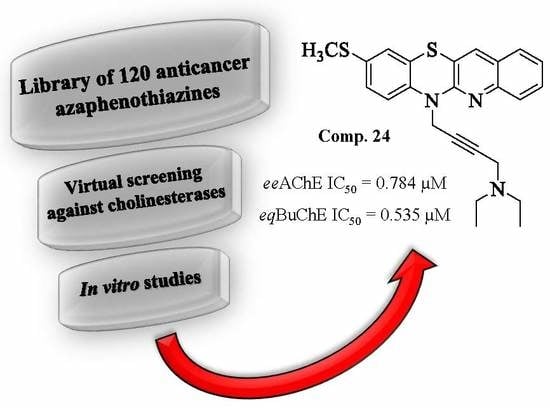
Dual Action of Dipyridothiazine and Quinobenzothiazine Derivatives—Anticancer and Cholinesterase-Inhibiting Activity
Abstract
1. Introduction
2. Results and Discussion
3. Methods
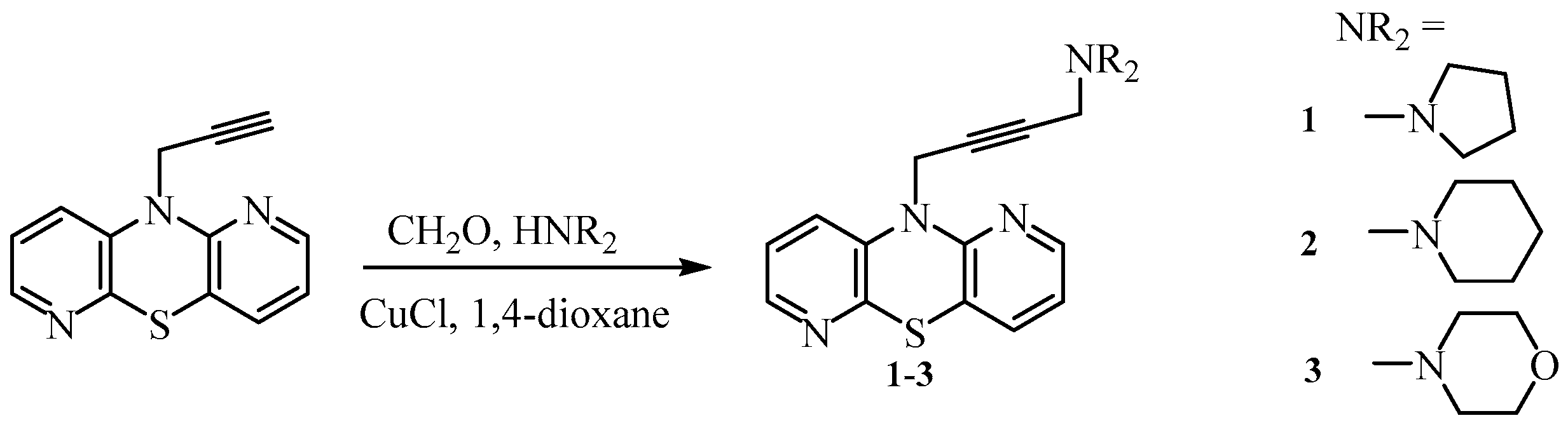
3.1. Chemistry
3.1.1. 10-(4-Pyrrolidin-1-yl-but-2-ynyl)-1,6-diazaphenothiazine (1)
3.1.2. 10-(4-Piperidin-1-yl-but-2-ynyl)- 1,6-diazaphenothiazine (2)
3.1.3. 10-(4-Morpholin-4-yl-but-2-ynyl)-1,6-diazaphenothiazine (3)
3.2. Molecular Modeling
3.3. Biological Tests
3.3.1. AChE/BuChE-Inhibitory Activity
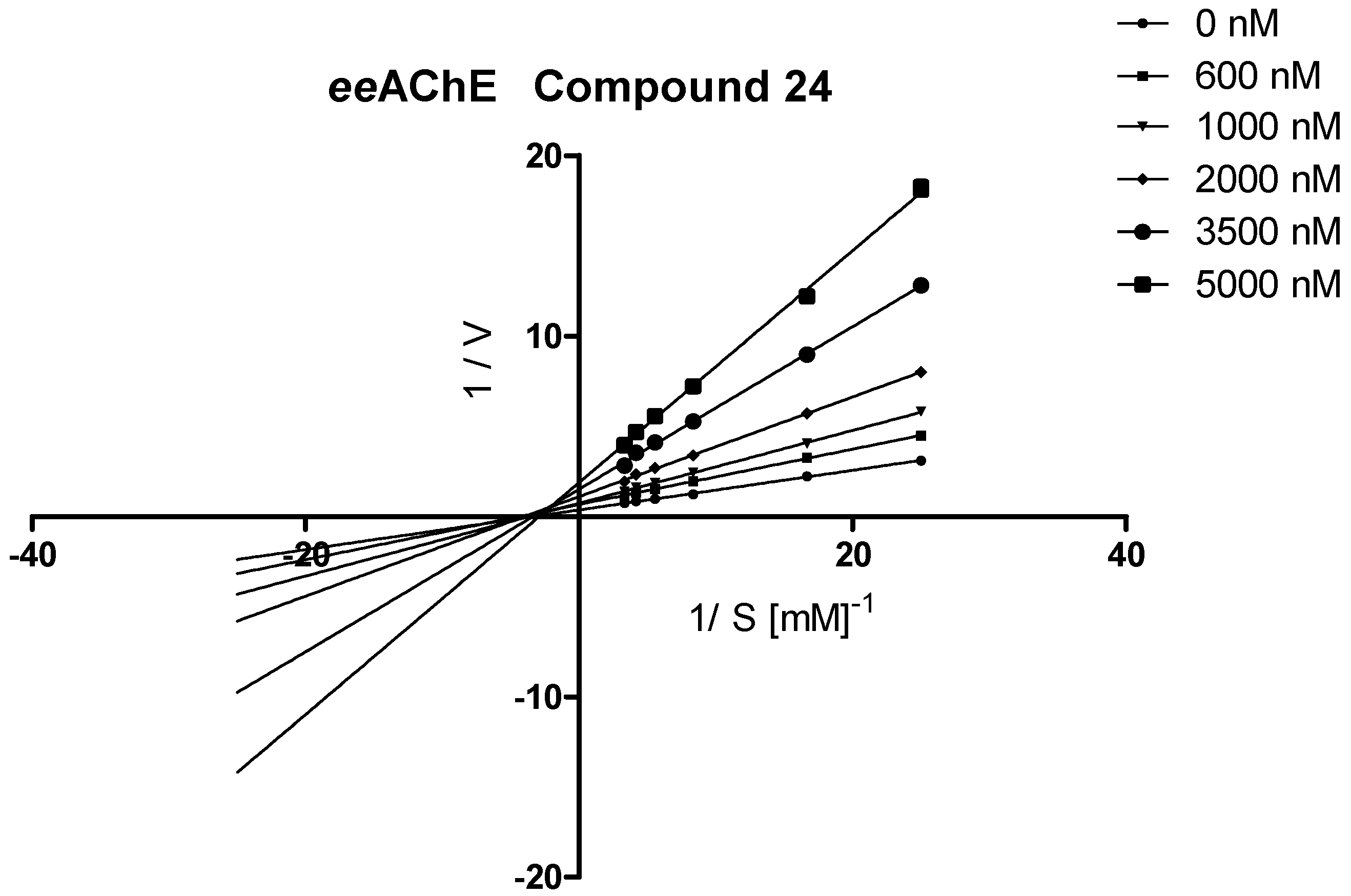
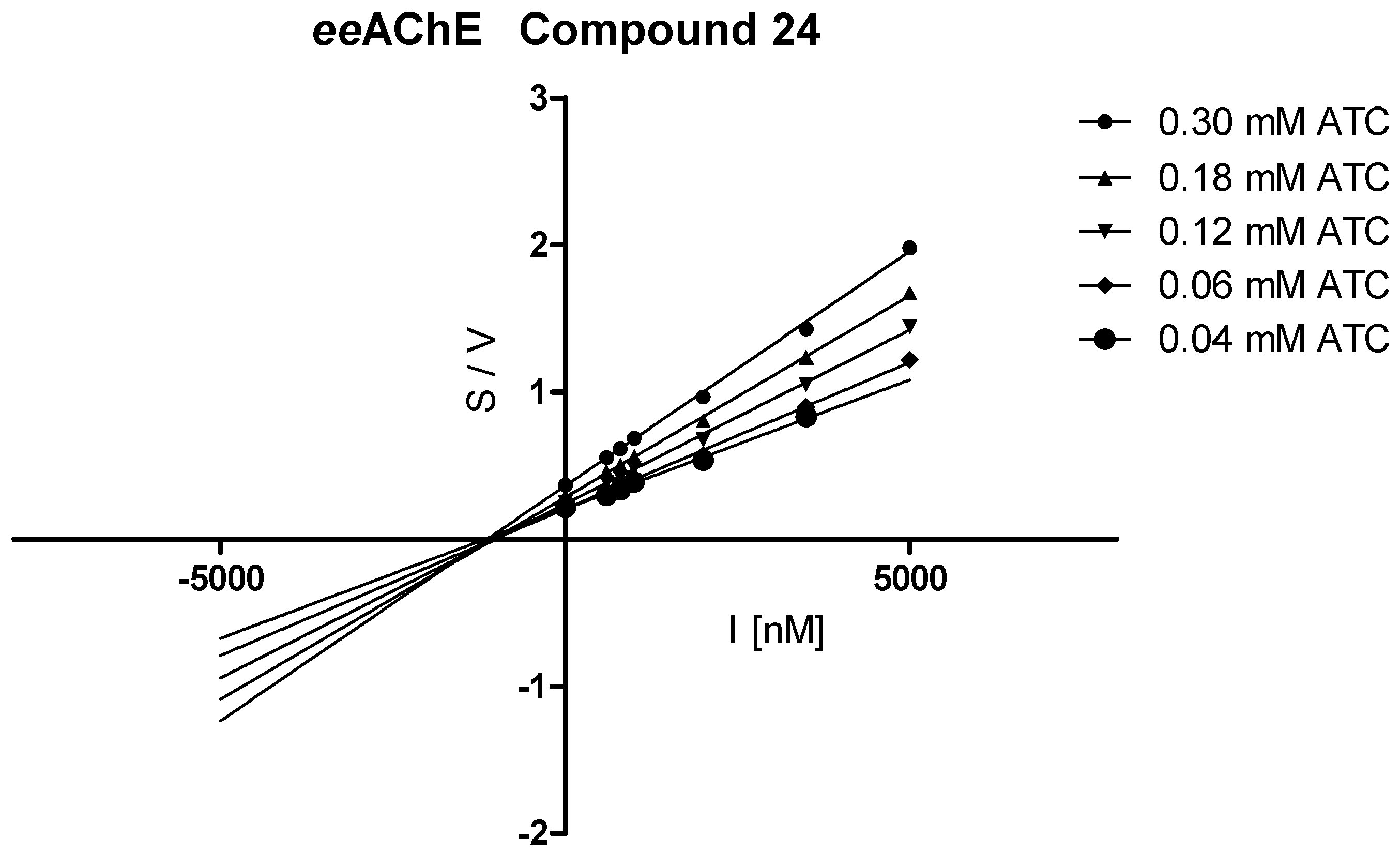
3.3.2. Kinetic Studies
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Driver, J.A.; Beiser, A.; Au, R.; Kreger, B.E.; Splansky, G.L.; Kurth, T.; Kiel, D.P.; Lu, K.P.; Seshadri, S.; Wolf, P.A. Inverse association between cancer and Alzheimer’s disease: Results from the Framingham Heart Study. BMJ 2012, 344, 19. [Google Scholar] [CrossRef] [PubMed]
- Catalá-López, F.; Crespo-Facorro, B.; Vieta, E.; Valderas, J.M.; Valencia, A.; Tabarés-Seisdedos, R. Alzheimer’s Disease and Cancer: Current Epidemiological Evidence for a Mutual Protection. Neuroepidemiology 2014, 42, 121–122. [Google Scholar] [CrossRef] [PubMed]
- Driver, J.A. Inverse association between cancer and neurodegenerative disease: Review of the epidemiologic and biological evidence. Biogerontology 2014, 15, 547–557. [Google Scholar] [CrossRef] [PubMed]
- White, R.S.; Lipton, R.B.; Hall, C.B.; Steinerman, J.R. Nonmelanoma skin cancer is associated with reduced Alzheimer disease risk. Neurology 2013, 80, 1966–1972. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.; Ahlberg, F.M.; Berk, M.; Ashley, D.M.; Khasraw, M. Emerging pharmacotherapy for cancer patients with cognitive dysfunction. BMC Neurol. 2013, 13, 153. [Google Scholar] [CrossRef] [PubMed]
- Aliev, G.; Obrenovich, M.E.; Tabrez, S.; Jabir, N.R.; Reddy, V.P.; Li, Y.; Burnstock, G.; Cacabelos, R.; Kamal, M.A. Link between cancer and Alzheimer disease via oxidative stress induced by nitric oxide-dependent mitochondrial DNA overproliferation and deletion. Oxid. Med. Cell. Longev. 2013, 2013, 1–19. [Google Scholar] [CrossRef]
- Demetrius, L.A.; Simon, D.K. The inverse association of cancer and Alzheimer’s: A bioenergetic mechanism. J. R. Soc. Interface 2013, 10, 20130006. [Google Scholar] [CrossRef]
