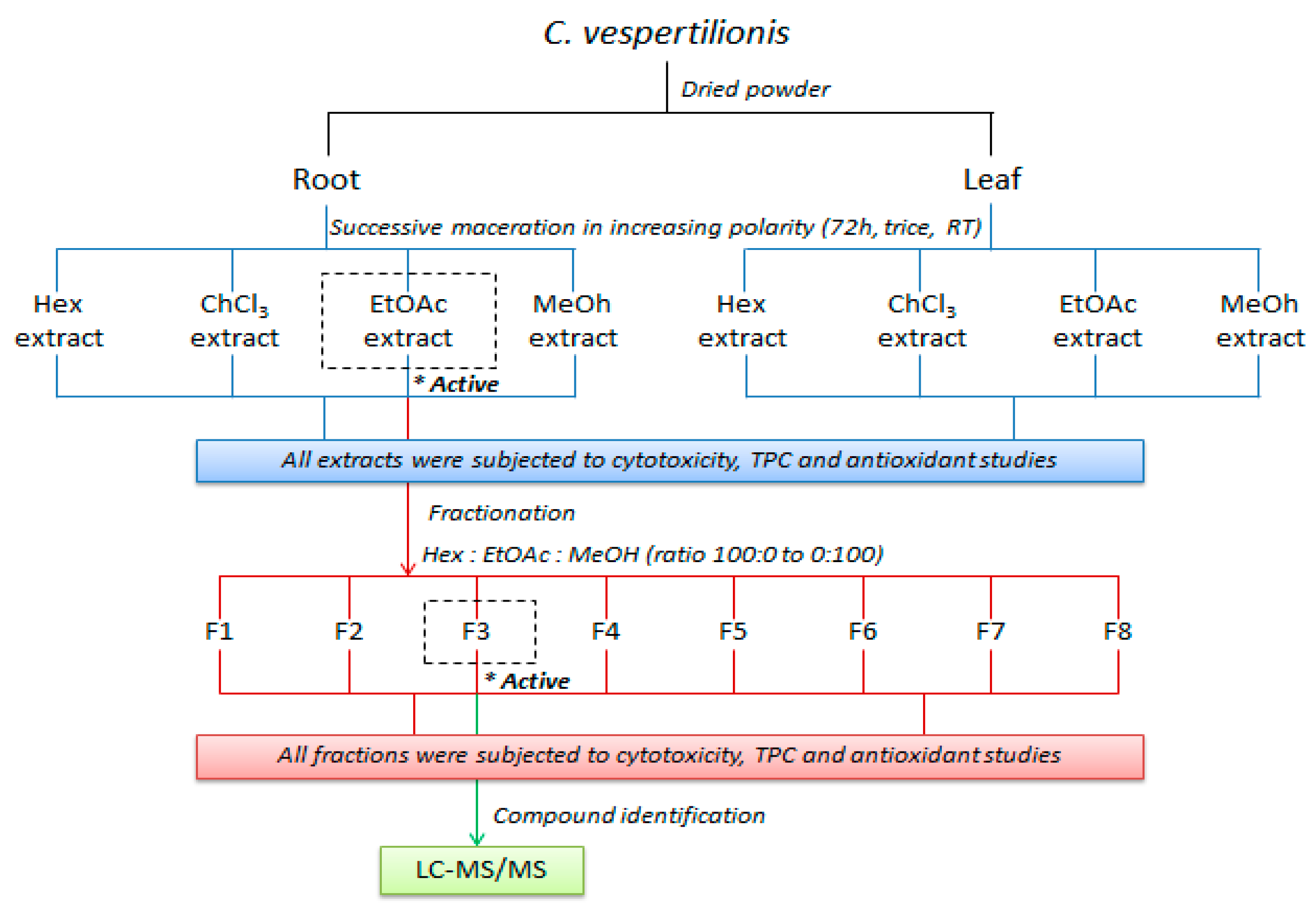
4.3. Preparation of Plant Extracts and Fractionation
4.4. Cell Culture
4.5. Determination of Cell Viability
4.6. Determination of Total Phenolic Content (TPC)
4.7. Determination of Antioxidant Activity
4.7.1. DPPH Free Radical Scavenging Activity
4.7.2. β-Carotene Bleaching Assay
4.8. LC-MS/MS Analysis
4.9. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-tieulent, J.; Jemal, A. Global cancer ctatistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Ching, S.M.; Hoo, F.K. Traditional medicinal plants and their therapeutic potential against major cancer types. In Anticancer Plants: Natural Products and Biotechnological Implements; Akhtar, M.S., Swamy, M.K., Eds.; Springer: Singapore, 2018; pp. 383–410. [Google Scholar]
- Zakaria, Z.; Gan, S.H.; Mohamed, M. In-vitro studies of Asian medicinal plants with potential activity against breast cancer. J. Appl. Biol. Biotechnol. 2018, 6, 49–55. [Google Scholar]
- Prakash, O.; Kumar, A.; Kumar, P. Anticancer potential of plants and natural products: A Review. Am. J. Pharmacol. Sci. 2013, 1, 104–115. [Google Scholar] [CrossRef]
- Greenwell, M.; Rahman, P.K.S.M. Medicinal plants: Their use in anticancer treatment. Int. J. Pharm. Pharm. Sci. 2015, 6, 4103–4112. [Google Scholar]
- Roy, A.; Jauhari, N.; Bharadvaja, N. Medicinal plants as a potential source of chemopreventive agents. In Anticancer Plants: Natural Products and Biotechnological Implements; Akhtar, M.S., Swamy, M.K., Eds.; Springer: Singapore, 2018; pp. 109–139. [Google Scholar]
- Hofer, D.; Schwach, G.; Tabrizi-Wizsy, N.G.; Sadjak, A.; Sturm, S.; Stuppner, H.; Pfragner, R. Christia vespertilionis plant extracts as novel antiproliferative agent against human neuroendocrine tumor cells. Oncol. Rep. 2013, 29, 2219–2226. [Google Scholar] [CrossRef]
- Arora, D.S.; Chandra, P. Antioxidant activity of Aspergillus fumigatus. IRSN Pharmacol. 2011, 2011, 619395. [Google Scholar]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 1–22. [Google Scholar] [CrossRef]