- Roe, C.M.; Behrens, M.I.; Xiong, C.; Miller, J.P.; Morris, J.C. Alzheimer disease and cancer. Neurology 2005, 64, 895–898. [Google Scholar] [CrossRef]
- Ganguli, M. A reduced risk of Alzheimer’s disease in those who survive cancer. BMJ 2012, 344, 9. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, O. Death from Alzheimer’s disease among cancer survivors: A population-based study. Curr. Med. Res. Opin. 2020, 1–7. [Google Scholar] [CrossRef]
- Majd, S.; Power, J.; Majd, Z. Alzheimer’s Disease and Cancer: When Two Monsters Cannot Be Together. Front. Neurosci. 2019, 13. [Google Scholar] [CrossRef] [PubMed]
- Okereke, O.I.; Meadows, M.E. More Evidence of an Inverse Association between Cancer and Alzheimer Disease. JAMA Netw. Open 2019, 2, e196167. [Google Scholar] [CrossRef] [PubMed]
- Mezencev, R.; Chernoff, Y.O. Risk of Alzheimer’s Disease in Cancer Patients: Analysis of Mortality Data from the US SEER Population-Based Registries. Cancers (Basel) 2020, 12, 796. [Google Scholar] [CrossRef] [PubMed]
- Sherzai, A.Z.; Parasram, M.; Haider, J.M.; Sherzai, D. Alzheimer Disease and Cancer: A National Inpatient Sample Analysis. Alzheimer Dis. Assoc. Disord. 2020. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.; Maloney, A.J.F. Selective loss of central cholinergic neurons in Alzheimer’s Disease. Lancet 1976, 308, 1403. [Google Scholar] [CrossRef]
- Mesulam, M.; Guillozet, A.; Shaw, P.; Quinn, B. Widely spread butyrylcholinesterase can hydrolyze acetylcholine in the normal and Alzheimer brain. Neurobiol. Dis. 2002, 9, 88–93. [Google Scholar] [CrossRef]
- Darvesh, S.; Hopkins, D.A.; Geula, C. Neurobiology of butyrylcholinesterase. Nat. Rev. Neurosci. 2003, 4, 131–138. [Google Scholar] [CrossRef]
- Paraoanu, L.E.; Layer, P.G. Acetylcholinesterase in cell adhesion, neurite growth and network formation. FEBS J. 2008, 275, 618–624. [Google Scholar] [CrossRef]
- Xi, H.-J.; Wu, R.-P.; Liu, J.-J.; Zhang, L.-J.; Li, Z.-S. Role of acetylcholinesterase in lung cancer. Thorac. Cancer 2015, 6, 390–398. [Google Scholar] [CrossRef]
- Reid, G.A.; Chilukuri, N.; Darvesh, S. Butyrylcholinesterase and the cholinergic system. Neuroscience 2013, 234, 53–68. [Google Scholar] [CrossRef]
- Dinamarca, M.C.; Sagal, J.P.; Quintanilla, R.A.; Godoy, J.A.; Arrzola, M.S.; Inestrosa, N.C. Amyloid-β-Acetylcholinesterase complexes potentiate neurodegenerative changes induced by the A peptide. Implications for the pathogenesis of Alzheimer’s disease. Mol. Neurodegener. 2010, 5, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Soreq, H. Acetylcholinesterase—New roles for an old actor. Nat. Rev. Neurosci. 2001, 2, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Toiber, D.; Berson, A.; Greenberg, D.; Melamed-Book, N.; Diamant, S.; Soreq, H. N-acetylcholinesterase-induced apoptosis in Alzheimer’s disease. PLoS ONE 2008, 3, e3108. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ratés, S.; Greenfield, S. Cancer and neurodegeneration: Two sides, same coin? Oncotarget 2017, 8, 22307–22308. [Google Scholar] [CrossRef] [PubMed]
- Hyatt, J.L.; Tsurkan, L.; Morton, C.L.; Yoon, K.J.P.; Harel, M.; Brumshtein, B.; Silman, I.; Sussman, J.L.; Wadkins, R.M.; Potter, P.M. Inhibition of acetylcholinesterase by the anticancer prodrug CPT-11. Chem. Biol. Interact. 2005, 157, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Lin, J.; Xiang, S.; Zhao, K.; Yu, J.; Zheng, J.; Xu, D.; Mak, S.; Hu, S.; Nirasha, S.; et al. Sunitinib, a Clinically Used Anticancer Drug, Is a Potent AChE Inhibitor and Attenuates Cognitive Impairments in Mice. ACS Chem. Neurosci. 2016, 7, 1047–1056. [Google Scholar] [CrossRef] [PubMed]
- Roldán-Peña, J.M.; Alejandre-Ramos, D.; López, Ó.; Maya, I.; Lagunes, I.; Padrón, J.M.; Peña-Altamira, L.E.; Bartolini, M.; Monti, B.; Bolognesi, M.L. New tacrine dimers with antioxidant linkers as dual drugs: Anti-Alzheimer’s and antiproliferative agents. Eur. J. Med. Chem. 2017, 138, 761–773. [Google Scholar] [CrossRef]
- Lodarski, K.; Jończyk, J.; Guzior, N.; Bajda, M.; Gładysz, J.; Walczyk, J.; Jeleń, M.; Morak-Młodawska, B.; Pluta, K.; Malawska, B. Discovery of butyrylcholinesterase inhibitors among derivatives of azaphenothiazines. J. Enzyme Inhib. Med. Chem. 2015, 30, 98–106. [Google Scholar] [CrossRef][Green Version]
- Ki, Y.S.; Park, E.Y.; Lee, H.-W.; Oh, M.S.; Cho, Y.-W.; Kwon, Y.K.; Moon, J.H.; Lee, K.-T. Donepezil, a Potent Acetylcholinesterase Inhibitor, Induces Caspase-Dependent Apoptosis in Human Promyelocytic Leukemia HL-60 Cells. Biol. Pharm. Bull. 2010, 33, 1054–1059. [Google Scholar] [CrossRef]
- Jeleń, M.; Pluta, K.; Zimecki, M.; Morak-Młodawska, B.; Artym, J.; Kocięba, M.; Kochanowska, I. Synthesis and biological evaluation of novel propargylquinobenzothiazines and their derivatives as potential antiproliferative, anti-inflammatory, and anticancer agents. J. Enzyme Inhib. Med. Chem. 2016, 31, 83–88. [Google Scholar] [CrossRef]