- Armania, N.; Yazan, L.S.; Musa, S.N.; Ismail, I.S.; Foo, J.B.; Chan, K.W.; Noreen, H.; Hisyam, A.H.; Zulfahmi, S.; Ismail, M. Dillenia suffruticosa exhibited antioxidant and cytotoxic activity through induction of apoptosis and G2/M cell cycle arrest. J. Ethnopharmacol. 2013, 146, 525–535. [Google Scholar] [CrossRef]
- Mishra, A.; Sharma, A.K.; Kumar, S.; Saxena, A.K.; Pandey, A.K. Bauhinia variegata leaf extracts exhibit considerable antibacterial, antioxidant, and anticancer activities. Biomed Res. Int. 2014, 2014, 915436. [Google Scholar]
- Chanda, S.; Dave, R. In-vitro models for antioxidant activity evaluation and some medicinal plants possessing antioxidant properties: An overview. Afr. J. Microbiol. Res. 2009, 3, 981–996. [Google Scholar]
- Nguyen-Pouplin, J.; Tran, H.; Tran, H.; Phan, T.A.; Dolecek, C.; Farrar, J.; Tran, T.H.; Caron, P.; Bodo, B.; Grellier, P. Antimalarial and cytotoxic activities of ethnopharmacologically selected medicinal plants from South Vietnam. J. Ethnopharmacol. 2007, 109, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, H.C.; Sisodia, B.S.; Cheema, H.S.; Agrawal, J.; Pal, A.; Darokar, M.P.; Srivastava, S.K. Novel antiplasmodial agents from Christia vespertilionis. Nat. Prod. Commun. 2013, 8, 1591–1594. [Google Scholar] [CrossRef] [PubMed]
- Dash, G.K. An appraisal of Christia vespertilionis (L. F.) bakh. F.: A promising medicinal plant. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 1037–1039. [Google Scholar]
- Muhammad Haffiz, J.; Nurhanan Murni, Y.; Nordati Akma, M.A. A preliminary study of chemical properties and in-vitro anti-cancer activities of Christia Sp. In Healing Power from Nature: Current Trends & Perspectives. In Proceedings of the 14th Seminar of Medicinal and Aromatic Plants, Institute Penyelidikan Perhutanan Malaysia, Kepong, Selangor Darul Ehsan, Malaysia, 11–12 October 2016; Chee, B.J., Mastura., M., Getha, K., Khoo, M.G.H., Mailina, J., Adiana, M.A., Rashan Jahn, M.S., Eds.; Forest Research Institute Malaysia (FRIM): Kuala Lumpur, Malaysia; pp. 118–122.