- Jeleń, M.; Pluta, K.; Zimecki, M.; Morak-Młodawska, B.; Artym, J.; Kocięba, M. 6-substituted 9-fluoroquino [3,2-b]benzo[1,4]thiazines display strong antiproliferative and antitumor properties. Eur. J. Med. Chem. 2015, 89, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Morak-Młodawska, B.; Pluta, K.; Latocha, M.; Jeleń, M.; Kuśmierz, D. Synthesis, Anticancer Activity, and Apoptosis Induction of Novel 3,6-Diazaphenothiazines. Molecules 2019, 24, 267. [Google Scholar] [CrossRef] [PubMed]
- Morak-Młodawska, B.; Pluta, K.; Latocha, M.; Jeleń, M. Synthesis, spectroscopic characterization, and anticancer activity of new 10-substituted 1,6-diazaphenothiazines. Med. Chem. Res. 2016, 25, 2425–2433. [Google Scholar] [CrossRef] [PubMed]
- Morak-Młodawska, B.; Pluta, K.; Zimecki, M.; Jeleń, M.; Artym, J.; Kocięba, M. Synthesis and selected immunological properties of 10-substituted 1,8-diazaphenothiazines. Med. Chem. Res. 2015, 24, 1408–1418. [Google Scholar] [CrossRef]
- Morak-Młodawska, B.; Pluta, K.; Latocha, M.; Jeleń, M.; Kuśmierz, D. Synthesis and anticancer and lipophilic properties of 10-dialkylaminobutynyl derivatives of 1,8- and 2,7-diazaphenothiazines. J. Enzyme Inhib. Med. Chem. 2016, 31, 1132–1138. [Google Scholar] [CrossRef]
- Morak-Młodawska, B.; Pluta, K.; Latocha, M.; Suwińska, K.; Jeleń, M.; Kuśmierz, D. 3,6-Diazaphenothiazines as potential lead molecules–synthesis, characterization and anticancer activity. J. Enzyme Inhib. Med. Chem. 2016, 31, 1512–1519. [Google Scholar] [CrossRef]
- Jeleń, M.; Pluta, K.; Zimecki, M.; Morak-Młodawska, B.; Artym, J.; Kociȩba, M. Synthesis and selected immunological properties of substituted quino[3,2-b]benzo[1,4]thiazines. Eur. J. Med. Chem. 2013, 63, 444–456. [Google Scholar] [CrossRef]
- Bajda, M.; Więckowska, A.; Hebda, M.; Guzior, N.; Sotriffer, C.; Malawska, B. Structure-Based Search for New Inhibitors of Cholinesterases. Int. J. Mol. Sci. 2013, 14, 5608–5632. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Jelen, M.; Pluta, K. Synthesis of quinobenzo-1, 4-thiazines from diquino-1,4-dithiin and 2,2′-dichloro-3-3′-diquinolinyl disulfide. Heterocycles 2009, 78, 2325–2336. [Google Scholar] [CrossRef]
- Online Demo-Fast 3D Structure Generation with CORINA Classic. Available online: https://www.mn-am.com/online_demos/corina_demo (accessed on 23 April 2020).
- SYBYL-X Suite, Version SYBYL-X 1.1; Tripos Associates Inc.: St. Louis, MO, USA, 2010.
- Gold 5.1; The Cambridge Crystallographic Data Centre: Cambridge, UK, 2011.
- Pymol 0.99rc6; Delano Scientific LLC: Palo Alto, CA, USA, 2006.
- Lazarevic-Pasti, T.; Leskovac, A.; Momic, T.; Petrovic, S.; Vasic, V. Modulators of Acetylcholinesterase Activity: From Alzheimer’s Disease to Anti-Cancer Drugs. Curr. Med. Chem. 2017, 24. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
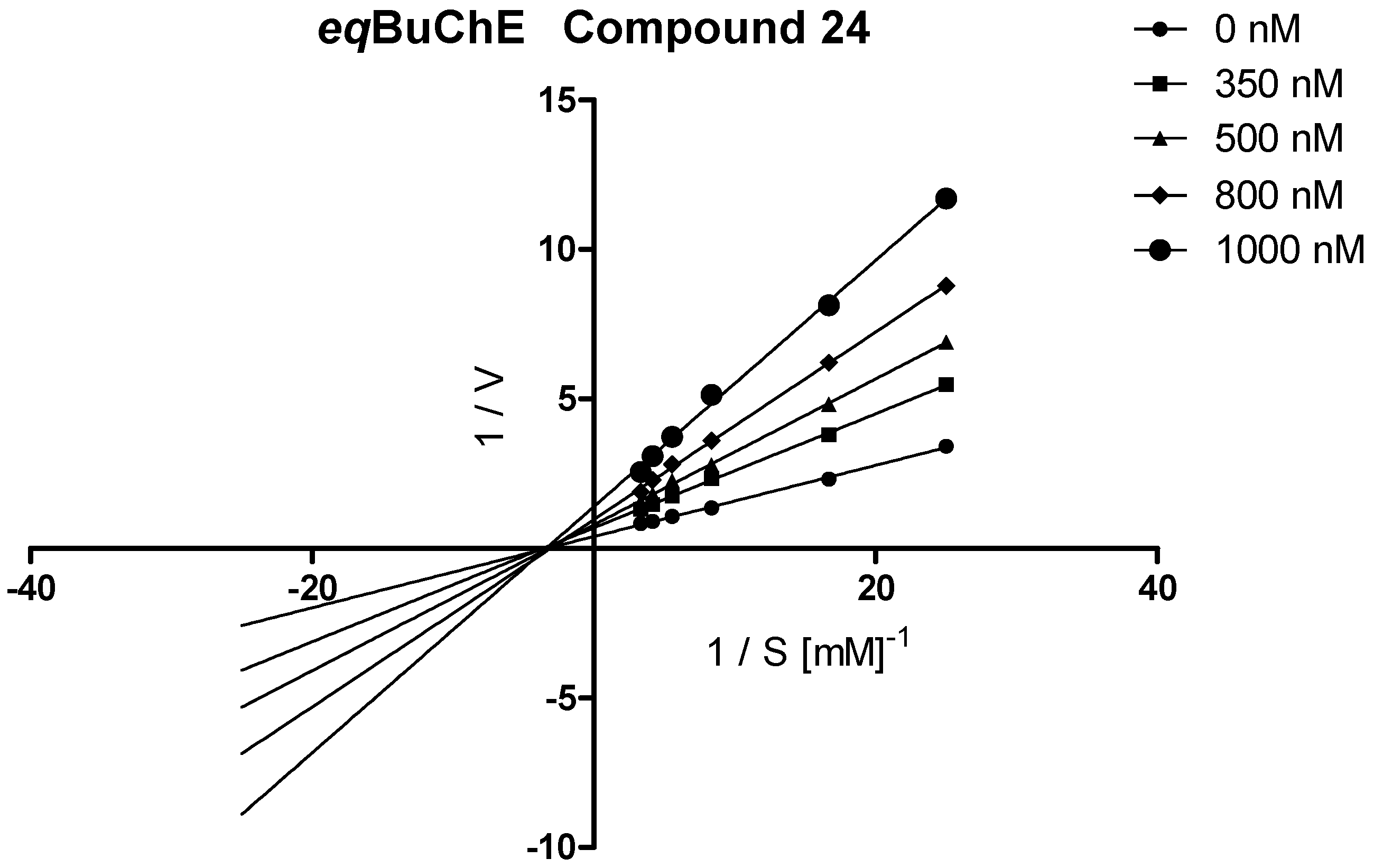
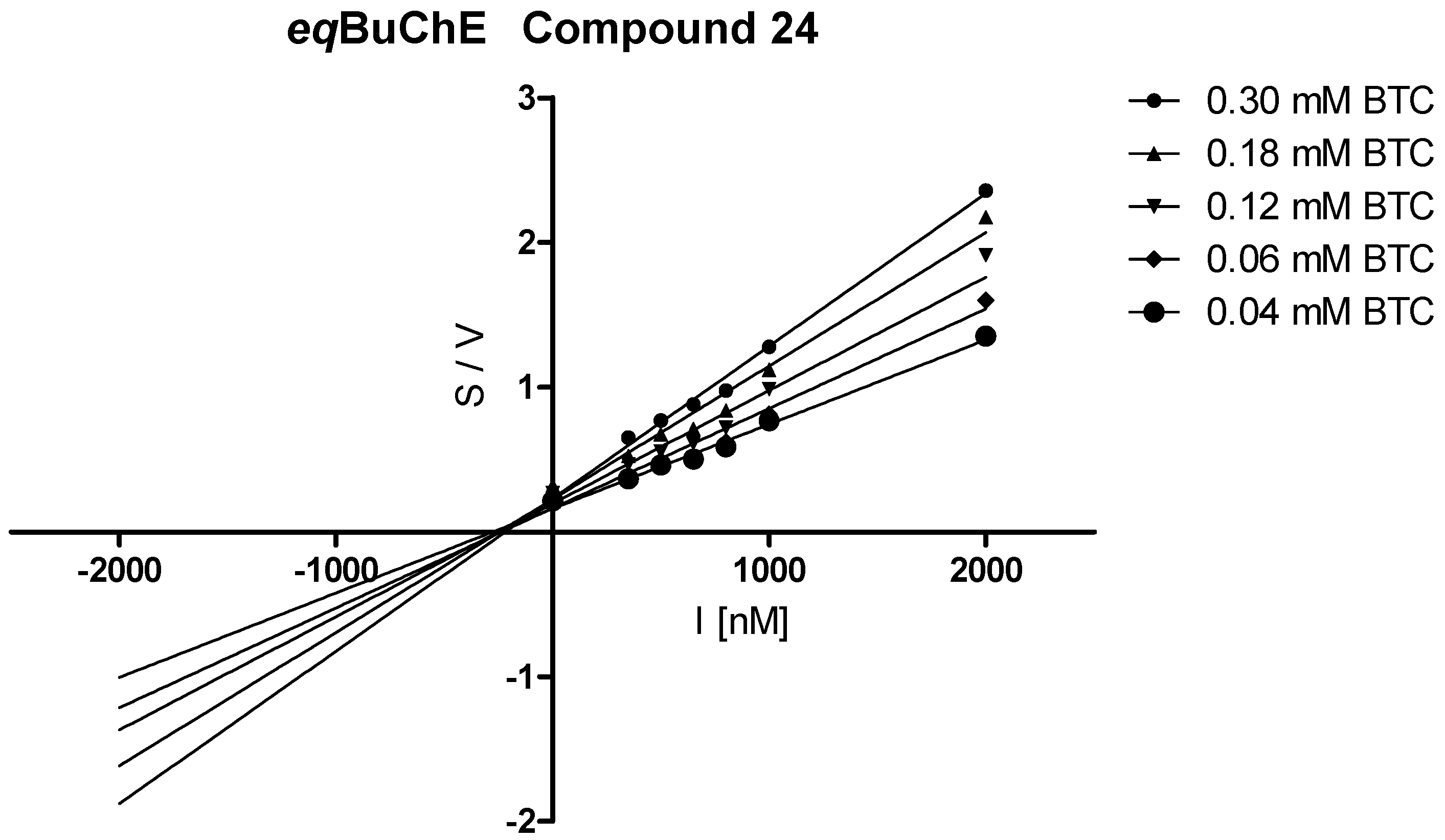
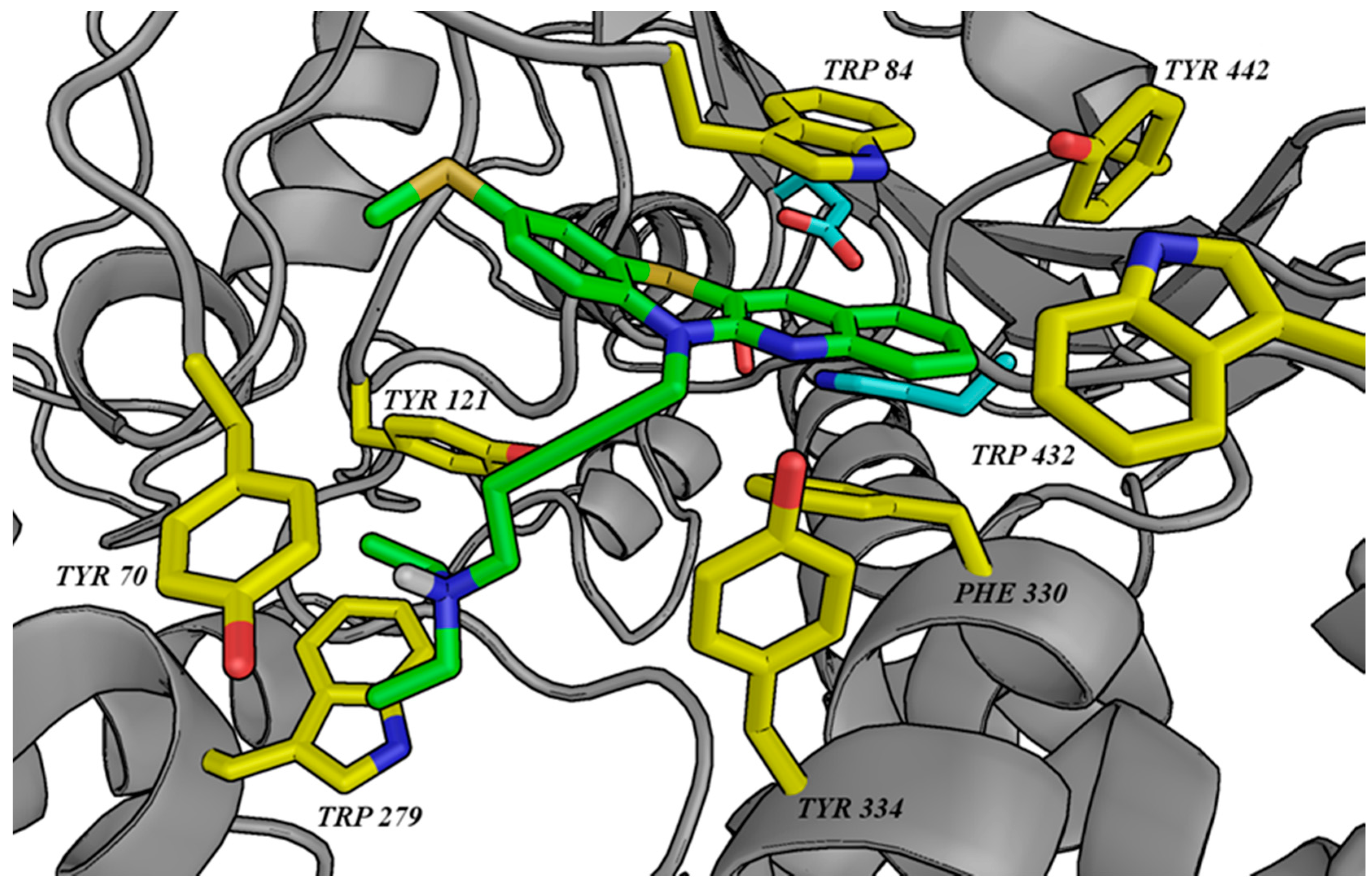
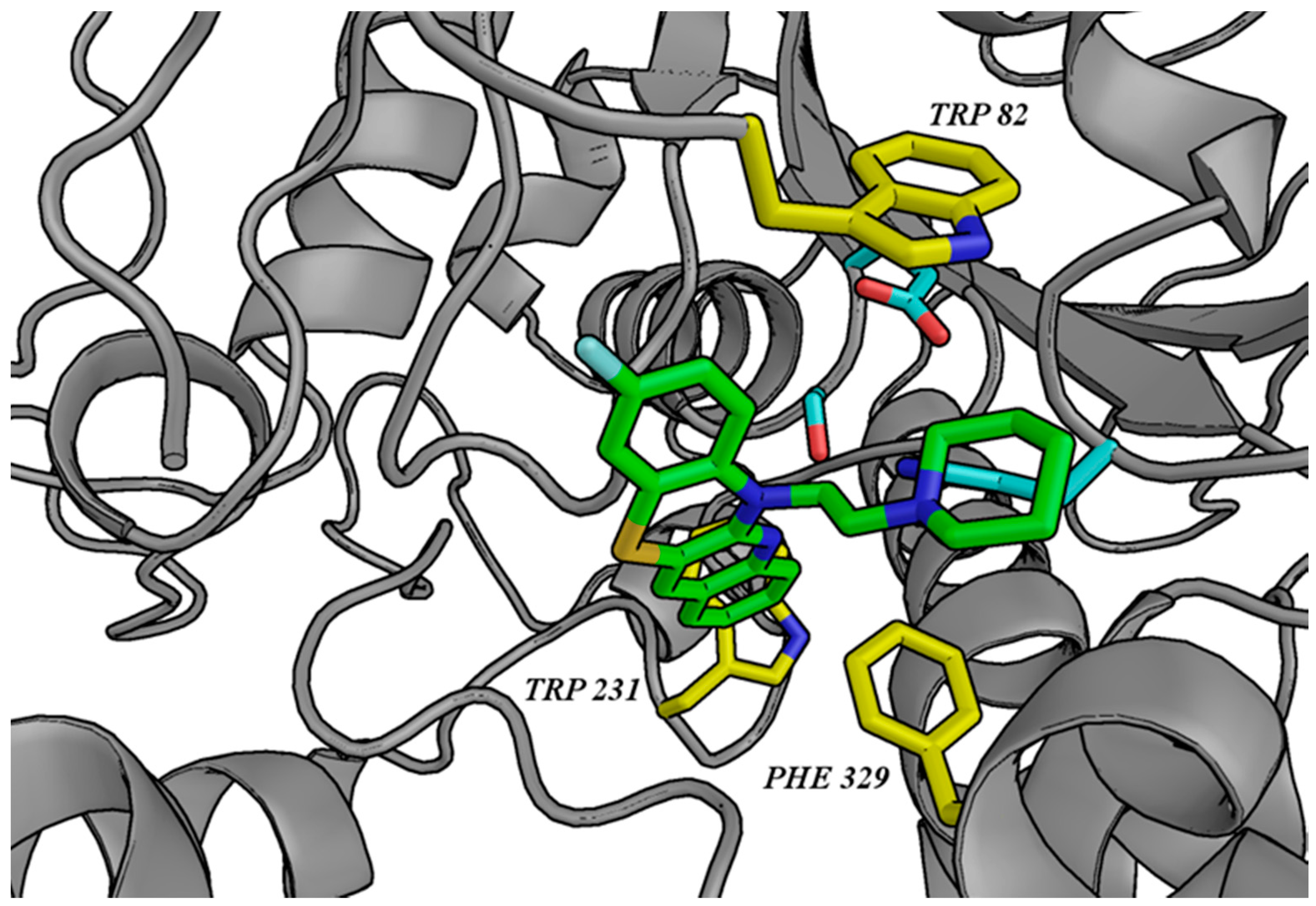
| Compound | R | % Inhibition +/− Ϭ a | IC50 eeAChE b [µM] +/− SEM c | % Inhibition +/− Ϭ | IC50 eqBuChE d [µM] +/− SEM | |
|---|---|---|---|---|---|---|
| Dipyridothiazines | ||||||
isomer 1,6-diaza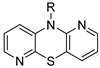 | 1 | 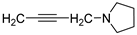 | 45.4 ± 9.8 | — | 65.4 ± 3.0 | 5.748 ± 0.164 |
| 2 | 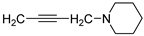 | 60.3 ± 8.8 | 8.309 ± 0.315 | 56.6 ± 2.4 | 8.942 ± 0.239 | |
| 3 | 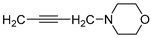 | 13.7 ± 1.5 | — | 63.6 ± 2.4 | 9.250 ± 0.275 | |