- Nurliana Abd, M.; Normala Abd, L. Synergistic interactions between Christia vespertilionis leaves extract and chemotherapy drug cyclophosphamide on WRL-68 cell line. Asian J. Pharm. Res. Dev. 2019, 7, 109–113. [Google Scholar]
- Osman, M.S.; Ghani, Z.A.; Ismail, N.F.; Razak, N.A.A.; Jaapar, J.; Ariff, M.A.M. Qualitative comparison of active compounds between red and green Mariposa Christia vespertillonis leaves extracts. In Proceedings of the 3rd Electronic and Green Material International Conference 2017; American Institute of Physics: College Park, MD, USA, 2017; Volume 1885. [Google Scholar]
- Mohd. Ghani, A.N.; Mat noor, S.; Zakaria, N.A.; Razali, M.A.; Abdul Rahim Siddiqe, A.S.K.; Abdul Wahab, M.F.; Hussin, S.; Mat Aris, N.F.S.; Hamir, N.; Mohd Ali Tan, Z. Mariposa tea enchanced with apple & mariposa crackers (Tea and crackers for anti-cancer). In E-proceedings of Food Innovation Expo, Dewan Banquet UiTM, 7 December; Razali, M.A., Mat noor, S., Zakaria, N.A., Tajizan, F.N., Mohd Zahari, M.S., Ramli, R.A.L., Wahab, J., Azmi, A., Md Nor, N., Eds.; Universiti Teknologi Mara (UiTM): Pulau Pinang, Malaysia, 2015; pp. 10–13, 33–36. [Google Scholar]
- Kaplánek, R.; Jakubek, M.; Rak, J.; Kejík, Z.; Havlík, M.; Dolenský, B.; Frydrych, I.; Hajdúch, M.; Kolář, M.; Bogdanová, K.; et al. Caffeine-hydrazones as anticancer agents with pronounced selectivity toward T-lymphoblastic leukaemia cells. Bioorg. Chem. 2015, 60, 19–29. [Google Scholar] [CrossRef]
- Blainski, A.; Lopes, G.C.; De Mello, J.C.P. Application and analysis of the folin-ciocalteu method for the determination of the total phenolic content from Limonium brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef]
- Kassim, N.K.; Rahmani, M.; Ismail, A.; Sukari, M.A.; Ee, G.C.L.; Nasir, N.M.; Awang, K. Antioxidant activity-guided separation of coumarins and lignan from Melicope glabra (Rutaceae). Food Chem. 2013, 139, 87–92. [Google Scholar] [CrossRef]
- Barchan, A.; Arakrak, A. The effects of solvents polarity on the phenolic contents and antioxidant activity of three Mentha species extracts. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 1–14. [Google Scholar]
- Zhang, P.; Omaye, S.T. Antioxidant and prooxidant roles for β-carotene, α-tocopherol and ascorbic acid in human lung cells. Toxicol. In Vitro 2001, 15, 13–24. [Google Scholar] [CrossRef]
- Boik, J. Natural Compounds in Cancer Therapy, 1st ed.; Silvine, F., Ed.; Oregon Medical Press: Princeton, NJ, USA, 2001. [Google Scholar]
- Solowey, E.; Lichtenstein, M.; Sallon, S.; Paavilainen, H.; Solowey, E.; Lorberboum-galski, H. Evaluating Medicinal Plants for Anticancer Activity. Sci. World J. 2014, 2014, 721402. [Google Scholar] [CrossRef] [PubMed]
- Razak, N.A.; Abu, N.; Ho, W.Y.; Zamberi, N.R.; Tan, S.W. Cytotoxicity of eupatorin in MCF-7 and MDA-MB-231 human breast cancer cells via cell cycle arrest, anti-angiogenesis and induction of apoptosis. Nature 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chang, N.; Chung, J.; Chen, K. Saikosaponin-A induces apoptotic mechanism in human breast MDA-MB-231 and MCF-7 cancer cells. Am. J. Chin. Med. 2003, 31, 363–377. [Google Scholar] [CrossRef]
- Kim, J.Y.; Dao, T.T.P.; Song, K.; Park, S.B.; Jang, H.; Park, M.K.; Gan, S.U.; Kim, Y.S. Annona muricata leaf extract triggered intrinsic apoptotic pathway to attenuate cancerous features of triple negative breast cancer MDA-MB-231 cells. Evidence-Based Complement. Altern. Med. 2018, 2018, 7972916. [Google Scholar] [CrossRef] [PubMed]
- Baba, S.A.; Malik, S.A. Determination of total phenolic and flavonoid content, antimicrobial and antioxidant activity of a root extract of Arisaema jacquemontii Blume. J. Taibah Univ. Sci. 2014, 9, 449–454. [Google Scholar] [CrossRef]
- Shin, S.; Moon, S.Y.; Kim, W.; Paek, S.; Park, H.H.; Lee, C.S. Structure-based classification and anti-cancer effects of plant metabolites. Int. J. Mol. Sci. 2018, 19, 2651. [Google Scholar] [CrossRef] [PubMed]
- Soobrattee, M.A.; Neergheen, V.S.; Luximon-Ramma, A.; Aruoma, O.I.; Bahorun, T. Phenolics as potential antioxidant therapeutic agents: Mechanism and actions. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2005, 579, 200–213. [Google Scholar] [CrossRef]
- Hyun, T.K.; Kim, H.C.; Kim, J.S. Antioxidant and antidiabetic activity of Thymus quinquecostatus Celak. Ind. Crops Prod. 2014, 52, 611–616. [Google Scholar] [CrossRef]
- Tor, Y.S.; Yazan, L.S.; Foo, J.B.; Armania, N.; Cheah, Y.K.; Abdullah, R. Induction of apoptosis through oxidative stress-related pathways in MCF-7, human breast cancer cells, by ethyl acetate extract of Dillenia suffruticosa. BMC Complement. Altern. Med. 2014, 14, 12. [Google Scholar] [CrossRef]
- Pandey, A.K.; Mishra, A.K.; Mishra, A. Antifungal and antioxidative potential of oil and extracts derived from leaves of Indian spice plant Cinnamomum tamala. Cell. Mol. Biol. 2012, 58, 142–147. [Google Scholar]
- Su, D.; Zhang, R.; Hou, F.; Zhang, M.; Guo, J.; Huang, F.; Deng, Y. Comparison of the free and bound phenolic profiles and cellular antioxidant activities of litchi pulp extracts from different solvents. BMC Complement. Altern. Med. 2014, 14, 9. [Google Scholar] [CrossRef] [PubMed]
- Barapatre, A.; Meena, A.S.; Mekala, S.; Das, A.; Jha, H. In-vitro evaluation of antioxidant and cytotoxic activities of lignin fractions extracted from Acacia nilotica. Int. J. Biol. Macromol. 2016, 84, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Noreen, H.; Semmar, N.; Farman, M.; McCullagh, J.S.O. Measurement of total phenolic content and antioxidant activity of aerial parts of medicinal plant Coronopus didymus. Asian Pac. J. Trop. Med. 2017, 10, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Emami, S.; Dadashpour, S. Current developments of coumarin-based anti-cancer agents in medicinal chemistry. Eur. J. Med. Chem. 2015, 102, 611–630. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Alam, B.; Quan, K.T.; Choi, H.; An, H.; Ju, M.; Lee, S.; Huh, Y.S.; Han, Y.; Na, M. Cytotoxic properties of the anthraquinone derivatives isolated from the roots of Rubia philippinensis. BMC Complement. Altern. Med. 2018, 18, 200. [Google Scholar] [CrossRef]