| 4 | 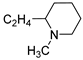 | 22.4 ± 1.1 | — | 57.0 ± 7.4 | 9.493 ± 0.330 | |
| 5 | 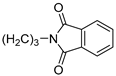 | 7.3 ± 0.9 | — | 52.5 ± 5.9 | 10.080 ± 0.441 | |
isomer 1,8-diaza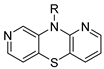 | 6 | (CH2)3N(CH3)2 | 20.4 ± 3.6 | — | 85.8 ± 0.5 | 0.865 ± 0.011 |
| 7 | 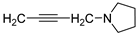 | 57.3 ± 0.4 | 10.020 ± 0.604 | 91.1 ± 0.8 | 0.929 ± 0.016 | |
| 8 | 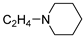 | 33.5 ± 1.6 | — | 66.0 ± 3.4 | 4.442 ± 0.049 | |
| 9 | 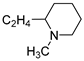 | 58.1 ± 4.1 | 6.546 ± 0.177 | 76.4 ± 0.2 | 3.034 ± 0.072 | |
| 10 | 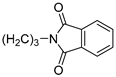 | 49.6 ± 1.3 | 9.410 ± 0.489 | 71.9 ± 4.3 | 3.666 ± 0.054 | |
isomer 2,7-diaza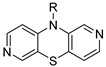 | 11 | 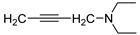 | 20.1 ± 1.4 | — | 20.7 ± 0.9 | — |
| 12 | 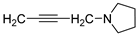 | 20.3 ± 1.0 | — | 31.4 ± 1.7 | — | |
| 13 | 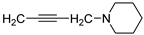 | 51.6 ± 3.2 | 11.330 ± 0.464 | 37.6 ± 1.6 | — | |
isomer 3,6-diaza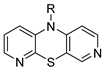 | 14 | 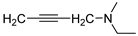 | 7.7 ± 2.1 | — | 20.4 ± 1.9 | — |