- Yeap, Y.S.Y.; Kassim, N.K.; Ng, R.C.; Ee, G.C.L.; Yazan, L.S.; Musa, K.H. Antioxidant properties of ginger (Kaempferia angustifolia Rosc.) and its chemical markers. Int. J. Food Prop. 2017, 20, 1158–1172. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Ng, W.K.; Yazan, L.S.; Yap, L.H.; Abd, W.; Wan, G.; Hafiza, N.; How, C.W.; Abdullah, R. Thymoquinone-loaded nanostructured lipid carrier exhibited cytotoxicity towards breast cancer cell lines (MDA-MB-231 and MCF-7) and cervical cancer cell lines (HeLa and SiHa). Biomed Res. Int. 2015, 2015, 263131. [Google Scholar] [CrossRef]
Sample Availability: Samples of the root and leaf crude extracts of the plant are available from the authors. |

| Cell Line | IC50 at 72 h Incubation (μg/mL) | ||||
|---|---|---|---|---|---|
| Root Extract | Ethyl Acetate Fraction | Doxorubicin (DOX) | |||
| Chloroform | Ethyl Acetate | F3 | F4 | ||
| MDA-MB-231 | 29.58 ± 3.80 | 11.34 ±1.20 | 5.72 ± 0.99 | 12.53 ± 1.44 | 0.05 ± 0.01 |
| MCF-7 | 54.55 ± 9.51 | 44.65 ± 5.78 | 8.98 ± 1.06 | 32.94 ± 2.72 | 0.06 ± 0.01 |
| 3T3 | 98.18 ± 13.35 | 77.38 ± 4.71 | 49.90 ± 8.63 | 68.30 ± 13.87 | 0.10 ± 0.01 |
| SI 3T3/MDA | 3.32 * | 6.82 * | 8.72 * | 5.45 * | 2.00 |
| SI 3T3/MCF | 1.80 | 1.73 | 5.56 * | 2.07 | 1.67 |
| DPPH IC50 (μg/mL) | |||
|---|---|---|---|
| Root Extract | Leaf Extract | ||
| Hexane | >2000 | >2000 | |
| Chloroform | 338.07 ± 3.32 b | 679.43 ± 4.72 a,A | |
| Ethyl acetate | 70.16 ± 1.49 a | 644.90 ± 20.09 a,A | |
| Methanol | 421.73 ± 5.40 c | 716.37 ± 16.46 a,B | |
| Ethyl Acetate Root Fraction | |||
| F1 | >2000 | F5 | 85.28 ± 1.13 a |
| F2 | 1786 ± 14.73 e | F6 | 76.71 ± 0.29 a |
| F3 | 169.63 ± 4.89 b | F7 | 235.63 ± 11.75 c |
| F4 | 101.53 ± 1.47 a | F8 | 1157.33 ± 22.50 d |
| Standards | |||
| Ascorbic acid | 16.99 ± 1.22 | α-tocopherol | 10.49 ± 0.98 |
| RT (m/z) | Component Name | Formula | Observed Neutral Mass (mDa) | Mass Error (mDa) | Observed m/z (ppm) | Mass Error (ppm) | Response | Adducts | Total Fragment Found |
|---|---|---|---|---|---|---|---|---|---|
| Flavonoids | |||||||||
| 13.34 | 3,4-Dihydro-4-(4′-hydroxyphenyl)-5,7-dihydroxycoumarin | C15H12O5 | 272.0685 | 0.1 | 271.0613 | 0.3 | 207136 | [M − H]− | 9 |
| 13.81 | Sternbin | C16H14O6 | 302.0792 | 0.2 | 301.0719 | 0.6 | 118792 | [M − H]− | 13 |
| 14.63 | Kaempferol | C15H10O6 | 286.0475 | −0.3 | 285.0402 | −1.0 | 2817 | [M − H]− | 6 |
| 14.63 | Quercetin | C15H10O7 | 301.0428 | 0.1 | 301.00828 | 0.4 | 29738 | [M − H]− | 3 |
| 14.64 | Kuchecarpins C | C17H16O7 | 332.0901 | 0.5 | 331.0828 | 1.5 | 1483888 | [M − H]− | 62 |
| 16.54 | 8-C-Prenyl kaempferol | C20H18O6 | 354.1107 | 0.1 | 353.1034 | 1.0 | 104746 | [M − H]− | 46 |