| 15 | 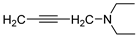 | 10.1 ± 3.1 | — | 17.2 ± 7.3 | — | |
| 16 | 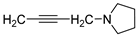 | 21.9 ± 5.7 | — | 58.5 ± 9.0 | 10.380 ± 0.308 | |
| 17 | 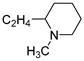 | 33.0 ± 4.5 | — | 16.1 ± 0.7 | — | |
| 18 | 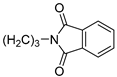 | 72.2 ± 0.7 | 4.263 ± 0.078 | 30.5 ± 5.7 | — | |
N-substituted 9-fluoroquinobenzothiazines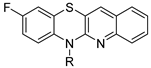 | ||||||
| 19 | H | 6.6 ± 1.3 | — | 81.0 ± 1.4 | 1.020 ± 0.028 | |
| 20 | CH3 | 3.0 ± 1.6 | — | 35.3 ± 12.3 | — | |
| 21 | (CH2)3NH2 | 6.5 ± 2.8 | — | 76.3 ± 1.2 | 2.511 ± 0.047 | |
| 22 | (CH2)4NH2 | 2.3 ± 2.1 | — | 64.7 ± 1.0 | 3.929 ± 0.196 | |
| 23 | 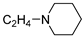 | 27.0 ± 2.8 | — | 97.7 ± 0.5 | 0.463 ± 0.010 | |
N-substituted 9-methylthioquinobenzothiazines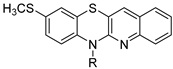 | ||||||
| 24 | 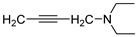 | 83.4 ± 1.1 | 0.784 ± 0.018 | 95.5 ± 0.8 | 0.535 ± 0.013 | |
| 25 | 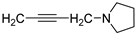 | 78.0 ± 1.4 | 2.218 ± 0.062 | 99.9 ± 0.7 | 0.506 ± 0.014 | |
| Reference | ||||||
| Tacrine | 0.024 ± 0.001 | 0.002 ± 0.0005 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jończyk, J.; Godyń, J.; Stawarska, E.; Morak-Młodawska, B.; Jeleń, M.; Pluta, K.; Malawska, B. Dual Action of Dipyridothiazine and Quinobenzothiazine Derivatives—Anticancer and Cholinesterase-Inhibiting Activity. Molecules 2020, 25, 2604. https://doi.org/10.3390/molecules25112604
Jończyk J, Godyń J, Stawarska E, Morak-Młodawska B, Jeleń M, Pluta K, Malawska B. Dual Action of Dipyridothiazine and Quinobenzothiazine Derivatives—Anticancer and Cholinesterase-Inhibiting Activity. Molecules. 2020; 25(11):2604. https://doi.org/10.3390/molecules25112604
Chicago/Turabian StyleJończyk, Jakub, Justyna Godyń, Ewelina Stawarska, Beata Morak-Młodawska, Małgorzata Jeleń, Krystian Pluta, and Barbara Malawska. 2020. "Dual Action of Dipyridothiazine and Quinobenzothiazine Derivatives—Anticancer and Cholinesterase-Inhibiting Activity" Molecules 25, no. 11: 2604. https://doi.org/10.3390/molecules25112604
APA StyleJończyk, J., Godyń, J., Stawarska, E., Morak-Młodawska, B., Jeleń, M., Pluta, K., & Malawska, B. (2020). Dual Action of Dipyridothiazine and Quinobenzothiazine Derivatives—Anticancer and Cholinesterase-Inhibiting Activity. Molecules, 25(11), 2604. https://doi.org/10.3390/molecules25112604