| 16.54 | Kuwanon L | C35H30O11 | 626.1796 | 0.8 | 625.1723 | 1.3 | 22479 | [M − H]− | 67 |
| Quinones | |||||||||
| 14.10 | Fallacinol | C16H12O6 | 300.0637 | 0.3 | 299.0564 | 0.9 | 1026234 | [M − H]− | 9 |
| 15.24 | Alizarin | C14H8O4 | 240.0421 | −0.1 | 239.0349 | −0.5 | 5774 | [M − H]− | 1 |
| 15.24 | Purpurin | C14H8O5 | 256.0372 | 1.0 | 255.0299 | 0.1 | 27010 | [M − H]− | 2 |
| 15.24 | Rhein | C15H8O6 | 284.0325 | 0.4 | 283.0252 | 1.3 | 925958 | [M − H]− | 8 |
| 16.54 | Denbinobin | C16H12O5 | 284.0686 | 0.1 | 283.0613 | 0.4 | 3957 | [M − H]− | 10 |
| Coumarins | |||||||||
| 8.14 | 5,7-Dihydroxychromone | C9H6O4 | 178.0267 | 0.1 | 177.0195 | 0.8 | 75787 | [M − H]− | 4 |
| 16.08 | Wedelolacetone | C15H12O5 | 314.0428 | 0.2 | 313.0356 | 0.6 | 232941 | [M − H]− | 8 |
| Phenolic acids | |||||||||
| 15.92 | Sanleng acid | C18H34O5 | 330.2408 | 0.2 | 329.2335 | 0.5 | 683016 | [M − H]− | 17 |
| Parameter | Condition | |||
|---|---|---|---|---|
| Ultra-Performance Liquid Chromatography (UPLC) | ACQUITY UPLC HSS T3 | |||
| Column | 100 mm × 2.1 mm × 1.8 μm, Waters | |||
| Column Temperature | 40 °C | |||
| Flow Rate | 0.6 mL/min | |||
| A (water + 0.1% formic acid) | ||||
| B (acetonitrile + 0.1% formic acid) | ||||
| Mobile Phase | Time | A (%) | B (%) | |
| 0 | 99 | 1 | ||
| 5 | 99 | 1 | ||
| 16 | 65 | 35 | ||
| 18 | 0 | 100 | ||
| 20 | 99 | 1 | ||
| Injection Volume | 1 μL | |||
| Metabolite Eluted | Vion IMS HDMS QTOF (Waters), positive and negative | |||
| Ion source | capillary voltage (kV) | 1.5 | ||
| reference capillary voltage (kV) | 3 | |||
| Collision energies | low-energy (eV) | 4 | ||
| high-energy (eV) | 10–40 | |||
| Scan range (Da) | 50–1500 | |||
| Scan time (s) | 0.1 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.J.; Saiful Yazan, L.; Kassim, N.K.; Che Abdullah, C.A.; Esa, N.; Lim, P.C.; Tan, D.C. Cytotoxic Activity of Christia vespertilionis Root and Leaf Extracts and Fractions against Breast Cancer Cell Lines. Molecules 2020, 25, 2610. https://doi.org/10.3390/molecules25112610
Lee JJ, Saiful Yazan L, Kassim NK, Che Abdullah CA, Esa N, Lim PC, Tan DC. Cytotoxic Activity of Christia vespertilionis Root and Leaf Extracts and Fractions against Breast Cancer Cell Lines. Molecules. 2020; 25(11):2610. https://doi.org/10.3390/molecules25112610
Chicago/Turabian StyleLee, Joanna Jinling, Latifah Saiful Yazan, Nur Kartinee Kassim, Che Azurahanim Che Abdullah, Nurulaidah Esa, Pei Cee Lim, and Dai Chuan Tan. 2020. "Cytotoxic Activity of Christia vespertilionis Root and Leaf Extracts and Fractions against Breast Cancer Cell Lines" Molecules 25, no. 11: 2610. https://doi.org/10.3390/molecules25112610
APA StyleLee, J. J., Saiful Yazan, L., Kassim, N. K., Che Abdullah, C. A., Esa, N., Lim, P. C., & Tan, D. C. (2020). Cytotoxic Activity of Christia vespertilionis Root and Leaf Extracts and Fractions against Breast Cancer Cell Lines. Molecules, 25(11), 2610. https://doi.org/10.3390/molecules25112610






